The Use of Cinnamon Essential Oils in Aquaculture: Antibacterial, Anesthetic, Growth-Promoting, and Antioxidant Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils
2.2. Quantification of Phytochemicals in EOs and Preparation of CCEO Nanoemulsion
2.3. Clinical Isolates
2.4. In Vitro Antibacterial Activity
2.5. Animals and Water Quality Parameters
2.6. Induction of Anesthesia and Recovery
2.7. Diet Supplementation with CCEO and Zootechnical Parameters
- Weight gain (WG) = final body weight − initial body weight
- Relative weight gain (RWG) = 100 × (final body weight − initial body weight)/initial body weight
- Specific growth rate (SGR) = 100 × (ln final weight − ln initial weight)/days of experiment
2.8. Oxidative Status Markers
2.9. Statistical Analysis
3. Results
3.1. Quantification of CZEO Compounds
3.2. In Vitro Antibacterial Activity
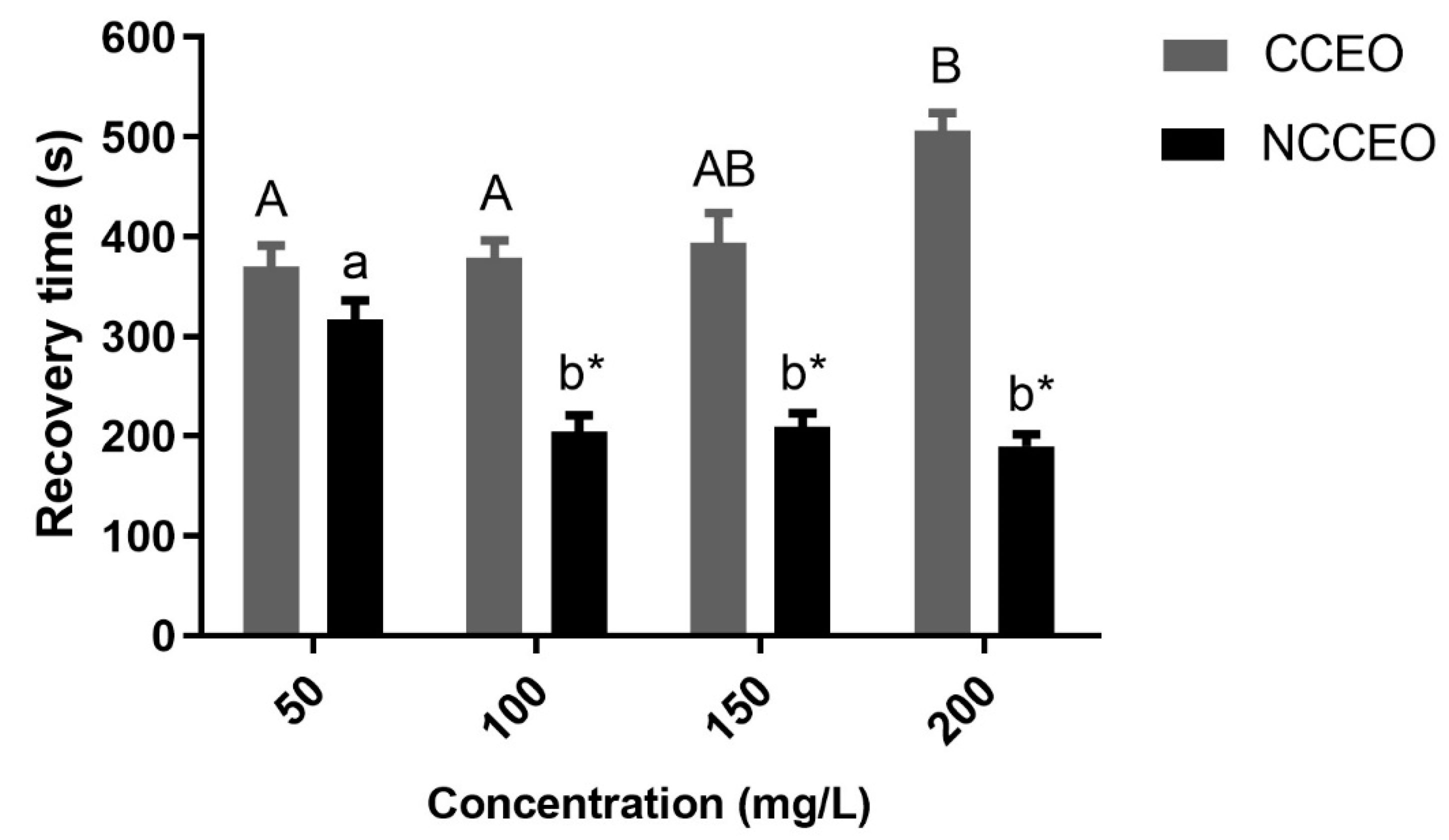
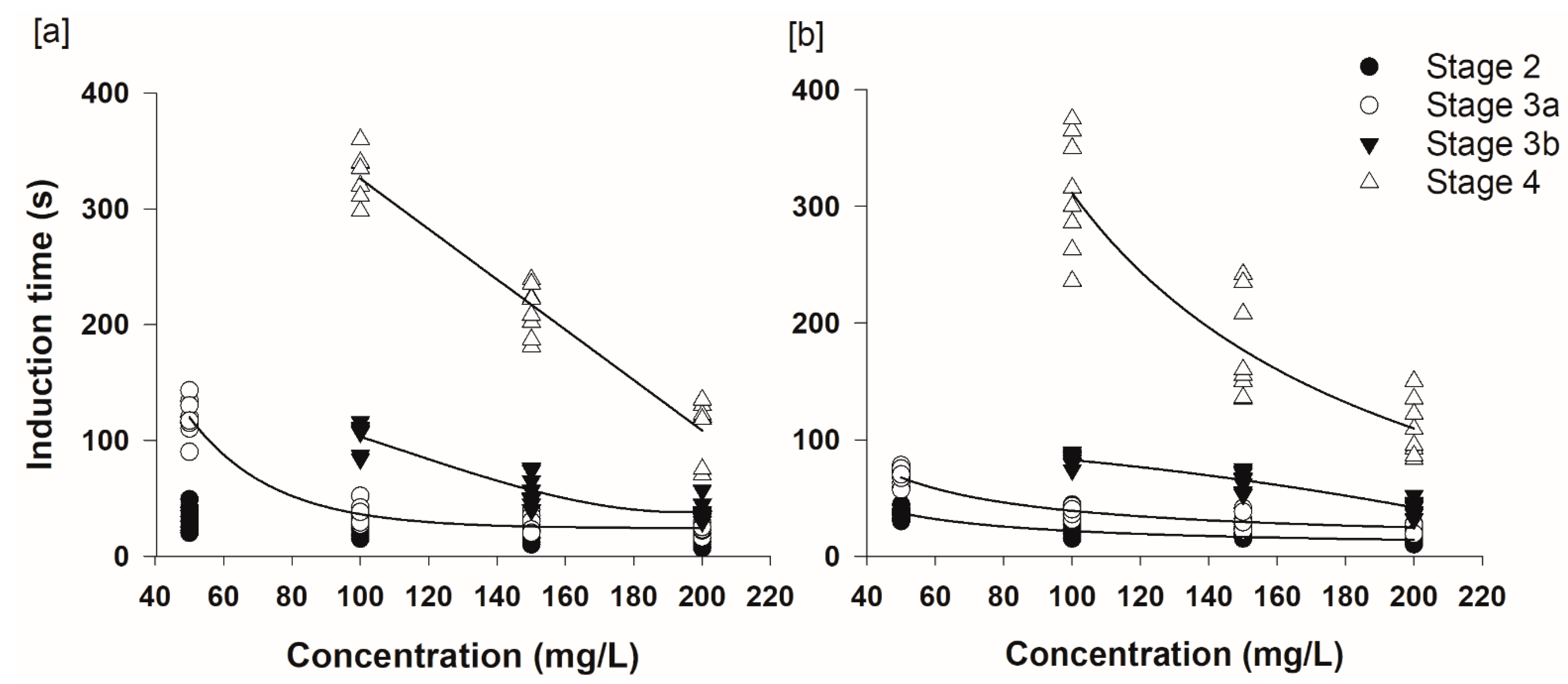
3.3. Induction of Anesthesia and Recovery
3.4. Zootechnical Parameters
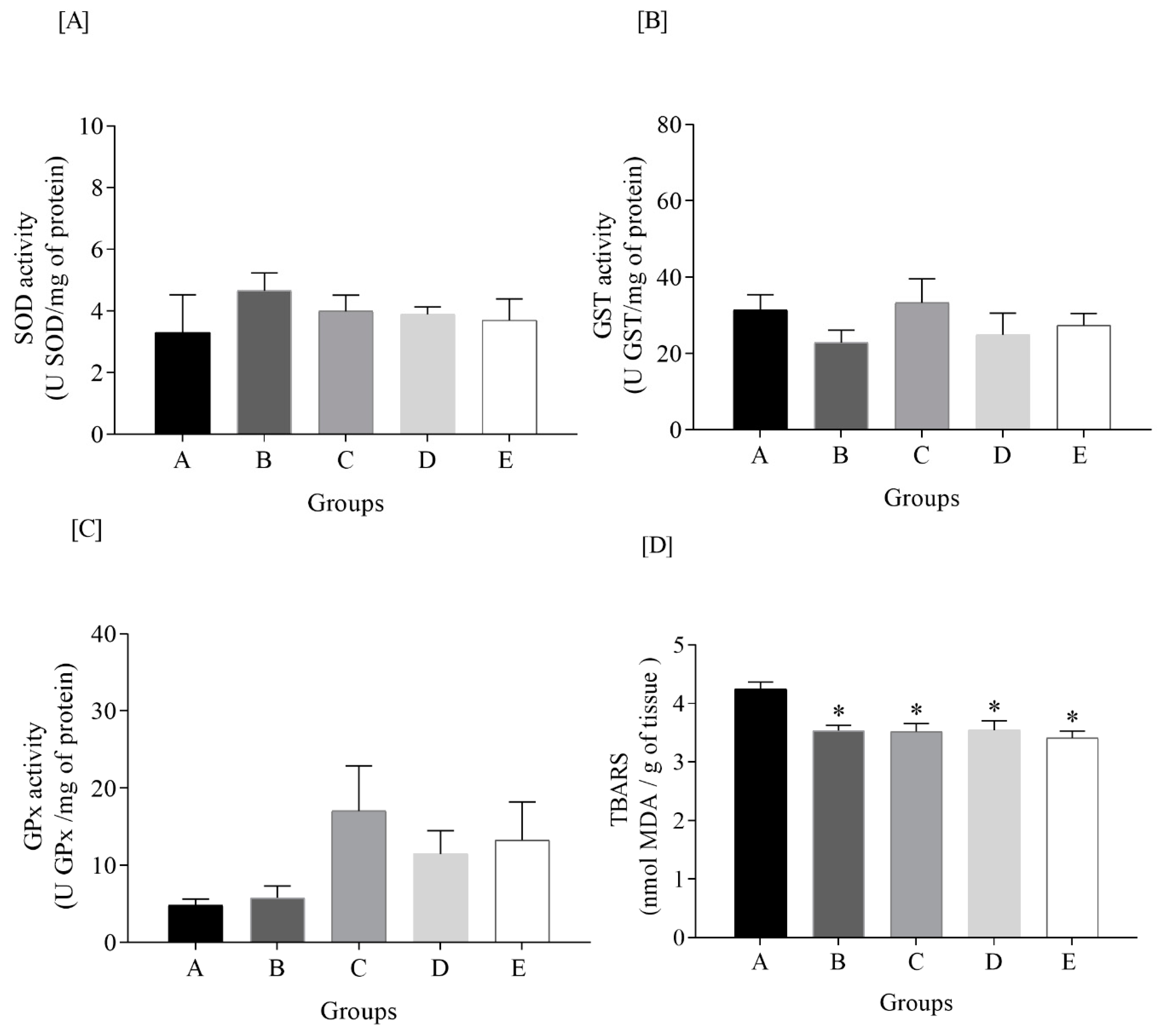
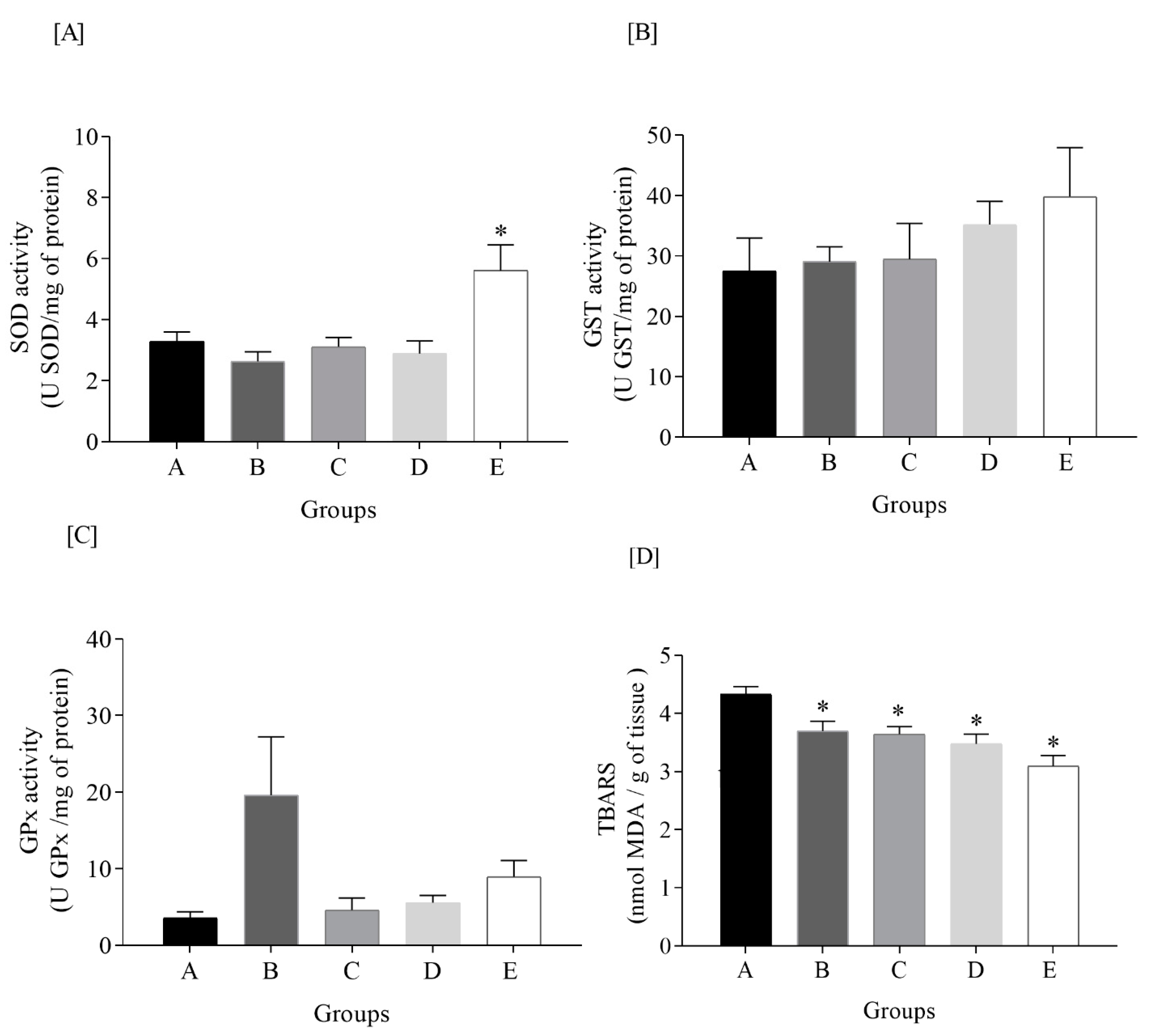
3.5. Oxidative Status Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aydın, B.; Barbas, L.A.L. Sedative and anesthetic properties of essential oils and their active compounds in fish: A review. Aquaculture 2020, 520, 734999. [Google Scholar] [CrossRef]
- Citarasu, T. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac. Int. 2010, 18, 403–414. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.A.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complementary Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fan, L.; Fan, S.; Wang, J.; Luo, T.; Tang, Y.; Yu, L. Cinnamomum cassia Presl: A review of its traditional uses, phytochemistry, pharmacology and toxicology. Molecules 2019, 24, 3473. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.; Zhou, Z.; Doan, H.V.; Davies, S.J.; Harikrishnan, R. Boosting immune function and disease bio-control through environment-friendly and sustainable approaches in finfish aquaculture: Herbal therapy scenarios. Rev. Fish. Sci. Aquac. 2020, 28, 303–321. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.T.; Ahmadifar, E.; Sewilam, H. Antiparasitic and antibacterial functionality of essential oils: An alternative approach for sustainable aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.A.; Heinzmann, B.M.; Baldisserotto, B. The effects of essential oils and their major compounds on fish bacterial pathogens—A review. J. Appl. Microbiol. 2018, 125, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Majolo, C.; Rocha, S.I.B.; Chagas, E.C.; Chaves, F.C.M.; Bizzo, H.R. Chemical composition of Lippia spp. essential oil and antimicrobial activity against Aeromonas Hydrophila. Aquac. Res. 2017, 48, 2380–2387. [Google Scholar] [CrossRef]
- Öntaş, C.; Baba, E.; Kaplaner, E.; Küçükaydin, S.; Öztürk, M.; Ercan, M.D. Antibacterial activity of Citrus limon peel essential oil and Argania spinosa oil against fish pathogenic bacteria. Kafkas Univ. Vet. Fak. Derg. 2016, 22, 741–749. [Google Scholar] [CrossRef]
- Snuossi, M.; Trabelsi, N.; Taleb, S.B.; Dehmeni, A.; Flamini, G.; De Feo, V. Laurus nobilis, Zingiber officinale and Anethum graveolens essential oils: Composition, antioxidant and antibacterial activities against bacteria isolated from fish and shellfish. Molecules 2016, 21, 1414. [Google Scholar] [CrossRef]
- Bandeira Junior, G.; Sutili, F.J.; Gressler, L.T.; Ely, V.L.; Silveira, B.P.; Tasca, C.; Baldisserotto, B. Antibacterial potential of phytochemicals alone or in combination with antimicrobials against fish pathogenic bacteria. J. Appl. Microbiol. 2018, 125, 655–665. [Google Scholar] [CrossRef]
- Ghatak, S.; Blom, J.; Das, S.; Sanjukta, R.; Puro, K.; Mawlong, M.; Ngachan, S.V. Pan-genome analysis of Aeromonas hydrophila, Aeromonas veronii and Aeromonas caviae indicates phylogenomic diversity and greater pathogenic potential for Aeromonas hydrophila. Antonie Van Leeuwenhoek 2016, 109, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Karunasagar, I.; Karunasagar, I.; Pai, R. Systemic Citrobacter freundii infection in common carp, Cyprinus carpio L., fingerlings. J. Fish Dis. 1992, 15, 95–98. [Google Scholar] [CrossRef]
- Kanki, M.; Yoda, T.; Tsukamoto, T.; Shibata, T. Klebsiella pneumoniae produces no histamine: Raoultella planticola and Raoultella omithinolytica strains are histamine producers. Appl. Environ. Microbiol. 2002, 68, 3462–3466. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.F.; Baldissera, M.D.; Baldisserotto, B.; Heinzmann, B.M.; Martos-Sitcha, J.A.; Mancera, J.M. Essential oils as stress-reducing agents for fish aquaculture: A review. Front. Physiol. 2019, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Mirghaed, A.T.; Yousefi, M. Application of herbal anaesthetics in aquaculture. Rev. Aquac. 2019, 11, 550–564. [Google Scholar] [CrossRef]
- Syahidah, A.; Saad, C.R.; Daud, H.M.; Abdelhadi, Y.M. Status and potential of herbal applications in aquaculture: A review. Iran. J. Fish. Sci. 2015, 14, 27–44. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Gomes, L.C.; Golombieski, J.I.; Gomes, A.R.C.; Baldisserotto, B. Biology of Rhamdia quelen (Teleostei, Pemelodidae). Ciência Rural. 2000, 30, 179–185. [Google Scholar] [CrossRef]
- Valenti, W.C.; Barros, H.P.; Moraes-Valenti, P.; Bueno, G.W.; Cavalli, R.O. Aquaculture in Brazil: Past, present and future. Aquac. Rep. 2021, 19, 100611. [Google Scholar] [CrossRef]
- Garlet, Q.I.; Souza, C.F.; Rodrigues, P.; Descovi, S.N.; Martinez-Rodríguez, G.; Baldisserotto, B.; Heinzmann, B.M. GABAa receptor subunits expression in silver catfish (Rhamdia quelen) brain and its modulation by Nectandra grandiflora Nees essential oil and isolated compounds. Behav. Brain Res. 2019, 376, 112178. [Google Scholar] [CrossRef] [PubMed]
- Volpato, A.; Baretta, D.; Zortéa, T.; Campigotto, G.; Galli, G.M.; Glombowsky, P.; Silva, A.S. Larvicidal and insecticidal effect of Cinnamomum zeylanicum oil (pure and nanostructured) against mealworm (Alphitobius diaperinus) and its possible environmental effects. J. Asia-Pac. Entomol. 2016, 19, 1159–1165. [Google Scholar] [CrossRef]
- Miller, R.; Carson, J.; Dalsgaard, I.; Gaunt, P.; Gieseker, C.; Hawke, J.; Wu, C. Methods for Broth Dilution Susceptibility Testing of Bacteria Isolated from Aquatic Animals, Approved Guideline, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Bandeira Junior, G.; Souza, C.F.; Baldissera, M.D.; Descovi, S.N.; Silveira, B.P.; Tasca, C.; Baldisserotto, B. Plant essential oils against bacteria isolated from fish: An in vitro screening and in vivo efficacy of Lippia origanoides. Ciência Rural. 2019, 49, e20190064. [Google Scholar] [CrossRef]
- Gomes, D.P.; Chaves, B.W.; Becker, A.G.; Baldisserotto, B. Water parameters affect anaesthesia induced by eugenol in silver catfish, Rhamdia quelen. Aquac. Res. 2011, 42, 878–886. [Google Scholar] [CrossRef]
- Zeppenfeld, C.C.; Hernández, D.R.; Santinón, J.J.; Heinzmann, B.M.; Cunha, M.A.; Schmidt, D.; Baldisserotto, B. Essential oil of Aloysia triphylla as feed additive promotes growth of silver catfish (Rhamdia quelen). Aquac. Nutr. 2016, 22, 933–940. [Google Scholar] [CrossRef]
- Evelson, P.; Travacio, M.; Repetto, M.; Escobar, J.; Llesuy, S.; Lissi, E.A. Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch. Biochem. Biophys. 2001, 388, 261–266. [Google Scholar] [CrossRef]
- Beutler, E. Superoxide dismutase. In Red Cell Metabolism: A Manual of Biochemical Methods; Beutler, E., Ed.; Grune & Stratton: Philadelphia, PA, USA, 1984. [Google Scholar]
- Mannervik, B.; Guthenberg, C. Glutathione transferase (human placenta). Methods Enzymol. 1981, 77, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.A.; Lopes, D.L.A.; Emerenciano, M.G.C.; Nora, L.; Souza, C.F.; Baldissera, M.D.; Silva, A.S. Phosphatidylcholine in diets of juvenile Nile tilapia in a biofloc technology system: Effects on performance, energy metabolism and the antioxidant system. Aquaculture 2020, 515, 734574. [Google Scholar] [CrossRef]
- Wendel, A. Glutathione peroxidase. Methods Enzymol. 1981, 77, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microssomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Read, S.M.; Northcote, D.H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal. Biochem. 1981, 116, 53–64. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporatio: Carol Stream, IL, USA, 2009. [Google Scholar]
- NIST/EPA/NIH. Mass Spectral Library; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008.
- Sutili, F.J.; Kreutz, L.C.; Noro, M.; Gressler, L.T.; Heinzmann, B.M.; Vargas, A.P.C.; Baldisserotto, B. The use of eugenol against Aeromonas hydrophila and its effect on hematological and immunological parameters in silver catfish (Rhamdia quelen). Vet. Immunol. Immunopathol. 2014, 157, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Atki, Y.E.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef]
- Huang, D.F.; Xu, J.G.; Liu, J.X.; Zhang, H.; Hu, Q.P. Chemical constituents, antibacterial activity and mechanism of action of the essential oil from Cinnamomum cassia Bark against four food related bacteria. Microbiology 2014, 83, 357–365. [Google Scholar] [CrossRef]
- Ooi, L.S.M.; Li, Y.; Kam, S.; Wang, H.; Wong, E.Y.L.; Ooi, V.E.C. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am. J. Chin. Med. 2006, 34, 511–522. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Wijesinghe, G.K.; Feiria, S.B.; Maia, F.C.; Oliveira, T.R.; Joia, F.; Barbosa, J.P.; Höfling, J.F. In-vitro antibacterial and antibiofilm activity of Cinnamomum verum leaf oil against Pseudomonas aeruginosa, Staphylococcus aureus and Klebsiella pneumoniae. An. Da Acad. Bras. De Ciências 2021, 93, e20201507. [Google Scholar] [CrossRef]
- Gogoi, R.; Sarma, N.; Loying, R.; Pandey, S.K.; Begum, T.; Lal, M. A comparative analysis of bark and leaf essential oil and their chemical composition, antioxidant, anti-inflammatory, antimicrobial activities and genotoxicity of north east Indian Cinnamomum zeylanicum Blume. Nat. Prod. J. 2021, 11, 74–84. [Google Scholar] [CrossRef]
- Choi, O.; Cho, S.K.; Kim, J.; Park, C.G.; Kim, J. In vitro antibacterial activity and major bioactive components of Cinnamomum verum essential oils against cariogenic bacteria, Streptococcus mutans and Streptococcus sobrinus. Asian Pac. J. Trop. Biomed. 2016, 6, 308–314. [Google Scholar] [CrossRef]
- Power, D.M.; Fuentes, J.; Harrison, A.P. A noninvasive monitoring device for anesthetics in fish. Open Access Anim. Physiol. 2010, 2, 17–23. [Google Scholar] [CrossRef][Green Version]
- Benovit, S.C.; Silva, L.L.; Salbego, J.; Loro, V.L.; Mallmann, C.A.; Baldisserotto, B.; Heinzmann, B.M. Anesthetic activity and bio-guided fractionation of the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. in silver catfish Rhamdia quelen. An. Da Acad. Bras. De Ciências 2015, 87, 1675–1689. [Google Scholar] [CrossRef]
- Kheawfu, K.; Pikulkaew, S.; Rades, T.; Müllertz, A.; Jørgensen, L.G.; Okonogi, S. Design and optimization of self-nanoemulsifying drug delivery systems of clove oil for efficacy enhancement in fish anesthesia. J. Drug Deliv. Sci. Technol. 2021, 61, 102241. [Google Scholar] [CrossRef]
- Khumpirapang, N.; Pikulkaew, S.; Müllertz, A.; Rades, T.; Okonogi, S. Self-microemulsifying drug delivery system and nanoemulsion for enhancing aqueous miscibility of Alpinia galanga oil. PLoS ONE 2017, 12, e0188848. [Google Scholar] [CrossRef]
- Rodrigues, P.; Ferrari, F.T.; Barbosa, L.B.; Righi, A.; Laporta, L.; Garlet, Q.I.; Heinzmann, B.M. Nanoemulsion boosts anesthetic activity and reduces the side effects of Nectandra grandiflora Nees essential oil in fish. Aquaculture 2021, 545, 737146. [Google Scholar] [CrossRef]
- Teixeira, R.R.; Souza, R.C.; Sena, A.C.; Baldisserotto, B.; Heinzmann, B.M.; Couto, R.D.; Copatti, C.E. Essential oil of Aloysia triphylla in Nile tilapia: Anaesthesia, stress parameters and sensory evaluation of fillets. Aquac. Res. 2017, 48, 3383–3392. [Google Scholar] [CrossRef]
- Sena, A.C.; Teixeira, R.R.; Ferreira, E.L.; Heinzmann, B.M.; Baldisserotto, B.; Caron, B.O.; Copatti, C.E. Essential oil from Lippia alba has anaesthetic activity and is effective in reducing handling and transport stress in tambacu (Piaractus mesopotamicus × Colossoma macropomum). Aquaculture 2016, 465, 374–379. [Google Scholar] [CrossRef]
- Amer, S.A.; Metwally, A.E.; Ahmed, S.A.A. The influence of dietary supplementation of cinnamaldehyde and thymol on the growth performance, immunity and antioxidant status of monosex Nile tilapia fingerlings (Oreochromis niloticus). Egypt. J. Aquat. Res. 2018, 44, 251–256. [Google Scholar] [CrossRef]
- El-Hamid, M.I.A.; Ibrahim, S.M.; Eldemery, F.; El-Mandrawy, S.A.M.; Metwally, A.S.; Khalifa, E.; Ibrahim, D. Dietary cinnamaldehyde nanoemulsion boosts growth and transcriptomes of antioxidant and immune related genes to fight Streptococcus agalactiae infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2021, 113, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Tartila, S.S.Q.; Jusadi, D.; Setiawati, M.; Fauzi, I.A. Evaluation of dietary supplementation with cinnamon products on growth, blood composition, liver structure, and meat quality of striped catfish (Pangasianodon hypophthalmus). Aquac. Int. 2021, 29, 2243–2257. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Xing, K.; Jiang, P.; Wang, J. Dietary cinnamaldehyde and Bacillus subtilis improve growth performance, digestive enzyme activity, and antioxidant capability and shape intestinal microbiota in tongue sole, Cynoglossus semilaevis. Aquaculture 2021, 531, 735798. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, W.; Zhang, J.; Feng, L.; Wu, P.; Liu, Y.; Zhou, X. Cinnamaldehyde improves the growth performance and digestion and absorption capacity in grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2020, 46, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Ribeiro, K.; Melo, J.F.B.; Teixeira, D.V.; Vidal, L.V.O.; Copatti, C.E. Essential oil from ginger influences the growth, haematological and biochemical variables and histomorphometry of intestine and liver of Nile tilapia juveniles. Aquaculture 2021, 534, 736325. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Aftabgard, M.; Van Doan, H. The improving role of savory (Satureja hortensis) essential oil for Caspian roach (Rutilus caspicus) fry: Growth, haematological, immunological, and antioxidant parameters and resistance to salinity stress. Aquaculture 2022, 548, 737653. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Miller, A. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Valková, V.; Tvrdá, E.; Terentjeva, M.; Žiarovská, J.; Kowalczewski, P.L. Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation. Open Chem. 2021, 19, 214–227. [Google Scholar] [CrossRef]
- Mousa, A.A.; Ramachandran, R.; Ozdemir, O.; Karsi, A.; Abdelhamed, H. Dietary trans-cinnamaldehyde improves oxidative stress response of channel catfish (Ictalurus punctatus) following Edwardsiella ictaluri infection. Aquaculture 2021, 532, 735985. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ghaderi, J.; Gómez-Guillén, M.C. Tailoring physico-mechanical and antimicrobial/antioxidant properties of biopolymeric films by cinnamaldehyde-loaded chitosan nanoparticles and their application in packaging of fresh rainbow trout fillets. Food Hydrocoll. 2022, 124, 107249. [Google Scholar] [CrossRef]
- Loke, X.; Chang, C.; Hou, C.; Cheng, K.; Hsieh, C. Plasma-treated polyethylene coated with polysaccharide and protein containing cinnamaldehyde for active packaging films and applications on tilapia (Orechromis niloticus) fillet preservation. Food Control 2021, 125, 108016. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Wang, Y. Development of antimicrobial active film containing CINnamaldehyde and its application to snakehead (Ophiocephalus argus) fish. J. Food Process Eng. 2017, 40, e12554. [Google Scholar] [CrossRef]

| Ingredients | g/kg |
|---|---|
| Soybean meal | 300 |
| Meat and bone meal | 350 |
| Rice bran | 120 |
| Corn | 150 |
| Canola oil | 30 |
| Salt | 10 |
| Vitamin and mineral premix * | 30 |
| Phosphate dicalcium | 10 |
| Analyzed proximate composition | g/kg |
| Dry matter content | 923.6 |
| Protein | 461.7 |
| Ether extract | 105.4 |
| Crude fiber | 29.4 |
| Mineral matter | 142.9 |
| Acid detergent fiber | 29.1 |
| Neutral detergent fiber | 164.1 |
| RT (min) | Constituent | Relative Percentage (%) | RI Cal | RI Ref |
|---|---|---|---|---|
| 14.8 | o-cymene | 0.6 | 1022.9 | 1022 |
| 17.835 | Linalool | 1.0 | 1098.5 | 1096 |
| 26.94 | Eugenol | 91.1 | 1351.6 | 1359/1370 |
| 29.134 | β-caryophyllene | 2.8 | 1418.6 | 1419 |
| 29.902 | E-cinnamyl acetate | 1.0 | 1443.0 | 1446 |
| 32.046 | Eugenol acetate | 1.8 | 1511.9 | 1514 |
| 39.209 | Benzyl benzoate or ascabiol | 1.8 | 1764.1 | 1762 |
| Aeromonas hydrophila (MF 372510) | Citrobacter freundii (MF 565839) | Raoultella ornithinolytica (MF 372511) | ||||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| CZEO | 50 | 100 | 400 | 800 | 800 | 800 |
| CCEO | 100 | 400 | 400 | 800 | 200 | 800 |
| NCCEO | 25 | 50 | 50 | 200 | 50 | 200 |
| Diet (mL EO per kg of Diet) | |||||
|---|---|---|---|---|---|
| 0 | 0.25 | 0.50 | 1.0 | 2.0 | |
| IW (g) | 4.38 ± 0.31 | 4.03 ± 0.06 | 4.32 ± 0.55 | 4.87 ± 0.46 | 4.45 ± 0.06 |
| ISL (cm) | 9.46 ± 0.07 | 9.08 ± 0.11 | 9.25 ± 0.38 | 9.75 ± 0.38 | 9.33 ± 0.04 |
| 30 days | |||||
| SL (cm) | 9.47 ± 0.08 | 9.08 ± 0.11 | 9.25 ± 0.38 | 9.33 ± 0.04 | 9.75 ± 0.38 |
| W (g) | 5.94 ± 0.32 | 5.83 ± 0.60 | 5.78 ± 0.53 | 6.64 ± 0.23 | 6.03 ± 0.57 |
| WG (g) | 1.55 ± 0.60 | 1.80 ± 0.55 | 1.46 ± 1.08 | 1.77 ± 0.70 | 1.58 ± 0.52 |
| RWG (%) | 37.73 ± 16.32 | 44.17 ± 12.83 | 42.68 ± 34.93 | 39.72 ± 18.04 | 35.32 ± 11.27 |
| SGR (%/day) | 0.51 ± 0.20 | 0.60 ± 0.14 | 0.50 ± 0.38 | 0.53 ± 0.22 | 0.49 ± 0.14 |
| 60 days | |||||
| SL (cm) | 12.3 ± 0.30 a | 12.44 ± 0.27 a | 13.03 ± 0.10 ab | 13.82 ± 0.38 b | 8.36 ± 0.14 c |
| W (g) | 8.16 ± 0.58 ab | 7.34 ± 0.35 a | 9.85 ± 0.15 bc | 11.07 ± 0.84 c | 8.35 ± 0.14 ab |
| WG (g) | 3.78 ± 0.37 a | 3.30 ± 0.30 a | 5.53 ± 0.54 bc | 5.94 ± 0.59 c | 3.91 ± 0.15 ab |
| RWG (%) | 85.34 ± 5.05 | 81.37 ± 6.62 | 138.34 ± 28.77 | 114.20 ± 7.91 | 88.09 ± 3.93 |
| SGR (%/day) | 1.03 ± 0.05 | 0.99 ± 0.06 | 1.40 ± 0.19 | 1.26 ± 0.08 | 1.05 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandeira Junior, G.; Bianchini, A.E.; de Freitas Souza, C.; Descovi, S.N.; da Silva Fernandes, L.; de Lima Silva, L.; Cargnelutti, J.F.; Baldisserotto, B. The Use of Cinnamon Essential Oils in Aquaculture: Antibacterial, Anesthetic, Growth-Promoting, and Antioxidant Effects. Fishes 2022, 7, 133. https://doi.org/10.3390/fishes7030133
Bandeira Junior G, Bianchini AE, de Freitas Souza C, Descovi SN, da Silva Fernandes L, de Lima Silva L, Cargnelutti JF, Baldisserotto B. The Use of Cinnamon Essential Oils in Aquaculture: Antibacterial, Anesthetic, Growth-Promoting, and Antioxidant Effects. Fishes. 2022; 7(3):133. https://doi.org/10.3390/fishes7030133
Chicago/Turabian StyleBandeira Junior, Guerino, Adriane Erbice Bianchini, Carine de Freitas Souza, Sharine Nunes Descovi, Liana da Silva Fernandes, Lenise de Lima Silva, Juliana Felipetto Cargnelutti, and Bernardo Baldisserotto. 2022. "The Use of Cinnamon Essential Oils in Aquaculture: Antibacterial, Anesthetic, Growth-Promoting, and Antioxidant Effects" Fishes 7, no. 3: 133. https://doi.org/10.3390/fishes7030133
APA StyleBandeira Junior, G., Bianchini, A. E., de Freitas Souza, C., Descovi, S. N., da Silva Fernandes, L., de Lima Silva, L., Cargnelutti, J. F., & Baldisserotto, B. (2022). The Use of Cinnamon Essential Oils in Aquaculture: Antibacterial, Anesthetic, Growth-Promoting, and Antioxidant Effects. Fishes, 7(3), 133. https://doi.org/10.3390/fishes7030133








