TRPV1 Receptor Identification in Rainbow Trout (Oncorhynchus mykiss) and Evaluation of the Effects Produced by Ocimum basilicum Super Critical Fluid Extract
Abstract
1. Introduction
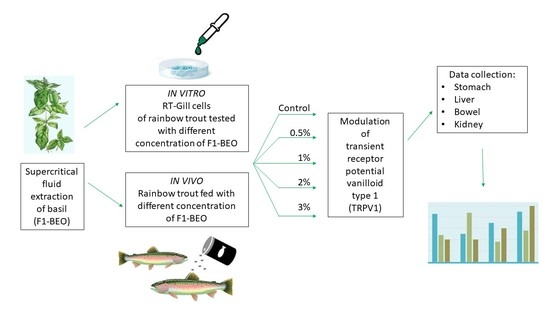
2. Materials and Methods
2.1. In Vitro Experiment
2.1.1. Cell Culture
2.1.2. Immunocytochemistry Protocol
2.2. In Vivo Trial
2.2.1. Ethical Statement
2.2.2. Chemical Profile of Supercritical Fluid Basil Extract (F1-BEO)
2.2.3. Diet Formulation and Rainbow Trout Nutrition
2.2.4. Rainbow Trout
2.2.5. Histology Protocol
2.3. Development of ICC and IHC Reaction and Image Acquisition
2.3.1. Development of Colorimetric Reactions of ICC and IHC
2.3.2. Image Acquisition, Software Processing and Statistical Analysis
3. Results
3.1. Cell Culture and ICC
3.2. Basil Extract Composition
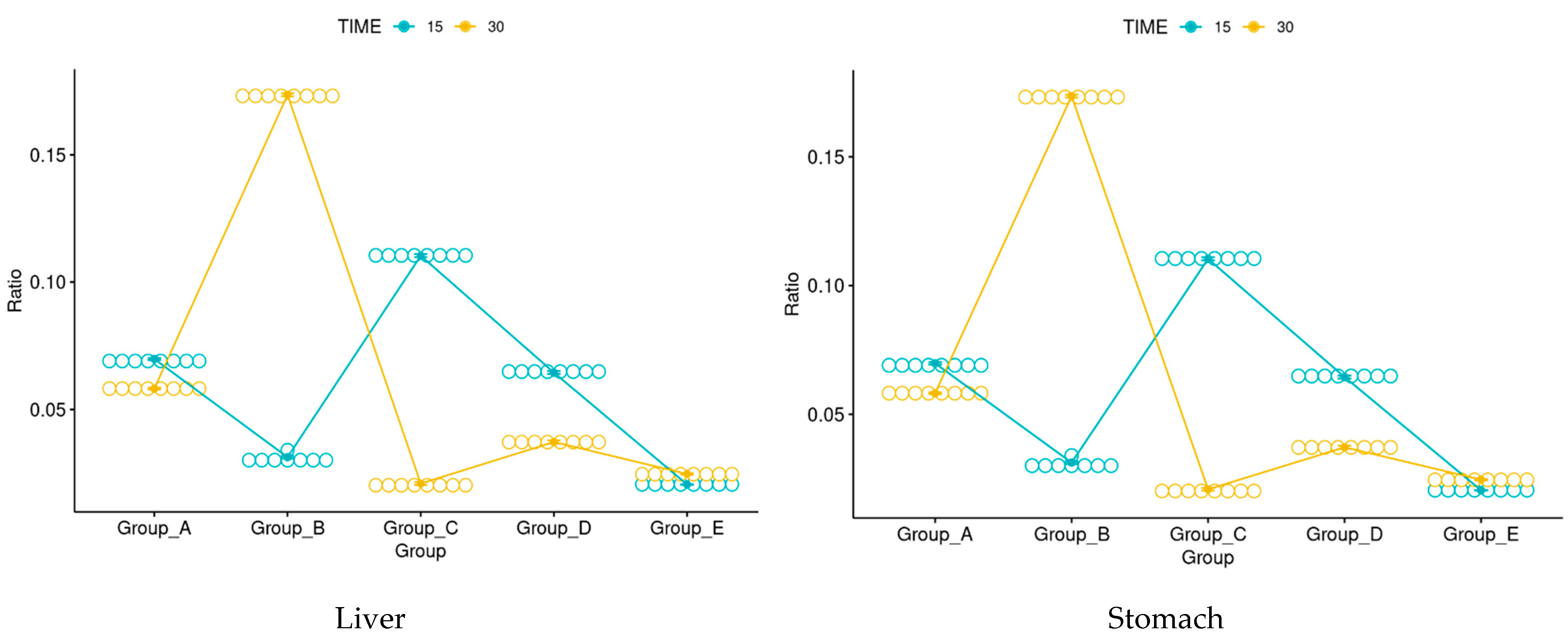
3.3. Rainbow Trout
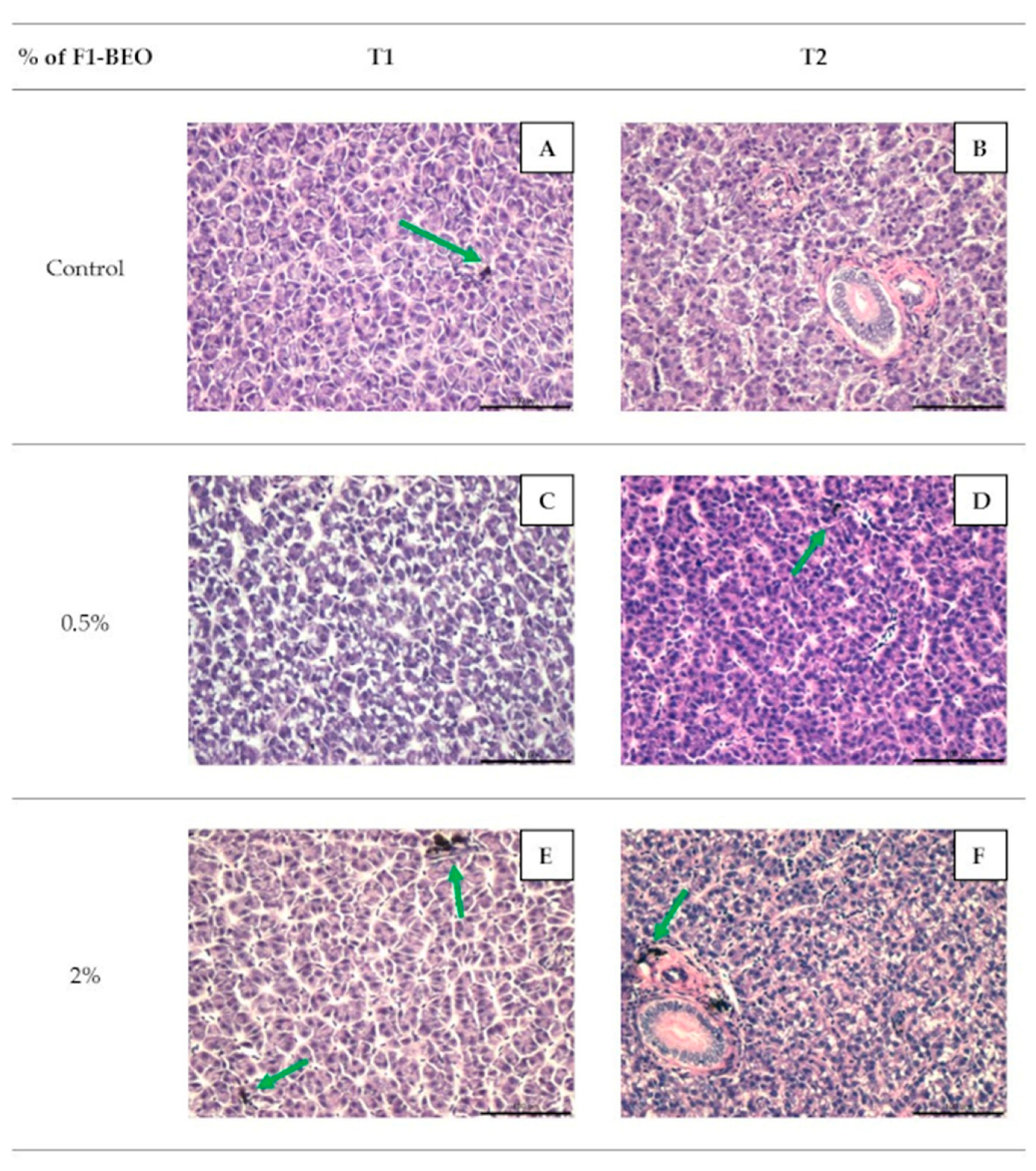
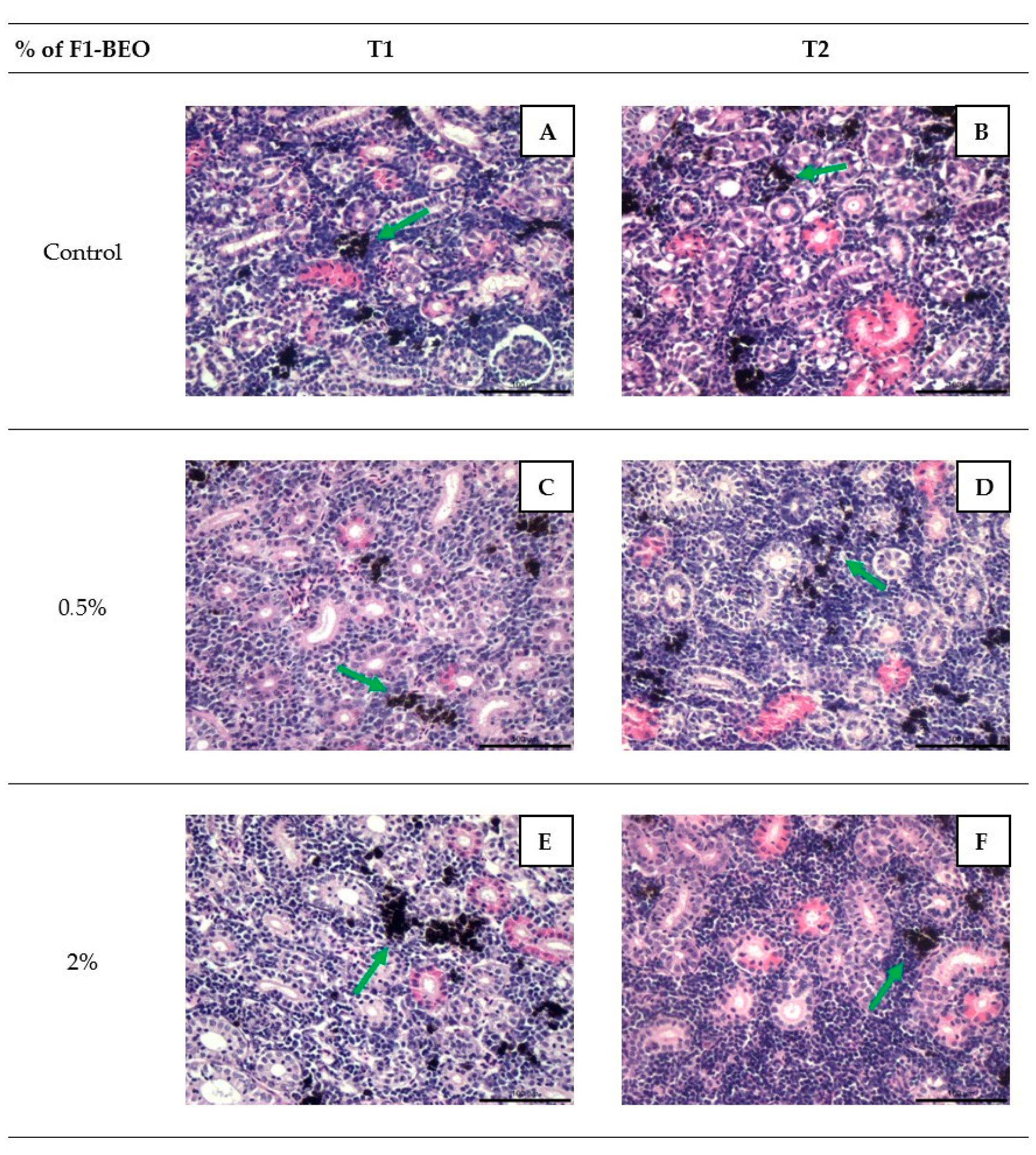
3.4. Histology
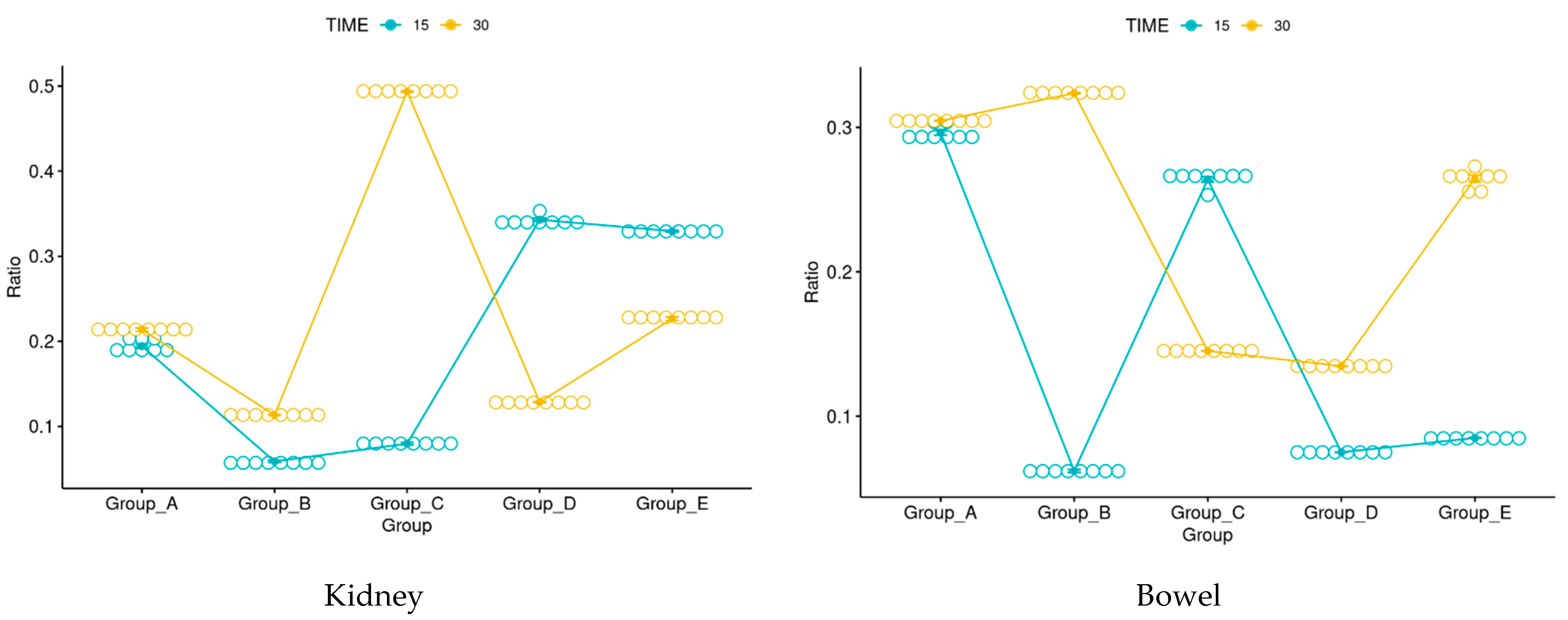
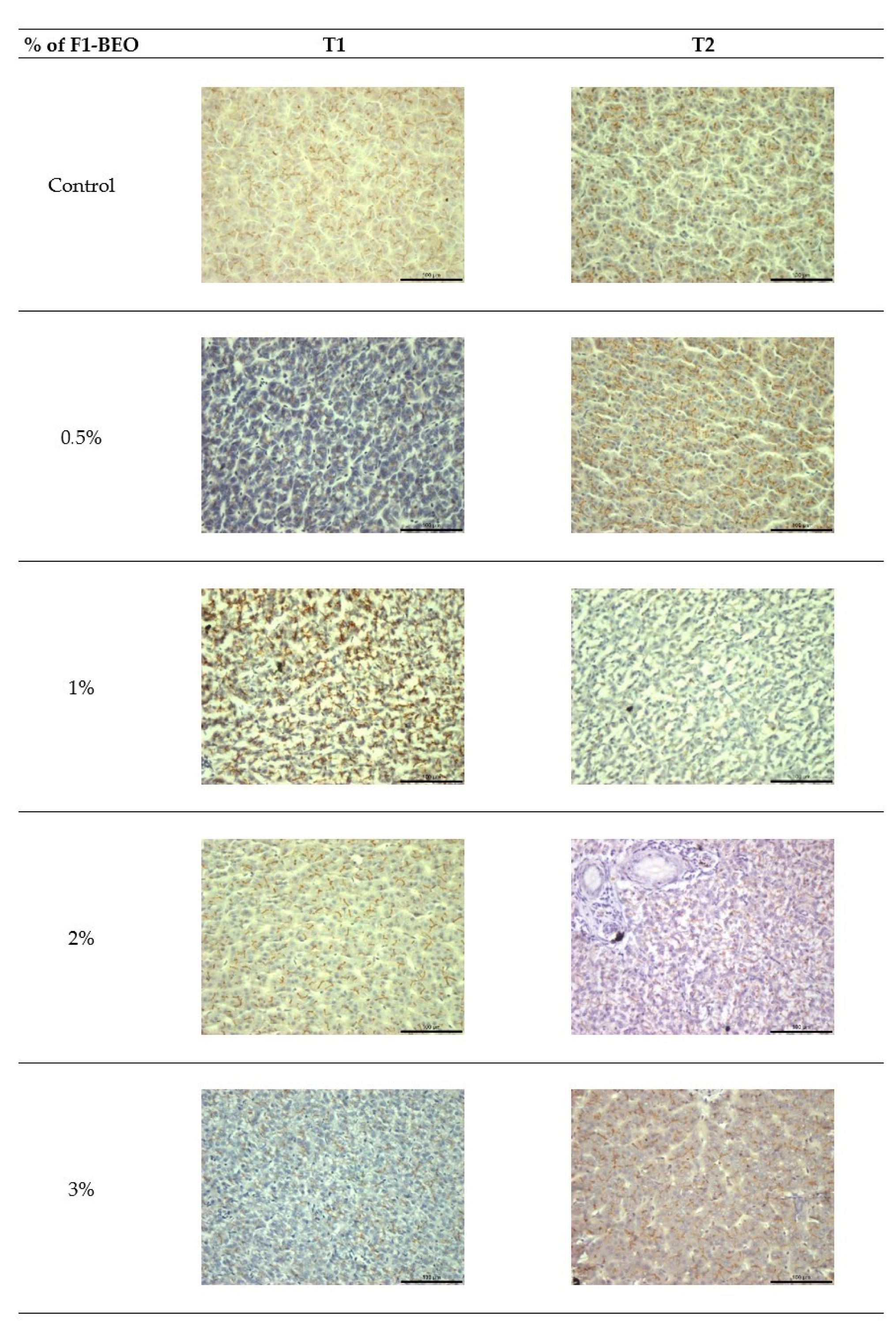
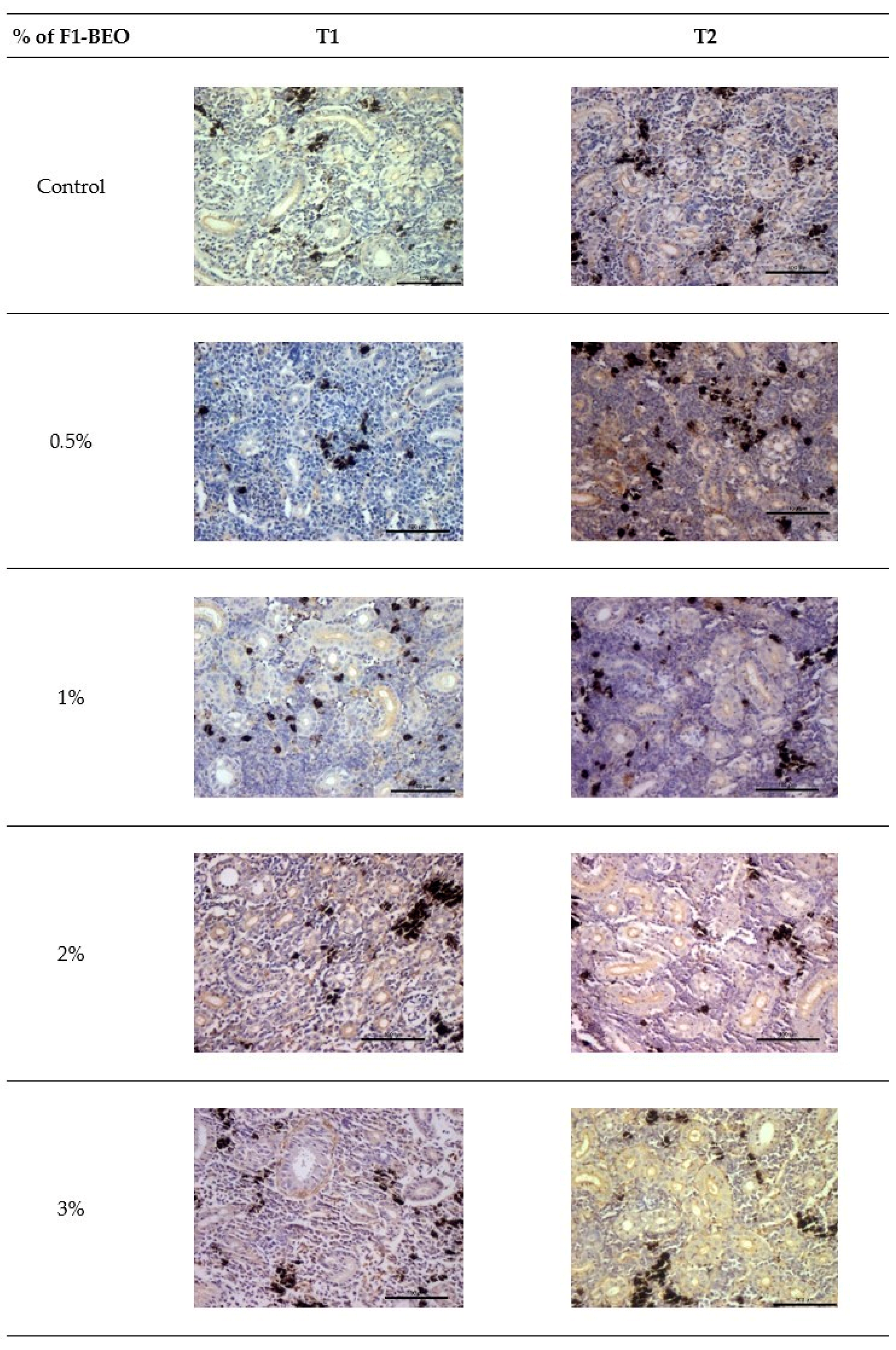
3.5. Immunohistochemistry
3.6. Morphological Evaluation and IHC of Liver and Kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Veronesi, B.; Oortgiesen, M. The TRPV1 receptor: Target of toxicants and therapeutics. Toxicol. Sci. 2006, 89, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Nigam, S.; Di Marzo, V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007, 114, 13–33. [Google Scholar] [CrossRef]
- Vandewauw, I.; Owsianik, G.; Voets, T. Systematic and quantitative MRNA expression analysis of trp channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci. 2013, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Patapoutian, A.; Peier, A.M.; Story, G.M.; Viswanath, V. ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat. Rev. Neurosci. 2003, 4, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, C.; Barbero, R.; Cuniberti, B.; Racca, S.; Abbadessa, G.; Piccione, F.; Re, G. Transient receptor potential vanilloid 1 expression and functionality in MCF-7 cells: A preliminary investigation. J. Breast Cancer 2014, 17, 332. [Google Scholar] [CrossRef]
- Vercelli, C.; Barbero, R.; Cuniberti, B.; Odore, R.; Re, G. Expression and functionality of TRPV1 receptor in human MCF-7 and canine CF.41 cells. Vet. Comp. Oncol. 2015, 13, 133–142. [Google Scholar] [CrossRef]
- Barbero, R.; Vercelli, C.; Cuniberti, B.; della Valle, M.F.; Martano, M.; Re, G. Expression of functional TRPV1 receptor in primary culture of canine keratinocytes. J. Vet. Pharmacol. Ther. 2018, 41, 795–804. [Google Scholar] [CrossRef]
- Vercelli, C.; Barbero, R.; Re, G. Transient receptor potential vanilloid 1 involvement in animal pain perception. Am. J. Anim. Vet. Sci. 2015, 10, 47–52. [Google Scholar]
- Vercelli, C.; Gambino, G.; Amadori, M.; Re, G.; Martignani, E.; Barberis, R.V.; Janus, I.; Tursi, M. TRPV1 Receptor identification in bovine and canine mitral valvular interstitial cells. Vet. Sci. 2021, 8, 183. [Google Scholar] [CrossRef]
- Brown, J.D.; Saeed, M.; Do, L.; Braz, J.; Basbaum, A.I.; Iadarola, M.J.; Wilson, D.M.; Dillon, W.P. CT-Guided Injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Sci. Transl. Med. 2015, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Viswanath, V.; Patapoutian, A. TRP ion channels and temperature sensation. Annu. Rev. Neurosci. 2006, 29, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.R.; Moiseenkova-Bell, V.Y. Structure of thermally activated TRP channels. Curr. Top. Membr. 2014, 74, 181–211. [Google Scholar]
- Li, T.; Li, J.-W.; Pang, C.-L.; An, H.; Geng, Y.-Z.; Wang, J.-Q. Oscillation of S5 helix under different temperatures in determination of the open probability of TRPV1 channel. Chin. Phys. B 2020, 29, 098701. [Google Scholar] [CrossRef]
- Bossus, M.; Charmantier, G.; Lorin-Nebel, C. Transient receptor potential vanilloid 4 in the European sea bass Dicentrarchus labrax: A candidate protein for osmosensing. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 160, 43–51. [Google Scholar] [CrossRef]
- Seale, A.P.; Watanabe, S.; Breves, J.P.; Lerner, D.T.; Kaneko, T.; Gordon Grau, E. Differential regulation of TRPV4 mRNA levels by acclimation salinity and extracellular osmolality in euryhaline tilapia. Gen. Comp. Endocrinol. 2012, 178, 123–130. [Google Scholar] [CrossRef]
- Liedtke, W.; Kim, C. Functionality of the TRPV subfamily of TRP ion channels: Add mechano-TRP and osmo-TRP to the lexicon! Cell. Mol. Life Sci. 2005, 62, 2985–3001. [Google Scholar] [CrossRef]
- Watanabe, S.; Seale, A.P.; Grau, E.G.; Kaneko, T. Stretch-activated cation channel TRPV4 mediates hyposmotically induced prolactin release from prolactin cells of mozambique tilapia Oreochromis mossambicus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1004–R1011. [Google Scholar] [CrossRef]
- Startek, J.B.; Boonen, B.; Talavera, K.; Meseguer, V. TRP channels as sensors of chemically-induced changes in cell membrane mechanical properties. Int. J. Mol. Sci. 2019, 20, 371. [Google Scholar] [CrossRef]
- Vriens, J.; Appendino, G.; Nilius, B. Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 2009, 75, 1262–1279. [Google Scholar] [CrossRef]
- Zheng, J. Molecular mechanism of TRP channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [PubMed]
- Raboune, S.; Stuart, J.M.; Leishman, E.; Takacs, S.M.; Rhodes, B.; Basnet, A.; Jameyfield, E.; McHugh, D.; Widlanski, T.; Bradshaw, H.B. Novel endogenous N-acyl amides activate TRPV1-4 receptors, BV-2 microglia, and are regulated in brain in an acute model of inflammation. Front. Cell. Neurosci. 2014, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- York, J.M.; Zakon, H.H. Evolution of transient receptor potential (TRP) ion channels in antarctic fishes (cryonotothenioidea) and identification of putative thermosensors. Genome Biol. Evol. 2022, 14, evac009. [Google Scholar] [CrossRef] [PubMed]
- Nisembaum, L.G.; Besseau, L.; Paulin, C.-H.; Charpantier, A.; Martin, P.; Magnanou, E.; Fuentès, M.; Delgado, M.-J.; Falcón, J. In the heat of the night: Thermo-TRPV channels in the salmonid pineal photoreceptors and modulation of melatonin secretion. Endocrinology 2015, 156, 4629–4638. [Google Scholar] [CrossRef] [PubMed]
- Nisembaum, L.G.; Loentgen, G.; L’Honoré, T.; Martin, P.; Paulin, C.-H.; Fuentès, M.; Escoubeyrou, K.; Delgado, M.J.; Besseau, L.; Falcón, J. Transient receptor potential-vanilloid (TRPV1-TRPV4) channels in the atlantic salmon, salmo salar. a focus on the pineal gland and melatonin production. Front. Physiol. 2022, 12, 784416. [Google Scholar] [CrossRef]
- Gau, P.; Poon, J.; Ufret-Vincenty, C.; Snelson, C.D.; Gordon, S.E.; Raible, D.W.; Dhaka, A. The zebrafish ortholog of TRPV1 is required for heat-induced locomotion. J. Neurosci. 2013, 33, 5249–5260. [Google Scholar] [CrossRef]
- Nobre Bezerra, A.J.N.; Silva, F.C.O.; da Silva, A.W.; Ferreira, M.K.A.; Marinho, E.M.; Marinho, M.M.; Magalhães, F.E.A.; Bandeira, P.N.; Rodrigues Teixeira, A.M.; Marinho, E.S.; et al. Antinociceptive effect of triterpene acetyl aleuritolic acid isolated from Croton zehntneri in adult zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2021, 534, 478–484. [Google Scholar] [CrossRef]
- Chen, S.; Chiu, C.N.; McArthur, K.L.; Fetcho, J.R.; Prober, D.A. TRP channel mediated neuronal activation and ablation in freely behaving zebrafish. Nat. Methods 2016, 13, 147–150. [Google Scholar] [CrossRef]
- Christie, S.; Wittert, G.A.; Li, H.; Page, A.J. Involvement of TRPV1 channels in energy homeostasis. Front. Endocrinol. 2018, 9, 420. [Google Scholar] [CrossRef]
- Díaz-Rúa, A.; Chivite, M.; Comesaña, S.; Velasco, C.; Valente, L.M.P.; Soengas, J.L.; Conde-Sieira, M. The endocannabinoid system is affected by a high-fat-diet in rainbow Trout. Horm. Behav. 2020, 125, 104825. [Google Scholar] [CrossRef]
- Boltana, S.; Sanhueza, N.; Donoso, A.; Aguilar, A.; Crespo, D.; Vergara, D.; Arriagada, G.; Morales-Lange, B.; Mercado, L.; Rey, S.; et al. The expression of TRPV channels, prostaglandin E2 and pro-inflammatory cytokines during behavioural fever in fish. Brain Behav. Immun. 2018, 71, 169–181. [Google Scholar] [CrossRef]
- Tamminen, L.-M.; Emanuelson, U.; Blanco-Penedo, I. Systematic review of phytotherapeutic treatments for different farm animals under european conditions. Front. Vet. Sci. 2018, 5, 140. [Google Scholar] [CrossRef]
- Vercelli, C.; Pasquetti, M.; Giovannetti, G.; Visioni, S.; Re, G.; Giorgi, M.; Gambino, G.; Peano, A. In vitro and in vivo evaluation of a new phytotherapic blend to treat acute externa otitis in Dogs. J. Vet. Pharmacol. Ther. 2021, 44, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, P.; Bergagna, S.; Vercelli, C.; Pagliasso, G.; Dellepiane, L.; Renzi, M.; Barbero, R.; Re, G.; Elia, A.C.; Dondo, A.; et al. Changes in serum blood parameters in farmed rainbow trout (Oncorhynchus mykiss) fed with diets supplemented with waste derived from supercritical fluid extraction of sweet basil (Ocimum basilicum). Fishes 2022, 7, 89. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.R.; Alagawany, M.; Kari, Z.A.; Abdul Razab, M.K.A.; Hamid, N.K.A.; Moonmanee, T.; Van Doan, H. Exploring the roles of dietary herbal essential oils in aquaculture: A review. Animals 2022, 12, 823. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 2011, 317, 1–15. [Google Scholar] [CrossRef]
- Kumar, C.B.; Kumar, A.; Rathore, G. Antibacterial activity of palmarosa oil significantly varies between Aeromonas veronii and Aeromonas caviae and exhibits selective action on tetracycline and sulfonamide resistant A. caviae. J. Appl. Microbiol. 2022, 132, 4321–4329. [Google Scholar] [CrossRef]
- Magara, G.; Prearo, M.; Vercelli, C.; Barbero, R.; Micera, M.; Botto, A.; Caimi, C.; Caldaroni, B.; Bertea, C.M.; Mannino, G.; et al. Modulation of antioxidant defense in farmed rainbow trout (Oncorhynchus mykiss) fed with a diet supplemented by the waste derived from the supercritical fluid extraction of basil (Ocimum basilicum). Antioxidants 2022, 11, 415. [Google Scholar] [CrossRef]
- Ledic-Neto, J.; Dotta, G.; Garcia, P.; Brum, A.; Gonçalves, E.L.T.; Martins, M.L. Haematology and melanomacrophage centers in Nile tilapia fed supplemented diet with propolis. Acta Sci. Biol. Sci. 2014, 36, 263–269. [Google Scholar] [CrossRef][Green Version]
- Brum, A.; Pereira, S.A.; Cardoso, L.; Chagas, E.C.; Chaves, F.C.M.; Mouriño, J.L.P.; Martins, M.L. Blood biochemical parameters and melanomacrophage centers in Nile tilapia fed essential oils of clove basil and ginger. Fish Shellfish Immunol. 2018, 74, 444–449. [Google Scholar] [CrossRef]
- Lacerda, J.T.; Gomes, P.R.L.; Zanetti, G.; Mezzalira, N.; Lima, O.G.; de Assis, L.V.M.; Guler, A.; Castrucci, A.M.; Moraes, M.N. Lack of TRPV1 channel modulates mouse gene expression and liver proteome with glucose metabolism changes. Int. J. Mol. Sci. 2022, 23, 7014. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Li, C.; Li, S.; Zhang, X.; He, Z.; Jiang, X.; He, Q.; Huang, R.; Wang, Y.; Liu, X. Capsaicin regulates dyslipidemia by altering the composition of bile acids in germ-free mice. Food Funct. 2022, 13, 10665–10679. [Google Scholar] [CrossRef] [PubMed]
- Aldossary, S.A.; Chohan, M.S.; Mohaini, M.A.; Tasleem Rasool, S. Capsaicin ameliorate the nephrotoxicity induced by methotrexate. Pak. J. Pharm. Sci. 2021, 34, 2191–2195. [Google Scholar] [PubMed]
- Liu, Z.; Wang, W.; Li, X.; Tang, S.; Meng, D.; Xia, W.; Wang, H.; Wu, Y.; Zhou, X.; Zhang, J. Capsaicin ameliorates renal fibrosis by inhibiting TGF-β1–Smad2/3 signaling. Phytomedicine 2022, 100, 154067. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kurogi, M.; Saitoh, O. The diversity in sensitivity of TRPA1 and TRPV1 of various animals to polyphenols. Biomed. Res. 2021, 42, 43–51. [Google Scholar] [CrossRef]
- Martella, A.; Sepe, R.M.; Silvestri, C.; Zang, J.; Fasano, G.; Carnevali, O.; De Girolamo, P.; Neuhauss, S.C.F.; Sordino, P.; Di Marzo, V. Important role of endocannabinoid signaling in the development of functional vision and locomotion in zebrafish. FASEB J. 2016, 30, 4275–4288. [Google Scholar] [CrossRef]
- Das, S.; Pradhan, C.; Pillai, D. Dietary coriander (Coriandrum sativum L) oil improves antioxidant and anti-inflammatory activity, innate immune responses and resistance to Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022, 132, 108486. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Doleski, P.H.; de Vargas, A.C.; Duarte, M.M.M.F.; Duarte, T.; Boligon, A.A.; Leal, D.B.R.; Baldisserotto, B. Melaleuca alternifolia essential oil prevents alterations to purinergic enzymes and ameliorates the innate immune response in silver catfish infected with Aeromonas hydrophila. Microb. Pathog. 2017, 109, 61–66. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.W.; Liu, L.L.; Cao, Y.C.; Zhu, H. Dietary oregano essential oil improved the immune response, activity of digestive enzymes, and intestinal microbiota of the koi carp Cyprinus carpio. Aquaculture 2020, 518, 734781. [Google Scholar] [CrossRef]
- Agius, C.; Roberts, R.J. Melano-macrophage centres and their role in fish pathology. J. Fish Dis. 2003, 26, 499–509. [Google Scholar] [CrossRef]
- Zimov, S.; Yazulla, S. Localization of vanilloid receptor 1 (TRPV1/VR1)-like immunoreactivity in goldfish and zebrafish retinas: Restriction to photoreceptor synaptic ribbons. J. Neurocytol. 2004, 33, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Kumar Majhi, R.; Kumar, A.; Yadav, M.; Swain, N.; Kumari, S.; Saha, A.; Pradhan, A.; Goswami, L.; Saha, S.; Samanta, L.; et al. Thermosensitive ion channel TRPV1 is endogenously expressed in the sperm of a fresh water teleost fish (Labeo rohita) and regulates sperm motility. Channels 2013, 7, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Tominaga, M. Evolutionary tuning of TRPA1 and TRPV1 thermal and chemical sensitivity in vertebrates. Temperature 2017, 4, 141–152. [Google Scholar] [CrossRef]
- Castillo, K.; Diaz-Franulic, I.; Canan, J.; Gonzalez-Nilo, F.; Latorre, R. Thermally activated TRP channels: Molecular sensors for temperature detection. Phys. Biol. 2018, 15, 021001. [Google Scholar] [CrossRef] [PubMed]
- Mizrak, S.C.; van Dissel-Emiliani, F.M.F. Transient receptor potential vanilloid receptor-1 confers heat resistance to male germ cells. Fertil. Steril. 2008, 90, 1290–1293. [Google Scholar] [CrossRef]
- Flores-Aldama, L.; Vandewege, M.W.; Zavala, K.; Colenso, C.K.; Gonzalez, W.; Brauchi, S.E.; Opazo, J.C. Evolutionary analyses reveal independent origins of gene repertoires and structural motifs associated to fast inactivation in calcium-selective TRPV channels. Sci. Rep. 2020, 10, 8684. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Tominaga, M. Functional diversity and evolutionary dynamics of thermoTRP channels. Cell Calcium 2015, 57, 214–221. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vercelli, C.; Amadori, M.; Tursi, M.; Gambino, G.; Pastorino, P.; Prearo, M.; Ala, U.; Barbero, R.; Re, G. TRPV1 Receptor Identification in Rainbow Trout (Oncorhynchus mykiss) and Evaluation of the Effects Produced by Ocimum basilicum Super Critical Fluid Extract. Fishes 2023, 8, 38. https://doi.org/10.3390/fishes8010038
Vercelli C, Amadori M, Tursi M, Gambino G, Pastorino P, Prearo M, Ala U, Barbero R, Re G. TRPV1 Receptor Identification in Rainbow Trout (Oncorhynchus mykiss) and Evaluation of the Effects Produced by Ocimum basilicum Super Critical Fluid Extract. Fishes. 2023; 8(1):38. https://doi.org/10.3390/fishes8010038
Chicago/Turabian StyleVercelli, Cristina, Michela Amadori, Massimiliano Tursi, Graziana Gambino, Paolo Pastorino, Marino Prearo, Ugo Ala, Raffaella Barbero, and Giovanni Re. 2023. "TRPV1 Receptor Identification in Rainbow Trout (Oncorhynchus mykiss) and Evaluation of the Effects Produced by Ocimum basilicum Super Critical Fluid Extract" Fishes 8, no. 1: 38. https://doi.org/10.3390/fishes8010038
APA StyleVercelli, C., Amadori, M., Tursi, M., Gambino, G., Pastorino, P., Prearo, M., Ala, U., Barbero, R., & Re, G. (2023). TRPV1 Receptor Identification in Rainbow Trout (Oncorhynchus mykiss) and Evaluation of the Effects Produced by Ocimum basilicum Super Critical Fluid Extract. Fishes, 8(1), 38. https://doi.org/10.3390/fishes8010038