Sporosarcina aquimarina MS4 Regulates the Digestive Enzyme Activities, Body Wall Nutrients, Gut Microbiota, and Metabolites of Apostichopus japonicus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Strains, Medium and Feed
2.3. Culture Trial
2.4. Growth Performance
2.5. Digestive Enzyme Activities
2.6. Nutrient Compositions
2.7. Gut Microbiota
2.8. Gut Untargeted Metabolomics Assay
2.9. Statistical Analysis
3. Results
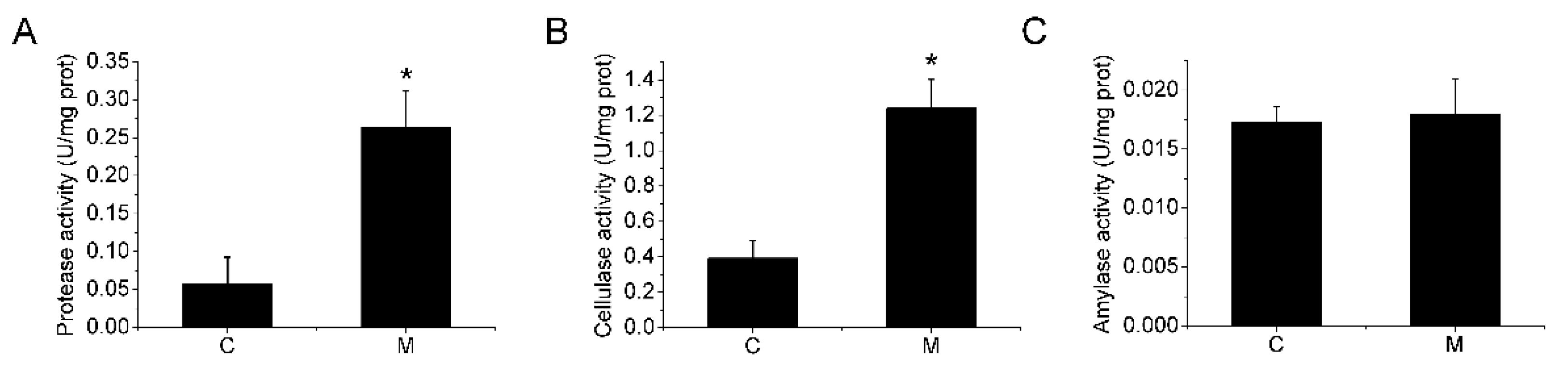
3.1. Growth Performance and Digestive Enzyme Activities
3.2. Body Wall Nutrients
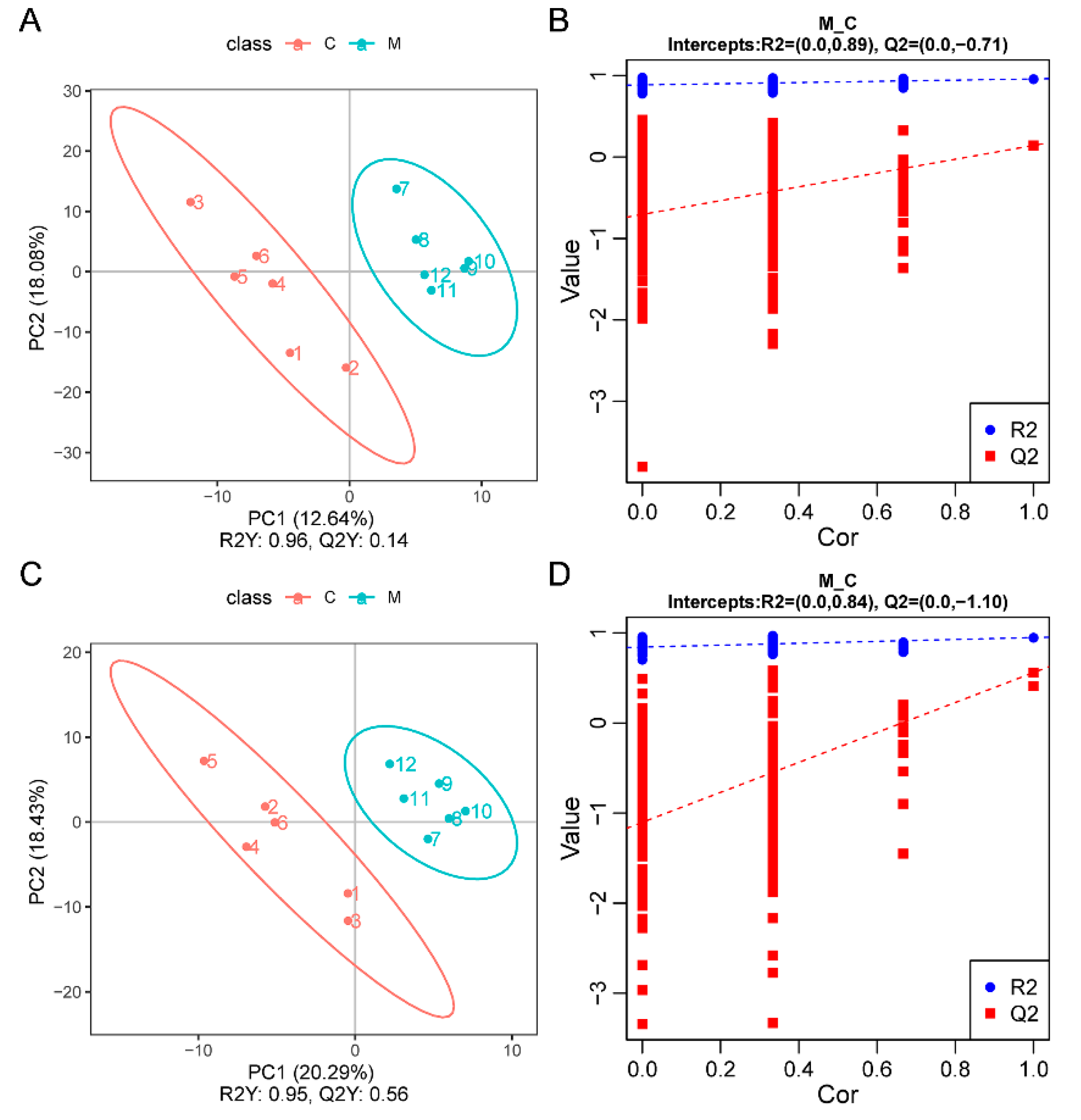
3.3. Gut Microbiota Analysis
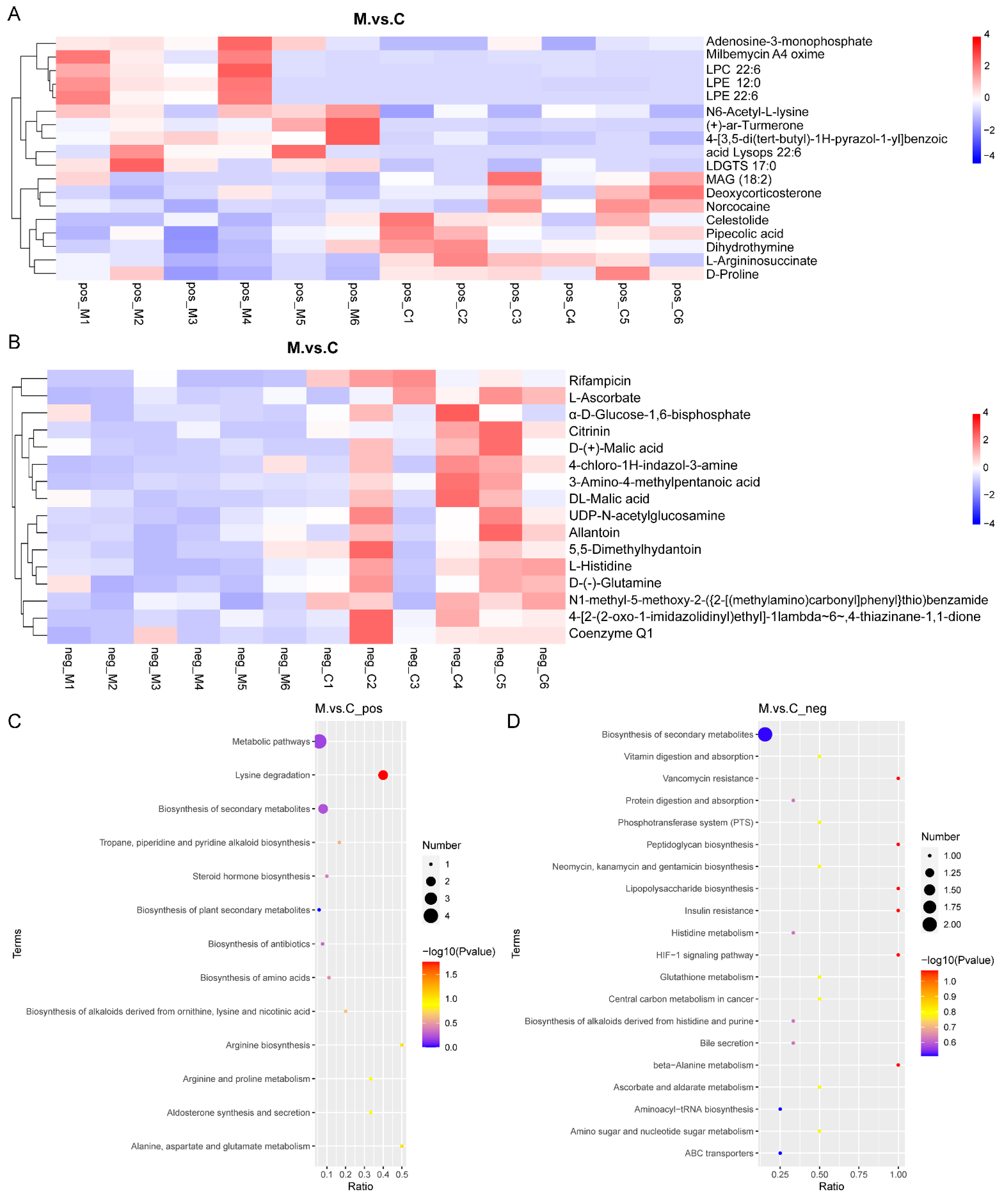
3.4. Differential Metabolites in the Gut
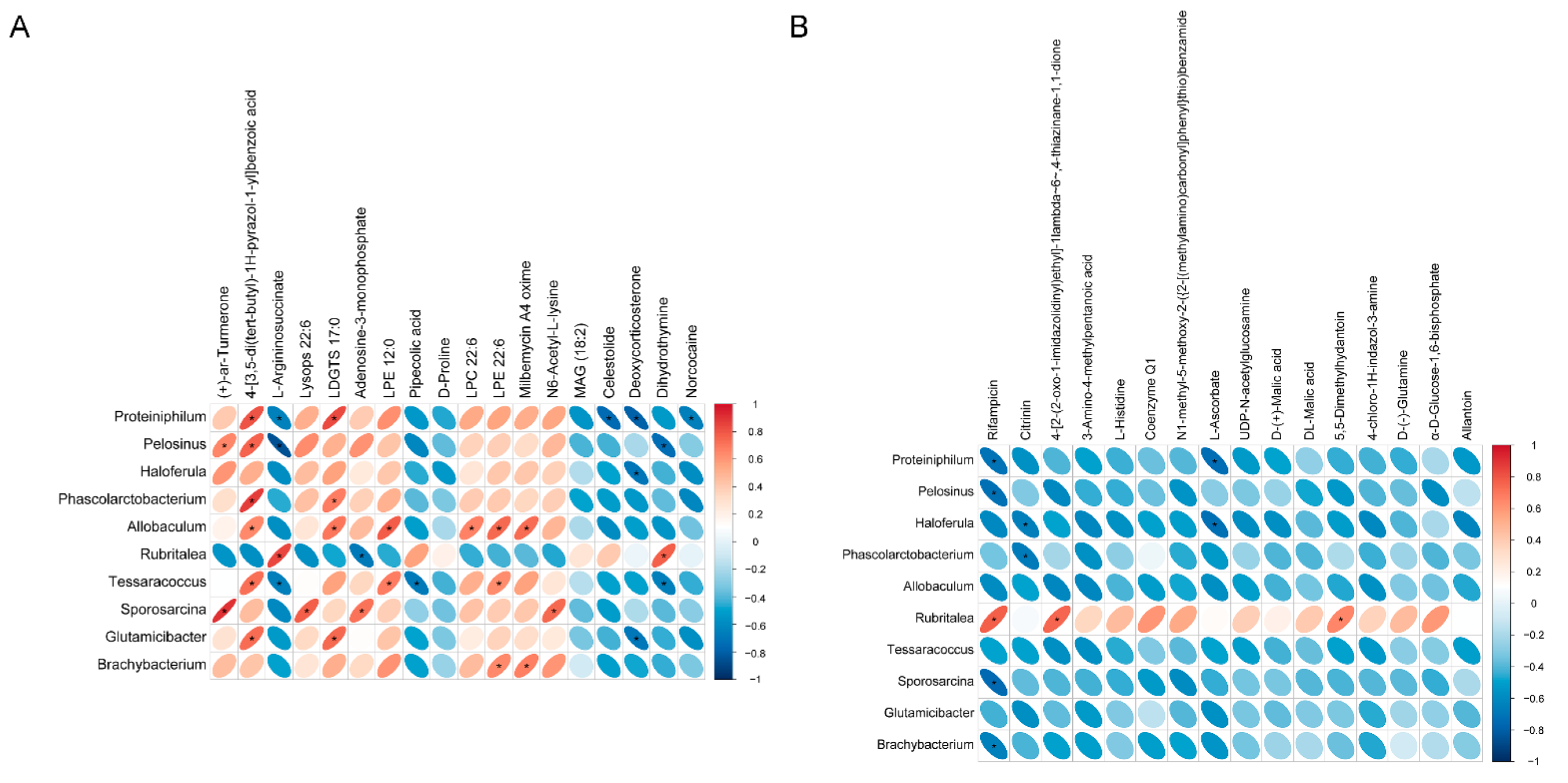
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pangestuti, R.; Arifin, Z. Medicinal and health benefit effects of functional sea cucumbers. J. Tradit. Complement. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Sun, L.; Liu, S.; Zhang, L.; Sun, J.; Yang, H. Metabolomic analysis of white, green and purple morphs of sea cucumber Apostichopus japonicus during body color pigmentation process. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 39, 100827. [Google Scholar] [CrossRef]
- Romero, J.; Feijoo, C.G.; Navarrete, P. Antibiotics in aquaculture—Use, abuse and alternatives. In Health and Environment in Aquaculture; Carvalho, E.D., David, G.S., Silva, R.J., Eds.; IntechOpen: Rijeka, Croatia, 2012; pp. 159–198. [Google Scholar]
- Xia, Y.; Wang, M.; Gao, F.; Lu, M.; Chen, G. Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Anim. Nutr. 2020, 6, 69–79. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, Y.; Jiang, J.; Chen, L. Effects of feed supplemented with probiotics on the culture of sea cucumber Apostichopus japonicus. Aquac. Nutr. 2021, 27, 2703–2711. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhao, L.Q.; Liu, J.F.; Wang, H.; Xiao, S. Effect of potential probiotic Rhodotorula benthica D30 on the growth performance, digestive enzyme activity and immunity in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2015, 43, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ren, Y.; Jiang, S.; Zhou, S.; Zhao, J.; Wang, R.; Li, Y. Effects of dietary supplementation of four strains of lactic acid bacteria on growth, immune-related response and genes expression of the juvenile sea cucumber Apostichopus japonicus Selenka. Fish Shellfish Immunol. 2018, 74, 69–75. [Google Scholar] [CrossRef]
- Feng, Z.; Song, X.; Zhao, L.; Zhu, W. Isolation of probiotics and their effects on growth, antioxidant and non-specific immunity of sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2020, 106, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-h.; Guo, H.; Zhang, T.-r.; Wang, H.; Liu, B.-n.; Xiao, S. Growth performance and digestion improvement of juvenile sea cucumber Apostichopus japonicus fed by solid-state fermentation diet. Aquac. Nutr. 2017, 23, 1312–1318. [Google Scholar] [CrossRef]
- Ning, Y.C.; Wu, X.Y.; Zhou, X.H.; Ding, J.; Chang, Y.Q.; Yang, Z.L.; Huang, Z.Q.; Zuo, R.T. An evaluation on the selenium yeast supplementation in the practical diets of early juvenile sea cucumber (Apostichopus japonicus): Growth performance, digestive enzyme activities, immune and antioxidant capacity, and body composition. Aquac. Nutr. 2021, 27, 2142–2153. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, B.; Li, Y.; Zhan, H.; Liu, Y.; Xiao, S.; Zhao, Q.; Wang, J. Dietary supplementation with Sporosarcina aquimarina MS4 enhances juvenile sea cucumber (Apostichopus japonicus) growth, immunity and disease resistance against Vibrio splendidus infection at low temperature. Aquac. Nutr. 2021, 27, 918–926. [Google Scholar] [CrossRef]
- Haide, M.S.; Sultana, R.; Jamil, K.; Tarar, O.M.; Afzal, W. A study on proximate composition, amino acid profile, fatty acid profile and some mineral contents in two species of sea cucumber. J. Anim. Plant Sci. 2015, 25, 168–175. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Leng, P.; Hu, Z. Feasible and effective reuse of municipal sludge for vegetation restoration: Physiochemical characteristics and microbial diversity. Sci. Rep. 2019, 9, 879. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yi, H.; Zhan, H.; Wang, L.; Wang, J.; Li, Y.; Liu, B. Gibberellic acid-induced fatty acid metabolism and ABC transporters promote astaxanthin production in Phaffia rhodozyma. J. Appl. Microbiol. 2022, 132, 390–400. [Google Scholar] [CrossRef]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.S.; Stenton-Dozey, J.; Zhang, J. Parameterisation and application of dynamic energy budget model to sea cucumber Apostichopus japonicus. Aquac. Environ. Interact. 2017, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhou, Z.; Dong, Y.; Jiang, B.; Chen, Z.; Gao, S.; Guan, X.; Han, L. Seasonal variations of immune parameters in the coelomic fluid of sea cucumber Apostichopus japonicus cultured in pond. Aquac. Res. 2017, 48, 1677–1687. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Pan, Y.; Lin, C.; Wang, F.; Kan, R.; Yang, H. Feeding behavior and digestive physiology in sea cucumber Apostichopus japonicus. Physiol. Behav. 2015, 139, 336–343. [Google Scholar] [CrossRef]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Cheng, Y.; Lv, D.; Li, M.; Qi, Y.; Lan, J.; Zhao, Q.; Li, Z. Marine sulfated polysaccharides: Preventive and therapeutic effects on metabolic syndrome: A review. Mar. Drugs 2021, 19, 608. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhou, Q.; Liu, B.; Chen, F.; Wang, M. Holothurian fucosylated chondroitin sulfates and their potential benefits for human health: Structures and biological activities. Carbohydr. Polym. 2022, 275, 118691. [Google Scholar] [CrossRef] [PubMed]
- Benevenga, N.J.; Blemings, K.P. Unique aspects of lysine nutrition and metabolism. J. Nutr. 2007, 137, 1610s–1615s. [Google Scholar] [CrossRef] [Green Version]
- Plotkin, D.L.; Delcastillo, K.; Van Every, D.W.; Tipton, K.D.; Aragon, A.A.; Schoenfeld, B.J. Isolated leucine and branched-chain amino acid supplementation for enhancing muscular strength and hypertrophy: A narrative review. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 292–301. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Dietary DHA and health: Cognitive function ageing. Nutr. Res. Rev. 2016, 29, 281–294. [Google Scholar] [CrossRef]
- Zeng, F.; Rabbi, M.H.; Hu, Y.; Li, Z.; Ren, X.; Han, Y.; Ren, T. Synergistic effects of dietary selenomethionine and vitamin C on the immunity, antioxidant status, and intestinal microbiota in sea cucumber (Apostichopus japonicus). Biol. Trace Elem. Res. 2021, 199, 3905–3917. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Lee, J.J.; Kim, B.S.; Choi, S.H. Whole-body microbiota of sea cucumber (Apostichopus japonicus) from south korea for improved seafood management. J. Microbiol. Biotechnol. 2017, 27, 1753–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of probiotics in aquaculture of China—A review of the past decade. Fish Shellfish Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, P.; Zhang, C.; Du, J.; Du, X.; Zhu, C.; Liu, J.; Xie, P.; Li, S. Comparative study of the gut microbiota among four different marine mammals in an aquarium. Front. Microbiol. 2021, 12, 769012. [Google Scholar] [CrossRef]
- Kang, H.; Traiwan, J.; Weerawongwiwat, V.; Jung, M.Y.; Jeong, J.H.; Myung, S.C.; Lee, K.C.; Lee, J.S.; Kim, W. Haloferula chungangensis sp. nov., isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2013, 63, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maukonen, J.; Ouwehand, A.C. Changes in the microbiota composition and function in relation to aging. In Comprehensive Gut Microbiota; Glibetic, M., Ed.; Elsevier: Oxford, UK, 2022; pp. 85–96. [Google Scholar] [CrossRef]
- McCarville, J.L.; Chen, G.Y.; Cuevas, V.D.; Troha, K.; Ayres, J.S. Microbiota metabolites in health and disease. Annu. Rev. Immunol. 2020, 38, 147–170. [Google Scholar] [CrossRef]
- Debnath, N.; Kumar, R.; Kumar, A.; Mehta, P.K.; Yadav, A.K. Gut-microbiota derived bioactive metabolites and their functions in host physiology. Biotechnol. Genet. Eng. Rev. 2021, 37, 105–153. [Google Scholar] [CrossRef] [PubMed]
- Kontara, E.K.; Djunaidah, I.S.; Coutteau, P.; Sorgeloos, P. Comparison of native, lyso and hydrogenated soybean phosphatidylcholine as phospholipid source in the diet of postlarval Penaeus japonicus bate. Arch. Anim. Nutr. 1998, 51, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wealleans, A.L.; Jansen, M.; di Benedetto, M. The addition of lysolecithin to broiler diets improves growth performance across fat levels and sources: A meta-analysis of 33 trials. Br. Poult. Sci. 2020, 61, 51–56. [Google Scholar] [CrossRef]

| Ingredients | Content (g/100 g) |
|---|---|
| Degumming kelp powder | 32 |
| Sargasso powder | 20 |
| Ulva powder | 20 |
| Cornmeal | 6 |
| Wall-broken yeast | 5 |
| Fermented soybean meal | 4 |
| Shrimp meal | 4 |
| Multidimensional premix | 4 |
| Fish meal | 3 |
| Scallop edge powder | 2 |
| Analyzed nutrients | |
| Moisture | 5.13 ± 0.12 |
| Crude protein | 14.96 ± 0.55 |
| Total sugar | 33.40 ± 0.63 |
| Crude fat | 1.52 ± 0.03 |
| Ash | 43.64 ± 1.21 |
| Growth Parameters | Group C | Group M |
|---|---|---|
| Initial weight (g) | 1.88 ± 0.02 a | 1.95 ± 0.13 a |
| Final weight (g) | 1.98 ± 0.05 a | 2.19 ± 0.10 a |
| Natural mortality (%) | 4.17 ± 1.18 a | 0.00 ± 0.00 b |
| WGR (%) | 5.48 ± 1.85 a | 12.22 ± 2.35 b |
| SGR (%/d) | 0.09 ± 0.03 a | 0.19 ± 0.03 b |
| FCR | 6.14 ± 0.17 a | 4.89 ± 0.56 b |
| Component | Group C (g/100 g) | Group M (g/100 g) |
|---|---|---|
| Moisture | 9.60 ± 0.21 a | 9.27 ± 0.24 a |
| Protein | 49.40 ± 0.53 a | 50.65 ± 0.49 a |
| Polysaccharide | 6.49 ± 0.14 a | 7.34 ± 0.24 b |
| Fat | 0.82 ± 0.04 a | 0.89 ± 0.08 a |
| Ash | 33.47 ± 1.01 a | 31.70 ± 1.35 a |
| Amino Acids | Group C (mg/g) | Group M (mg/g) |
|---|---|---|
| Asp | 40.29 ± 2.18 | 43.09 ± 1.65 |
| Thr | 19.74 ± 0.91 | 21.42 ± 0.86 |
| Ser | 18.69 ± 0.90 | 19.37 ± 0.38 |
| Glu | 60.37 ± 2.29 | 62.73 ± 1.08 |
| Gly | 38.87 ± 3.35 | 40.42 ± 3.21 |
| Ala | 23.53 ± 1.85 | 24.67 ± 1.53 |
| Cys | 5.00 ± 0.37 | 4.53 ± 1.65 |
| Val | 16.18 ± 0.86 | 17.64 ± 1.09 |
| Met | 6.10 ± 0.23 | 6.34 ± 0.35 |
| Ile | 13.03 ± 0.66 | 14.54 ± 0.43 |
| Leu * | 21.61 ± 0.85 | 23.65 ± 0.50 |
| Tyr | 11.32 ± 0.65 | 12.37 ± 0.24 |
| Phe * | 13.81 ± 0.67 | 15.51 ± 0.41 |
| His | 13.21 ± 0.38 | 14.11 ± 0.56 |
| Lys * | 18.97 ± 0.33 | 20.23 ± 0.34 |
| Arg | 24.59 ± 2.18 | 26.99 ± 1.19 |
| Pro | 19.06 ± 1.42 | 20.21 ± 1.59 |
| Total | 364.37 ± 19.46 | 387.82 ± 15.50 |
| Fatty Acids | Control | MS1 | |
|---|---|---|---|
| Saturated fatty acids | Tridecanoic acid (C13:0) | 1.72 ± 0.24 | - |
| Tetradecanoic acid (C14:0) | - | 2.81 ± 0.13 | |
| Pentadecanoic acid (C15:0) * | 9.08 ± 3.05 | 1.36 ± 0.05 | |
| Hexadecanoic acid (C16:0) | 0.38 ± 0.11 | 3.10 ± 1.39 | |
| Stearate (C18:0) | 5.94 ± 0.66 | 5.49 ± 0.37 | |
| Nonadecanoic acid (C19:0) | 0.75 ± 0.35 | 1.19 ± 0.32 | |
| Eicosanoic acid (C20:0) | 2.19 ± 0.55 | 1.20 ± 0.17 | |
| Heneicosanoic acid (C21:0) | 1.94 ± 0.30 | 2.00 ± 0.21 | |
| Docosanoic acid (C22:0) | 0.97 ± 0.45 | 1.83 ± 0.39 | |
| Heptacosanoic acid (C27:0) * | 0.61 ± 0.09 | 2.51 ± 0.20 | |
| Subtotal | 23.58 ± 4.07 | 21.48 ± 0.63 | |
| Monounsaturated fatty acids | 7-Hexadecenoic acid (C16:1) | - | 5.02 ± 0.73 |
| 9-Hexadecenoic acid (16:1) * | 11.62 ± 0.91 | 0.55 ± 0.16 | |
| 9-Octadecenoic acid (C18:1) * | 15.93 ± 3.21 | 0.25 ± 0.03 | |
| 12-Octadecenoic acid (C18:1) | - | 8.89 ± 1.96 | |
| 13-Octadecenoic acid (C18:1) | - | 7.44 ± 0.58 | |
| cis-11-Eicosenoic acid (C20:1) | 11.75 ± 0.70 | 11.18 ± 2.21 | |
| Total | 13-Docosenoic acid (C22:1) | 1.21 ± 1.05 | 2.26 ± 0.88 |
| 15-Tetracosenoic acid (C24:1) | 2.60 ± 0.68 | 2.42 ± 1.03 | |
| Subtotal Polyunsaturated fatty acids | 43.11 ± 2.05 | 38.01 ± 4.95 | |
| 9,12-Octadecadienoic acid (C18:2) | 4.91 ± 0.36 | 4.33 ± 0.23 | |
| 11,14-Eicosadienoic acid (C20:2) | 3.83 ± 0.36 | 3.71 ± 0.47 | |
| 5,8,11,14-Eicosatetraenoic acid (C20:4) | 16.75 ± 1.44 | 18.34 ± 3.82 | |
| 5,8,11,14,17-Eicosapentaenoic acid, methyl ester (C20:5) | 5.76 ± 4.04 | 4.87 ± 0.52 | |
| 4,7,10,13,16,19-Docosahexaenoic acid (C22:6) * | 2.06 ± 0.79 | 9.27 ± 1.78 | |
| Subtotal | 33.31 ± 6.09 | 40.51 ± 5.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, H.; Bai, Q.; Li, Y.; Zhan, H.; Liu, Y.; Liu, B.; Wang, J. Sporosarcina aquimarina MS4 Regulates the Digestive Enzyme Activities, Body Wall Nutrients, Gut Microbiota, and Metabolites of Apostichopus japonicus. Fishes 2022, 7, 134. https://doi.org/10.3390/fishes7030134
Yi H, Bai Q, Li Y, Zhan H, Liu Y, Liu B, Wang J. Sporosarcina aquimarina MS4 Regulates the Digestive Enzyme Activities, Body Wall Nutrients, Gut Microbiota, and Metabolites of Apostichopus japonicus. Fishes. 2022; 7(3):134. https://doi.org/10.3390/fishes7030134
Chicago/Turabian StyleYi, Hong, Qinglu Bai, Ying Li, Honglei Zhan, Yujia Liu, Bingnan Liu, and Jihui Wang. 2022. "Sporosarcina aquimarina MS4 Regulates the Digestive Enzyme Activities, Body Wall Nutrients, Gut Microbiota, and Metabolites of Apostichopus japonicus" Fishes 7, no. 3: 134. https://doi.org/10.3390/fishes7030134
APA StyleYi, H., Bai, Q., Li, Y., Zhan, H., Liu, Y., Liu, B., & Wang, J. (2022). Sporosarcina aquimarina MS4 Regulates the Digestive Enzyme Activities, Body Wall Nutrients, Gut Microbiota, and Metabolites of Apostichopus japonicus. Fishes, 7(3), 134. https://doi.org/10.3390/fishes7030134






