Isolation, Identification and Characteristics of Aeromonas caviae from Diseased Largemouth Bass (Micropterus salmoides)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Specimens
2.2. Pathogen Detection and Identification
2.3. 16S Ribosomal DNA Sequencing Analysis
2.4. Bacterial Biochemical Identification
2.5. Screening of Virulence Genes
2.6. Histopathological Analysis
2.7. Antibiotic Susceptibility Test
2.8. Pathogenicity Assays
3. Results
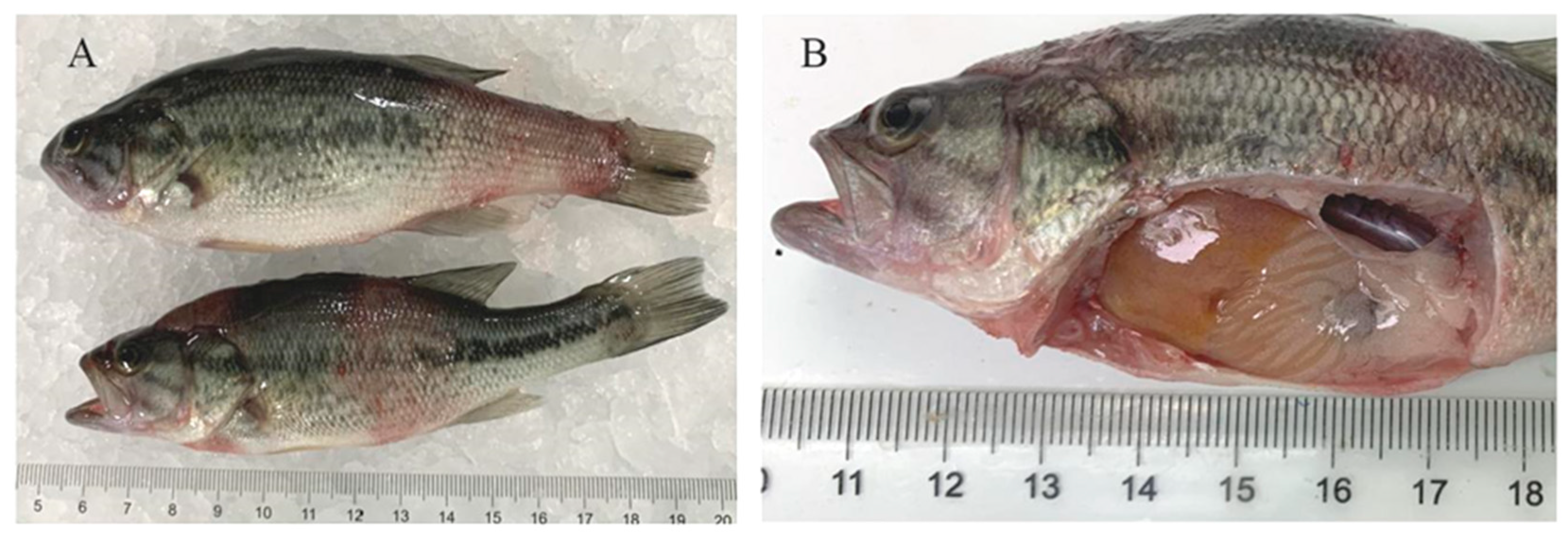
3.1. Clinical Symptoms
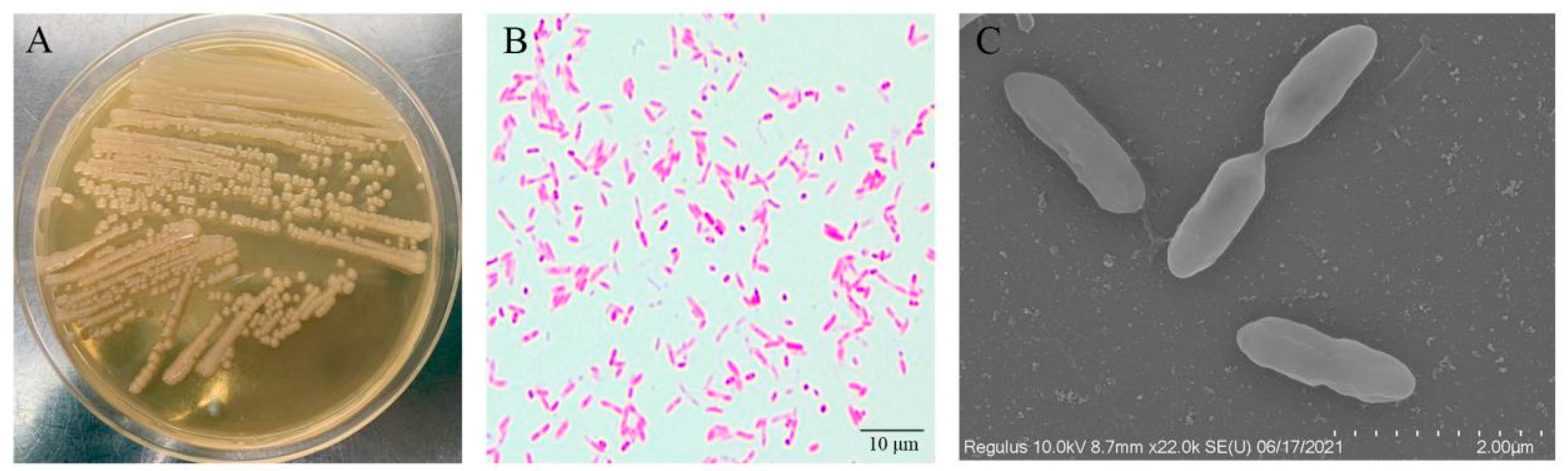
3.2. Pathogen Isolation and Characterization
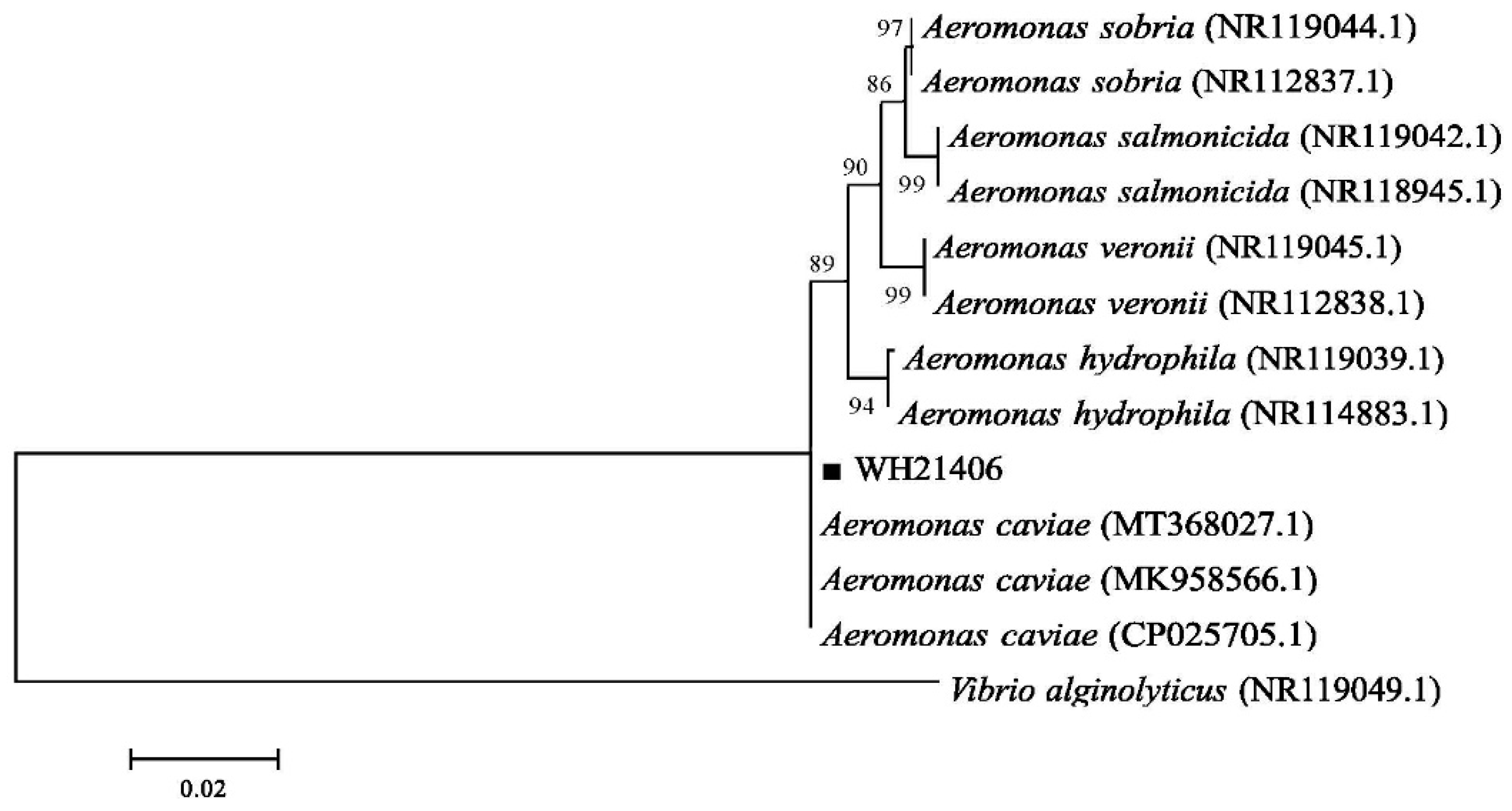
3.3. Bacterial 16S rDNA Sequence Analysis
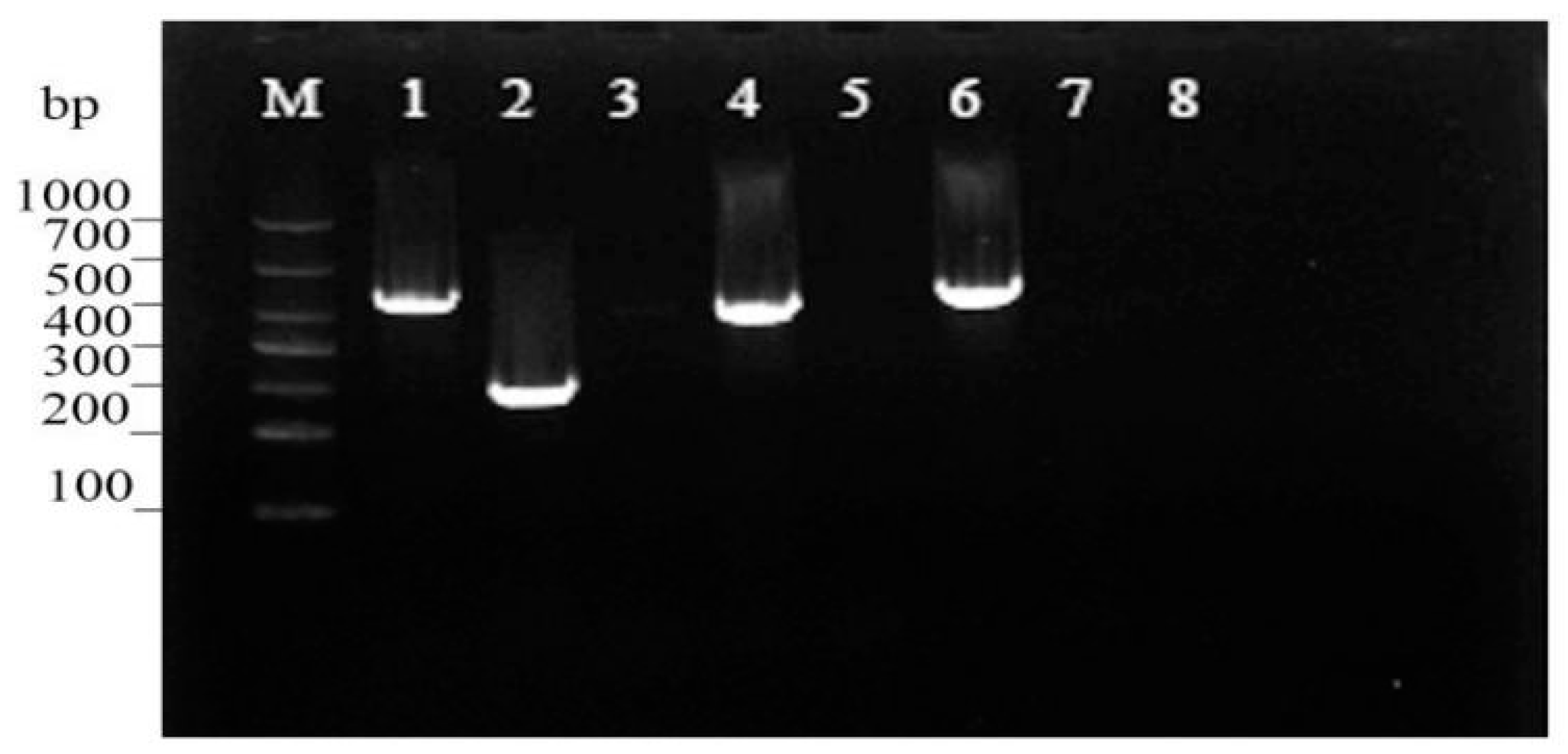
3.4. Virulence Gene Assessment
3.5. Histopathological Observations
3.6. Antibiotic Susceptibility Test
3.7. Pathogenicity Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coyle, S.D.; Tidwell, J.H.; Webster, C.D. Response of Largemouth Bass Micropterus salmoides to Dietary Supplementation of Lysine, Methionine, and Highly Unsaturated Fatty Acids. J. World Aquac. Soc. 2010, 31, 89–95. [Google Scholar] [CrossRef]
- Mizanur, R.M.; Li, X.; Sharifuzzaman, S.; He, M.; Leng, X. Dietary threonine requirement of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2021, 543, 736884. [Google Scholar]
- Gong, Y.; Yang, F.; Hu, J.; Liu, C.; Xie, S. Effects of dietary yeast hydrolysate on the growth, antioxidant response, immune response and disease resistance of largemouth bass (Micropterus salmoides). Fish Shellfish. Immunol. 2019, 94, 548–557. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, X.; Poolsawat, L.; Guo, Z.; Yao, W.; Zhang, C.; Leng, X. Effects of fish meal replaced by fermented soybean meal on growth performance, intestinal histology and microbiota of largemouth bass (Micropterus salmoides). Aquac. Nutr. 2020, 26, 1058–1071. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Zhua, X.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Huang, X.; Chen, D.; Yang, S.-Y.; et al. Dietary methionine hydroxy analogue supplementation benefits on growth, intestinal antioxidant status and microbiota in juvenile largemouth bass Micropterus salmoides. Aquaculture 2022, 556, 738279. [Google Scholar] [CrossRef]
- Guo, Z.R.; Zhao, Z.; Zhang, C.; Jia, Y.J.; Wang, G.X. Carbon nanotubes-loaded subunit vaccine can increase protective immunity against rhabdovirus infections of largemouth bass (Micropterus Salmoides). Fish Shellfish. Immunol. 2020, 99, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Deng, G.; Bai, J.; Li, S.; Yu, L.; Quan, Y.; Yang, X.; Jiang, X.; Zhu, Z.; Ye, X. A Strain of Siniperca chuatsi Rhabdovirus Causes High Mortality among Cultured Largemouth Bass in South China. J. Aquat. Anim. Health 2013, 25, 197–204. [Google Scholar] [CrossRef]
- Shimahara, Y.; Huang, Y.F.; Tsai, M.A.; Wang, P.C.; Chen, S.C. Immune response of largemouth bass, Micropterus salmoides, to whole cells of different Nocardia seriolae strains. Fish. Sci. 2010, 76, 489–494. [Google Scholar] [CrossRef]
- Huizinga, H.W.; Esch, G.W.; Hazen, T.C. Histopathology of red-sore disease (Aeromonas hydrophila) in naturally and experimentally infected largemouth bass Micropterus salmoides (Lacepede). J. Fish Dis. 2010, 2, 263–277. [Google Scholar] [CrossRef]
- Pei, C.; Song, H.; Zhu, L.; Qiao, D.; Yan, Y.; Li, L.; Zhao, X.; Zhang, J.; Jiang, X.; Kong, X. Identification of Aeromonas veronii isolated from largemouth bass Micropterus salmoides and histopathological analysis. Aquaculture 2021, 540, 736707. [Google Scholar] [CrossRef]
- Camus, A.; Griffin, M.; Armwood, A.; Soto, E. A Spontaneous Outbreak of Systemic Edwardsiella piscicida Infection in Largemouth Bass Micropterus salmoides (Lacépède, 1802) in California, USA. J. Fish Dis. 2019, 42, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Jianchou, L.W.W.A.C. Study on Antioxidation Function of Micropterus salmoides Tissues by Aeromonas Sobria Artificial Infection. Hubei Agric. Sci. 2009, 48, 1719–1721. [Google Scholar] [CrossRef]
- Francis-Floyd, R.; Reed, P.; Bolon, B.; Estes, J.; Mckinney, S. An epizootic of Edwardsiella tarda in largemouth bass (Micropterus salmoides). J. Wildl. Dis. 1993, 29, 334. [Google Scholar] [CrossRef] [Green Version]
- Bowker, J.D.; Carty, D.; Trushenski, J.T.; Bowman, M.P.; Wandelear, N.; Matthews, M. Controlling Mortality Caused by External Columnaris in Largemouth Bass and Bluegill with Chloramine-T or Hydrogen Peroxide. N. Am. J. Aquac. 2013, 75, 342–351. [Google Scholar] [CrossRef]
- Martinez-Murcia, A.J.; Saavedra, M.J.; Mota, V.R.; Maier, T.; Stackebrandt, E.; Cousin, S. Aeromonas aquariorum sp. nov., isolated from aquaria of ornamental fish. Int. J. Syst. Evol. Microbiol. 2008, 58, 1169–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, B.C.J.; Hossain, S.; Dahanayake, P.S.; Heo, G.J. Aeromonas spp. from marketed Yesso scallop (Patinopecten yessoensis): Molecular characterization, phylogenetic analysis, virulence properties and antimicrobial susceptibility. J. Appl. Microbiol. 2019, 126, 288–299. [Google Scholar] [CrossRef]
- Williams, M.A.; Addo, S.; Terhune, J.S.; Davis, D.A.; Carrias, A.A.; Liles, M.R. Effects of Bacillus subtilis strains and the prebiotic Previda((R)) on growth, immune parameters and susceptibility to Aeromonas hydrophila infection in Nile tilapia, Oreochromis niloticus. Aquac. Res. 2017, 48, 4798–4810. [Google Scholar]
- Lim, Y.L.; Ee, R.; Yin, W.F.; Chan, K.G. Quorum Sensing Activity of Aeromonas Caviae Strain YL12, A Bacterium Isolated from Compost. Sensors 2014, 14, 7026–7040. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.; Madan, N.; Nambi, K.; Majeed, S.A.; Basha, A.N.; Hameed, A.S. Studies on ulcerative disease caused by Aeromonas caviae-like bacterium in Indian catfish, Clarias batrachus (Linn). Aquaculture 2013, 376, 146–150. [Google Scholar] [CrossRef]
- Zepeda-Velázquez, A.P.; Vega-Sánchez, V.; Ortega-Santana, C.; Rubio-Godoy, M.; de Oca-Mira, D.M.; Soriano-Vargas, E. Pathogenicity of Mexican isolates of Aeromonas sp. in immersion experimentally-infected rainbow trout (Oncorhynchus mykiss, Walbaum 1792). Acta Trop. J. Biomed. Sci. 2017, 169, 122–124. [Google Scholar] [CrossRef]
- Xiankai, Z.; Haipeng, C.; Chunhua, M. Solation and antibiotic susceptibility of an Aeromonas caviae pathogen from yellow leg disease-infected Penaeus vannamei. J. Aquac. 2020, 41, 6. [Google Scholar]
- Haipeng, C.; Lefu, W.; Yibin, Y.; Tingting, S.; Shan, H. Isolation and biological characterisitics of an Aeromonas caviae pathogen from Procambarus clarkii. Acta Hydrobiol. Sin. 2014, 38, 7. [Google Scholar]
- Baldissera, M.; Souza, C.F.; Júnior, G.; Verdi, C.M.; Moreira, K.; Rocha, M.; Veiga, M.; Santos, R.; Vizzotto, B.S.; Baldisserotto, B. Aeromonas caviae alters the cytosolic and mitochondrial creatine kinase activities in experimentally infected silver catfish: Impairment on renal bioenergetics. Microb. Pathog. 2017, 110, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Sunniva, H.; Olav, V.; Jakobsen, A.N. Species Distribution and Prevalence of Putative Virulence Factors in Mesophilic Aeromonas spp. Isolated from Fresh Retail Sushi. Front. Microbiol. 2017, 8, 931. [Google Scholar]
- Sen, K.; Rodgers, M. Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: A PCR identification. J. Appl. Microbiol. 2004, 97, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Dallal, M.; Fard, R.; Talkhabi, M.K.; Aghaiyan, L.; Salehipour, Z. Prevalence, virulence and antimicrobial resistance patterns of Aeromonas spp. isolated from children with diarrhea. Germs 2016, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Jensen, S.; Bergh, Ø.; Enger, Ø.; Hjeltnes, B. Use of PCR-RFLP for genotyping 16S rRNA and characterizing bacteria cultured from halibut fry. Can. J. Microbiol. 2002, 48, 379–386. [Google Scholar] [CrossRef]
- Hu, M.; Wang, N.; Pan, Z.H.; Lu, C.P.; Liu, Y.J. Identity and virulence properties of Aeromonas isolates from diseased fish, healthy controls and water environment in China. Lett. Appl. Microbiol. 2012, 55, 224–233. [Google Scholar] [CrossRef]
- Gui-Hong, F.U.; Xiao, D.; Kun, H.U.; Yang, X.L. Multiplex PCR and ERIC-PCR genotype in virulence genes of Aeromonas hydrophila in Crucian carp. Mar. Fish. 2014, 36, 549. [Google Scholar]
- de Pádua, S.B.; Jerônimo, G.T.; de Menezes-Filho, R.N.; Taboga, S.R.; Martins, M.L.; Belo, M.A.A. Pathological assessment of farmed yellowtail tetra Astyanax altiparanae infested by Acusicola sp. (Ergasilidae). Aquac. Rep. 2015, 2, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.; Khan, S.A.; Khan, A.A.; Sung, K.; Tran, Q.; Kerdahi, K.; Steele, R. Detection and characterization of virulence genes and integrons in Aeromonas veronii isolated from catfish. Food Microbiol. 2010, 27, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Clark, C.G.; Liu, C.; Pucknell, C.; Munro, C.K.; Kruk, T.; Caldeira, R.; Woodward, D.L.; Rodgers, F.G. Detection and Characterization of the Hemolysin Genes in Aeromonas hydrophila and Aeromonas sobria by Multiplex PCR. J. Clin. Microbiol. 2003, 41, 1048–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zeng, X.; Jiang, N.; Zhou, Y.; Zeng, L. Pseudomonas alcaligenes infection and mortality in cultured Chinese sturgeon, Acipenser sinensis. Aquaculture 2015, 446, 37–41. [Google Scholar] [CrossRef]
- Jiang, N.; Jin, X.U.; Jie, M.A.; Fan, Y.; Zhou, Y.; Liu, W.; Zeng, L.; Disease, D.; Institute, Y.; Sciences, C. Histopathology and Ultrastructural Pathology of Cyprinid Herpesvirus Ⅱ(CyHV-2) Infection in Gibel Carp, Carassius auratus gibelio. Wuhan Univ. J. Nat. Sci. 2015, 20, 413–420. [Google Scholar] [CrossRef]
- Barry, A.L.; Coyle, M.B.; Thornsberry, C.; Gerlach, E.H.; Hawkinson, R.W. Methods of measuring zones of inhibition with the Bauer-Kirby disk susceptibility test. J. Clin. Microbiol. 1979, 10, 885. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Gu, Y.; Zhou, H.; Xu, L.; Gai, C. Acinetobacter venetianus, a potential pathogen of red leg disease in freshwater-cultured whiteleg shrimp Penaeus vannamei. Aquac. Rep. 2020, 18, 100543. [Google Scholar] [CrossRef]
- Santos, Y.; Laixier, R.; Bandin, I.; Lamas, J.; Toranzo, A. Susceptibility of turbot (Scophthalmus maximus), coho salmon (Oncorhynchus kisutch, and rainbow trout of. my kiss) to strains of Vibrio anguillarum and their exotoxins. J. Appl. Ichthyol. 2010, 7, 160–167. [Google Scholar]
- Zou, Y.; Zhang, P.; Mo, Z.; Liu, T.; Xu, Y. Isolation and identification of the pathogenic bacteria from Scophthamus maximus. Gaojishu Tongxun 2004, 14, 89–93. [Google Scholar]
- Saitou, N. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [Green Version]
- Mary, L.R.; Kurcheti, P.P.; Babu, G.; Purushothaman, C.S. Effect of Aeromonas hydrophila Infection on Caspase-3 Expression and Activity in Rohu, Labeo rohita. J. Aquac. Res. Dev. 2013, 4, 370–386. [Google Scholar]
- Woo, S.J.; Kim, M.S.; Jeong, M.G.; Do, M.Y.; Hwang, S.D.; Kim, W.J. Establishment of Epidemiological Cut-Off Values and the Distribution of Resistance Genes in Aeromonas hydrophila and Aeromonas veronii Isolated from Aquatic Animals. Antibiotics 2022, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.J.; Martínez-Murcia, A.; Esteves, A.C.; Correia, A.; Saavedra, M.J. Phylogenetic diversity, antibiotic resistance and virulence traits of Aeromonas spp. from untreated waters for human consumption. Int. J. Food Microbiol. 2012, 159, 230–239. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Bottari, N.B.; Verdi, C.M.; Santos, R.C.V.; Vizzotto, B.S.; Baldisserotto, B. Purinergic signalling displays an anti-inflammatory profile in the spleen of fish experimentally infected with Aeromonas caviae: Modulation of the immune response. J. Fish Dis. 2018, 41, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Guo, C.; Cao, H.; Yang, X. Isolation, identification, pathogenicity and in vitro antibacterial drugs of pathogenic Aeromonas hydrophila from Micropterus salmoides with hemorrhagic disease. Freshw. Fish. 2018, 48, 54–60. [Google Scholar]
- Senderovich, Y.; Ken-Dror, S.; Vainblat, I.; Blau, D.; Izhaki, I.; Halpern, M. A Molecular Study on the Prevalence and Virulence Potential of Aeromonas spp. Recovered from Patients Suffering from Diarrhea in Israel. PLoS ONE 2012, 7, e30070. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Gao, X.; Jiang, Q.; Wen, Y.; Lin, L. Characterization of Virulence Properties of Aeromonas veronii Isolated from Diseased Gibel Carp (Carassius gibelio). Int. J. Mol. Sci. 2016, 17, 496. [Google Scholar] [CrossRef]
- Abrami, L.; Fivaz, M.; Glauser, P.E.; Sugimoto, N.; Zurzolo, C.; van der Goot, F.G. Sensitivity of Polarized Epithelial Cells to the Pore-Forming Toxin Aerolysin. Infect. Immun. 2003, 71, 739. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Zhao, N.; Amer, S.; Qian, M.; Lv, M.; Zhao, Y.; Su, X.; Cao, J.; He, H.; Zhao, B. Protective efficacy of PLGA microspheres loaded with divalent DNA vaccine encoding the ompA gene of Aeromonas veronii and the hly gene of Aeromonas hydrophila in mice. Vaccine 2013, 31, 5754–5759. [Google Scholar] [CrossRef]
- Azzam-Sayuti, M.; Ina-Salwany, M.Y.; Zamri-Saad, M.; Yusof, M.T.; Amal, M. The prevalence, putative virulence genes and antibiotic resistance profiles of Aeromonas spp. isolated from cultured freshwater fishes in peninsular Malaysia. Aquaculture 2021, 540, 736719. [Google Scholar] [CrossRef]
- Wang, K.; Xiao, D.; He, Y.; Chen, D.; Geng, Y.; Liu, T.; Huang, G. Isolation, Purification and pathogenicity of the haemolysin produced by Aeromonas caviae from Silurus meridionalis Chen. Oceanol. Et Limnol. Sin. 2012, 43, 1122–1127. [Google Scholar]
- Guo, Y.; Zhou, M.; Li, Y.; Wang, W. Aeromonas caviae from Macrobrachium rosenbergii: Isolation and identification. Chin. Agric. Sci. Bull. 2020, 36, 147–153. [Google Scholar]


| Gene | Primer Sequence (5′−3′) | Product Size (bp) | Optimal Annealing Temperature (°C) | References |
|---|---|---|---|---|
| 16SrRNA-F | AGAGTTTGATCATGGCTCAG | 1500 | 55 | Jensen et al. [27] |
| 16SrRNA-R | TACGGTTACCTTGTTACGACTT | |||
| ahp-F | ATTGGATCCCTGCCTATCGCTTCAGTTCA | 911 | 55 | Hu et al. [28] |
| ahp-R | GCTAAGCTTGCATCCGTGCCGTATTCC | |||
| ela-F | ACACGGTCAAGGAGATCAAC | 513 | 55 | Sen and Rodgers [25] |
| ela-R | CGCTGGTGTTGGCCAGCAGG | |||
| act-F | ATCGTCAGCGACAGCTTCTT | 500 | 55 | Fu et al. [29] |
| act-R | CTCATCCCTTGGCTTGTTGT | |||
| fla-F | TCCAACCGTYTGACCTC | 608 | 55 | Hu et al. [28] |
| fla-R | GMYTGGTTGCGRATGGT | |||
| hly-F | GGCCGGTGGCCCGAAGATACGGG | 597 | 65 | Heuzenroeder et al. [30] |
| hly-R | GGCGGCGCCGGACGAGACGGG | |||
| alt-F | TGACCCAGTCCTGGCACGGC | 442 | 64 | Nawaz et al. [31] |
| alt-R | GGTGATCGATCACCACCAGC | |||
| aer-F | CAAGAACAAGTTCAAGTGGCCA | 309 | 57 | Wang et al. [32] |
| aer-R | ACGAAGGTGTGGTTCCAGT | |||
| lip-F | ATCTTCTCCGACTGGTTCGG | 382 | 55 | Sen and Rodgers [25] |
| lip-R | CCGTGCCAGGACTGGGTCTT |
| Reagent | Result * | Reagent | Result * |
|---|---|---|---|
| A1 Negative Control | N | E1 Gelatin | P |
| A2 Dextrin | P | E2 Glycyl-l-Proline | P |
| A3 d-Maltose | P | E3 l-Alanine | P |
| A4 d-Trehalose | P | E4 l-Arginine | P |
| A5 d-Cellobiose | P | E5 l-Aspartic Acid | P |
| A6 Gentiobiose | B | E6 l-Glutamic Acid | P |
| A7 Sucrose | P | E7 l-Histidine | P |
| A8 d-Turanose | B | E8 l-Pyroglutamic Acid | P |
| A9 Stachyose | B | E9 l-Serine | P |
| A10 Positive Control | P | E10 Lincomycin | B |
| A11 PH6 | P | E11 Guanidine HCl | B |
| A12 PH5 | N | E12 Niaproof 4 | N |
| B1 d-Raffinose | P | F1 Pectin | P |
| B2 α-d-Lactose | P | F2 d-Galacturonic Acid | P |
| B3 d-Melibiose | P | F3 l-Galactonic Acid Lactone | P |
| B4 β-Methyl-d-Glucoside | P | F4 d-Gluconic Acid | P |
| B5 d-Salicin | P | F5 d-Glucuronic Acid | P |
| B6 N-Acetyl-d-Glucosamine | P | F6 Glucuronamide | N |
| B7 N-Acetyl-β-d-Mannosamine | P | F7 Mucic Acid | P |
| B8 N-Acetyl-d-Galactosamine | P | F8 Quinic Acid | P |
| B9 N-Acetyl Neuraminic Acid | B | F9 d-Saccharic Acid | P |
| B10 1% NaCl | P | F10 Vancomycin | P |
| B11 4% NaCl | B | F11 Tetrazolium Violet | P |
| B12 8% NaCl | N | F12 Tetrazolium Blue | P |
| C1 α-d-Glucose | P | G1 P-Hydroxy-Phenylacetic Acid | N |
| C2 d-Mannose | P | G2 Methyl Pyruvate | P |
| C3 d-Fructose | P | G3 d-Lactic Acid Methyl Ester | P |
| C4 d-Galactose | B | G4 l-Lactic Acid | P |
| C5 3-Methyl Glucose | P | G5 Citric Acid | P |
| C6 d-Fucose | P | G6 6α-Keto-Glutaric Acid | P |
| C7 l-Fucose | P | G7 d-Malic Acid | L |
| C8 l-Rhamnose | P | G8 l-Malic Acid | P |
| C9 Inosine | P | G9 Bromo-Succinic Acid | P |
| C10 1%Sodium Lactate | P | G10 Nalidixic Acid | P |
| C11 Fusidic Acid | N | G11 Lithium Chloride | P |
| C12 d-Serine | P | G12 Potassium Tellurite | N |
| D1 d-Sorbitol | P | H1 Tween 40 | P |
| D2 d-Mannitol | P | H2 γ-Amino-Butyric Acid | P |
| D3 d-Arabitol | B | H3 α-Hydroxy-Butyric Acid | P |
| D4 Myo-inositol | B | H4 β-Hydroxy-d, l-Butyric Acid | P |
| D5 Glycerol | P | H5 α-Keto-Butyric Acid | P |
| D6 d-Glucose-6-PO4 | P | H6 Acetoacetic Acid | P |
| D7 d-Fructose-6-PO4 | P | H7 Propionic Acid | P |
| D8 d-Aspartic Acid | P | H8 Acetic Acid | P |
| D9 d-Serine | P | H9 Formic Acid | B |
| D10 Troleandomycin | P | H10 Aztreonam | B |
| D11 Rifamycin SV | P | H11 Sodium Butyrate | B |
| D12 Minocycline | N | H12 Sodium Bromate | N |
| Drug Name | Inhibition Zone (mm) | Sensitivity * |
|---|---|---|
| Florfenicol | 6 | R |
| Enrofloxacin | 22 | S |
| Neomycin sulfate | 6 | R |
| Doxycycline | 6 | R |
| Norfloxacin | 18 | S |
| Gentamicin | 12 | I |
| Streptomycin | 22 | S |
| Compound Sulfamethoxazole | 6 | R |
| Tetracyclines | 6 | R |
| Amikacin | 17 | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, M.; Xiao, Z.; Li, Y.; Jiang, N.; Liu, W.; Meng, Y.; Fan, Y.; Zeng, L.; Zhou, Y. Isolation, Identification and Characteristics of Aeromonas caviae from Diseased Largemouth Bass (Micropterus salmoides). Fishes 2022, 7, 119. https://doi.org/10.3390/fishes7030119
Xue M, Xiao Z, Li Y, Jiang N, Liu W, Meng Y, Fan Y, Zeng L, Zhou Y. Isolation, Identification and Characteristics of Aeromonas caviae from Diseased Largemouth Bass (Micropterus salmoides). Fishes. 2022; 7(3):119. https://doi.org/10.3390/fishes7030119
Chicago/Turabian StyleXue, Mingyang, Zidong Xiao, Yiqun Li, Nan Jiang, Wenzhi Liu, Yan Meng, Yuding Fan, Lingbing Zeng, and Yong Zhou. 2022. "Isolation, Identification and Characteristics of Aeromonas caviae from Diseased Largemouth Bass (Micropterus salmoides)" Fishes 7, no. 3: 119. https://doi.org/10.3390/fishes7030119
APA StyleXue, M., Xiao, Z., Li, Y., Jiang, N., Liu, W., Meng, Y., Fan, Y., Zeng, L., & Zhou, Y. (2022). Isolation, Identification and Characteristics of Aeromonas caviae from Diseased Largemouth Bass (Micropterus salmoides). Fishes, 7(3), 119. https://doi.org/10.3390/fishes7030119







