The Opportunistic Pathogen Chryseobacterium balustinum WLT: Pathogenicity and Antibiotic Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Sampling
2.2. Bacterial Isolation
2.3. Biochemical Characterization
2.4. Bacterial Identification
2.5. Phylogenetic Analysis
2.6. Histopathological Analysis
2.7. Antibiotic Susceptibility Test
2.8. Pathogenicity Challenge Trials
3. Results
3.1. Clinical Symptoms and Bacterial Isolation
3.2. Biochemical Characterization
3.3. Phylogenetic Analysis
3.4. Pathogenicity Challenge Trials
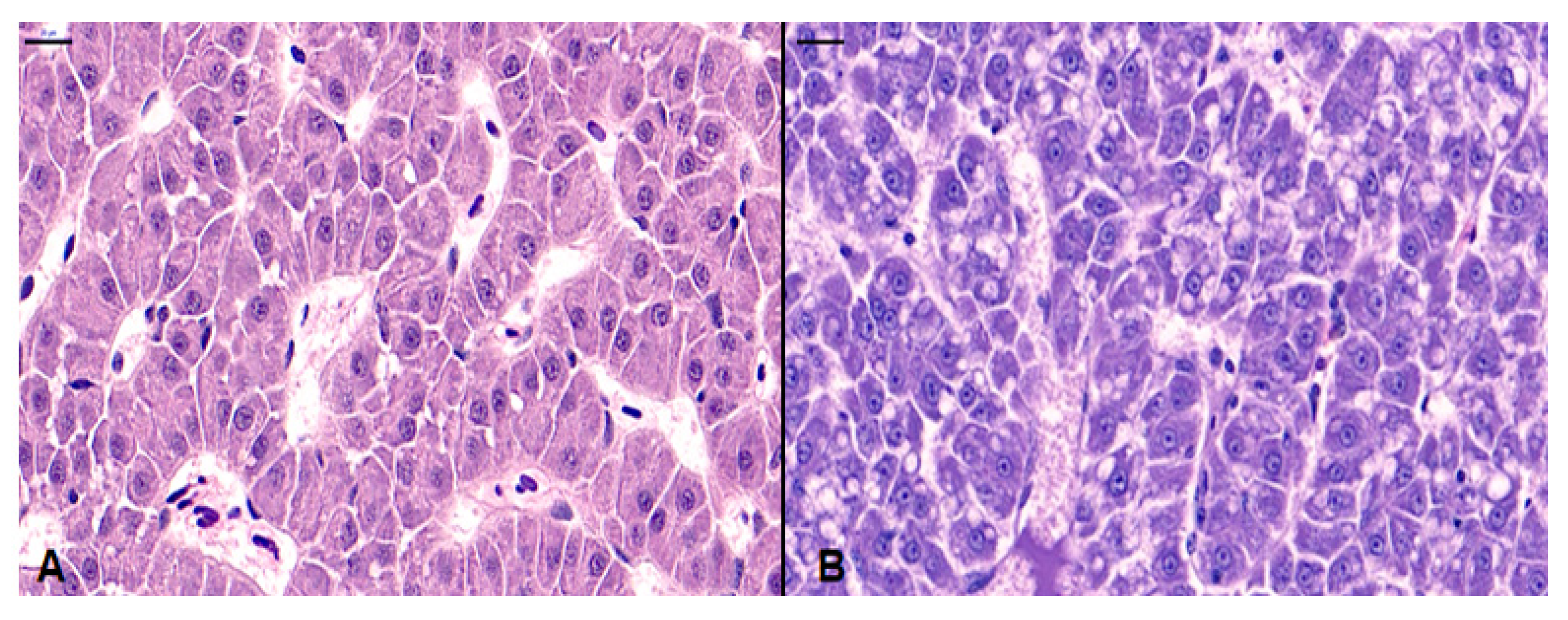
3.5. Histopathological Analysis
3.6. Antibiotic Susceptibility Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Vandamme, P.; Bernardet, J.F.; Segers, P.; Kersters, K.; Holmes, B. New Perspectives in the Classification of the Flavobacteria: Description of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int. J. Syst. Evol. 1994, 44, 827–831. [Google Scholar] [CrossRef]
- García-López, M.; Meier-Kolthoff, J.P.; Tindall, B.J.; Gronow, S.; Woyke, T.; Kyrpides, N.C.; Göker, M. Analysis of 1000 type-strain genomes improves taxonomic classification of Bacteroidetes. Front. Microbiol. 2019, 10, 2083. [Google Scholar] [CrossRef] [Green Version]
- del Carmen Montero-Calasanz, M.; Göker, M.; Rohde, M.; Spröer, C.; Schumann, P.; Busse, H.J.; Camacho, M. Chryseobacterium hispalense sp. nov., a plant-growth-promoting bacterium isolated from a rainwater pond in an olive plant nursery, and emended descriptions of Chryseobacterium defluvii, Chryseobacterium indologenes, Chryseobacterium wanjuense and Chryseobacterium gregarium. Int. J. Syst. Evol. 2013, 63, 4386–4395. [Google Scholar]
- Kim, B.Y.; Weon, H.Y.; Cousin, S.; Yoo, S.H.; Kwon, S.W.; Go, S.J.; Stackebrandt, E. Flavobacterium daejeonense sp. nov. and Flavobacterium suncheonense sp. nov., isolated from greenhouse soils in Korea. Inst. J. Syst. Evol. 2006, 56, 1645–1649. [Google Scholar] [CrossRef]
- Harrison, F.C. The discoloration of halibut. Can. J. Res. 1929, 1, 214–239. [Google Scholar] [CrossRef]
- Ingrid Tsôeu, L.; Jooste, P.J.; Charimba, G.; Hugo, C.J. Spoilage potential of a novel group of bacteria isolated from dairy products. S. Afr. J. Sci. 2016, 112, 01–08. [Google Scholar]
- Behrendt, U.; Ulrich, A.; Schumann, P. Chryseobacterium gregarium sp. nov., isolated from decaying plant material. Int. J. Syst. Evol. 2008, 58, 1069–1074. [Google Scholar] [CrossRef] [Green Version]
- de Beer, H.; Hugo, C.J.; Jooste, P.J.; Willems, A.; Vancanneyt, M.; Coenye, T.; Vandamme, P.A. Chryseobacterium vrystaatense sp. nov., isolated from raw chicken in a chicken-processing plant. Int. J. Syst. Evol. 2005, 55, 2149–2153. [Google Scholar] [CrossRef] [Green Version]
- Hantsis-Zacharov, E.; Shaked, T.; Senderovich, Y.; Halpern, M. Chryseobacterium oranimense sp. nov., a psychrotolerant, proteolytic and lipolytic bacterium isolated from raw cow’s milk. Int. J. Syst. Evol. 2008, 58, 2635–2639. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.H.; Waddingdon, M.; Greenberg, D.; Schreckenberger, P.C.; Carnahan, A.M. Atypical Chryseobacterium meningosepticum and meningitis and sepsis in newborns and the immunocompromised, Taiwan. Emerg. Infect. Dis. 2000, 6, 481. [Google Scholar] [CrossRef]
- Bloch, K.C.; Nadarajah, R.; Jacobs, R. Chryseobacterium meningosepticum: An emerging pathogen among immunocompromised adults. Report of 6 cases and literature review. Medicine 1997, 76, 30–41. [Google Scholar] [CrossRef] [PubMed]
- McKew, G. Severe sepsis due to Chryseobacterium indologenes in an immunocompetent adventure traveler. J. Clin. Microbiol. 2014, 52, 4100–4101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, C.; Matte-Tailliez, O.; Kerouault, B.; Bernardet, J.F. Resistance pattern and assessment of phenicol agents’ minimum inhibitory concentration in multiple drug resistant Chryseobacterium isolates from fish and aquatic habitats. J. Appl. Microbiol. 2005, 99, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Saticioglu, I.B.; Duman, M.; Altun, S. Genome analysis and antimicrobial resistance characteristics of Chryseobacterium aquaticum isolated from farmed salmonids. Aquaculture 2021, 535, 736364. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jung, W.J.; Kim, S.W.; Giri, S.S.; Kim, H.J.; Kim, S.G.; Kang, J.W.; Park, S.C. Janthinobacterium tructae sp. nov., Isolated from Kidney of Rainbow Trout (Oncorhynchus mykiss). Pathogens 2021, 10, 229. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Wayne, P.A. Clinical and laboratory standards institute. Performance Standards for Antimicrobial Susceptibility Testing. 2011. Available online: https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=450165 (accessed on 23 December 2021).
- Nematollahi, A.; Decostere, A.; Pasmans, F.; Haesebrouck, F. Flavobacterium psychrophilum infections in salmonid fish. J. Fish Dis. 2003, 26, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Altinok, I. Multiplex PCR assay for detection of four major bacterial pathogens causing rainbow trout disease. Dis. Aquat. Org. 2011, 93, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, M.; Rodrıguez, S.; Prieto, S.I.P. Nested PCR improves detection of infectious hematopoietic necrosis virus in cells coinfected with infectious pancreatic necrosis virus. J. Virol. Methods 1999, 81, 1–9. [Google Scholar] [CrossRef]
- Garver, K.A.; Hawley, L.M.; McClure, C.A.; Schroeder, T.; Aldous, S.; Doig, F.; Richard, J. Development and validation of a reverse transcription quantitative PCR for universal detection of viral hemorrhagic septicemia virus. Dis. Aquat. Org. 2011, 95, 97–112. [Google Scholar] [CrossRef] [Green Version]
- Inglis, V.; Aoki, T. Rapid identification of Aeromonas salmonicida subspecies salmonicida by the polymerase chain reaction. Aquaculture 1996, 141, 13–24. [Google Scholar]
- Cipriano, R.C. Aeromonas hydrophila and Motile Aeromonad Septicemias of Fish; US Department of the Interior, Fish and Wildlife Service, Division of Fishery Research: Washington, DC, USA, 1984; Volume 68. [Google Scholar]
- Scarpellini, M.; Franzetti, L.; Galli, A. Development of PCR assay to identify Pseudomonas fluorescens and its biotype. FEMS Microbiol. Lett. 2004, 236, 257–260. [Google Scholar] [CrossRef]
- Barnes, M.E.; Brown, M.L. A review of Flavobacterium psychrophilum biology, clinical signs, and bacterial cold water disease prevention and treatment. Open Fish Sci. J. 2011, 4, 40. [Google Scholar] [CrossRef]
- Loch, T.P.; Faisal, M. Emerging flavobacterial infections in fish: A review. J. Adv. Res. 2015, 6, 283–300. [Google Scholar] [CrossRef]
- Fraser, S.L.; Jorgensen, J.H. Reappraisal of the antimicrobial susceptibilities of Chryseobacterium and Flavobacterium species and methods for reliable susceptibility testing. Antimicrob. Agents Chemother. 1997, 41, 2738–2741. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.G.; Giri, S.S.; Kim, S.W.; Kwon, J.; Lee, S.B.; Park, S.C. First Isolation and Characterization of Chryseobacterium cucumeris SKNUCL01, Isolated from Diseased Pond loach (Misgurnus anguillicaudatus) in Korea. Pathogens 2020, 9, 397. [Google Scholar] [CrossRef]
- Sundell, K.; Landor, L.; Nicolas, P.; Jørgensen, J.; Castillo, D.; Middelboe, M.; Wiklund, T. Phenotypic and genetic predictors of pathogenicity and virulence in Flavobacterium psychrophilum. Front. Microbiol. 2019, 10, 1711. [Google Scholar] [CrossRef] [PubMed]
- Karami, E.; Alishahi, M.; Molayemraftar, T.; Ghorbanpour, M.; Tabandeh, M.R.; Mohammadian, T. Study of pathogenicity and severity of Lactococcus garvieae isolated from rainbow trout (Oncorhynchus mykiss) farms in Kohkilooieh and Boyerahmad province. Fish. Aquat. Sci. 2019, 22, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pridgeon, J.W.; Klesius, P.H.; Garcia, J.C. Identification and virulence of Chryseobacterium indologenes isolated from diseased yellow perch (Perca flavescens). J. Appl. Microbiol. 2013, 114, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Shahi, N.; Sharma, P.; Pandey, J.; Bisht, I.; Mallik, S.K. Characterization and pathogenicity study of Chryseobacterium scophthalmum recovered from gill lesions of diseased golden mahseer, Tor putitora (Hamilton, 1822) in India. Aquaculture 2018, 485, 81–92. [Google Scholar] [CrossRef]
- Oh, W.T.; Jun, J.W.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Han, S.J.; Kwon, J.; Park, S.C. Isolation of Chryseobacterium siluri sp. nov., from liver of diseased catfish (Silurus asotus). Heliyon 2020, 6, e03454. [Google Scholar] [CrossRef]



| Antibiotics | Susceptibility | Inhibition Zone Diameter (mm) | Antibiotics | Susceptibility | Inhibition Zone Diameter (mm) |
|---|---|---|---|---|---|
| Ampicillin | R | 7 | Piperacillin | R | 9 |
| Cefazolin | R | 10 | Cefepime | R | 9 |
| Cefotaxime | R | 10 | Cefoxitin | S | 22 |
| Ceftazidime | R | 12 | Ceftizoxime | R | 3 |
| Aztreonam | R | 2 | Imipenem | S | 26 |
| Meropenem | S | 28 | Gentamicin | R | 11 |
| Amikacin | R | 4 | Kanamycin | R | 5 |
| Streptomycin | R | 2 | Tetracycline | R | 8 |
| Doxycycline | R | 11 | Ciprofloxacin | S | 24 |
| Nalidixic acid | R | 7 | Norfloxacin | S | 27 |
| Trimethoprim-sulfamethoxazole | R | 9 | Ofloxacin | S | 25 |
| Chloramphenicol | R | 13 | Erythromycin | R | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, W.J.; Kim, S.G.; Giri, S.S.; Kim, S.W.; Kang, J.W.; Kwon, J.; Oh, W.T.; Lee, S.B.; Lee, Y.M.; Jo, S.J.; et al. The Opportunistic Pathogen Chryseobacterium balustinum WLT: Pathogenicity and Antibiotic Resistance. Fishes 2022, 7, 26. https://doi.org/10.3390/fishes7010026
Jung WJ, Kim SG, Giri SS, Kim SW, Kang JW, Kwon J, Oh WT, Lee SB, Lee YM, Jo SJ, et al. The Opportunistic Pathogen Chryseobacterium balustinum WLT: Pathogenicity and Antibiotic Resistance. Fishes. 2022; 7(1):26. https://doi.org/10.3390/fishes7010026
Chicago/Turabian StyleJung, Won Joon, Sang Guen Kim, Sib Sankar Giri, Sang Wha Kim, Jeong Woo Kang, Jun Kwon, Woo Taek Oh, Sung Bin Lee, Young Min Lee, Su Jin Jo, and et al. 2022. "The Opportunistic Pathogen Chryseobacterium balustinum WLT: Pathogenicity and Antibiotic Resistance" Fishes 7, no. 1: 26. https://doi.org/10.3390/fishes7010026
APA StyleJung, W. J., Kim, S. G., Giri, S. S., Kim, S. W., Kang, J. W., Kwon, J., Oh, W. T., Lee, S. B., Lee, Y. M., Jo, S. J., Chi, C., Jun, J. W., & Park, S. C. (2022). The Opportunistic Pathogen Chryseobacterium balustinum WLT: Pathogenicity and Antibiotic Resistance. Fishes, 7(1), 26. https://doi.org/10.3390/fishes7010026








