Abstract
The present paper represents the first description of abnormal hermaphroditism in Galeus melastomus (Rafinesque, 1810). The black mouth cat shark specimen, collected in summer from southern Tyrrhenian Sea at the entrance of Salerno gulf, showed a basic intersexuality characterized by the presence of male secondary and female primary sexual characters. The reproductive system has been described with an accurate morphological analysis, also including a histological assessment of oocytes. Results showed the presence of only right clasper, not exceeding the pelvic fin in length, with the absence of internal males’ sexual organs. Concerning the female primary characters, the analysis of histological assessment showed the structure of mature oocyte, confirming the maturity stage of a mature female in active extruding stage, as highlighted by the macroscopic morphological analysis. Despite in many marine organisms’ hermaphroditism is a physiological condition showed by specimens during their lifecycle, in Elasmobranchs this is considered an abnormal condition, with some exceptions. The lack of information on hermaphroditism in elasmobranchs form the Mediterranean Sea requires an increase of scientific community’s attention, improving the knowledge on the reproductive biology of this sensitive taxon. This is essential to enhance the conservation of elasmobranchs populations in the entire Mediterranean basin.
1. Introduction
The congenital morphological variations are widely reported in elasmobranchs worldwide. According to literature, these abnormalities mainly involve the skeleton morphology [1,2,3,4,5,6,7], the reproductive system [8,9,10,11,12,13,14], and the skin color [15,16,17,18,19]. The most severe morphological malformations are those concerning the skeleton (e.g., deformities in skeleton structure, missing or additional fins, anomalies on cephalic horns, alterations in the cranium), which are mainly observed in embryos, being often fatal beyond birth, as confirmed by the relatively few observations on free swimming individuals [20]. The alterations on skin color (e.g., albinism, leucism and irregular skin pigmentation) or on reproductive system (e.g., abnormal hermaphroditism and anomalies on claspers structure) are considered less severe, having been widely observed in several free-swimming adult specimens [20].
According to previous literature [8], hermaphroditism is defined as the presence in the same individual of male and females primary or secondary sexual characters. Despite in many taxa (teleost fishes included) hermaphroditism is a physiological condition shared by individuals of a species during their ontogeny [21,22,23,24]. In Chondrichthyans this condition is usually defined as “abnormal″, with few exceptions, such as records of normal hermaphroditism in Apristurus longicephalus, Nakaya, 1975, Notorynchus cepedianus, Péron, 1807, Hexanchus griseus, Bonnaterre, 1788, Etmopterus granulosus, Günther, 1880, Centroscymnus coelolepis, Barbosa du Bocage & de Brito Capello, 1864, Heterodontus portusjacksoni, Meyer, 1793, Prionace glauca, Linnaeus, 1758, Scyliorhinus canicula, Linnaeus, 1758, and Scyliorhinus stellaris, Linnaeus, 1758 [25,26,27,28,29,30,31,32]. Difference between normal and abnormal hermaphroditism lies in the uniform presence of both male and female functional gonads in many of all individuals of a species at a given point of their lifetime [33,34]. In abnormal hermaphroditism, also called intersexes, the presence of both sexes characters is not uniform in the individuals of a species and one or both sexes are unfunctional, despite the presence of primary and/or secondary sexual morphological features of either.
Even if hermaphroditism is considered an adaptative way to increase the reproductive yield in teleost fishes [33], intersexuality in Chondrichthyans has usually been considered as an “un-adaptative characteristic″ [8]. Indeed, all the cartilaginous fishes are considered gonochoristic species, i.e., the individuals’ sex is genetically determined, with very evident secondary sexual characters, without sexual inversions during their entire life cycle and with a wide range of reproductive strategies (oviparity, aplacental and placental viviparity) [35,36]. Despite its un-functionality, according to literature, hermaphroditism was recorded worldwide in many families of sharks, such as Triakidae, Squalidae, Somniosidae, Carcharhinidae, Etmopteridae, Centrophoridae, Heterodontidae, Hexanchidae and Scyliorhinidae [14,27,29,31,32,34,37,38,39].
Concerning the Pentanchidae family, no reports are available from literature regarding intersexuality. The blackmouth catshark, Galeus melastomus, (Rafinesque, 1810), is the most common species in Mediterranean Sea belonging to this family. It is ubiquitous in the entire basin [40,41], and it is also widely reported on the Eastern Coast of the Atlantic Ocean [42,43]. It is a demersal generalist opportunist meso-predator [44,45,46] which inhabits the benthic environments with a wide bathymetric range (from 55 to 1400 m) [47]. In its entire distribution area, it is mainly found at a depth range from 300 to 800 m, while in the Tyrrhenian Sea it is mainly distributed from 500 to 800 m [41,48]. It is among the most common Elasmobranch species reported in trawl fisheries by-catch [49,50], but thanks to its bathymetric distribution (extending in depth less exploited by trawling) and to its peculiar life cycle, common among Pentanchidae and Scylliorhinidae species, populations of G. melastomus have maintained their structure despite the high fishing efforts all over the Mediterranean Sea [51,52,53]. G. melastomus is an oviparous species, with a continuous reproductive cycle in almost all the distribution areas and a lifecycle characterized by an early sexual maturation, a shorter generation time and a faster population increment than other Chondrichthyans families.
The aim of this study was to report the first case of abnormal hermaphroditism in G. melastomus from the Southern Tyrrhenian Sea (Central Mediterranean Sea), with an accurate morphological description of the primary and secondary sexual characters.
2. Materials and Methods
Specimens of G. melastomus were regularly collected using trawl nets during the annual scientific trawl-survey MEDITS (International Trawl Survey in the Mediterranean) [54,55]. The blackmouth catshark pool, to which the hermaphrodite specimen belonged, came from the southern Tyrrhenian Sea (Geographical Subarea, GSA-10) (Figure 1a,b). The GSA-10 extends from the coastal area of Campania (41°14′38.7″ N 13°37′04.2″ E) to San Vito lo Capo (38°11′41.0″ N 12°44′29.8″ E), including the entire Sicilian north coast [56].
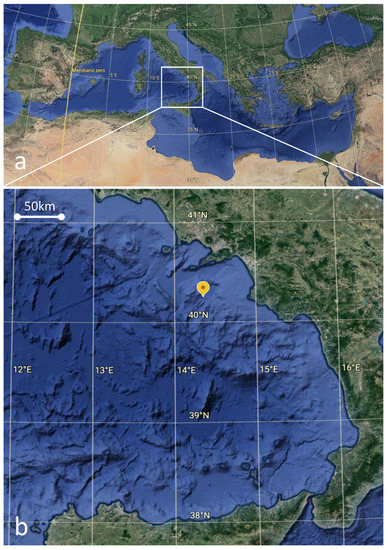
Figure 1.
General view of the Mediterranean basin (a), with a particular of the studied area (GSA 10) (b). The yellow mark indicates the sampling point with coordinates (40°24.087″ N 14°35.396″ E).
Samples collected during autumn 2021 at 316 m of depth were frozen on board to preserve the integrity of biological tissues and were immediately transferred to the laboratory after landing. During the gross necropsy performed in laboratory, each specimen was measured (total length in mm) weighted (weight in g) and sex maturation degree was determined according to Follesa, and Carbonara [57]. Concerning the hermaphrodite specimen, the maturation degree of both expressed sexes was evaluated. Measurements of the reproductive system were also performed: the Inner clasper length (i.e., distance in mm between the distal clasper tip to the insertion point at the cloaca), the Outer clasper length (distance in mm between the clasper tip and the outside insertion point in the pelvic fin), the Uteri (left and right) length (mm), maximum oviducal gland (left and right) diameter (mm) and weight (g), length (mm), width (mm) and weight (g) of ovaries and oocytes diameter (mm). Measurements were taken with an analog caliper (0.01 mm precision) and weight with an analytical balance (accuracy of 0.01 g).
Histological Assessment
Presumed mature eggs were immediately fixed 4% paraformaldehyde in phosphate buffered saline (PBS) 0.1 mol/L (pH 7.4) for 4 h, dehydrated in graded ethanol, cleared in xylene, embedded in Paraplast® (McCormick Scientific, St. Louis, MO, USA) and cut into 5 μm serial sections. The sections were stained with Masson trichrome, and AB pH 2.5 staining followed by PAS (AB/PAS) [58]. Sections were examined under a Leica DM6B microscope equipped with a Leica DFC7000T.
3. Results
Galeus melastomus hermaphrodite specimen was caught in September 2021 at the entrance of Salerno Gulf (40°24.087″ N 14°35.396″ E) (Figure 1b), in a total catch of 15 specimens, ranging in size from 101 to 440 mm. Specimen’s total length and weight were 440 mm and 260 g, respectively (Figure 2a). It showed only the right clasper, which did not exceed the pelvic fin in length (Figure 2b), characterized by a skeleton still flexible, an Inner length of 22.5 mm and an Outer length of 11.6 mm. According to literature [57], the maturation stage of the secondary male sexual character, represented in the studied individual by the single clasper, (in males elasmobranchs claspers normally are two and they are peculiar appendices on the pelvic fins, used during the copulation to transfer sperms inside females’ reproductive system), was attributable to a maturing specimen (Stage 2: presence of robust but still flexible claspers, as long or longer than pelvic fins). Moreover, males’ primary sexual characters (e.g., epididymis, testis, spermiducts) were totally absent.
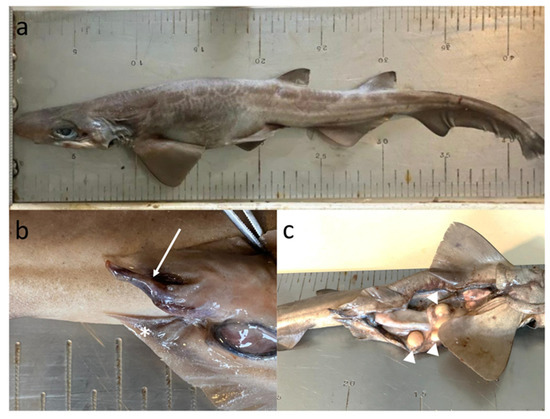
Figure 2.
External lateral view (a) of the Galeus melastomus specimen caught in southern Tyrrhenian Sea, with a particular view of secondary male sexual organs (b) and primary female sexual organs (c). Arrow indicates the external right clasper; asterisk indicates the location of the absent right clasper and arrow heads indicate the large yellow oocytes.
Concerning the females’ sexual characters, according to Follesa and Carbonara [57] the maturation stage was of a mature female in active extruding stage (Stage 3b). As showed in Figure 2c, the ovary was transparent with a lenght of 55 mm and a width of 33 mm. Inside the ovary, the 12 oocytes detected were yellow and different in size (with a diameter from 3 to 17 mm and a weight from 0.13 to 0.46 g). The oviducts, 120 mm in length, showed the presence of large oviducal glands (with a length of 25 and 32.8 mm, and a weight of 0.52 and 1.18 g, respectively) in the second portions and eggs well formed, ready for the emission, in the last portion nearest the cloaca.
The analysis of histological assessment showed the structure of mature oocyte with four different layers from inside to outside as follow: 1-pellucid zone, 2-granular layer, 3-basal surface, 4-theca cells (Figure 3a,b).
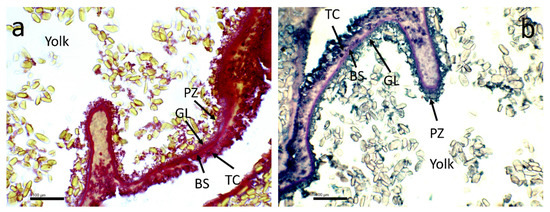
Figure 3.
(a,b) Representative figures of mature oocyte showing four different layers: PZ = pellucid 149 zone, GL = granular layer, BS = basal surface, TC = theca cells, highlighted using Masson trichrome (a) and AB/PAS (b). Scale bars: 100 mm.
4. Discussion
According to the terminology present in [59] and the classification introduced by [34] the elasmobranchs intersexuality can be categorized as follow: (i) basic intersexuality, (ii) incomplete intersexuality and (iii) complete intersexuality. The basic intersexuality is the most reported among Elasmobranchs, resulting in the presence of external claspers and female gonads internally [11,13,39,60,61] or no clasper externally and internal male gonads occurrence [62,63]. Incomplete intersexuality is reported as the category of intersexuality showing the most frequent association of both sexual characters (e.g., the contemporary presence of ovaries, oviduct, testes and claspers; the presence of clasper and testis in gravid females; the presence of both male and female tissue in a gonad, called “ovotestis″; the presence in the mesovarium of a rudimentary testis in females individuals; the absence of claspers with the contemporary presence of ovary, oviduct and a testis) [64,65,66,67,68,69,70]. Finally, in the complete intersexuality, the individuals exhibit both sexes with the most developmental degree and a very frequent improper maturation/development resulting in a no functionality of one or both sexes (e.g., a peritoneal cavity exhibiting both male and female tract in left and right sides; presence of ovotestes; peritoneal cavity exhibiting both male gonad and tract, and female gonad and tract) [29,31,32,37,71].
The specimen of G. melastomus analyzed in present paper showed a basic intersexuality, characterized externally by the presence of the left clasper, while internally by the presence of a well-developed female reproductive tract and the totally absence of any male reproductive organs, such as epididymis or testis. The female reproductive tract was fully functional, also showing the presence of mature big and yellow oocytes and well-formed eggs ready to be deposited. Also, histological analyses highlighted the maturity of oocytes, with a well-developed follicular epithelium differentiated by four layers [72,73].
On our best knowledge, this represents the first description of hermaphroditism in G. melastomus. According to literature, there were other reports of abnormalities only at morphological level in this species. Previous literature [74] have reported a male specimen from the Algerian coast with an abnormal morphology of claspers and pelvic fins (the right pelvic fin smaller than the left, with an inconspicuous right clasper, smaller than the left one). While in 2020 [18] it was reported the first case of a G. melastomus specimen without skin-related structures.
Concerning other elasmobranchs species, several cases of basic intersexuality have been recorded worldwide [34, and references therein]. In the Mediterranean Sea basic intersexuality has been reported in a specimen of Squalus blainville, Risso, 1827, from eastern basin [62], in Torpedo torpedo, Linnaeus, 1758, [68] and in Rayformes species, such as Raja asterias, Delaroche, 1809, [75]. Despite the high occurrence of reported hermaphroditism cases in S. canicula, from the Atlantic Ocean [37,76,77,78], and its high distribution in the Mediterranean basin, only one case has been recorded in Mediterranean population, with no occurrence of basic intersexuality [31]. According to literature reported below, the highest occurrence of hermaphrodites S. canicula in the Atlantic Ocean was recorded in the English Channel, during the first decades of 1900; while, regarding the Mediterranean Sea, the only reported case of hermaphroditism in this species was recorded in Tunisia. Further analysis and monitoring are required to study the occurrence of hermaphroditism in Scyliorhinidae family, especially in Mediterranean Sea.
5. Conclusions
Although the constant monitoring of Mediterranean Sea fisheries and stocks, and the growing interest on elasmobranchs biology and conservation, the lack of knowledge about the occurrence of hermaphroditism in this taxon especially in the Mediterranean basin, requires an increase in effort and attention of the entire marine scientific community. Increase the knowledge about intersexuality in cartilaginous fish is essential to fully understand their population dynamics, biology, and life history traits. These are essentials information to improve their conservation through better fisheries management policies. Moreover, focusing the attention on these peculiar cases, deepening the knowledge with accurate histological assessments and timely reports and descriptions, can clarify the possible causes and ecological dynamics underlying them, improving monitoring and evaluation about their condition in a heavily exploited environments such as the Mediterranean Sea. Further analysis on elasmobranchs populations and stocks are required to understand and monitoring the occurrence of hermaphroditism and other “abnormalities” or modification on the reproductive biology in this sensitive and ecologically essential taxon.
Author Contributions
Methodology, C.D. and S.F.; investigation, M.A.; resources, N.S.; data curation, S.S. and G.C.; writing—original draft preparation, C.D. and M.A.; writing—review and editing, S.S.; visualization, G.C.; supervision, G.C.; project administration, P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. Fish specimen collection was authorized by the MEDITS project as part of annual research surveys, all involving lethal sampling. No experiments were conducted, nor were surgical procedures performed. No procedures caused lasting harm to sentient fish, nor were sentient fish subjected to chemical agents. The care and use of collected animals complied with animal welfare guidelines, laws and regulations set by the Italian Government.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
This study was supported by project MEDITS (International bottom Trawl Survey in the Mediterranean).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bornatowski, H.; Abilhoa, V. Record of an anomalous embryo of Rhinobatos percellens (Elasmobranchii: Rhinobatidae) in the southern coast of Brazil. Mar. Biodivers. Rec. 2009, 2, e36. [Google Scholar] [CrossRef]
- Capapé, C.; Ali, M. Record of dicephalous embryo in Longnose Spurdog Squalus blainvillei (Chondrichthyes: Squalidae) from the Syrian coast (Eastern Mediterranean). In ANNALES Series Historia Naturalis; Scientific and Research Center of the Republic of Slovenia: Pirano, Slovenia, 2017; Volume 27, pp. 59–62. [Google Scholar]
- Castro-Aguirre, J.L.; Torres-Villegas, J.R. Sobre un Caso de Bicefalia Funcional en Rhinoptera Steindachneri Evermann y Jenkins (Chondricthys, Elasmobranchii, Batoidei), Capturado en la Costa Occidental de Baja California, Mexico. Cienc. Mar. 1979, 6, 27–41. [Google Scholar] [CrossRef]
- Delpiani, S.M.; Deli Antoni, M.Y.; Barbini, S.A.; Figueroa, D.E. First record of a dicephalic specimen of tope Galeorhinus galeus (Elasmobranchii: Triakidae). J. Fish Biol. 2011, 78, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Ehemann, N.; Marín-Sanz, J.; Barany-González, M. Nota: Two cases of two-head shark embryos, smalleye smooth-hound Mustelus higmani and the blue shark Prionace glauca. Bol. Investig. Mar. Y Costeras 2016, 45, 149–153. [Google Scholar] [CrossRef]
- Escobar-Sánchez, O.; Galván-Magaña, F.; Downton-Hoffmann, C.A.; Carrera-Fernández, M.; Alatorre-Ramírez, V.G. First record of a morphological abnormality in the longtail stingray Dasyatis longa (Myliobatiformes: Dasyatidae) in the Gulf of California, Mexico. Mar. Biodivers. Rec. 2009, 2, e26. [Google Scholar] [CrossRef]
- Galván-Magaña, F.; Escobar-Sánchez, O.; Carrera-Fernández, M. Embryonic bicephaly in the blue shark, Prionace glauca, from the Mexican Pacific Ocean. Mar. Biodivers. Rec. 2011, 4, e1. [Google Scholar] [CrossRef]
- Atz, J.W. Intersexuality in fishes. In Intersexuality in Vertebrates Including Man; Marshall, A.J., Armstrong, C.N., Eds.; Academic Press: New York, NY, USA, 1964; pp. 145–232. [Google Scholar]
- Capapé, C.; El Kamel-Moutalibi, O.; Mnasri, N.; Boumaïza, M.; Reynaud, C. A case of hermaphroditism in tortonese’s stingray, Dasyatis tortonesei (Elasmobranchii: Rajiformes: Dasyatidae) from the lagoon of bizerte, Tunisia. Acta Ichthyol. Piscat. 2012, 42, 141–149. [Google Scholar] [CrossRef]
- Fields, A.T.; Feldheim, K.A.; Poulakis, G.R.; Chapman, D.D. Facultative parthenogenesis in a critically endangered wild vertebrate. Curr. Biol. 2015, 25, R446–R447. [Google Scholar] [CrossRef]
- Gianeti, M.D.; Vooren, C.M. A hermaphrodite guitarfish, Rhinobatos horkelii (Müller & Henle, 1841) (Rajiformes: Rhinobatidae), from southern Brazil. Cah. Biol. Mar. 2007, 48, 407–409. [Google Scholar]
- González-gonzález, L.V. Primer Reporte de Mono Clasper en Zapteryx Exasperata Para El Golfo de California, México Genéticas O. Compendio de Resúmenes Orales Y Carteles del XV Congreso Nacional de Ictiología, V Simposio Latinoamericano de Ictiología; I Simposio Internacional de Genómica de Peces: Aguascalientes, Mexico, 2016; Volume 9, pp. 213–214. [Google Scholar]
- Haas, D.L.; Ebert, D.A. First Record of Hermaphroditism in the Bering Skate, Bathyraja interrupta. Northwest Nat. 2008, 89, 181–185. [Google Scholar] [CrossRef]
- Costa, M.E.; Borges, T.C.; Capapé, C. Cases of abnormal hermaphroditism in velvet belly Etmopterus spinax (Chondrichthyes: Etmopteridae) from the southern coast of Portugal. Cah. Biol. Mar. 2013, 54, 309–317. [Google Scholar] [CrossRef]
- Ball, R.E.; Jones, C.S.; Lynghammar, A.; Noble, L.R.; Griffiths, A.M. The first confirmed cases of full albinism in rajid species. J. Fish Biol. 2013, 82, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Becerril-García, E.E.; Tamburin, E.; González-Armas, R.; Galván-Magaña, F. First record of albinism in the swell shark, Cephaloscyllium ventriosum (Elasmobranchii: Carcharhiniformes: Scyliorhinidae). Acta Ichthyol. Piscat. 2017, 47, 201–204. [Google Scholar] [CrossRef]
- Souissi, J.B.; Golani, D.; Méjri, H.; Salem, M.B.; Capapé, C. First confirmed record of the Halave’s Guitarfish, Rhinobatos halavi (Forsskål, 1775) (Chondrichthyes: Rhinobatidae) in the Mediterranean Sea with a description of a case of albinism in elasmobranchs. Cah. Biol. Mar. 2007, 48, 67–75. [Google Scholar]
- Mulas, A.; Bellodi, A.; Porcu, C.; Cau, A.; Coluccia, E.; Demurtas, R.; Marongiu, M.F.; Pesci, P.; Follesa, M.C. Living naked: First case of lack of skin-related structures in an elasmobranch, the blackmouth catshark (Galeus melastomus). J. Fish Biol. 2020, 97, 1252–1256. [Google Scholar] [CrossRef]
- Bottaro, M.; Ferrando, S.; Gallus, L.; Girosi, L.; Vacchi, M. First record of albinism in the deep-water shark Dalatias licha. Mar. Biodivers. Rec. 2008, 1, e10. [Google Scholar] [CrossRef]
- Moore, A.B.M. Morphological abnormalities in elasmobranchs. J. Fish Biol. 2015, 87, 465–471. [Google Scholar] [CrossRef]
- Smith, C.L.; Armstrong, C.N.; Marshall, A.J. Intersexuality in Vertebrates Including Man. Copeia 1967, 1967, 692. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Greco, S.; Sophronidis, K.; Lembo, G.; Giordano, D.; Argyri, A. Geographical distribution, abundance and some population characteristics of the species of the genus Pagellus (Osteichthyes: Perciformes) in different areas of the Mediterranean. Sci. Mar. 2002, 66, 65–82. [Google Scholar] [CrossRef]
- Busalacchi, B.; Bottari, T.; Giordano, D.; Profeta, A.; Rinelli, P. Distribution and biological features of the common pandora, Pagellus erythrinus (Linnaeus, 1758), in the southern Tyrrhenian Sea (Central Mediterr). Helgol. Mar. Res. 2014, 68, 491–501. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; Di Paola, D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spanò, N.; et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 16315. [Google Scholar] [CrossRef] [PubMed]
- Iglésias, S.P.; Sellos, D.Y.; Nakaya, K. Discovery of a normal hermaphroditic chondrichthyan species: Apristurus longicephalus. J. Fish Biol. 2005, 66, 417–428. [Google Scholar] [CrossRef]
- Daniel, J.F. The Elasmobranch Fishes. [With Plates]; University of California Press: Berkeley, CA, USA, 1928. [Google Scholar]
- Irvine, S.B.; Laurenson, L.J.B.; Stevens, J.D.; Martin, R.A.; MacKinlay, D. Hermaphroditism in the Southern Lanternshark, Etmopterus granulosus. Biology of Deep Sea Elasmobranchs. In Biology of Deep Sea Elasmobranchs, Proceedings of the International Congress on the Biology of Fish, Vancouver, BC, Canada, 21-25 July 2002; University of British Columbia: Vancouver, BC, Canada, 2002; Volume 1, pp. 49–54. [Google Scholar]
- Jones, A.A.; Potter, I.C. Description of the reproductive tract and gonad histology of a second form of hermaphroditism in the Port Jackson shark Heterodontus portusjacksoni. J. Mar. Biol. Assoc. UK 2009, 89, 1403–1407. [Google Scholar] [CrossRef]
- Jones, A.A.; White, W.T.; Potter, I.C. A hermaphroditic Port Jackson shark, Heterodontus portusjacksoni, with complete and separate female and male reproductive tracts. J. Mar. Biol. Assoc. UK 2005, 85, 1171–1172. [Google Scholar] [CrossRef]
- Pratt, H.L. Reproduction in the Blue Shark, Prionace Glauca. Fish. Bull. 1979, 77, 445–470. [Google Scholar]
- Capapé, C.; Zahnd, J. Cas d’hermaphrodisme chez Scyliorhinus canicula (Linné, 1758). Bull. Inst. Océanographique 1974, 3, 131–138. [Google Scholar]
- Capapè, C.; Chadli, A.; Baouendi, A. A case of hermaphroditism in Scyliorhinus stellaris (Linné, 1758) (Pisces, Scyliorhinidae): Morphological and histological study. Arch. Inst. Pasteur Tunis 1979, 56, 343–351. [Google Scholar]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Hendon, J.M.; Koester, D.M.; Hoffmayer, E.R.; Driggers, W.B.; Cicia, A.M. Occurrence of an Intersexual Blacktip Shark in the Northern Gulf of Mexico, with Notes on the Standardization of Classifications for This Condition in Elasmobranchs. Mar. Coast. Fish. 2013, 5, 174–180. [Google Scholar] [CrossRef]
- Dodd, J.M. Reproduction in cartilaginous fishes (chondrichthyes). In Fish Physiology; Elsevier: Amsterdam, Netherlands, 1983; Volume 9, pp. 31–95. ISBN 1546-5098. [Google Scholar]
- Luer, C.A. Sharks, Skates, and Rays: The Biology of Elasmobranch Fishes; JHU Press: Baltimore, MD, USA, 2000; Volume 2000, ISBN 0801860482. [Google Scholar]
- King, A.D. Hermaphroditism in the Common dogfish (Scyliorhinus caniculus). J. Zool. 1966, 148, 312–314. [Google Scholar] [CrossRef]
- Irvine, S.B. Age, Growth and Reproduction of Deepwater Dogfishes from Southeastern Australia; Deakin University: Victoria, Australia, 2004; p. 397. [Google Scholar]
- Zhao, Y.; Chen, M.; Jiang, C.; Yang, S.; Xiao, J. Occurrence of an Intersexual Pacific Spadenose Shark Scoliodon macrorhynchos from the Southern Taiwan Strait. Mar. Coast. Fish. 2017, 9, 573–576. [Google Scholar] [CrossRef]
- D’Iglio, C.; Savoca, S.; Rinelli, P.; Spanò, N. Diet of the Deep-Sea Shark Galeus melastomus Rafinesque, 1810, in the Mediterranean Sea: What We Know and What We Should Know. Sustainability 2021, 13, 3962. [Google Scholar] [CrossRef]
- Rinelli, P.; Bottari, T.; Florio, G.; Romeo, T.; Giordano, D.; Greco, S. Observations on distribution and biology of Galeus melastomus (Chondrichthyes, Scyliorhinidae) in the southern Tyrrhenian Sea (central Mediterranean). Cybium 2005, 29, 41–46. [Google Scholar]
- Van der Land, J.; Costello, M.J.; Zavodnik, D.; Santos, R.S.; Porteiro, F.M.; Bailly, N.; Eschmeyer, W.N.; Froese, R. Pisces. Collect. Patrim. Nat. 2001, 50, 357–374. [Google Scholar]
- Compagno, L.J. V Sharks of the world. An annotated and illustrated catalouge of shark species to date. Part II (Carcharhiniformes). FAO Fish. Synopsis 1984, 4, 250–655. [Google Scholar]
- D’Iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and Ecological Aspects of the Blackmouth Catshark (Galeus melastomus Rafinesque, 1810) in the Southern Tyrrhenian Sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- Albo-Puigserver, M.; Navarro, J.; Coll, M.; Aguzzi, J.; Cardona, L.; Sáez-Liante, R. Feeding ecology and trophic position of three sympatric demersal chondrichthyans in the northwestern Mediterranean. Mar. Ecol. Prog. Ser. 2015, 524, 255–268. [Google Scholar] [CrossRef]
- Yemisken, E.; Navarro, J.; Forero, M.; Megalofonou, P.; Eryilmaz, L. Trophic partitioning between abundant demersal sharks coexisting in the North Aegean Sea. J. Mar. Biol. Assoc. UK 2019, 99, 1213–1219. [Google Scholar] [CrossRef]
- Serena, F.; Mancusi, C.; Ungaro, N.; Hareide, N.R.; Guallart, J.; Coelho, R.; Crozier, P. Galeus melastomus. IUCN Red List Threat. Species 2009, e.T161398A124477972. [Google Scholar] [CrossRef]
- Psomadakis, P.N.; Giustino, S.; Vacchi, M. Mediterranean fish biodiversity: An updated inventory with focus on the Ligurian and Tyrrhenian seas. Zootaxa 2012, 3263, 1–46. [Google Scholar] [CrossRef]
- Sion, L.; Bozzano, A.; D’Onghia, G.; Capezzuto, F.; Panza, M. Chondrichthyes species in deep waters of the Mediterranean Sea. Sci. Mar. 2004, 68, 153–162. [Google Scholar] [CrossRef]
- Ragonese, S.; Di Stefano, L.; Bianchini, M.L. Catture e selettività di pesci cartilaginei nella pesca dei gamberi rossi nello Stretto di Sicilia. Biol. Mar. Medit. 2000, 7, 400–401. [Google Scholar]
- Rey, J.; de Sola, L.G.; Massutí, E. Distribution and biology of the blackmouth catshark Galeus melastomus in the Alboran Sea (Southwestern Mediterranean). J. Northwest Atl. Fish. Sci. 2005, 35, 215–223. [Google Scholar] [CrossRef]
- Ramírez-Amaro, S.; Ordines, F.; Terrasa, B.; Esteban, A.; García, C.; Guijarro, B.; Massutí, E. Demersal chondrichthyans in the western Mediterranean: Assemblages and biological parameters of their main species. Mar. Freshw. Res. 2016, 67, 636–652. [Google Scholar] [CrossRef]
- Gouraguine, A.; Hidalgo, M.; Moranta, J.; Bailey, D.M.; Ordines, F.; Guijarro, B.; Valls, M.; Barberá, C.; De Mesa, A. Elasmobranch spatial segregation in the western Mediterranean. Sci. Mar. 2011, 75, 653–664. [Google Scholar] [CrossRef]
- Bertrand, J.; Teresa Spedicato, M. International Bottom Trawl Survey in the Mediterranean Instruction Manual. 2017, p. 106. Available online: https://archimer.ifremer.fr/doc/00002/11321/ (accessed on 4 May 2022).
- Bertrand, J.A.; Relini, L.O.; Papaconstantinou, C.; Jukic-Peladic, S.; Souplet, A.; de Sola, L.G.; Piccinetti, C.; Kavadas, S.; Rossi, M. Mediterranean marine demersal resources: The Medits international trawl survey (1994–1999). Sci. Mar. 2002, 66 (Suppl. 2), 103–124. [Google Scholar]
- Perdichizzi, A.; D’Iglio, C.; Giordano, D.; Profeta, A.; Ragonese, S.; Rinelli, P. Comparing life-history traits in two contiguous stocks of the deep-water rose shrimp Parapenaeus longirostris (H. Lucas, 1846) (Crustacea: Decapoda) in the Southern Tyrrhenian Sea (Central Mediterranean Sea). Fish. Res. 2022, 248, 106206. [Google Scholar] [CrossRef]
- Follesa, M.C.; Carbonara, P. Atlas of the Maturity Stages of Mediterranean Fishery Resources. In Studies and Reviews n. 99; FAO: Rome, Italy, 2019; ISBN 9789251319758. [Google Scholar]
- Lauriano, E.R.; Pergolizzi, S.; Aragona, M.; Montalbano, G.; Guerrera, M.C.; Crupi, R.; Faggio, C.; Capillo, G. Intestinal immunity of dogfish Scyliorhinus canicula spiral valve: A histochemical, immunohistochemical and confocal study. Fish Shellfish Immunol. 2019, 87, 490–498. [Google Scholar] [CrossRef]
- Compagno, L.J.V. Checklist of living Chondrichthyes. In Reproductive Biology and Phylogeny of Chondrichthyes; Hamlett, W.C., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 513–517. [Google Scholar]
- Yano, K.; Tanaka, S. Hermaphroditism in the lantern shark Etmopterus unicolor (Squalidae, chondrichthyes). Jpn. J. Ichthyol. 1989, 36, 338–345. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.C.; Sosa-Nishizaki, O. Reproductive biology of the brown smoothhound shark Mustelus henlei, in the northern Gulf of California, México. J. Fish Biol. 2008, 73, 782–792. [Google Scholar] [CrossRef]
- Kousteni, V.; Megalofonou, P. Reproductive Biology of Squalus Blainvillei (Risso, 1826) in the Eastern Mediterranean Sea. Rapp. Comm. Int. Mer Médit. 2010, 39, 562. [Google Scholar]
- Springer, S.; Lowe, R.H. A New Smooth Dogshark, Mustelus higmani, from the Equatorial Atlantic Coast of South America. Copeia 1963, 1963, 245. [Google Scholar] [CrossRef]
- Veríssimo, A.; Gordo, L.; Figueiredo, I. Reproductive biology and embryonic development of Centroscymnus coelolepis in Portuguese mainland waters. ICES J. Mar. Sci. 2003, 60, 1335–1341. [Google Scholar] [CrossRef]
- Templeman, R. Characteristics Between Populations of Thorny Skate (Raja radiata) in the Northwest Atlantic. J. Northwest Atl. Fish. Sci. 1987, 7, 155–167. [Google Scholar] [CrossRef]
- Castro, J.I. Biology of the blacktip shark, Carcharhinus limbatus, off the southeastern United States. Bull. Mar. Sci. 1996, 59, 508–522. [Google Scholar]
- Yano, K. Studies on Morphology, Phylogeny, Taxonomy and Biology of Japanese Squaloid Sharks, Order Squaliformes. Doctoral Dissertation, Tokai University, Shimizu, Shizouka, Japan, 1985. [Google Scholar]
- Dalù, M.; Consalvo, G.C.; Romanelli, M. A hermaphrodite specimen of Torpedo torpedo (Chondrichthyes, Torpedinidae). Biol. Mar. Mediterr. 2003, 10, 792–794. [Google Scholar]
- Semper, C. Das Urogenitalsystem der Plagiostomen Und Seine Bedeutung Für das Uebrigen Wirbelthiere; Druck und Verlag der Stahel’schen Buch-und Kunsthandlung: Würzburg, Germany, 1875. [Google Scholar]
- Daniel, J.F. The Elasmobranch Fishes; University of California: Oakland, CA, USA, 1934. [Google Scholar]
- Braccini, J.M. An abnormal hermaphrodite piked spurdog, Squalus megalops, schooling with mature males. Mar. Biodivers. Rec. 2009, 2, e132. [Google Scholar] [CrossRef]
- Soto-López, K.; Ochoa-Báez, R.I.; Tovar-Ávila, J.; Galván-Magaña, F. Reproductive biology of the brown smooth-hound shark, Mustelus henlei (Chondrichthyes: Triakidae), off northwestern Mexico based on macroscopic and histological analyses. Ciencias Mar. 2018, 44, 125–139. [Google Scholar] [CrossRef]
- Serrano-López, J.N.; Soto-López, K.; Ochoa-Báez, R.I.; O’Sullivan, J.; Galván-Magaña, F. Morphometry and histology to assess the maturity stage of three endangered devil ray species (Elasmobranchii: Mobulidae) from the Gulf of California. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1624–1635. [Google Scholar] [CrossRef]
- Capapé, C.; Kassar, A.; Reynaud, C.; Hemida, F. Atypical characteristics in blackmouth catshark, Galeus melastomus (Chondrichthyes: Scyliorhinidae) from the Algerian coast (southern Mediterranean Sea). Thalass. Salentina 2019, 41, 23–32. [Google Scholar] [CrossRef]
- Quignard, J.P.; Negla, N. Anomalies au niveau du système génital chez les sélaciens rajiformes. Trav. Du Lab. Biol. Halieut. L’université Rennes 1971, 5, 121–124. [Google Scholar]
- Arthur, D.R. Abnormalities in the Sexual Apparatus of the Common Dogfish (Scyliorhinus caniculus). In Proceedings of the Linnean Society of London; Oxford University Press: New York, NY, USA, 1950; Volume 162, pp. 52–56. [Google Scholar]
- Baker, J.R.; Oxon, B.A. An Hermaphrodite Dogfish (Scyliorhinus canicula). J. Anat. 1919, 58, 335. [Google Scholar]
- Fuller, A.S.; Zacharov, J.M. Abnormalities in the Urinogenital System of the Common Dogfish. Nature 1960, 185, 50. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).