Abstract
Fermented soybean meal (FSM) is an important feed material that can replace fish meal to solve the shortage of animal protein. To improve the utilization of FSM, we optimized the co-fermentation conditions of soybean meal using Bacillus subtilis and Enterococcus faecium and studied the effects of replacing fish meal with different proportions of FSM on serum antioxidant indices and gut microbiota (GM) composition of crucian carp (Carassius auratus). Our results showed that the co-fermentation of soybean meal was the most effective when the ratio of B. subtilis X-2 and E. faecium X-4 was 2:3, glucose addition was 4.5%, KH2PO4 addition was 0.15%, MgSO4·7H2O addition was 0.1%, anhydrous sodium acetate addition was 0.4%, fermentation time was 120 h, and the solid–water ratio was 1:1. Replacing 40% fish meal with FSM in the feed significantly improved the serum T-AOC, POD, and IgM levels in C. auratus. Although there were significant differences in the midgut and hindgut microbiota structures of C. auratus, the addition of FSM to the feed did not cause significant differences in the GM structure, whether in the midgut or hindgut. Therefore, 40% FSM is the most suitable substitute for fish meal in the feed of C. auratus.
1. Introduction
Aquaculture has emerged as the major acquisition method for aquatic products owing to a decrease in wild fishery resources [1]. According to the latest worldwide statistics on aquaculture compiled by FAO, global aquatic animal production reached 82.1 million metric tons and global aquaculture production of farmed aquatic animals showed an average annual growth of 5.3% during the period from 2011 to2018 [2]. Although aquaculture provides a large amount of high-quality protein for human consumption, the increased market demand for animal protein feed such as fish meal, owing to the development of aquaculture, feed processing, and other industries, has resulted in an increasingly serious shortage of such resources [3]. Although plant proteins are an important substitute for animal proteins [3,4,5], most plant protein feeds contain a greater number of anti-nutritional factors, and an unbalanced proportion of amino acids and vitamins, which affects their utilization in aquaculture [3].
Microbial fermentation of plant protein feed can degrade its constituent biological macromolecules such as polysaccharides and proteins into easily absorbed small molecules, reduce its anti-nutritional factors, and improve its palatability [6]. Moreover, fermented feed can regulate the microecological balance of aquaculture animal intestines, thereby improving the production of such animals [7].
Soybean meal has 40–60% crude protein content, and is rich in minerals, vitamins, crude fiber, and functional factors (such as soybean isoflavones). Therefore, it can be used as a high-quality plant protein source to replace animal protein components, such as fish meal in feed [8]. However, untreated soybean meal has poor palatability and contains several anti-nutritional factors; thus, direct intake can lead to adverse reactions such as pancreatic swelling, intestinal structure damage, and allergy, as well as cause intestinal tissue abnormalities, hinder nutrient absorption, and reduce animal growth performance [9,10]. These factors limit the application of soybean meal to animal feed.
Microbial fermentation is an important means of improving the nutrient utilization efficiency of soybean meal [11,12], which improves soybean meal palatability and reduces the anti-nutritional factors. For instance, Refstie et al. [13] reported that lactic acid bacterial fermentation significantly reduced the content of trypsin inhibitors in soybean powder. Wang et al. [14] used fermented soybean meal (FSM) instead of fish meal and found that an increase in substitution levels led to a gradual increase in the feeding rate and feed conversion rate of juvenile large yellow croaker (Larimichthys crocea). However, FSM can replace only up to 45% of fish meal in the diets of yellow croaker. The dominant microbiota taxa in the intestines of L. crocea were Proteobacteria and Bacteroidetes. The gut microbiota (GM) is known to specifically influence the growth rate, antioxidant capacity, and immunity. FSM had no negative effect on the growth performance and intestinal integrity of L. crocea. Yan et al. [15] fed Phoxinus lagowskii with FSM instead of fish meal and found that an increase in the substitution ratio caused a decrease in the final body weight, as well as weight gain, specific growth, and protein sedimentation rates. These indices were significantly lower than those in the control group when 49.5–66% FSM was substituted for fish meal, along with significantly lower muscle crude protein, serum superoxide dismutase, and lysozyme. Simultaneously, they found that less than 33% substitution had insignificant effect on growth performance, feed utilization, and nonspecific immune response. Rahimnejad et al. [16] found the best results at 26.9–37.1% FSM substitution levels. Li et al. [17] compared the substitution effects of soybean meal with those of FSM fermented using Enterococcus faecium over fish meal on the growth, antioxidant levels, GM, morphology, and inflammatory response of turbot (Scophthalmus maximus). They observed a worsened growth performance with soybean meal. Compared with the control group, the FSM group had no significant differences; the highest values of lysozyme, total antioxidant capacity, complement 3, superoxide dismutase, and catalase were observed in the control group, followed by those in the FSM group, and the lowest in the soybean meal group. However, the result for malondialdehyde content was the opposite. There was obvious intestinal inflammation (villi and microvilli became shorter and wider; inflammatory cells appeared) in the soybean meal group, which was alleviated in the FSM group. The expression levels of interleukin-1β, tumor necrosis factor α, and IL-8 genes were the highest in the intestines of the soybean meal group, followed by that in the FSM group and the lowest in the control group. The GM of the control group showed greater similarity with that of the FSM group than with that of the soybean meal group.
Crucian carp (Carassius auratus) is an important freshwater aquaculture species in China. It has the advantages of a wide variety of feeds, strong adaptability and disease resistance, delicious meat, and high nutritional value. C. auratus is also an important ornamental fish [18]. Therefore, a study on the substitution of fish meal in C. auratus feed is of great significance to reduce its feeding cost and improve the comprehensive breeding performance.
In this study, two bacterial species with high protease activity were isolated from the soil, and the aerobic-anaerobic solid raw meal fermentation method was used to optimize the fermentation conditions. In addition, the effects of replacing fish meal with FSM in C. auratus feed on the serum antioxidation and GM of C. auratus were also studied. The results of this study can provide a basis for a rational utilization of soybean meal, improving animal gut microecological environment, and promoting animal health.
2. Materials and Methods
2.1. Source of Fermentation Strain and Fermentation Process
Bacillus subtilis X-2 and E. faecium X-4 were isolated from the soil in a peanut-planted experimental field of Hunan Agricultural University. Because the degradation rate of soybean globulin (DRSG) of soybean meal co-fermented with both B. subtilis X-2 and E. faecium X-4 was higher than that fermented with B. subtilis X-2 or E. faecium X-4, the soybean meal was co-fermented with both B. subtilis X-2 and E. faecium X-4 in this study. The fermentation conditions of strain ratio of B. subtilis X-2 and E. faecium X-4, glucose addition, KH2PO4 addition, MgSO4·7H2O addition, anhydrous sodium acetate addition, solid–water ratio, and fermentation time were optimized sequentially according to the DRSG. The soybean globulin content was determined using a kit (Shanghai Enzyme-linked Biotechnology Co., Ltd., China). First, B. subtilis X-2 and E. faecium X-4 were mixed in the proportions of 1:1, 2:3, 3:2, and 2:1 after which 10% of each mixture was inoculated into soybean meal solid fermentation medium by maintaining a solid–water ratio of 1:1, and then fermented at 37 °C for 120 h under natural pH conditions (pH = 7.0 ± 0.1). The soybean globulin content in the FSM was determined and DRSG was calculated. The proportion of strains with the highest DRSG was selected as the optimal strain ratio for the next experiment. Then, under optimal strain ratio, 3%, 3.5%, 4%, 4.5%, and 5% glucose was added to the solid fermentation medium of the basic fermented soybean meal. After fermentation for 120 h under the same conditions, the glucose content with the highest DRSG was selected as the optimal glucose concentration for subsequent experiments. Finally, under the optimal strain ratio and glucose addition conditions, 0.05%, 0.1%, 0.15%, 0.2%, and 0.25% KH2PO4 were added to the solid fermentation medium of the basic soybean meal. After fermentation for 120 h under the same conditions, the KH2PO4 percentage with the highest DRSG was considered to be the best KH2PO4 strength. Under the above optimized conditions, 0.05%, 0.1%, 0.15%, 0.2%, and 0.25% MgSO4·7H2O were added to the solid fermentation medium of the basic soybean meal. After fermentation for 120 h under the same conditions, MgSO4·7H2O concentration with the highest DRSG was considered as the optimal addition amount. Then, under the optimized conditions, 0.3%, 0.4%, 0.5%, 0.6%, and 0.7% anhydrous sodium acetate was added to the solid fermentation medium of the basic soybean meal. After fermentation for 120 h, the anhydrous sodium acetate amount with the highest DRSG was assumed as optimal addition. Under the above optimized conditions, the solid–water ratio of basic soybean meal solid fermentation medium was changed to 1:0.7, 1:0.8, 1:0.9, 1:1, and 1:1.1, respectively. After fermentation for 120 h, the solid–water ratio with the highest DRSG was used as the optimal solid–water ratio for subsequent experiments. Finally, considering all the above optimized conditions, the first sampling was started after fermenting the medium for 48 h, and then sampling it every 24 h until 144 h of fermentation. The time with the highest DRSG was used as the optimal fermentation time.
2.2. Preparation of FSM
Soybean meal was purchased from Hunan Agricultural University Pursuit Feed Co., Ltd., Changsha, China. The FSM was fermented using B. subtilis X-2 and E. faecium X-4 under the optimized fermentation conditions already mentioned. The crude protein and crude fat contents of FSM were 41.7% and 0.7%, respectively.
2.3. Experimental Design and Experimental Feed Preparation
The experimental feed formula and nutritional composition were based on our previous report [19]. The composition and nutritional levels of the basic feed are shown in Table 1. Twenty percent increment of fish meal substitution (0%, 20%, 40%, 60%, and 80%) with FSM were prepared (labelled FSM0, FSM20, FSM40, FSM60, and FSM80, respectively) with equal nitrogen and energy. After being fully crushed, the raw materials were sifted through a 0.425 mm aperture screen, weighed, mixed according to the feed formula, granulated, dried, and stored in a cool storeroom.

Table 1.
Ingredients and proximate composition of experimental diets (% dry-matter basis).
2.4. Experimental Conditions
The experiment was conducted in fish pond culture cages (1.5 × 1.5 × 1.5 m3) in the Yunyuan Aquaculture Experimental Base of Hunan Agricultural University. The fish used in the experiment were hybrid C. auratus (carp ♂ × white C. auratus ♀). Six hundred C. auratus (29.24 ± 0.07 g) with healthy physique and similar weight were selected and divided into five groups, with four repetitions in each group and 30 fish in each repetition. The experiment was started after feeding the fish with basic feed for one week. The daily feeding amount was 3% of the body weight of the fish. The fish were fed twice a day (7:30 and 17:30), and the experiment lasted 56 days. During the experiment, the water temperature was 28.0 ± 1.82 °C (between 26 °C and 30 °C), pH was 8.0 ± 0.63 (between 7 and 9), dissolved oxygen was 4.0 ± 0.59 mg L–1 (between 3 and 5 mg L–1), ammonia nitrogen content was 0.04 ± 0.02 mg L–1 (less than 0.05 mg L–1), and nitrite nitrogen content was 0.004 ± 0.001 mg L–1 (less than 0.005 mg L–1).
2.5. Sample Collection
At the end of the experiment, the fish in each cage were weighed and counted. Six fish of uniform size were randomly collected from each cage. After anaesthetizing them with MS-222 and weighing, blood was collected from their tail vein using a 2 mL disposable syringe and then placed in a 2 mL centrifuge tube. After incubation at 4 °C for 2 h, serum was collected, centrifuged at 1800× g for 10 min, and used for the determination of serum biochemical indices. Then, four fish were randomly selected and the contents of the midgut and hindgut were collected into freezing tubes and stored at −80 °C for GM composition analysis.
2.6. Determination of Serum Indices of C. auratus
Total antioxidant capacity (T-AOC), peroxidase (POD), total superoxide dismutase (T-SOD), malondialdehyde (MDA), catalase (CAT), and immunoglobulin M (IgM) in the serum were measured using kits (Nanjing Jiancheng Bioengineering Co., Ltd., Nanjing, China) according to the manufacturer’s instructions.
2.7. Analysis of GM Composition
The total DNA in the intestinal contents was extracted using a Magabio soil/fecal genomic DNA purification kit (Bioer, Hangzhou, China). Prokaryotic universal primers 338F (5′- ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were used to amplify the V3-V4 region of the 16S rRNA gene, as previously described [20]. The sequenced paired-end raw reads were filtered using fastp version 0.14.1 software to remove the splice sequences and low-quality sequences (quality score <20, or length <200 bp) and then spliced into raw tags. Subsequently, the quality of raw tags was quality controlled using USEARCH version 10.0.240 and low-quality tags (sequence mismatched bases >5 bp, alignment similarity <90%, or sequence overlap <16 bp) were removed. The remaining high-quality tags were then clustered into operational taxonomic units (OTUs) at 97% identity using UPARSE [21]. The taxonomic assignment of each OTU was determined using the Ribosomal Database Project classifier [22] by referring to the Greengenes gg_13_8_otus database [23]. DNA extraction and high-throughput sequencing were performed by the Guangdong Meige Gene Technology Co., Ltd., Guangdong, China.
2.8. Data Analysis
Data were expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) with the Tukey–Kramer post-hoc test was conducted using R 2.5.1 [24]. Nonparametric multivariate analysis of variance (PERMANOVA) [25] was conducted using the vegan package [26] in R 3.5.1 [24]. A heatmap profile was drawn using the pheatmap package in R. Differences with p < 0.05 were considered significant.
3. Results
3.1. Optimization Results of Fermentation Conditions
The ratio of B. subtilis X-2 and E. faecium X-4 had no significant effect on the DRSG; however, the highest DRSG, up to 30.41%, was observed at a ratio of 2:3 (Figure 1A). Therefore, the ratio was set as 2:3 for the follow-up experiment. With increasing glucose addition, the DRSG in the FSM increased, with the highest DRSG (33.78%) observed at a glucose concentration of 4.5% (Figure 1B), which was determined as the optimum glucose concentration. DRSG increased with the addition of KH2PO4, with the highest value (34.73%) observed at a KH2PO4 concentration of 0.15%. When KH2PO4 concentration increased more than 0.15%, DRSG decreased (Figure 1C). Therefore, 0.15% was the most appropriate amount of added KH2PO4. DRSG increased after the addition of MgSO4·7H2O, and reached 34.83% at a MgSO4·7H2O concentration of 0.1%. However, it decreased with continued addition of MgSO4·7H2O (Figure 1D). Therefore, the amount of MgSO4·7H2O added was set as 0.1% for the subsequent tests. The highest DRSG (35.24%) was observed at 0.4% anhydrous sodium acetate content. However, it decreased when amounts higher or lower than this concentration (0.4%) were added (Figure 1E). Therefore, the most suitable amount of added anhydrous sodium acetate was determined as 0.4%. DRSG increased with increased fermentation time and attained the highest value (36.15%) for a fermentation time of 120 h (Figure 1F), which was determined as the optimum fermentation time. With an increase in the solid–water ratio of the solid medium for soybean meal fermentation, the DRSG first increased and then decreased. DRSG reached the highest point (36.85%) for a solid–water ratio of 1:1 (Figure 1G), which was the most suitable solid–water ratio. Based on the above observations, the optimal fermentation conditions were determined as follows: B. subtilis X-2 and E. faecium X-4 in the ratio 2:3; 4.5% added glucose; 0.15% added KH2PO4; 0.1% added MgSO4·7H2O; 0.4% added anhydrous sodium acetate; fermentation time of 120 h; a solid–water ratio of 1:1. Under these conditions, DRSG was 36.85%.
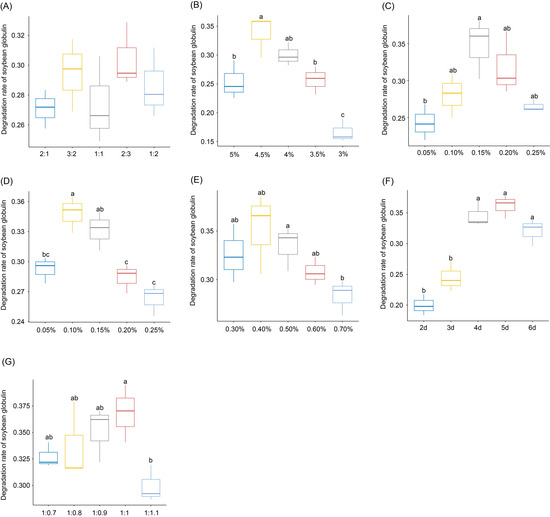
Figure 1.
Effects of different fermentation conditions on the degradation rate of soybean globulin (DRSG) in fermented soybean meal (FSM). (A) Effect of the proportion of Bacillus subtilis X-2 and Enterococcus faecium X-4 on the DRSG in FSM; (B) Effect of glucose addition on the DRSG in FSM; (C) Effect of KH2PO4 addition on the DRSG in FSM; (D) Effect of MgSO4·7H2O addition on the DRSG in FSM; (E) Effect of anhydrous sodium acetate on the DRSG in FSM; (F) Effect of fermentation time on the DRSG in FSM; (G) Effect of solid water ratio on the DRSG in FSM. The difference in the lower-case letters above the boxes indicates that there was a significant difference between the two groups (p < 0.05).
3.2. Effects of Substituting FSM for Fish Meal on Serum Antioxidant Indices of C. auratus
The T-AOC and POD of each group first increased and then decreased, reaching the highest levels in the FSM40 group. There were no significant differences among the FSM20, FSM40, FSM60, and FSM80 groups (p > 0.05), but the T-AOC levels of the FSM40 and FSM60 groups were significantly higher than those of the FSM0 group (p < 0.05). T-SOD first increased and then decreased in each group, with no significant difference (p > 0.05), and reached the highest level in the FSM40 group. There were no significant differences in MDA among the groups (p > 0.05), which showed a trend of decreasing first and then increasing. Similarly, no significant differences were seen in CAT in each group (p > 0.05), which showed a trend of increasing first and then decreasing, and the highest values in the FSM60 group. The IgM levels in the FSM40 and FSM60 groups were significantly higher than those in the FSM0 group (p < 0.05), and there was no significant difference between the two groups (p > 0.05; Table 2).

Table 2.
Effects of fermented soybean meal instead of fish meal on serum antioxidant indices of crucian carp. T-AOC, total antioxidant capacity; POD, peroxidase; T-SOD, total superoxide dismutase; MDA, malondialdehyde; CAT, catalase; IgM, immunoglobulin M. The data are expressed as mean ± standard deviation. Different lowercase letters in the upper right of the data indicate that there was a significant difference between the two groups (p < 0.05).
3.3. Effects of Replacing Fish Meal with FSM on GM Composition of C. auratus
To analyze the effect of substituting fish meal with different FSM concentrations on the GM composition of C. auratus, 3691,616 high-quality 16S rRNA gene sequences were obtained from 40 C. auratus midgut and hindgut content samples (five groups, four replicates in each group). Finally, 53,899 sequences were randomly selected from each sample for subsequent analyses. In total, 4130 OTUs were identified. The number of OTUs and Chao1 indices of midgut microbiota in the FSM0 and FSM20 groups were significantly higher than those of the hindgut microbiota (Kruskal–Wallis test, p < 0.05); however, the same indices for the midgut microbiota in the FSM40, FSM60, and FSM80 groups did not show any significant difference from those of hindgut microbiota (Kruskal–Wallis test, p > 0.05; Figure 2A,D). Although the Simpson index did not detect any significant difference in each group (Kruskal–Wallis test, p > 0.05; Figure 2C), the Shannon index of midgut microbiota in the FSM80 group was significantly higher than that of the hindgut microbiota (Kruskal–Wallis test, p < 0.05), while no significant difference was observed in other groups (Kruskal-Wallis test, p > 0.05; Figure 2B).
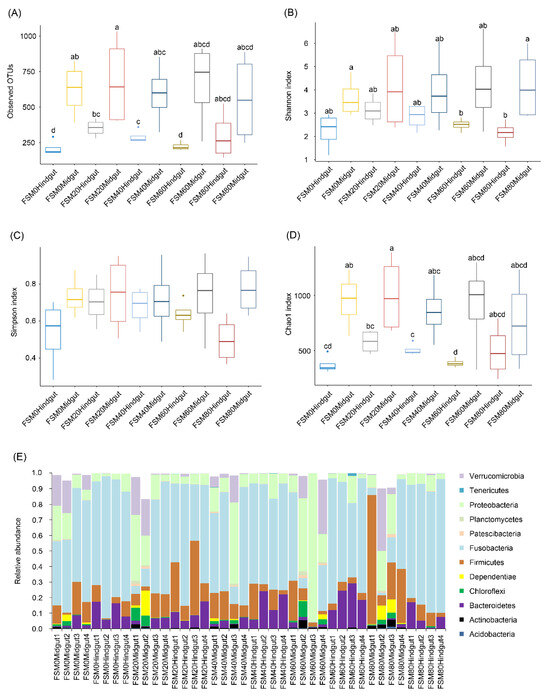
Figure 2.
α-diversity indices and dominant phylum compositions of gut microbiota of crucian carp (Carassius auratus). (A) number of observed OTUs; (B) Shannon index; (C) Simpson index; (D) Chao1 index; (E) relative abundance of dominant phyla in the gut microbiota of C. auratus.
Except for a few, OTUs were divided into 37 phyla (one archaeal phylum and 36 bacterial phyla), the dominant phyla being Acidobacteria, Actinobacteria, Bacteroidetes, Chloroflexi, Dependentiae, Firmicutes, Fusobacteria, Patescibacteria, Planctomycetes, Proteobacteria, Tenericutes, and Verrucomicrobia, all of which belonged to bacteria (Figure 2E).
PCA results showed significant differences in the midgut and hindgut microbiota structures of C. auratus (PERMANOVA, F = 11.782, p < 0.001; Figure 3A). However, the addition of FSM to the feed did not cause significant differences in the GM structure, regardless of midgut (PERMANOVA, F = 0.872, p = 0.622; Figure 3B) or hindgut (PERMANOVA, F = 1.337, p = 0.179; Figure 3C). A heatmap with complete clustering also showed that the GM of C. auratus was generally divided into two branches, the midgut and hindgut, but a small number of midgut samples were clustered into the hindgut samples (Figure 3D).
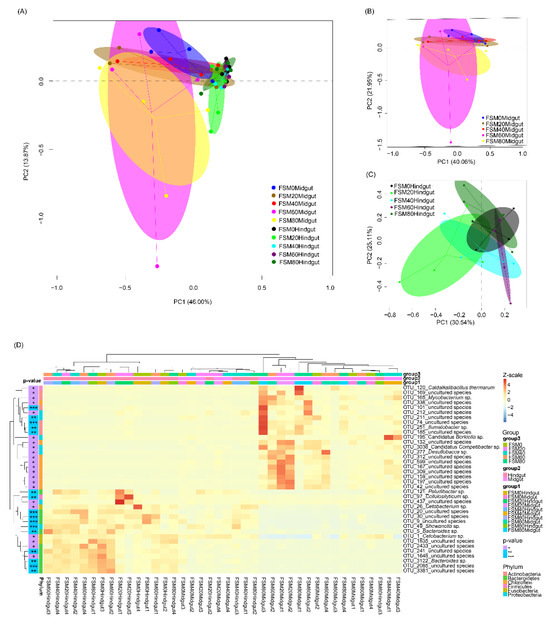
Figure 3.
PCA and heatmap profiles of all samples. (A) midgut samples; (B) and hindgut samples; (C) heatmap profile of significantly different dominant OTUs in all samples. The numbers in the sample names indicate the proportion of fermented soybean meal (FSM) instead of fish meal. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Interestingly, the UniFrac distances and Bray Curtis distances of midgut microbiota in the FSM60 and FSM80 groups were significantly higher than those in the FSM0 group (Kruskal–Wallis test, p < 0.05; Figure 4A,B), but no significant difference in the UniFrac distances of the hindgut microbiota was detected among the groups (Kruskal–Wallis test, p > 0.05; Figure 4C,D), with the exception that the UniFrac distance of hindgut microbiota in the FSM20 group was significantly higher than that in the FSM0 group (Kruskal–Wallis test, p < 0.05; Figure 4C). These results indicated that when the FSM replaced more than 60% of fish meal in the feed, it significantly increased the β-diversity of midgut microbiota among C. auratus individuals.
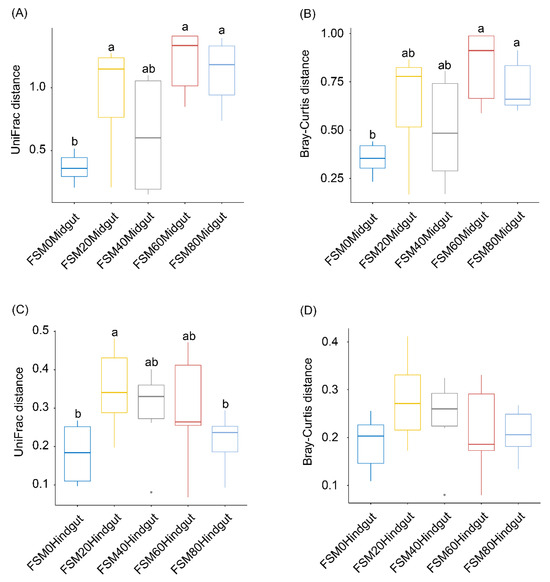
Figure 4.
β-diversity indices of crucian carp (Carassius auratus) gut microbiota. (A) UniFrac distances of C. auratus midgut microbiota; (B) Bray–Curtis distances of C. auratus midgut microbiota; (C) UniFrac distances of C. auratus hindgut microbiota; (D) Bray–Curtis distances of C. auratus hindgut microbiota. The numbers in the sample names indicate the proportion of fermented soybean meal instead of fish meal. The difference in the lower case letters above the boxes indicates that there was a significant difference between the two groups (p < 0.05).
4. Discussion
Fermentation is an important method to effectively remove anti-nutritional factors from soybean meal and improve its utilization in feed [13,14]. Commonly used strains for soybean meal fermentation include lactic acid bacteria, B. subtilis, B. cereus, B. licheniformis, B. coagulans, and E. faecium [13,17,27,28]. In this study, we co-fermented soybean meal with B. subtilis and E. faecium and optimized the fermentation conditions. Under the optimized fermentation condition, the DRSG was 36.85%, which was higher than previously reported [18,19].
Previous studies on the substituting fish meal with FSM in fish culture showed that the latter, when used for replacing less than 40% of fish meal did not affect the growth and immunological indices of fish [14,15,16]. In our previous study, we showed that replacing 60% fish meal with FSM significantly increased not only the weight gain rate and specific growth rate of C. auratus, but also the intestinal body ratio. Using FSM in a ratio of 40% exerted no significant effect on the weight gain rate, specific growth rate, and intestinal body ratio of C. auratus [19]. Wang et al. [14] used fermented soybean meal (FSM) instead of fish meal and found that an increase in substitution levels led to a gradual increase in the feeding rate and feed conversion rate of juvenile large yellow croaker (Larimichthys crocea). However, FSM can replace only up to 45% of fish meal in the diets of yellow croaker. Yan et al. [15] fed Phoxinus lagowskii with FSM instead of fish meal and found that an increase in the substitution ratio caused a decrease in the final body weight, as well as weight gain, specific growth, and protein sedimentation rates. These indices were significantly lower than those in the control group when 49.5–66% FSM was substituted for fish meal. Rahimnejad et al. [16] found the best results at 26.9–37.1% FSM substitution levels. Although FSM substitution levels may be affected by fermentation conditions and the fermentation degree of soybean meal, the above results show that less than 40% FSM substitution levels may not have a significant adverse effect on fish growth. The 40% FSM substitution level was also consistent with our results of serum antioxidant indices and gut microbiota in this study.
Oxygen free radicals produced by fish metabolism can be eliminated by enzyme and non-enzyme systems. POD and T-SOD are the key enzymes involved in redox reactions [29]. T-SOD is the only enzyme in the antioxidant system with free radicals acting as a substrate, which can remove free radicals in the body through a disproportionation reaction. The generated hydrogen and oxygen are removed by CAT to avoid damage to the fish body owing to oxidation [30]. T-AOC is a comprehensive index for characterizing antioxidant performance. IgM is the first type of immunoglobulin found in fish, and its content can reflect the strength of immunity to various pathogens [31]. Our results showed that replacing 40% fish meal with FSM in the feed significantly improved the serum T-AOC, POD, and IgM levels of C. auratus, indicating that the use this combination can enhance the antioxidant and immune capacities of C. auratus.
Fish intestines contain a large number of microorganisms, which play an important role in their growth, nutrition, development, and immunity [32,33,34,35]. According to our results, the dominant phyla in the GM of C. auratus were Acidobacteria, Actinobacteria, Bacteroidetes, Chloroflexi, Dependentiae, Firmicutes, Fusobacteria, Patescibacteria, Planctomycetes, Proteobacteria, Tenericutes, and Verrucomicrobia, similar to those observed in previous reports on omnivorous fish [36]. Moreover, our results showed significant differences between the midgut and hindgut microbiota compositions of C. auratus, again similar to a previous report [36]. Nevertheless, although previous studies documented that food composition significantly affected the composition of GM [33,34,37], our results showed that the use of FSM instead of fish meal did not significantly change the GM composition in C. auratus; however, a higher than 60% substitution rate significantly increased the beta-diversity of midgut microbiota. However, the effect of this change on the growth and immunity of C. auratus remains unclear.
5. Conclusions
The best effect of soybean meal co-fermentation was observed for a B. subtilis X-2 and E. faecium X-4 ratio of 2:3, 4.5% glucose addition, 0.15% KH2PO4 addition, 0.1% MgSO4·7H2O addition, 0.4% anhydrous sodium acetate addition, fermentation time of 120 h, and a 1:1 solid–water ratio. Replacing 40% fish meal with FSM in the feed significantly improved the serum T-AOC, POD, and IgM levels in C. auratus. Although there were significant differences in the midgut and hindgut microbiota structure of C. auratus, the addition of FSM to the feed did not produce significant differences in the GM structure, whether in the midgut or hindgut.
Author Contributions
Q.X., H.W. and S.L.; methodology, Q.X., Z.Y., S.C. and W.Z.; software, Q.X. and S.X.; validation, J.L.; formal analysis, Q.X. and Z.Y.; investigation, Q.X., S.C., W.Z., S.X. and J.L.; resources, H.W. and S.L.; data curation, J.L.; writing—original draft preparation, Q.X.; writing—review and editing, S.L.; visualization, Q.X. and Z.Y.; supervision, H.W.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Special Funds for the Construction of Innovative Provinces in Hunan Province, China, grant number 2020NK2029.
Institutional Review Board Statement
The study protocol was approved by the Biomedical Research Ethics Committee of Hunan Agricultural University (approval No. 2021(063)).
Data Availability Statement
Raw DNA sequences were deposited in the NCBI Sequence Read Archive database with accession number PRJNA756401.
Acknowledgments
We would like to thank Jiajia Ni at Guangdong Meilikang Bio-Science Ltd., China for assistance with data analysis and manuscript revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gong, W.; Gao, S.; Zhu, Y.; Wang, G.; Zhang, K.; Li, Z.; Yu, E.; Tian, J.; Xie, Y.; Xie, J.; et al. Effect of the aerobic denitrifying bacterium Pseudomonas furukawaii ZS1 on microbiota compositions in grass carp culture water. Water 2021, 13, 1329. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Ji, H.; Zhu, T.; Shan, S. Current status of dietary fish meal replacement research for non-carnivorous fish species. J. Dalian Fish. Univ. 2009, 24, 343–349. [Google Scholar]
- El-Saidy, D.M.S.D.; Gaber, M.M.A. Replacement of fish meal with a mixture of different plant protein sources in juvenile Nile tilapia, Oreochromis niloticus (L.) diets. Aquacul. Res. 2003, 34, 1119–1127. [Google Scholar] [CrossRef]
- Gaylord, T.G.; Barrows, F.T.; Teague, A.M.; Johansen, K.A.; Overturf, K.E.; Shepherd, B. Supplementation of taurine and methionine to all-plant protein diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 269, 514–524. [Google Scholar] [CrossRef]
- Sakarika, M.; Delmoitié, B.; Ntagia, E.; Chatzigiannidou, I.; Gabet, X.; Ganigué, R.; Rabaey, K. Production of microbial protein from fermented grass. Chem. Eng. J. 2021, 433, 133631. [Google Scholar] [CrossRef]
- Zhu, W.; Xiao, S.; Chen, S.; Xu, Q.; Yang, Z.; Liu, J.; Wang, H.; Lan, S. Effects of fermented mulberry leaves on growth, serum antioxidant capacity, digestive enzyme activities and microbial compositions of the intestine in crucian (Carassius carassius). Aquacul. Res. 2021, 52, 6356–6366. [Google Scholar] [CrossRef]
- Tacon, A.G.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Urán, P.A.; Gonçalves, A.A.; Taverne-Thiele, J.J.; Schrama, J.W.; Verreth, J.A.; Rombout, J.H.W.M. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2008, 25, 751–760. [Google Scholar] [CrossRef]
- Suárez, J.A.; Gaxiola, G.; Mendoza, R.; Cadavid, S.; Garcia, G.; Alanis, G.; Suárez, A.; Faillace, J.; Cuzon, G. Substitution of fish meal with plant protein sources and energy budget for white shrimp Litopenaeus vannamei (Boone, 1931). Aquaculture 2009, 289, 118–123. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Y.; Ye, J.; Du, Z.; Kong, Y. An evaluation of replacing fish meal with fermented soybean meal in the diet of Macrobrachium nipponense: Growth, nonspecific immunity, and resistance to Aeromonas hydrophila. Fish Shellfish Immunol. 2015, 44, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, R.-L.; Ren, Z.-Q.; Fan, Y.-W.; Hu, S.-B.; Zhuo, C.-F.; Deng, Z.-Y. Improvement of protein quality and degradation of allergen in soybean meal fermented by Neurospora crassa. LWT 2019, 101, 220–228. [Google Scholar] [CrossRef]
- Refstie, S.; Sahlström, S.; Bråthen, E.; Baeverfjord, G.; Krogedal, P. Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture 2005, 246, 331–345. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Q.; Feng, J.; He, J.; Lou, Y.; Zhu, J. Effect of dietary fermented soybean meal on growth, intestinal morphology and microbiota in juvenile large yellow croaker, Larimichthys crocea. Aquacul. Res. 2019, 50, 748–757. [Google Scholar] [CrossRef]
- Yan, L.; Wu, L.; Quan, Y.; Xing, X.; Lin, J.; Wang, H. Effects of replacing fish meal with fermented soybean on growth performance, feed utilization and non-specific immune indexes of Phoxinus lagowskii Dybowski. J. Northwest A F Univ. Nat. Sci. Ed. 2017, 45, 7–13. [Google Scholar]
- Rahimnejad, S.; Zhang, J.-J.; Wang, L.; Sun, Y.; Zhang, C. Evaluation of Bacillus pumillus SE5 fermented soybean meal as a fish meal replacer in spotted seabass (Lateolabrax maculatus) feed. Aquaculture 2021, 531, 735975. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Liu, C.; Zhou, H.; Wang, X.; Mai, K.; He, G. Effects of dietary raw or Enterococcus faecium fermented soybean meal on growth, antioxidant status, intestinal microbiota, morphology, and inflammatory responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 100, 261–271. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, T.; Deng, C.; Li, X. Development of phylogenetic relationship of different crucian carp (Carassius auratus) strains in China. Henan Aquacul. 2018, 3, 25–27. [Google Scholar]
- Xu, Q.; Yang, Z.; Zhu, W.; Chen, S.; Xiao, S.; Liu, J.; Wang, H.; Lan, S. Effects of fermented soybean meal instead of fish meal on growth, serum biochemical indexes and intestinal structure of Carassius auratus. Feed Ind. Mag. 2021, 42, 31–37. [Google Scholar]
- Castrillo, G.; Teixeira, P.J.P.L.; Paredes, S.H.; Law, T.F.; de Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Mieczkowski, P.; Jones, C.D.; et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OUT sequences from microbial applicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 21 October 2021).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Chi, C.-H.; Cho, S.-J. Improvement of bioactivity of soybean meal by solid-state fermentation with Bacillus amyloliquefaciens versus Lactobacillus spp. and Saccharomyces cerevisiae. LWT-Food Sci. Technol. 2016, 68, 619–625. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Yang, G.; Guo, L. Research on the enzyme activity of neutral protease from Bacillus subtilis L1 strain fermenting the soybean meal. Food Ferment. Sci. Technol. 2018, 54, 55–59. [Google Scholar]
- Li, Y.; Shi, F.; Ma, L.; Liu, J.; Meng, Q. Preliminary study on the relationship between activity of POD and SOD in hyphae and pathogenicity of Sclerotinia sclerotiorum on soybean. J. Heilongjiang Bayi Agricul. Univ. 2021, 33, 1–6, 14. [Google Scholar]
- Yin, H.; Huang, J.; Li, X.; Zheng, X. Effect of replacing fish meal with fermented and unfermented soybean meal on growth performance, serum antioxidant capacity and digestive enzymes of juvenile Cyprinus carpio-haematopterus. Feed Indust. 2019, 40, 46–52. [Google Scholar]
- Zhang, P.; Lin, H.; Chen, G. Research progress on the effects of oral immunoglobulin in animal health. China Feed 2019, 22, 20–23. [Google Scholar]
- Ganguly, S.; Prasad, A. Microflora in fish digestive tract plays significant role in digestion and metabolism. Rev. Fish Biol. Fish. 2012, 22, 11–16. [Google Scholar] [CrossRef]
- Ni, J.; Yu, Y.; Zhang, T.; Gao, L. Comparison of intestinal bacterial communities in grass carp, Ctenopharyngodon idellus, from two different habitats. Chin. J. Oceanol. Limnol. 2012, 30, 757–765. [Google Scholar] [CrossRef]
- Ni, J.; Yan, Q.; Yu, Y.; Zhang, T. Factors influencing the grass carp gut microbiome and its effect on metabolism. FEMS Microbiol. Ecol. 2014, 87, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.R.; Ran, C.; Ringø, E.; Zhou, Z.G. Progress in fish gastrointestinal microbiota research. Rev. Aquacul. 2018, 10, 626–640. [Google Scholar] [CrossRef]
- Li, J.; Ni, J.; Li, J.; Wang, C.; Li, X.; Wu, S.; Zhang, T.; Yu, Y.; Yan, Q. Comparative study on gastrointestinal microbiota of eight fish species with different feeding habits. J. Appl. Microbiol. 2014, 117, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.R.; Snowberg, L.K.; Caporaso, J.G.; Knight, R.; Bolnick, D.I. Dietary input of microbes and host genetic variation shape among-population differences in stickleback gut microbiota. ISME J. 2015, 9, 2515–2526. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).