Preliminary Insight into Winter Native Fish Assemblages in Guadiana Estuary Salt Marshes Coping with Environmental Variability and Non-Indigenous Fish Introduction
Abstract
1. Introduction
2. Results
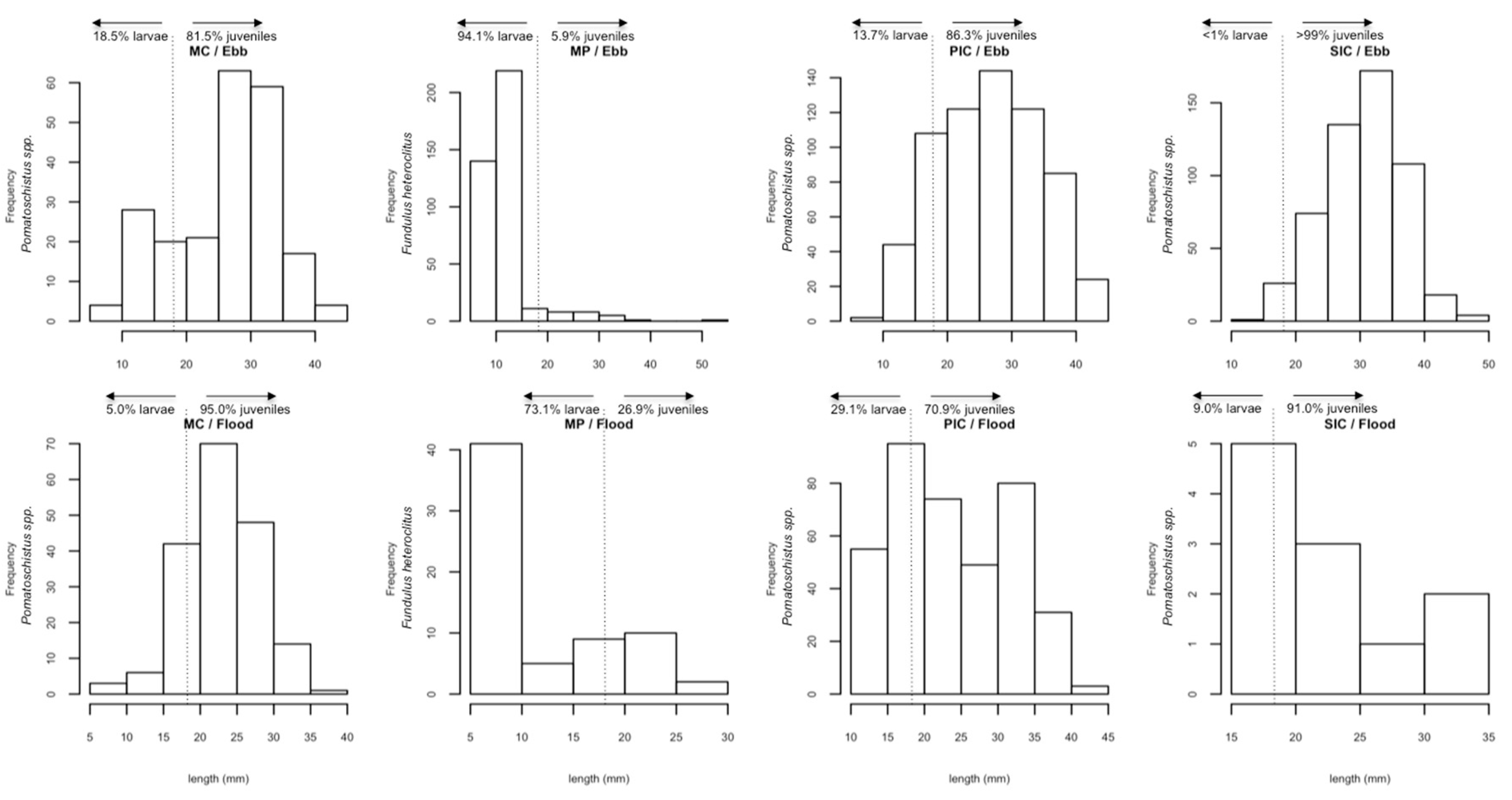
2.1. Composition of Fish Communities
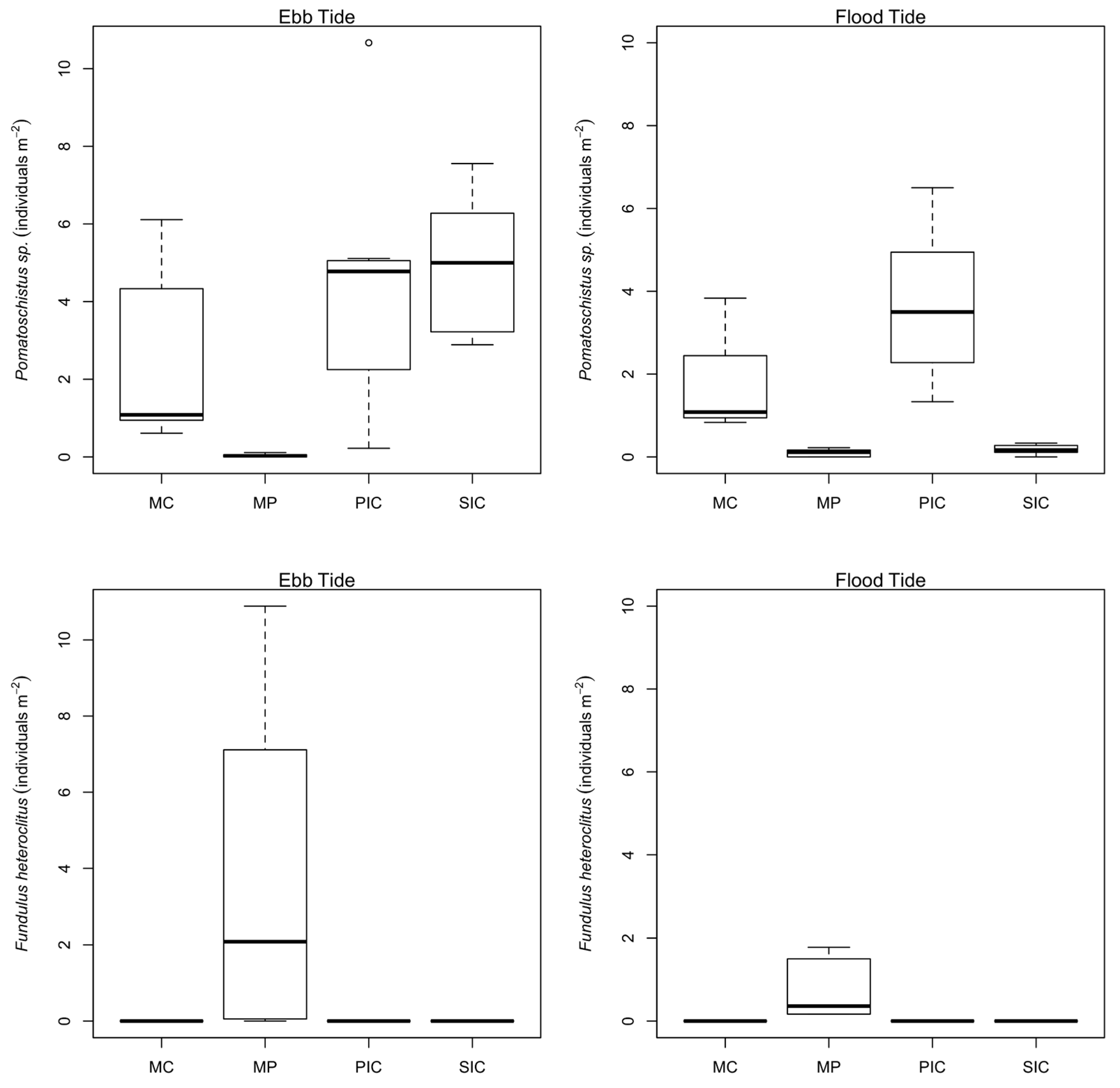
2.2. Fish Distribution among Habitats
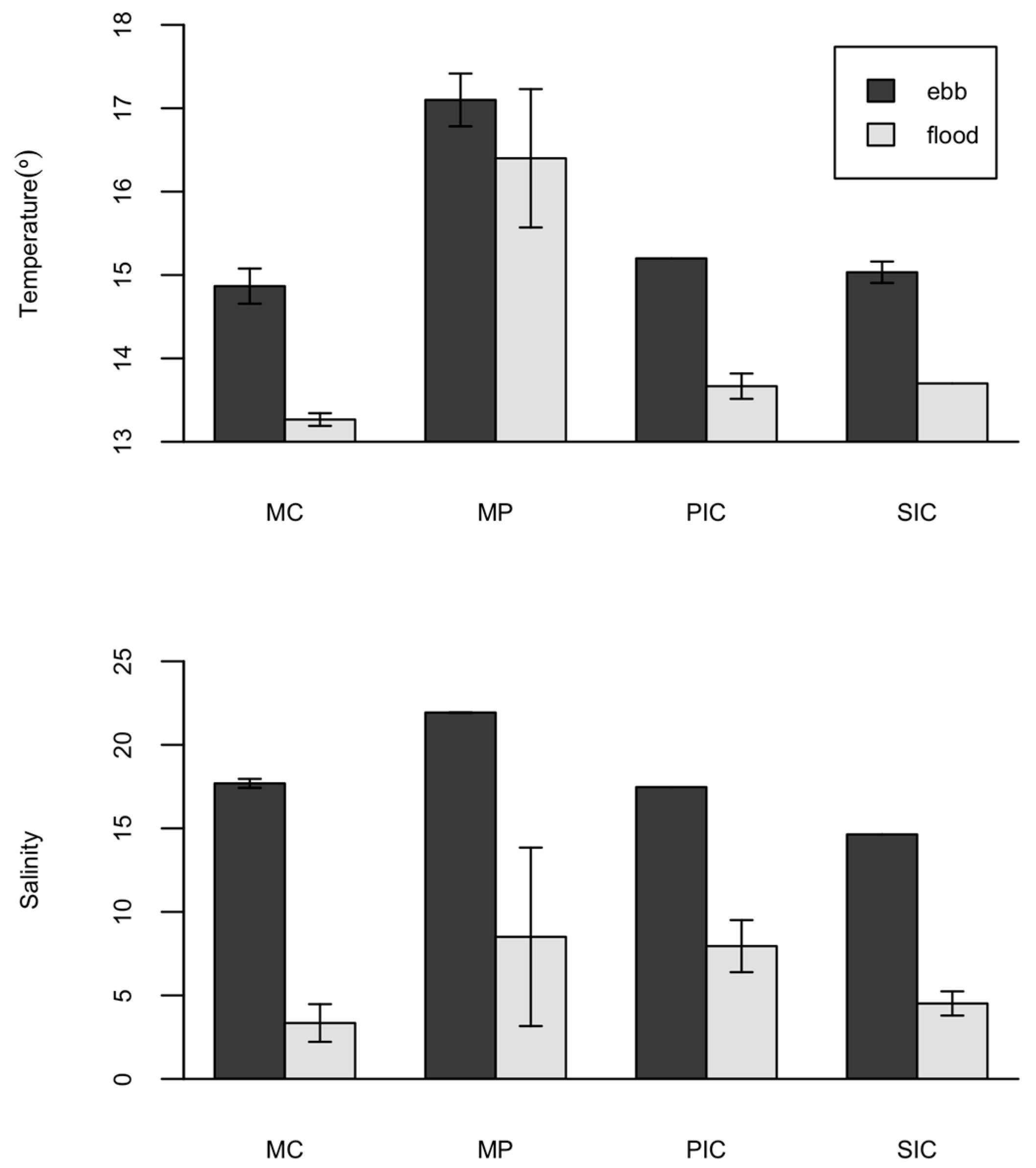
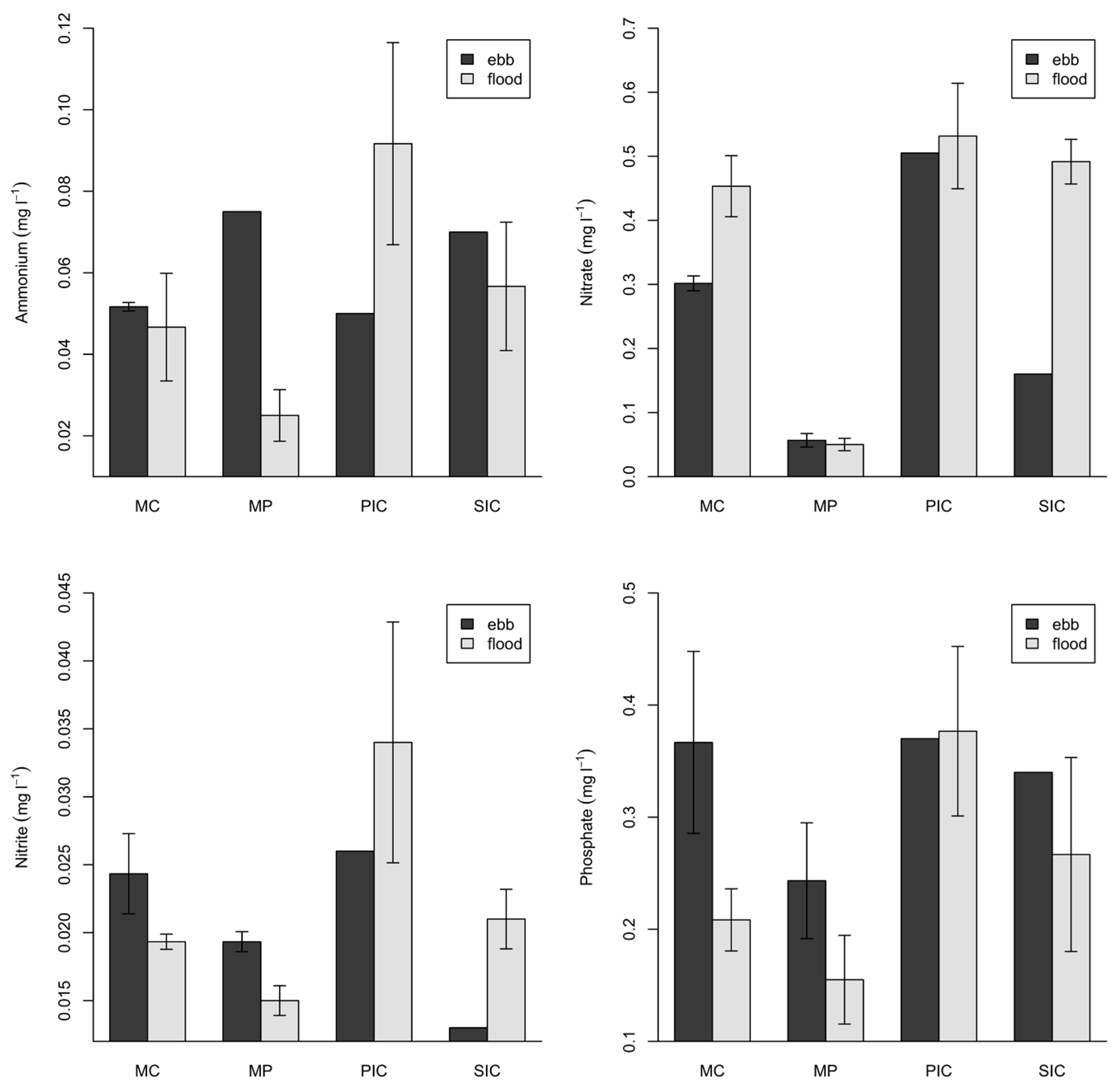
2.3. Environmental Parameters
3. Discussion
4. Materials and Methods
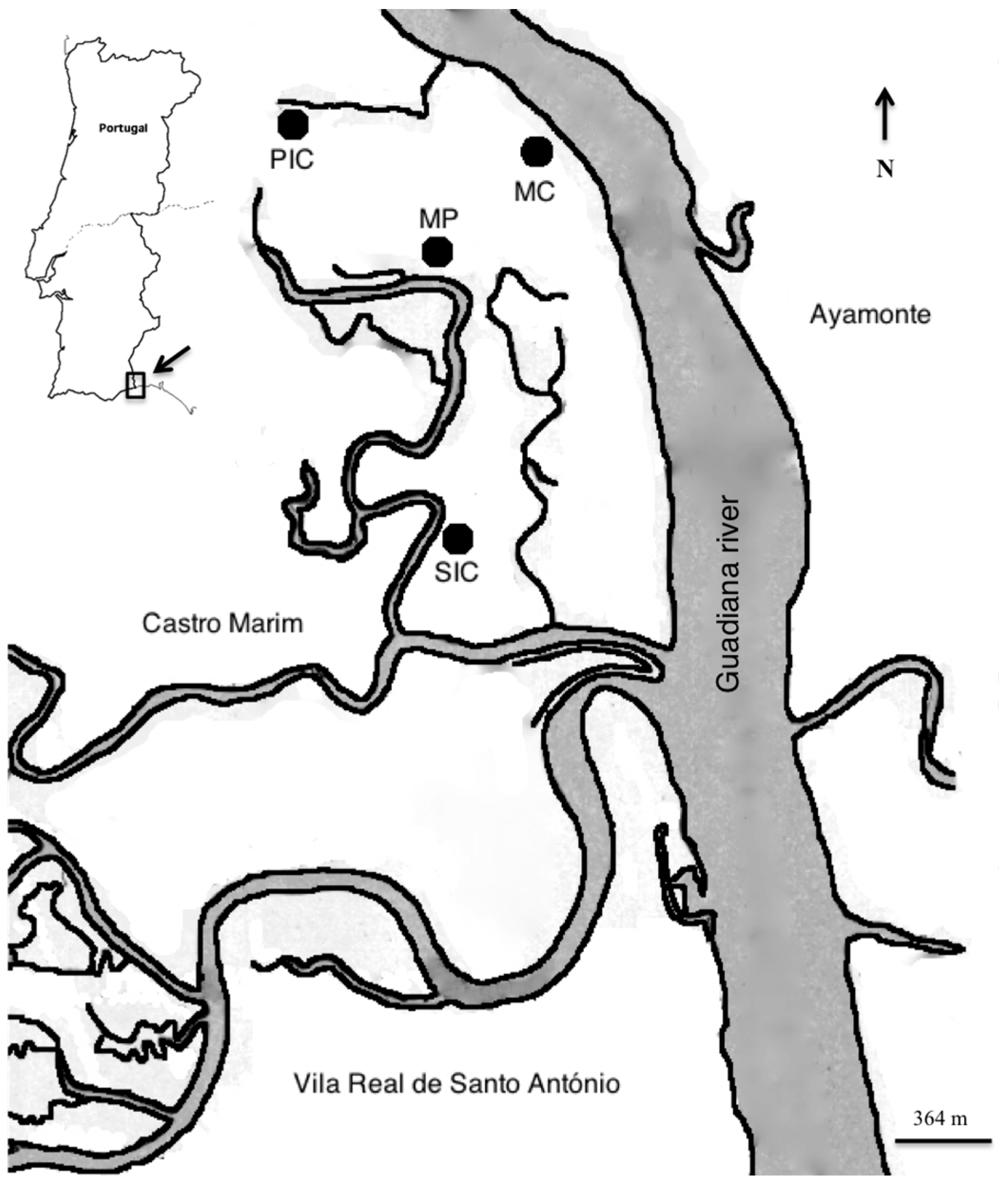
4.1. Sampling and Field Methodology
4.2. Laboratory Analysis
4.3. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vasconcelos, R.P.; Reis-Santos, P.; Fonseca, V.; Maia, A.; Ruano, M.; França, S.; Vinagre, C.; Costa, M.J.; Cabral, H. Assessing anthropogenic pressures on estuarine fish nurseries along the Portuguese coast: A multi-metric index and conceptual approach. Sci. Total Environ. 2007, 374, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Beck, M.W.; Heck, K.L.; Able, K.W.; Childers, D.L.; Eggleston, D.B.; Gillanders, B.M.; Halpern, B.; Hays, C.G.; Hoshino, K.; Minello, T.J.; et al. The Identification, Conservation, and Management of Estuarine and Marine Nurseries for Fish and Invertebrates. Bioscience 2001, 51, 633. [Google Scholar] [CrossRef]
- Able, K.W. A re-examination of fish estuarine dependence: Evidence for connectivity between estuarine and ocean habitats. Estuar. Coast. Shelf Sci. 2005, 64, 5–17. [Google Scholar] [CrossRef]
- Veiga, P.; Vieira, L.; Bexiga, C.; Sa, R.; Erzini, K. Structure and temporal variations of fish assemblages of the Castro Marim salt marsh, southern Portugal. Estuar. Coast. Shelf Sci. 2006, 70, 27–38. [Google Scholar] [CrossRef]
- Gonçalves, R.; Correia, A.D.; Atanasova, N.; Teodósio, M.A.; Ben-Hamadou, R.; Chícharo, L. Environmental factors affecting larval fish community in the salt marsh area of Guadiana estuary (Algarve, Portugal). Sci. Mar. 2015, 79, 25–34. [Google Scholar] [CrossRef]
- Santos, A.M.P.; Chícharo, A.; Dos Santos, A.; Moita, T.; Oliveira, P.B.; Peliz, A.; Ré, P. Physical-biological interactions in the life history of small pelagic fish in the Western Iberia Upwelling Ecosystem. Prog. Oceanogr. 2007, 74, 192–209. [Google Scholar] [CrossRef]
- Zwolinski, J.; Stratoudakis, Y.; Soares, E. Intraannual variation in the batch fecundity of sardine off Portugal. J. Fish Biol. 2001, 58, 1633–1645. [Google Scholar] [CrossRef]
- Sogard, S.M.; Able, K.W. A comparison of eelgrass, sea lettuce macro-algae, and marsh creeks as habitats for epibenthic fishes and decapods. Estuar. Coast. Shelf Sci. 1991, 33, 501–519. [Google Scholar] [CrossRef]
- Guest, M.A.; Connolly, R.M.; Loneragan, N.R. Seine nets and beam trawls compared by day and night for sampling fish and crustaceans in shallow seagrass habitat. Fish Res. 2003, 64, 185–196. [Google Scholar] [CrossRef]
- Ribeiro, J.; Bentes, L.; Coelho, R.; Gonçalves, J.; Lino, P.; Monteiro, P.; Erzini, K. Seasonal, tidal and diurnal changes in fish assemblages in the Ria Formosa lagoon (Portugal). Estuar. Coast. Shelf Sci. 2006, 67, 461–474. [Google Scholar] [CrossRef]
- Cravo, A.; Lopes, B.; Serafim, A.; Company, R.; Barreira, L.; Gomes, T.; Bebianno, M.J. A multibiomarker approach in Mytilus galloprovincialis to assess environmental quality. J. Environ. Monit. 2009, 11, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Cushing, D.H. Plankton production and year-class strength in fish populations: An update of the match/mismatch hypothesis. Adv. Mar. Biol. 1990, 26, 249–293. [Google Scholar] [CrossRef]
- Crooks, J.A.; Chang, A.L.; Ruiz, G.M. Aquatic pollution increases the relative success of invasive species. Biol. Invasions 2010, 13, 165–176. [Google Scholar] [CrossRef]
- Chícharo, M.A.; Chícharo, L.; Morais, P. Inter-annual differences of ichthyofauna structure of the Guadiana estuary and adjacent coastal area (SE Portugal/SW Spain): Before and after Alqueva dam construction. Estuar. Coast. Shelf Sci. 2006, 70, 39–51. [Google Scholar] [CrossRef]
- Chícharo, M.A.; Leitão, T.; Range, P.; Gutierrez, C.; Morales, J.; Morais, P.; Chícharo, L. Alien species in the Guadiana Estuary (SE-Portugal/SW-Spain): Blackfordia virginica (Cnidaria, Hydrozoa) and Palaemon macrodactylus (Crustacea, Decapoda): Potential impacts and mitigation measures. Aquat. Invasions 2009, 4, 501–506. [Google Scholar] [CrossRef]
- Faria, A.; Morais, P.; Chícharo, M.A. Ichthyoplankton dynamics in the Guadiana estuary and adjacent coastal area, South-East Portugal. Estuar. Coast. Shelf Sci. 2006, 70, 85–97. [Google Scholar] [CrossRef]
- Domingues, R.B.; Sobrino, C.; Galvão, H. Impact of reservoir filling on phytoplankton succession and cyanobacteria blooms in a temperate estuary. Estuar. Coast. Shelf Sci. 2007, 74, 31–43. [Google Scholar] [CrossRef]
- Bunn, S.E.; Arthington, A.H. Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef]
- Hagan, S.M.; Brown, S.A.; Able, K.W. Production of mummichog (Fundulus heteroclitus): Response in marshes treated for common reed (Phragmites australis) removal. Wetlands 2007, 27, 54–67. [Google Scholar] [CrossRef]
- Fernández-Delgado, C. Life-history patterns of the salt-marsh killifish Fundulus heteroclitus (L.) introduced in the estuary of the guadalquivir river (South West Spain). Estuar. Coast. Shelf Sci. 1989, 29, 573–582. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase; World Wide Web Electronic Publication: Kiel, Germany, 2017; Available online: www.fishbase.org (accessed on 11 October 2017).
- Sierra, J. La aparición del pez momia en el Delta del Ebro amenaza al samaruc valenciano. Las Provincias 2006, 6. (In Spanish) [Google Scholar]
- Gutiérrez-Estrada, J.C.; Prenda, J.; Oliva, F.; Fernández-Delgado, C. Distribution and Habitat Preferences of the Introduced Mummichog Fundulus heteroclitus (Linneaus) in South-western Spain. Estuar. Coast. Shelf Sci. 1998, 46, 827–835. [Google Scholar] [CrossRef]
- Koutsogiannopoulou, V.; Wilson, J.G. The fish assemblage of the intertidal salt marsh creeks in North Bull Island, Dublin Bay: Seasonal and tidal changes in composition, distribution and abundance. Hydrobiologia 2007, 588, 213–224. [Google Scholar] [CrossRef]
- Green, B.C.; Smith, D.J.; Earley, S.E.; Hepburn, L.J.; Underwood, G.J.C. Seasonal changes in community composition and trophic structure of fish populations of five salt marshes along the Essex coastline, United Kingdom. Estuar. Coast. Shelf Sci. 2009, 85, 247–256. [Google Scholar] [CrossRef]
- Desmond, J.S.; Zedler, J.B.; Williams, G.D. Fish use of tidal creek habitats in two southern California salt marshes. Ecol. Eng. 2000, 14, 233–252. [Google Scholar] [CrossRef]
- West, J.M.; Zedler, J.B. Marsh-Creek Connectivity: Fish Use of a Tidal Salt Marsh in Southern California. Estuaries 2000, 23, 699. [Google Scholar] [CrossRef]
- Laffaille, P. Composition of Fish Communities in a European Macrotidal Salt Marsh (the Mont Saint-Michel Bay, France). Estuar. Coast. Shelf Sci. 2000, 51, 429–438. [Google Scholar] [CrossRef]
- Leitão, R.; Martinho, F.; Neto, J.M.; Cabral, H.; Marques, J.C.; Pardal, M.A. Feeding ecology, population structure and distribution of Pomatoschistus microps (Krøyer, 1838) and Pomatoschistus minutus (Pallas, 1770) in a temperate estuary, Portugal. Estuar. Coast. Shelf Sci. 2006, 66, 231–239. [Google Scholar] [CrossRef]
- Mathieson, S.; Cattrijsse, A.; Costa, M.; Drake, P.; Elliott, M.; Gardner, J.; Marchand, J. Fish assemblages of European tidal marshes: A comparison based on species, families and functional guilds. Mar. Ecol. Prog. Ser. 2000, 204, 225–242. [Google Scholar] [CrossRef]
- Basos, N. GIS as a Tool to Aid Pre- and Post-Processing of Hydrodynamic Models. Application to the Guadiana Estuary. Master’s Thesis, Algarve University, Faro, Portugal, 2013. [Google Scholar]
- Faria, A.M.; Borges, R.; Gonçalves, E.J. Critical swimming speeds of wild-caught sand-smelt Atherina presbyter larvae. J. Fish Biol. 2014, 85, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.M.; Muha, T.; Morote, E.; Chícharo, M.A. Influence of starvation on the critical swimming behaviour of the Senegalese sole (Solea senegalensis) and its relationship with RNA/DNA ratios during ontogeny. Sci. Mar. 2011, 75, 87–94. [Google Scholar] [CrossRef]
- Silva, L.; Faria, A.M.; Teodósio, M.A.; Garrido, S. Ontogeny of swimming behaviour in sardine Sardina pilchardus larvae and effect of larval nutritional condition on critical speed. Mar. Ecol. Prog. Ser. 2014, 504, 287–300. [Google Scholar] [CrossRef]
- Gisbert, E.; López, M.A. First record of a population of the exotic mummichog Fundulus heteroclitus (L., 1766) in the Mediterranean Sea basin (Ebro River delta). J. Fish Biol. 2007, 71, 1220–1224. [Google Scholar] [CrossRef]
- Feldmeth, C.R.; Waggoner, J.P. Field measurements of tolerance to extreme hypersalinity in the California killifish, Fundulus parvipinnis. Copeia 1972, 3, 592–594. [Google Scholar] [CrossRef]
- Lockfield, K.C.; Fleeger, J.W.; Deegan, L. Mummichog, Fundulus heteroclitus, Responses to Long-Term, Whole-Ecosystem Nutrient Enrichment. Mar. Ecol. Prog. Ser. 2013, 492, 211–222. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Posey, M.H.; Alphin, T.D.; Cahoon, L.; Lindquist, D.; Becker, M.E. Interactive effects of nutrient additions and predation on infaunal communities. Estuaries 1999, 22, 785–792. [Google Scholar] [CrossRef]
- Lefeuvre, J.C.; Laffaille, P.; Feunteun, E. Do fish communities function as biotic vectors of organic matter between salt marshes and marine coastal waters? Aquat. Ecol. 1999, 33, 293–299. [Google Scholar] [CrossRef]
- Elliot, M.; Hemingway, K.; Costello, M.J.; Duhamel, S.; Hostens, K.; Labropoulou, M.; Marshall, S.; Winkler, H. Links between fish and other trophic levels. In Fishes in Estuaries; Elliot, M., Hemingway, K.L., Eds.; Blackwell Science: Oxford, UK, 2002; pp. 54–123. ISBN 9780632057337. [Google Scholar]
- Abraham, B.J. Species profiles: Life histories and environmental requirements of coastal fishes and invertebrates (Mid-Atlantic) mummichog and striped killifish. USFWS Biol. Rep. 1989, 82. [Google Scholar]
- Hermoso, V.; Blanco-Garrido, F.; Prenda, J. Spatial distribution of exotic fish species in the Guadiana river basin, with two new records. Limnetica 2008, 27, 189–194. [Google Scholar]
- Rocha, C.; Galvão, H.; Barbosa, A. Role of transient silicon limitation in the development of cyanobacteria blooms in the Guadiana estuary, south-western Iberia. Mar. Ecol. Prog. Ser. 2002, 228, 35–45. [Google Scholar] [CrossRef]
- Secretariat of the Convention on Biological Diversity. Assessment and management of alien species that threaten ecosystems, habitats and species. Presented at the 6th Meeting of the Subsidiary Body on Scientific, Technical and Technological Advice, Montreal, QC, Canada, 12–16 March 2001. [Google Scholar]
- Agência Portuguesa do Ambiente. Plano de Gestão da Região Hidrográfica do Guadiana 2016–2021. Personal communication, 2015. [Google Scholar]
- Muus, B.J.; Dahlstrom, P. Guide Des Poissons D’eau Douce et Pêche; Quartier, A.A., Ed.; Delachaux & Niestlé: Neuchâtel-Paris, France, 1968; 248p. [Google Scholar]
- Whitehead, P.J.P.; Bauchot, M.L.; Hureau, J.C.; Nielsen, J.; Tortonese, E. Fishes of the North-Esatern Atlantic and Mediterranean; Whitehead, P.J.P., Ed.; UNESCO: London, UK, 1986; Volume 2, 1473p, ISBN 9230023086. [Google Scholar]
- Russell, F.S. The Eggs and Planktonic Stages of British Marine Fishes; Academic Press: New York, NY, USA, 1976; 539p, ISBN 0126040508. [Google Scholar]
- Ré, P. Ictioplânton Estuarino Da Península Ibérica (Guia de Identifição Dos Ovos E Estados Larvares Planctónicos; Câmara Municipal de Cascais: Cascais, Portugal, 1999; 163p, ISBN 972-637-065-5. [Google Scholar]
- Elliott, M.; Dewailly, F. The structure and components of european estuarine fish assemblages. Aquat. Ecol. 1995, 29, 397–417. [Google Scholar] [CrossRef]
- Pianka, E.R. The structure of Lizard Communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Zhang, J. Miscellaneous Functions for Analysing Species Association and Niche Overlap, version 0.2.2. Species Association Analysis Package (SPAA). 2016.
| Species | N | F (%) | Larvae (%) | Juveniles (%) | Mean Size (mm) | Size Range (mm) | Spatial Occupancy |
|---|---|---|---|---|---|---|---|
| Atherina sp. | 1 | 0.04 | - | 100.0 | 36.0 | - | MC |
| Diplodus sp. | 1 | 0.04 | 100.0 | - | 16.0 | - | PIC |
| Fundulus heteroclitus | 479 | 18.93 | 90.6 | 9.4 | 12.1 | 1.6–52.0 | MP |
| Pomatoschistus sp. | 2043 | 80.75 | 13.0 | 87.0 | 27.1 | 7.0–47.0 | MC, PIC, SIC, MP |
| Sardina pilchardus | 2 | 0.08 | 100.0 | - | 27.0 | 26.0–28.0 | MC, SIC |
| Solea spp. | 3 | 0.12 | 66.7 | 33.3 | 13.2 | 6.5–26.0 | MC |
| Syngnathus sp. | 1 | 0.04 | - | 100.0 | 92.0 | - | MP |
| Degrees of Freedom (df) | Mean Square | F Ratio | |
|---|---|---|---|
| Habitat | 3 | 7.333 | 40.59 * |
| Tide | 1 | 4.098 | 22.68 * |
| Habitat × Tide | 3 | 2.243 | 12.42 * |
| Error | 40 | 0.181 |
| Tide | Habitat | p Value |
|---|---|---|
| Ebb | MC vs. PIC | 0.009 ** |
| MP vs. PIC | 0.000 *** | |
| SIC vs. PIC | 0.999 ns | |
| MP vs. MC | 0.000 *** | |
| SIC vs. MC | 0.041 * | |
| SIC vs. MP | 0.000 *** | |
| Flood | MC vs. PIC | 0.236 ns |
| MP vs. PIC | 0.000 *** | |
| SIC vs. PIC | 0.000 *** | |
| MP vs. MC | 0.005 ** | |
| SIC vs. MC | 0.020 * | |
| SIC vs. MP | 0.999 ns |
| Density of Pomatoschistus sp. | Density of Fundulus heteroclitus | |||
|---|---|---|---|---|
| Juveniles | Larvae | Juveniles | Larvae | |
| Temperature (°) | −0.244 | −0.332 * | 0.564 * | 0.517 * |
| Salinity | −0.105 | −0.143 | 0.060 | 0.211 |
| Dissolved Oxygen (mg·L−1) | −0.208 | −0.133 | −0.084 | −0.137 |
| Cha (mg·L−1) | 0.109 | 0.214 | 0.160 | 0.031 |
| Ammonium (mg·L−1) | 0.017 | −0.103 | 0.048 | −0.131 |
| Nitrate (mg·L−1) | 0.467 * | 0.645 * | −0.508 * | −0.703 * |
| Nitrite (mg·L−1) | 0.190 | 0.493 * | −0.185 | −0.213 |
| Phosphate (mg·L−1) | 0.489 * | 0.361 ** | −0.310 * | −0.250 |
| Species | Ucrit Range (cms−1) | Size Range (mm) | Reference |
|---|---|---|---|
| Atherina presbyter | 3.6–18.7 | 6.6–21.0 | [34] |
| Solea senegalensis | 0.0–5.0 | 3.5–7.5 | [35] |
| Sardina pilchardus | 1.6–9.5 | 7.9–23.4 | [36] |
| Ecological Guild | Definition | Reference |
|---|---|---|
| Estuarine resident | Spend their entire lives in the estuary | [53] |
| Marine seasonal | Have regular seasonal visits to the estuary, mainly as adults | [53] |
| Marine juvenile | Use the estuary as nursery ground, usually spawning and spending much of their adult life at sea with seasonal visits to the estuary | [53] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, R.; Teodósio, M.A.; Cruz, J.; Ben-Hamadou, R.; Correia, A.D.; Chícharo, L. Preliminary Insight into Winter Native Fish Assemblages in Guadiana Estuary Salt Marshes Coping with Environmental Variability and Non-Indigenous Fish Introduction. Fishes 2017, 2, 19. https://doi.org/10.3390/fishes2040019
Gonçalves R, Teodósio MA, Cruz J, Ben-Hamadou R, Correia AD, Chícharo L. Preliminary Insight into Winter Native Fish Assemblages in Guadiana Estuary Salt Marshes Coping with Environmental Variability and Non-Indigenous Fish Introduction. Fishes. 2017; 2(4):19. https://doi.org/10.3390/fishes2040019
Chicago/Turabian StyleGonçalves, Renata, Maria Alexandra Teodósio, Joana Cruz, Radhouan Ben-Hamadou, Ana Dulce Correia, and Luís Chícharo. 2017. "Preliminary Insight into Winter Native Fish Assemblages in Guadiana Estuary Salt Marshes Coping with Environmental Variability and Non-Indigenous Fish Introduction" Fishes 2, no. 4: 19. https://doi.org/10.3390/fishes2040019
APA StyleGonçalves, R., Teodósio, M. A., Cruz, J., Ben-Hamadou, R., Correia, A. D., & Chícharo, L. (2017). Preliminary Insight into Winter Native Fish Assemblages in Guadiana Estuary Salt Marshes Coping with Environmental Variability and Non-Indigenous Fish Introduction. Fishes, 2(4), 19. https://doi.org/10.3390/fishes2040019






