Chlorella vulgaris as Protein Source in the Diets of African Catfish Clarias gariepinus
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolation and Cultivation of Algae
4.2. Feed Formulation
4.3. Experimental Set Up
4.4. Feeding of Fish and Hygiene
4.4.1. Weight Measurement of Fish
4.4.2. Growth and Nutritional Parameters Analyzed
4.4.3. Calculations and Statistical Analyses
5. Conclusions
Conflicts of Interest
References
- Francis, G.; Makkar, H.P.S.; Becker, K. Anti-nutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Gatlin, D.M., III; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, G.T.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, Å.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Vielma, J.; Ruohonen, K.; Gabaudan, J.; Vogel, K. Top-spraying soybean meal-based diets with phytase improves protein and mineral digestibilities but not lysine utilization in rainbow trout Oncorhynchus mykiss (Walbum). Aquac. Res. 2004, 31, 955–964. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Uchechukwu, E.; Pirhonen, J.; Vielma, J. Effects of sesame seed meal and bambaranut meal on growth and feed utilization and body composition of Juvenile African catfish Clarias gariepinus. Iran. J. Fish. Sci. 2014, 13, 998–1013. [Google Scholar]
- Hardy, R.W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Mustafa, M.G.; Nakagawa, H. A review: Dietary benefits of algae as an additive in fish feed. Isr. J. Aquac. Bamid. 1995, 47, 155–162. [Google Scholar]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Guccione, A.; Biondi, N.; Sampietro, G.; Rodolfi, L.; Bassi, N.; Tredici, M.R. Chlorella for protein and biofuels: from strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol. Biofuels 2014, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Kallqvist, T.; Olsen, E.; Vogt, G.; Gislerod, H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Bai, S.C.; Koo, J.-W.; Kim, K.W.; Kim, S.K. Effects of Chlorella powder as a feed additive on growth performance in juvenile Korean rockfish, Sebastes schlegeli (Hilgendorf). Aquac. Res. 2001, 32 (Suppl. S1), 92–98. [Google Scholar] [CrossRef]
- Nakagawa, H. Effect of dietary algae on improvement of lipid metabolism in fish. Biomed. Pharmacother. 1997, 51, 345–348. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Gouveia, A.; Rema, P.; Matos, J.; Gomes, E.F.; Pinto, I.S. Evaluation of three seaweeds Graci-laria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2006, 252, 85–91. [Google Scholar] [CrossRef]
- Görs, M.; Rhena Schumann, R.; Hepperle, D.; Karsten, U. Quality analysis of commercial Chlorella products used as dietary supplement in human nutrition. J. Appl. Phycol. 2010, 22, 265. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarretea, E.; Osorioa, A.; Riosa, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Sardesa, V.M. Nutritional role of polyunsaturated fatty acid. J. Nutr. Biochem. 1992, 3, 154–166. [Google Scholar] [CrossRef]
- Catarina, G.A.; Meireles, L.A.; Amaro, H.M.; Xavier, M.F. Changes in lipid class and fatty acid composition of cultures of Pavlova lutheri, in response to light intensity. J. Am. Oil Chem. Soc. 2010, 87, 791–801. [Google Scholar]
- Amaya, E.A.; Davis, D.A.; Rouse, D.B. Replacement of fishmeal in practical diets for the Pacific white shrimp (Litopenaeus vannamei) reared under pond conditions. Aquaculture 2007, 262, 393–401. [Google Scholar] [CrossRef]
- Azaza, M.S.; Mensi, F.; Ksouri, J.; Dhraief, M.N.; Brini, B.; Abdelmouleh, A.; Kraiem, M.M. Growth of Nile tilapia (Oreochromis niloticus L.) fed with diets containing graded levels of green algae ulva meal (Ulvarigida) reared in geothermal waters of southern Tunisia. J. Appl. Ichthyol. 2008, 24, 202–207. [Google Scholar] [CrossRef]
- Norambuena, F.; Hermon, K.; Skrzypczyk, V.; Emery, J.A.; Sharon, Y.; Beard, A.; Turchini, G.M. algae in fish feed: Performances and fatty acid metabolism in juvenile atlantic salmon. PLoS ONE 2015, 10, e0124042. [Google Scholar] [CrossRef] [PubMed]
- Gallego, I.; Jesús Casas, J.; Fuentes-Rodríguez, F.; Juan, M.; Sánchez-Castillo, P.; Pérez-Martínez, C. Culture of Spirogyra africana from farm ponds for long-term experiments and stock maintenance. Biotechnol. Agron. Soc. Environ. 2013, 17, 423–430. [Google Scholar]
- Kim, J.-H.; Kim, Y.H.; Lee, I.K. Morphotaxonomy of the genus Spirogyra (Zygnemataceae, Chlorophyta) in Korea. Algae 2004, 19, 91–105. [Google Scholar] [CrossRef]
- Ghazala, B.; Hena, L.; Zarina, A.; Shameel, M. Taxonomic survey of fresh water algae at the campus of BZ University of Multan, Pakistan. Int. J. Phycol. Phycochem. 2009, 5, 77–99. [Google Scholar]
- Masud-ul-Hasan, A.Z.; Shameel, M. Microtaxonomical studies on Chlorophycota and Vaucherophycota from Jauharabad District, Pakistan. Int. J. Phycol. Phycochem. 2010, 6, 141–154. [Google Scholar]
- Trifa, F.K.; Othman, F.A.; Omer, A.T. Oil and fatty acid composition of spirogyra and chara species from Bestan SWR spring water in Sulaimani-Kurdistan Region of Iraq. Egypt. J. Exp. Biol. 2013, 9, 159–162. [Google Scholar]
- Kay, R.A. Microalgae as food and supplement. Critical reviews. Food Sci. Nutr. 1991, 30, 555–573. [Google Scholar]
- Safi, C.; Charton, M.; Pignolet, O.; Silvestre, F.; Vaca-Garcia, C.; Pontalier, P.-Y. Influence of microalgae cell wall characteristics on protein extractability and determination of nitrogen-to-protein conversion factors. J. Appl. Phycol. 2013, 25, 523–529. [Google Scholar] [CrossRef]
- Daniel, N.; Sivaramakrishnan, T.; Saravanan, K.; Shalini, B.; Arunjyoti, B.; Sankar, R.; Dann Roy, S. A review on microalgae as potential fish feed ingredient. J. Andeman Sci. Assoc. 2016, 1, 140–144. [Google Scholar]
- Bakhtiyar, Y.; Langer, S.; Karlopia, S.K.; Ahmed, I. Growth, survival and proximate body composition of Labeo rohita larvae fed artificial food and natural food organisms under laboratory condition. Int. J. Fish. Aquac. 2011, 3, 114–117. [Google Scholar]
- Radhakrishnan, S.; Saravana Bhavan, P.; Seenivasan, C.; Muralisankar, T. Effect of dietary replacement of fishmeal with Chlorella vulgaris on growth performance, energy utilization and digestive enzymes in Macrobrachium rosenbegii postlarvae. Int. J. Fish. Aquac. 2015, 7, 62–70. [Google Scholar]
- Berliner, M.D. Proteins in Chlorella vulgaris. Microbios 1986, 46, 199–203. [Google Scholar]
- Seyfabadi, J.; Ramezanpour, Z.; Amini Khoeyi, Z. Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J. Appl. Phycol. 2011, 23, 721–726. [Google Scholar] [CrossRef]
- Lubitz, J.A. The protein quality, digestibility, and composition of micro-algae, Chlorella 71105. J. Food Sci. 1963, 28, 229–232. [Google Scholar] [CrossRef]
- Zeinhom, M.M. Nutritional and Physiological Studies on Fish. Ph.D. Thesis, Faculty of Agriculture, Zagazig University, Zagazig, Egypt, 2004. [Google Scholar]
- Shaaban, M. Green microalgae water extracts as foliar feeding to wheat plants. Pak. J. Biol. Sci. 2001, 4, 628–632. [Google Scholar]
- Shi, X.; Chen, F.; Chen, G.-H.; Pan, Y.-X.; Zhu, X.-M.; Liu, X.; Luo, Z. Fishmeal can be totally replaced by a mixture of rapeseed meal and chlorella meal in diets for crucian carp (Carassius auratus gibelio). Aquac. Res. 2017, 1–9. [Google Scholar] [CrossRef]
- Pakravan, S.; Akbarzadeh, A.; Sajjadi, M.M.; Hajimoracloo, A.; Noori, F. Chlorella vulgaris meal improved growth performance, digestive enzyme activities, fatty acid composition and tolerance of hypoxia and ammonia stress in juvenile Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2017. [Google Scholar] [CrossRef]
- Dawah, M.A.; Ibrahim, A.N.; Eladel, H.M. Amino acids content of Chlorella vulgaris and Scenedesmus bijuga raised on domestic sewage and agricultural drainage water. In Proceedings of the 1st Scientific Conference of the Egyptian Aquaculture Society, El-Arish, Egypt, 13–15 December 2002. [Google Scholar]
- Dawah, M.A.; Khater, A.M.; Shaker, I.M.A.; Ibrahim, N.A. Production of Scenedesmus bijuga (Chlorophyceae) in large scale in outdoor tanks and its use in feeding monosex Nile tilapia (Oreochromis niloticus) fry. J. Egypt. Acad. Soc. Environ. Dev. 2002, 2, 113–125. [Google Scholar]
- Van Der Meeren, T.; Mangor-Jensen, A.; Pickova, J. The effect of green water and light intensity on survival, growth and lipid composition in Atlantic cod (Gadus morhua) during intensive larval rearing. Aquaculture 2007, 265, 206–2173. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Cao, L.; Yang, Y.; Wang, W. Effects of daphnia (Moina micrura) plus chlorella (Chlorella pyrenoidosa) or microparticle diets on growth and survival of larval loach (Misgurnus anguillicaudatus). Aquac. Int. 2008, 16, 361–368. [Google Scholar] [CrossRef]
- Cahu, C.L.; Zambonino Infante, J.L.; Péres, A.; Quazuguel, P.; Le Gall, M.M. Algal addition in sea bass (Dicentrarchus labrax) larvae rearing: Effect on digestive enzymes. Aquaculture 1998, 161, 479–489. [Google Scholar] [CrossRef]
- Güroy, D.; Güroy, B.; Merrifield, D.L.; Ergün, S.; Tekinay, A.A.; Yiğit, M. Effect of dietary Ulva and Spirulina on weight loss and body composition of rainbow trout, Oncorhynchus mykiss(Walbaum), during a starvation period. J. Anim. Physiol. Anim. Nutr. 2011, 95, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Nandeesha, M.C.; Gangadhar, B.; Varghese, T.J.; Keshavanath, P. Effect of feeding Spirulina platensis on the growth, proximate composition and organoleptic quality of common carp, Cyprinus carpio L. Aquac. Res. 1998, 29, 305–312. [Google Scholar] [CrossRef]
- Badwy, T.M.; Ibrahim, E.M.; Zeinhom, M.M. Partial replacement of fishmeal with dried microalga (Chlorella spp. and Scenedesmus spp.) in Nile tilapia (Oreochromis niloticus) diets. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 801–811. [Google Scholar]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. n-3 Oil sources for use in aquaculture—Alternatives to the unsustainable harvest of wild fish. Nutr. Res. Rev. 2008, 21, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Z.; Jauncey, K. Optimal dietary carbohydrate to lipid ratio in African catfish Clarias gariepinus (Burchell 1822). Aquac. Int. 2004, 12, 169–180. [Google Scholar] [CrossRef]
- Ematipour, G.R.; Brown, M.L.; Gatlin, D.M., III. Effects of dietary carbohydrate: Lipid ratio on growth and body composition of hybrid striped bass. J. World Aquac. Soc. 1992, 23, 128–132. [Google Scholar] [CrossRef]
- Enyidi, U.; Pirhonen, J.; Kettunen, J.; Vielma, J. Effect of Feed Protein:Lipid Ratio on Growth Parameters of African Catfish Clarias gariepinus after Fish Meal Substitution in the Diet with Bambaranut (Voandzeia subterranea) Meal and Soybean (Glycine max) Meal. Fishes 2017, 2, 1. [Google Scholar] [CrossRef]
- Tan, Q.; Xie, S.; Zhu, X.; Lei, W.; Yang, Y. Effect of dietary carbohydrate- to-lipid ratios on the growth and feed utilization in Chinese longsnout catfish (Leiocassis longirostris Gunther). J. Appl. Ichthyol. 2007, 23, 605–610. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, X. Effects of high carbohydrate and high lipid diets on growth, body composition and glucose metabolism in southern catfish at two temperatures. Aquac. Res. 2010, 41, e431–e437. [Google Scholar] [CrossRef]
- Satoh, K.-I.; Nakagawa, H.; Kasahara, S. Effect of ulva meal supplementation on disease resistance of red sea bream. Nippon Suisan Gakkaishi 1987, 53, 1115–1120. [Google Scholar] [CrossRef]
- Rocha, R.J.; Ribeiro, L.; Costa, R.; Dinis, M.T. Does the presence of microalgae influence fish larvae prey capture? Aquac. Res. 2008, 39, 362–369. [Google Scholar] [CrossRef]
- Ogawa, T.; Aiba, S. Bioenergetic analysis of mixotrophic growth in Chlorella Vulgaris and Scenedesmus acutus. Biotechnol. Bioeng. 1981, 23, 1121–1132. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.C. Determination of total lipid classes and fatty acids in aquatic samples. In Lipids in Freshwater Ecosystems; Wetzel, R.G., Art, M.T., Wainmann, B.C., Eds.; Springer: New York, NY, USA, 1999; pp. 4–20. [Google Scholar]
- Kainz, M.; Arts, M.; Mazumder, A. Essential fatty acids in the planktonic food web and their ecological role for higher trophic level. Limnol. Oceanogr. 2004, 49, 1784–1793. [Google Scholar] [CrossRef]
| Feed | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Ini. no | 30 | 30 | 30 | 30 |
| Final no | 30 | 30 | 30 | 30 |
| Ini. Av. Wt | 1.09 ± 0.05 | 1.09 ± 0.05 | 1.09 ± 0.05 | 1.09 ± 0.05 |
| Fin. Av. Wt | 122.11 ± 2.51 a | 109.32 ± 1.09 b | 90.34 ± 0.55 c | 63.59 ± 1.30 d |
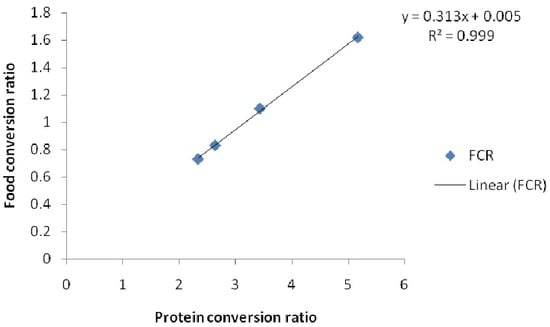
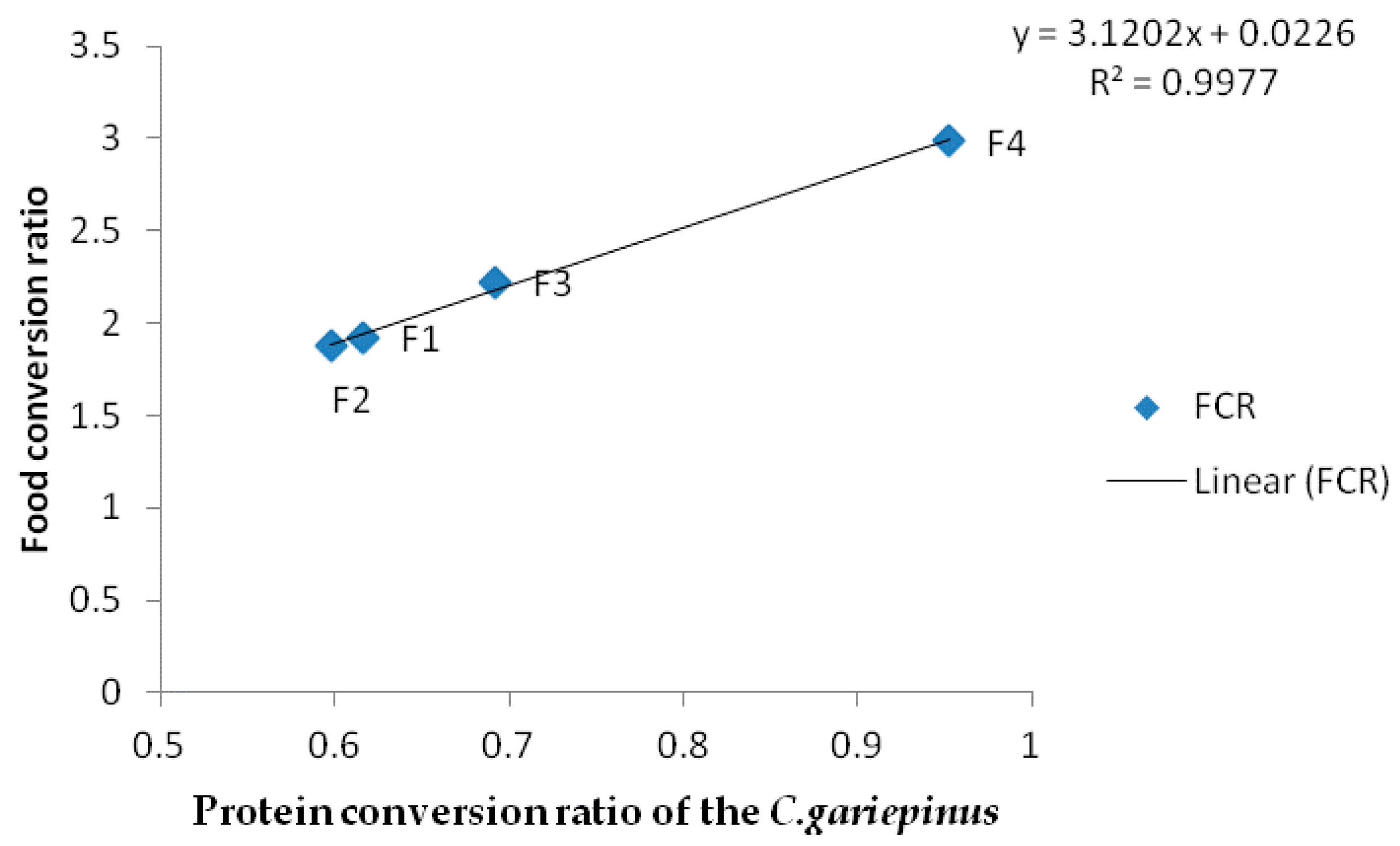
| FCR | 1.92 ± 0.05 a | 1.88±0.02 a | 2.22 ± 0.11 b | 2.98 ± 0.01 c |
| AWG | 121.02 ± 0.04 a | 108.23 ± 0.06 b | 89.25 ± 0.02 c | 62.50 ± 0.01 d |
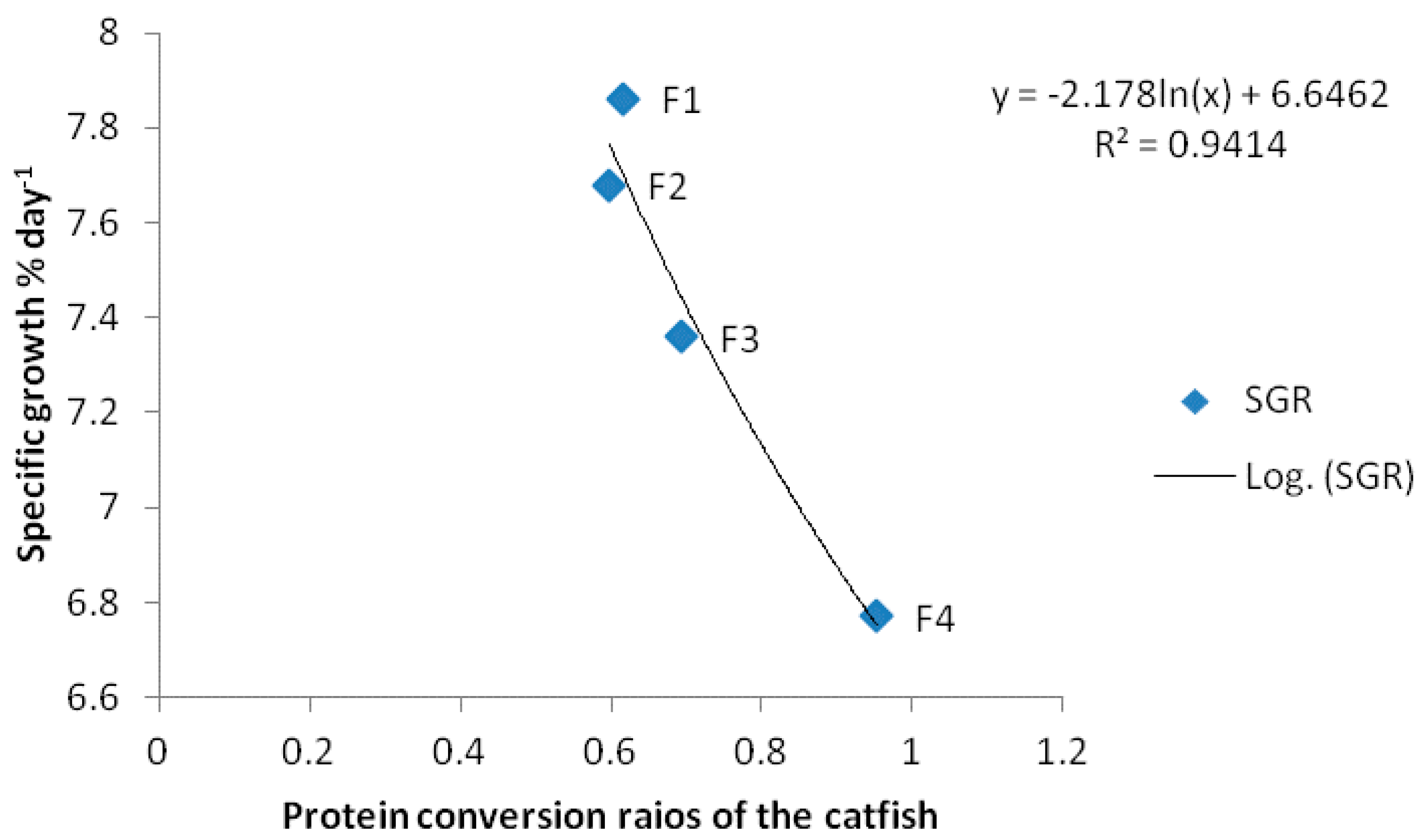
| SGR | 7.86 ± 0.45 a | 7.68 ± 0.21 a | 7.36 ± 0.44 a | 6.77 ± 0.07 b |
| HSI | 1.48 ± 0.01 a | 1.53 ± 0.1 a | 1.87 ± 0.08 b | 2.50 ± 0.59 c |
| PCR | 0.61 ± 0.02 a | 0.60 ± 0.03 a | 0.69 ± 0.11 b | 0.95 ± 0.23 c |
| PER | 2.46 ± 0.22 b | 2.30 ± 0.13 b | 2.07 ± 0.03 c | 2.02 ± 0.09 a |
| DFI | 3.88 ± 0.15 a | 3.39 ± 0.04 b | 3.30 ± 0.24 b | 3.11 ± 0.08 b |
| DMR | 7.95 ± 0.12 a | 8.11 ± 0.43 a | 8.84 ± 0.37 b | 12.34 ± 0.22 c |
| WPR | 1.54 ± 0.10 a | 1.5 ± 0.22 a | 1.81 ± 0.32 b | 2.50 ± 0.21 c |
| Items | Feeds | |||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| Fishmeal | 0 | 0 | 0 | 15 |
| Algae | 25 | 15 | 5 | 0 |
| Corn meal | 40 | 43 | 53 | 43 |
| Millet meal | 23 | 30 | 30 | 30 |
| Bone meal | 5 | 5 | 5 | 5 |
| Palm oil | 5 | 5 | 5 | 5 |
| Vitamin premix | 2 | 2 | 2 | 2 |
| Total | 100 | 100 | 100 | 100 |
| Proximate composition of diets | ||||
| Crude Protein % | 32.0 | 31.8 | 31.2 | 31.9 |
| Starch g | 200 | 198 | 204 | 204 |
| Crude lipids (%) | 11.9 | 11.8 | 11.6 | 11.2 |
| Ash g | 89 | 92 | 89 | 84 |
| Moisture% | 8.7 | 8.5 | 8.0 | 8.7 |
| Protein | Carbohydrate | Lipids | |
|---|---|---|---|
| Millet | 20.68 ± 1.55 | 49.50 ± 3.17 | 3.64 ± 0.06 |
| C. vulgaris | 54.65 ± 0.07 | 12.09 ± 3.17 | 24.40 ± 0.09 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enyidi, U.D. Chlorella vulgaris as Protein Source in the Diets of African Catfish Clarias gariepinus. Fishes 2017, 2, 17. https://doi.org/10.3390/fishes2040017
Enyidi UD. Chlorella vulgaris as Protein Source in the Diets of African Catfish Clarias gariepinus. Fishes. 2017; 2(4):17. https://doi.org/10.3390/fishes2040017
Chicago/Turabian StyleEnyidi, Uchechukwu D. 2017. "Chlorella vulgaris as Protein Source in the Diets of African Catfish Clarias gariepinus" Fishes 2, no. 4: 17. https://doi.org/10.3390/fishes2040017
APA StyleEnyidi, U. D. (2017). Chlorella vulgaris as Protein Source in the Diets of African Catfish Clarias gariepinus. Fishes, 2(4), 17. https://doi.org/10.3390/fishes2040017