Visual Characterization of Male and Female Greenshell™ Mussels (Perna canaliculus) from New Zealand Using Image-Based Shape and Color Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Greenshell™ Mussel Samples
2.2. Acquiring Whole Mussel Images
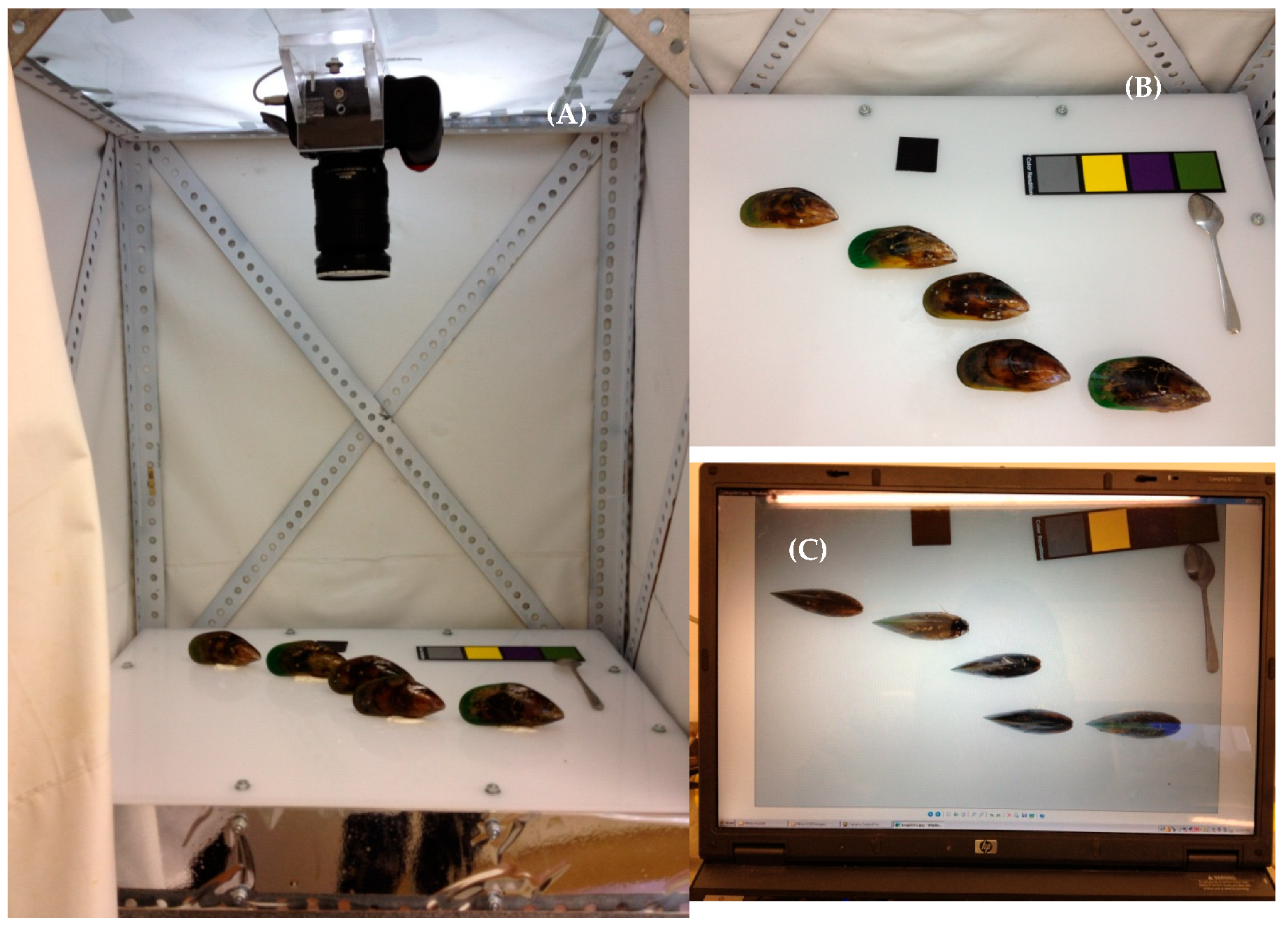
2.3. Determination of Gender
2.4. Color Analysis
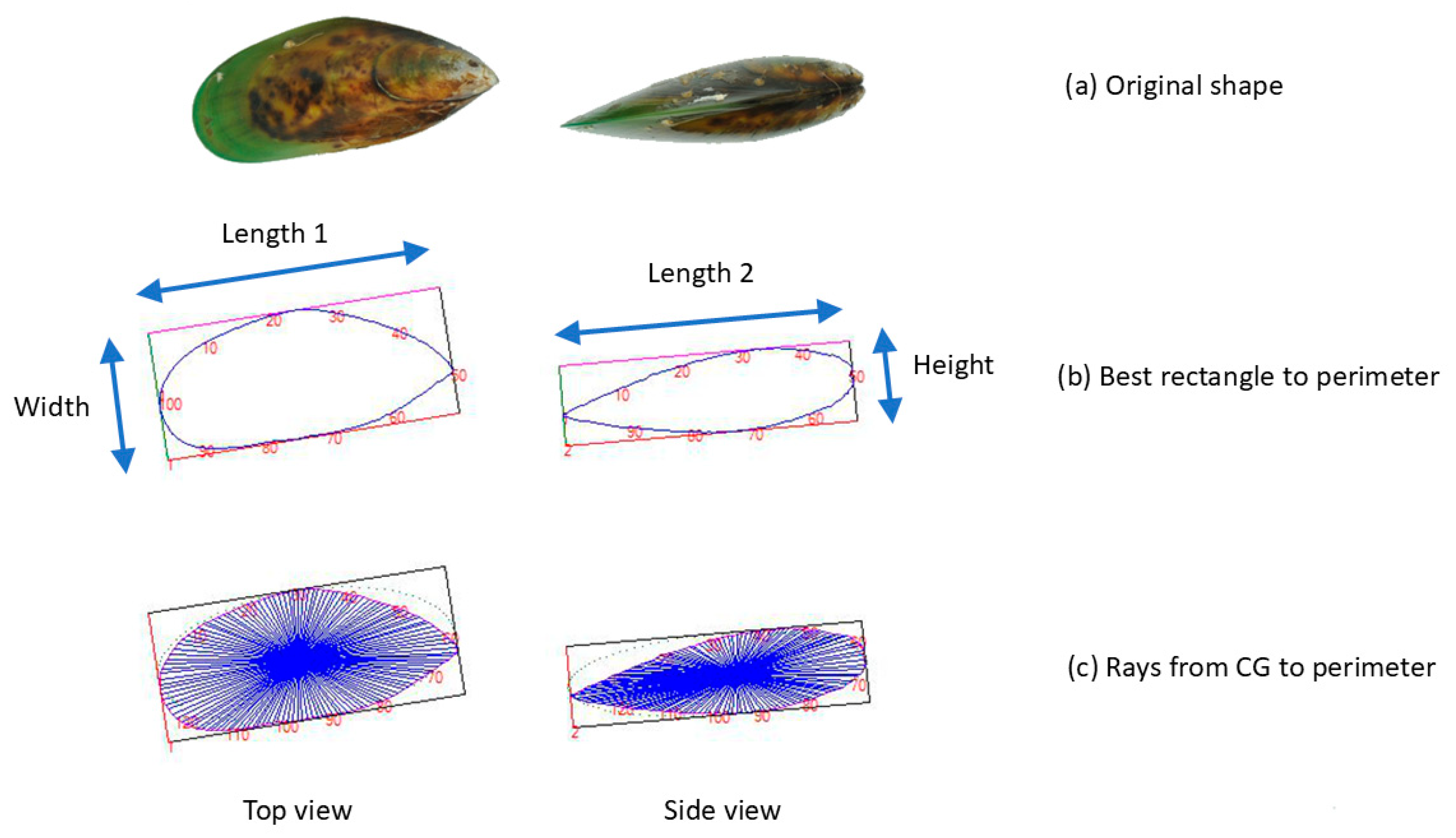
2.5. Image Analysis: Determination of Dimensions and Shape
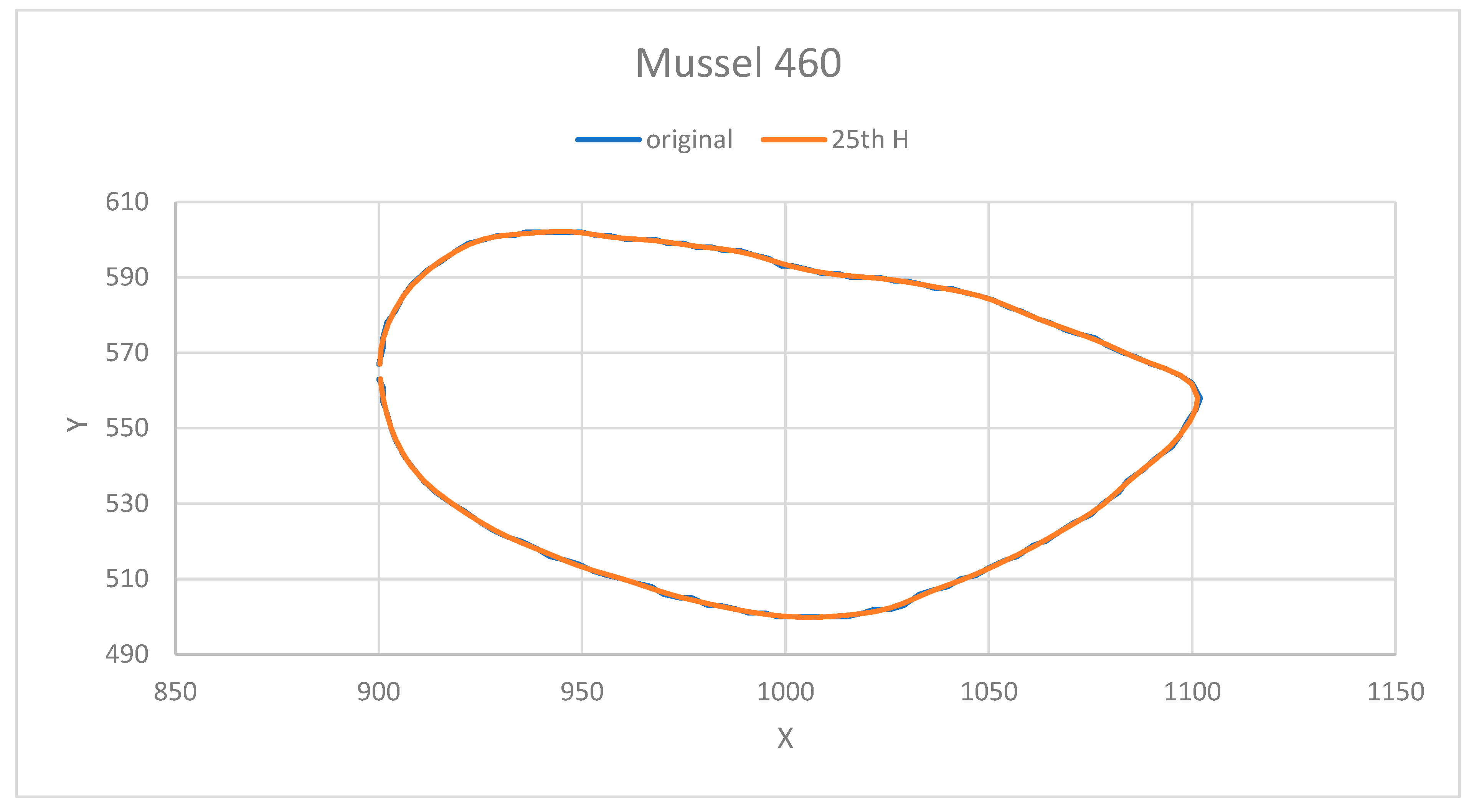
2.6. Elliptic Fourier Analysis
2.7. Neural Networks Analysis
2.8. Principal Component Analysis
2.9. Canonical Discriminant Analysis
2.10. Random Forests Analysis
3. Results and Discussion
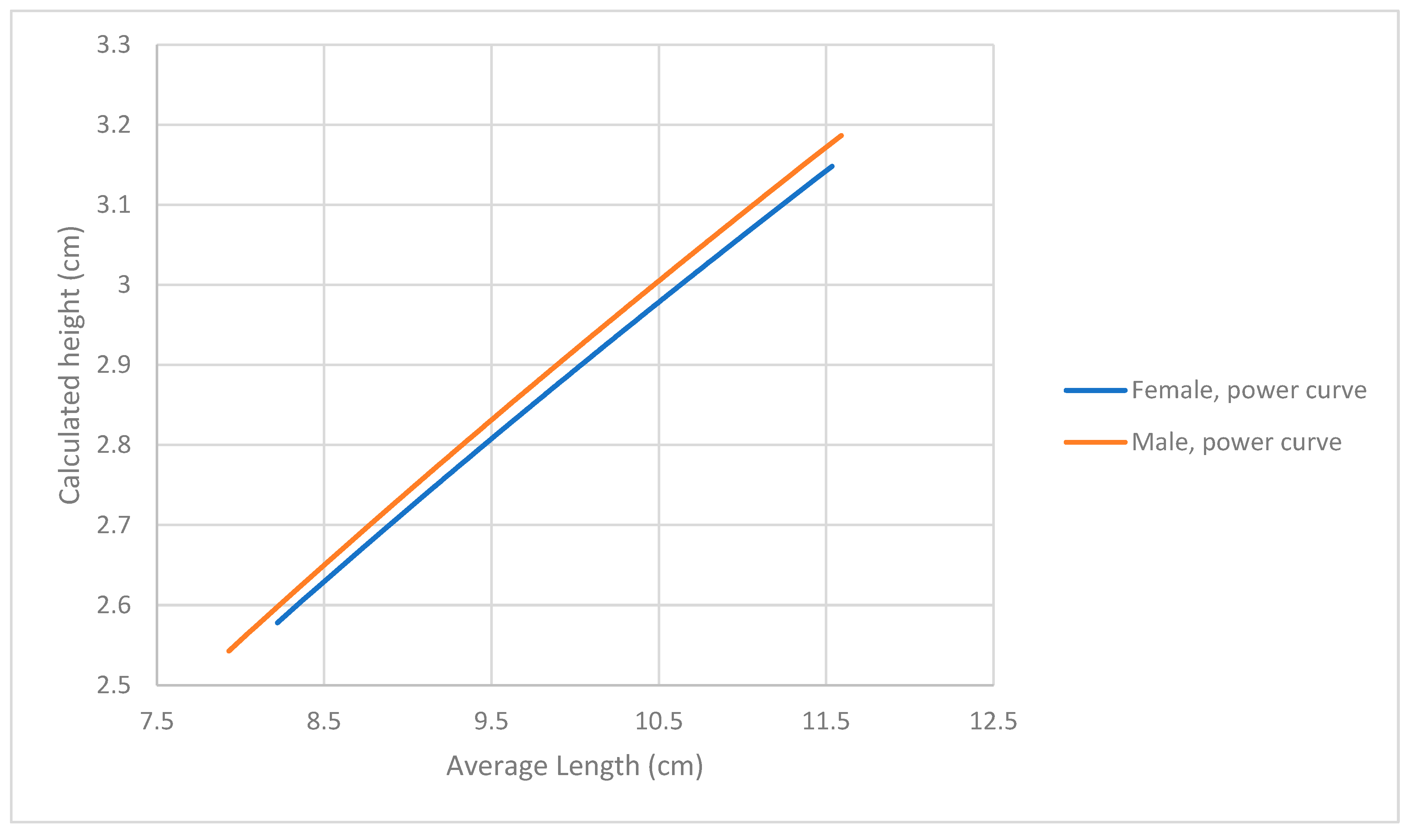
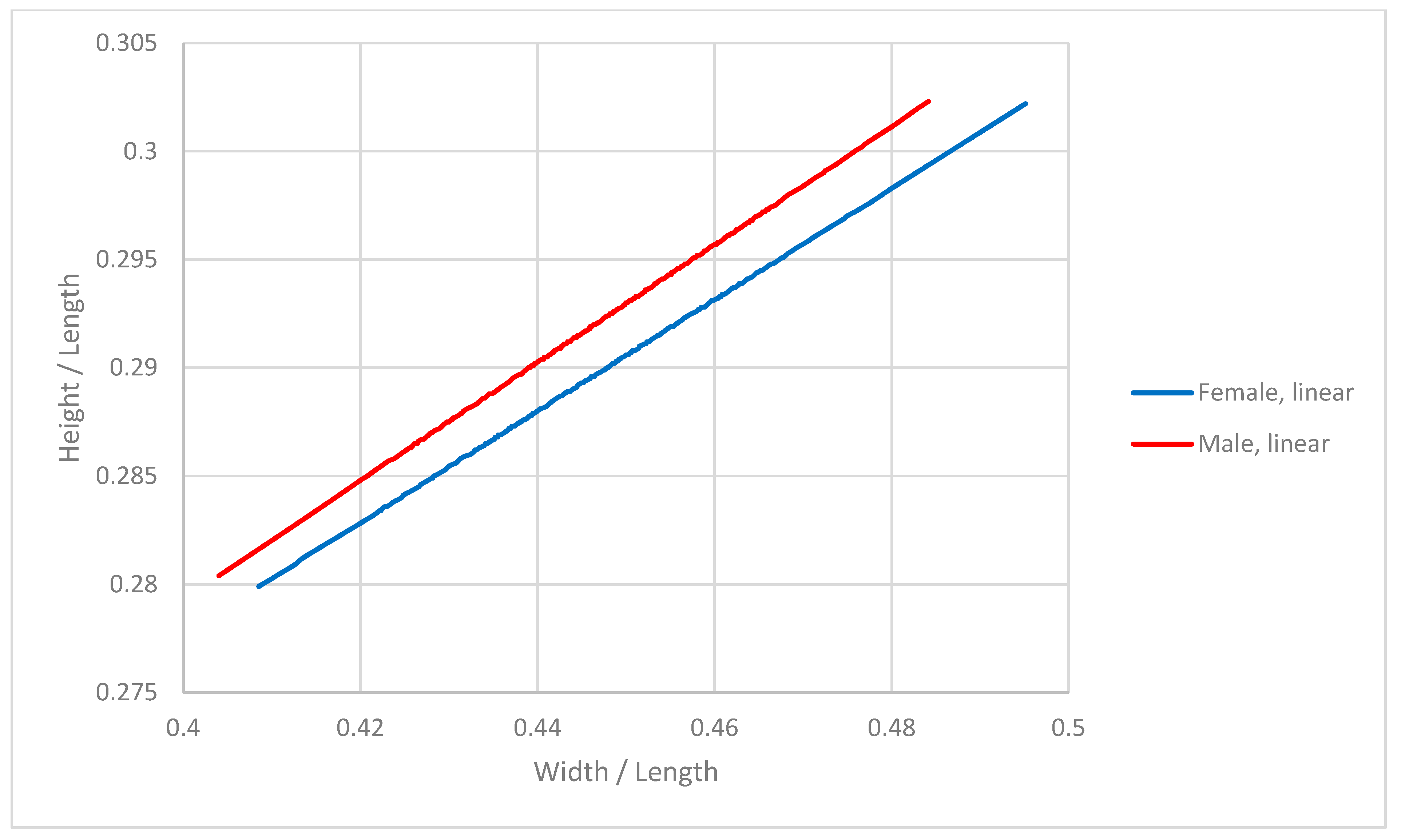
3.1. Analysis of Dimensions
3.2. Analysis of Weight vs. Area
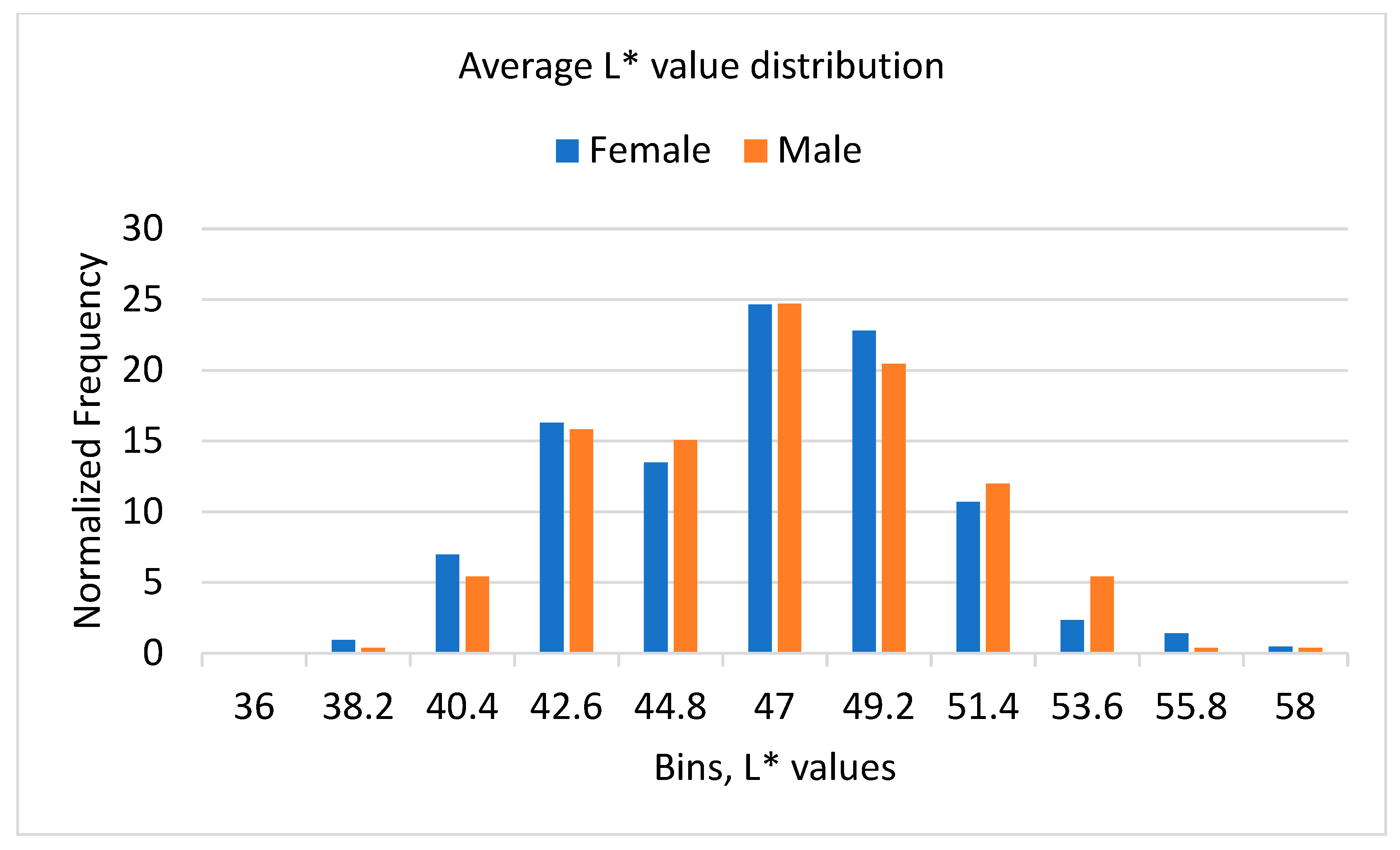
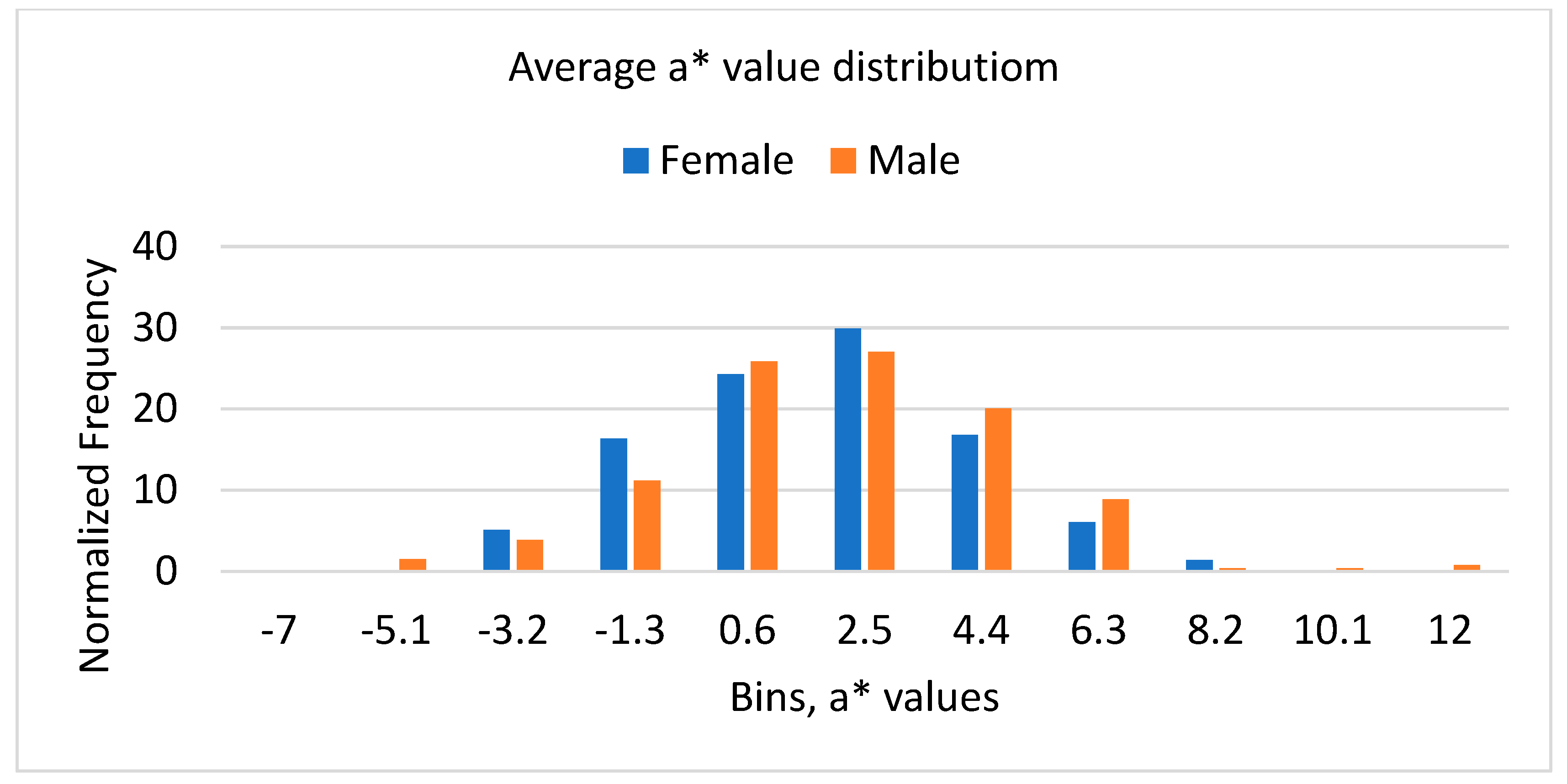
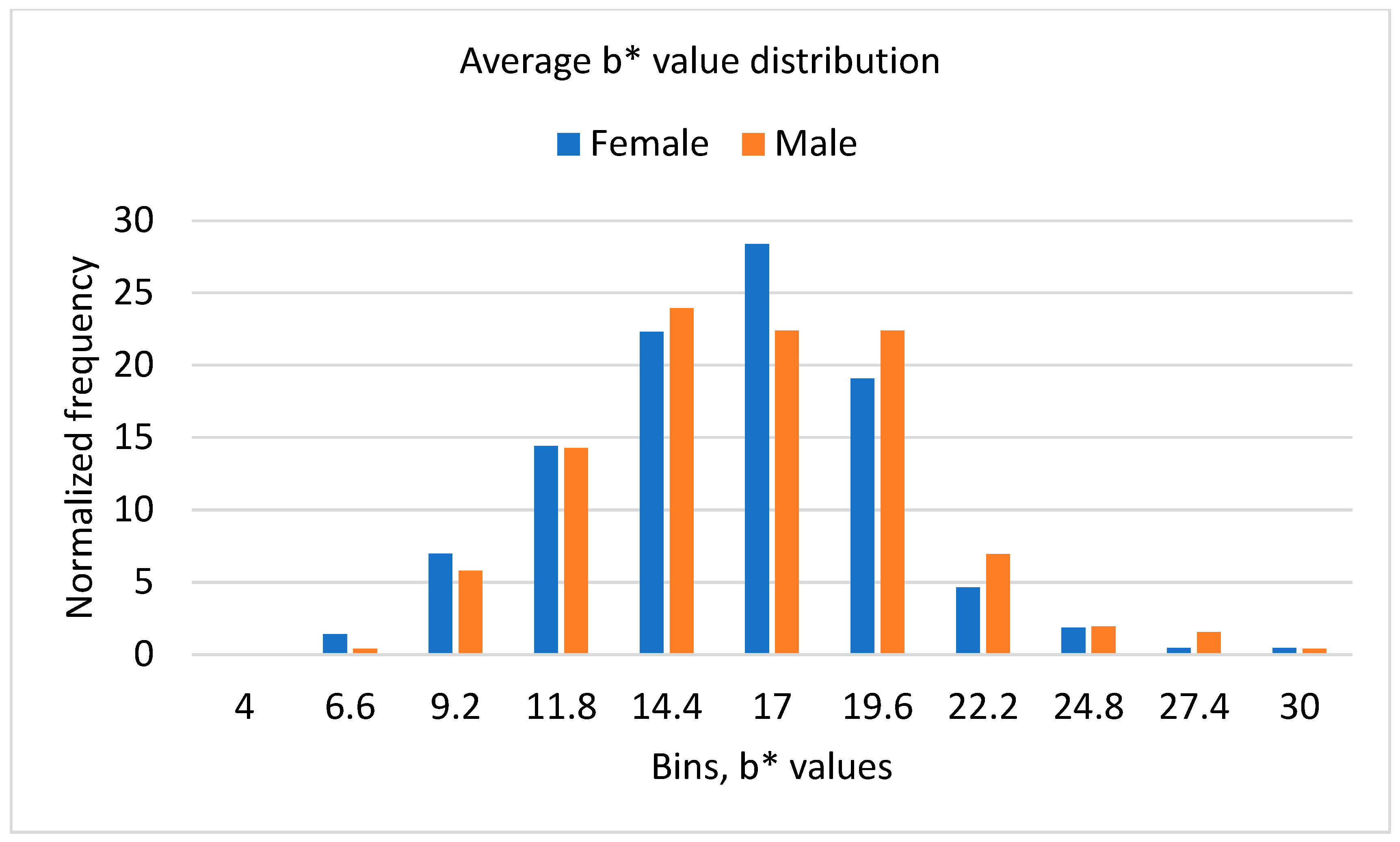
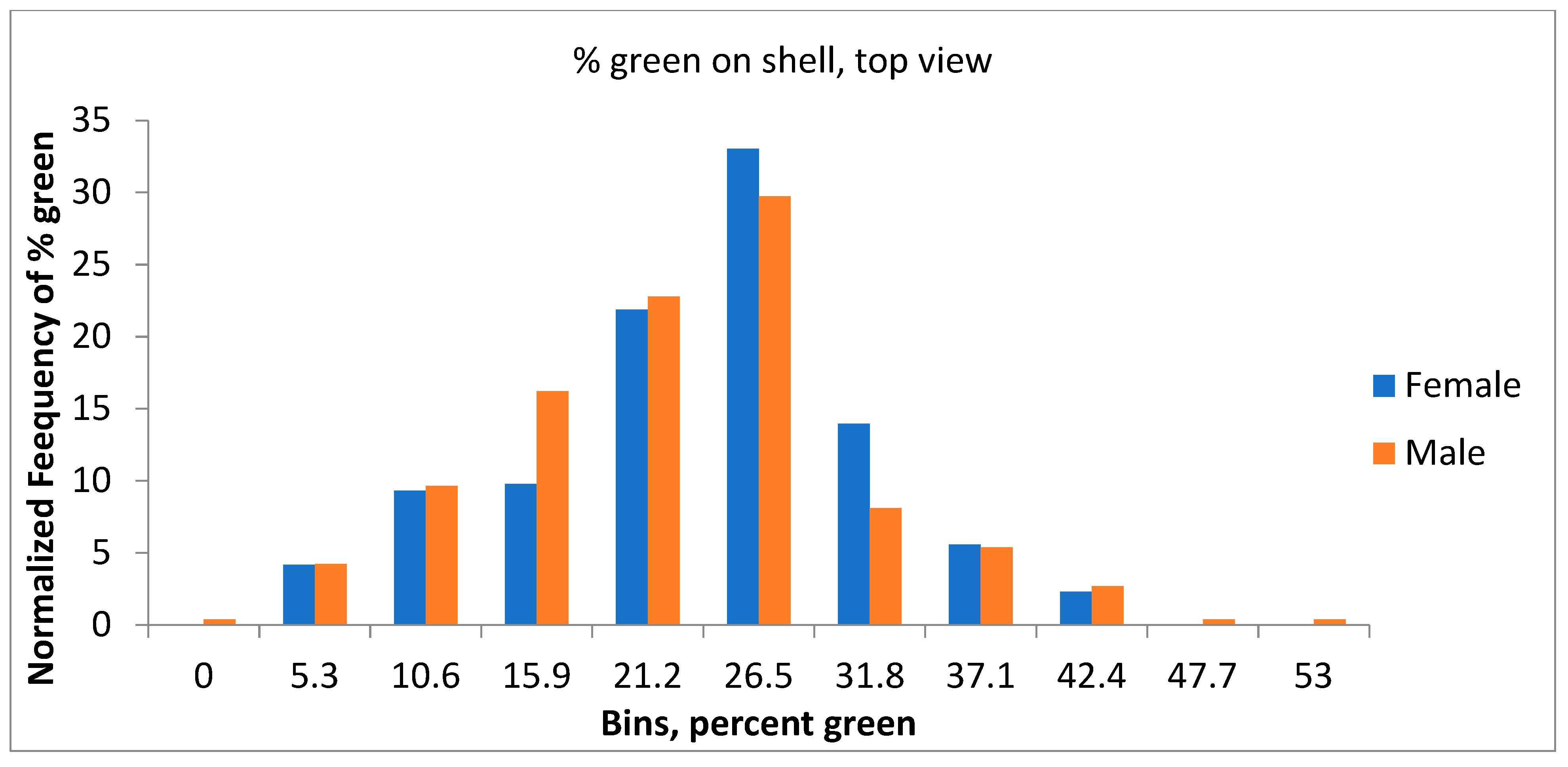
3.3. Results of Color Analysis
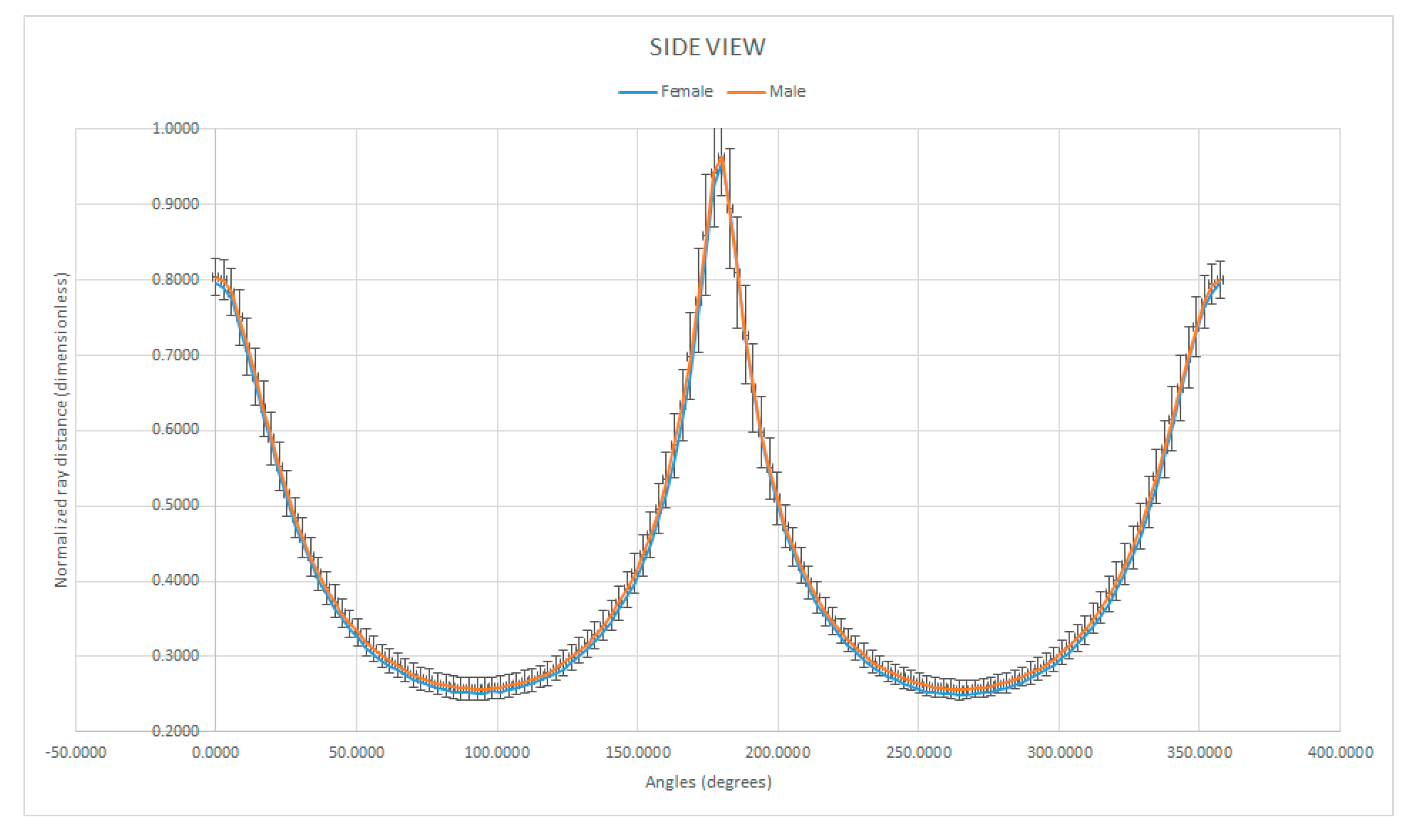
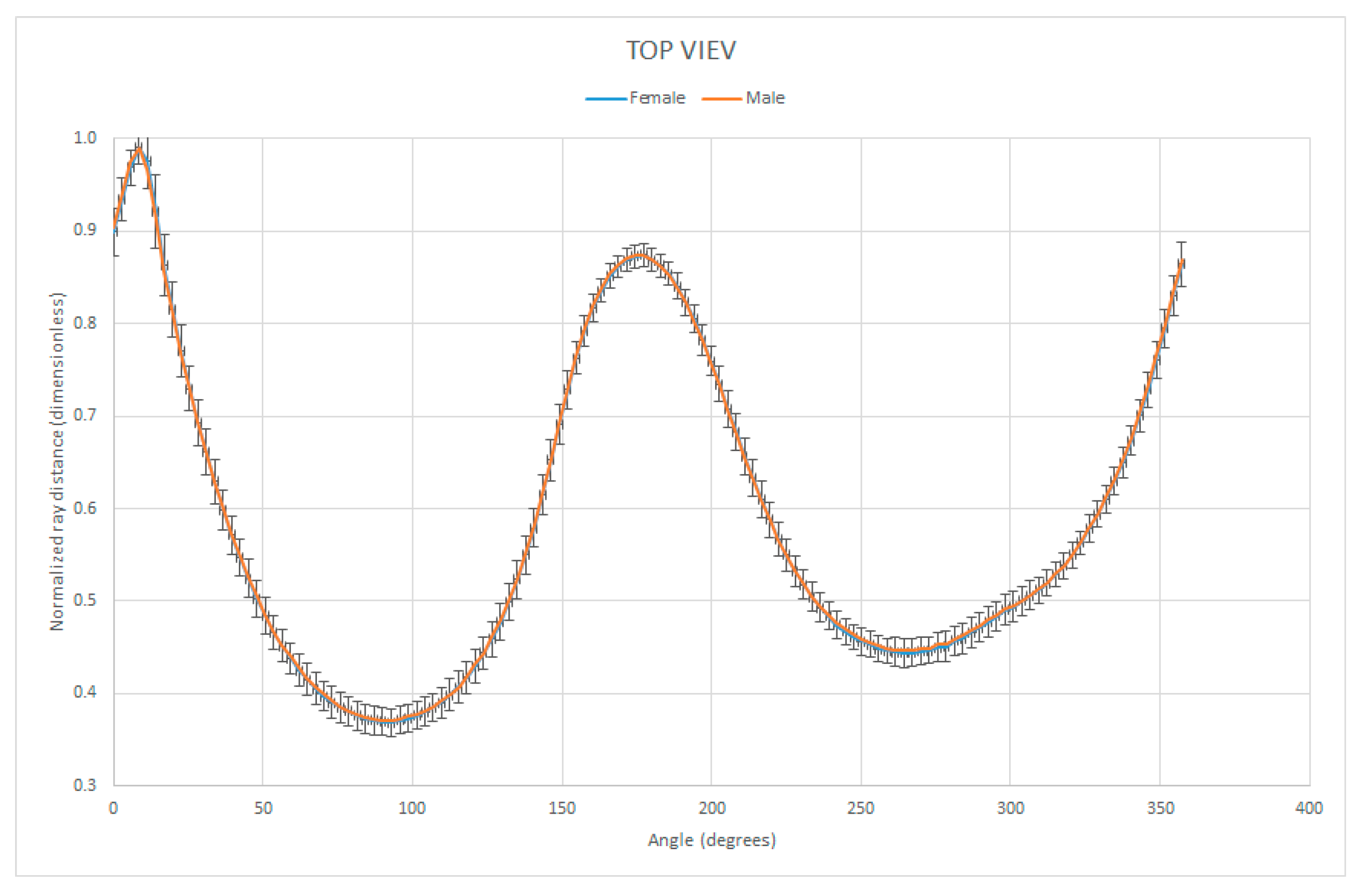
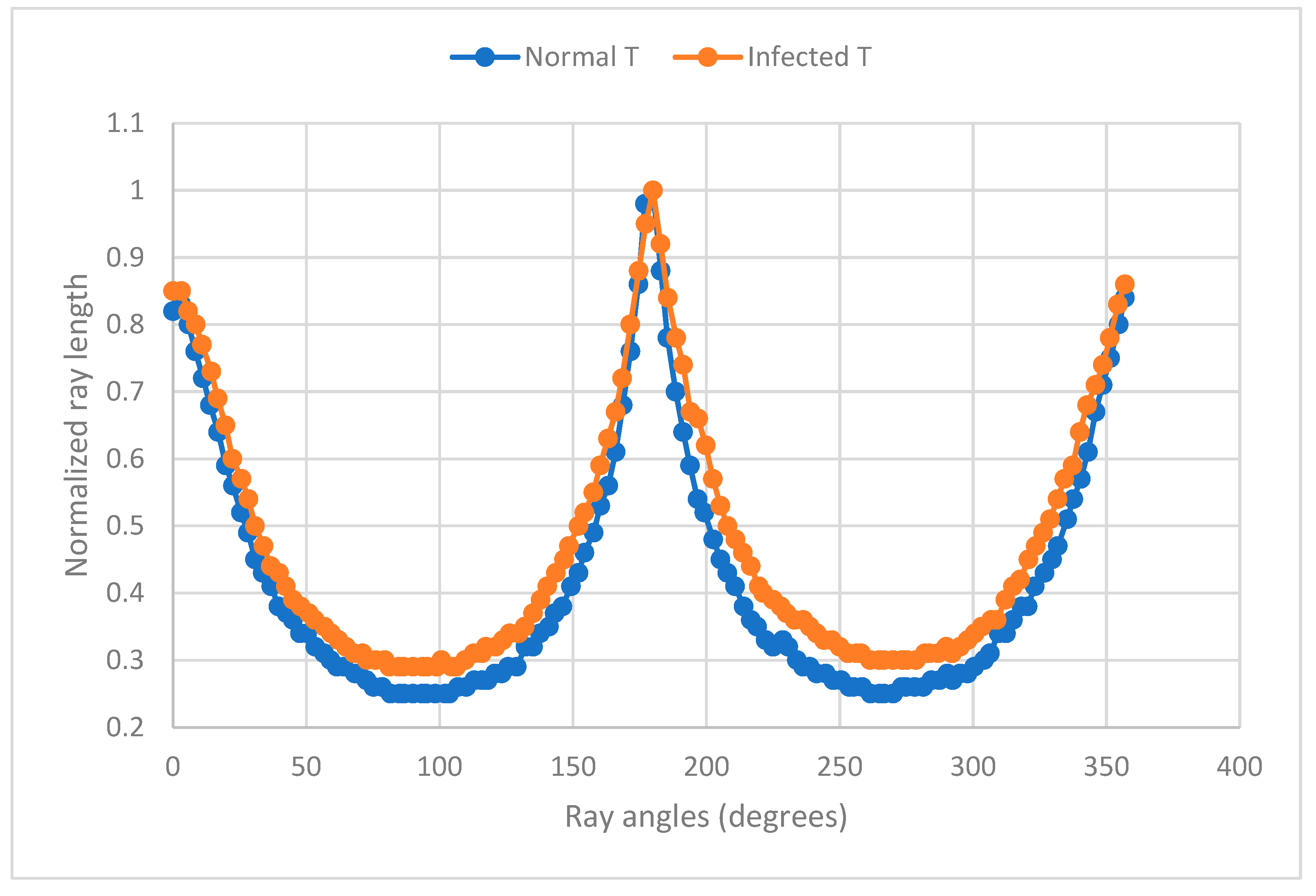
3.4. Shape Analysis by Normalized Ray Length vs. Ray Angle
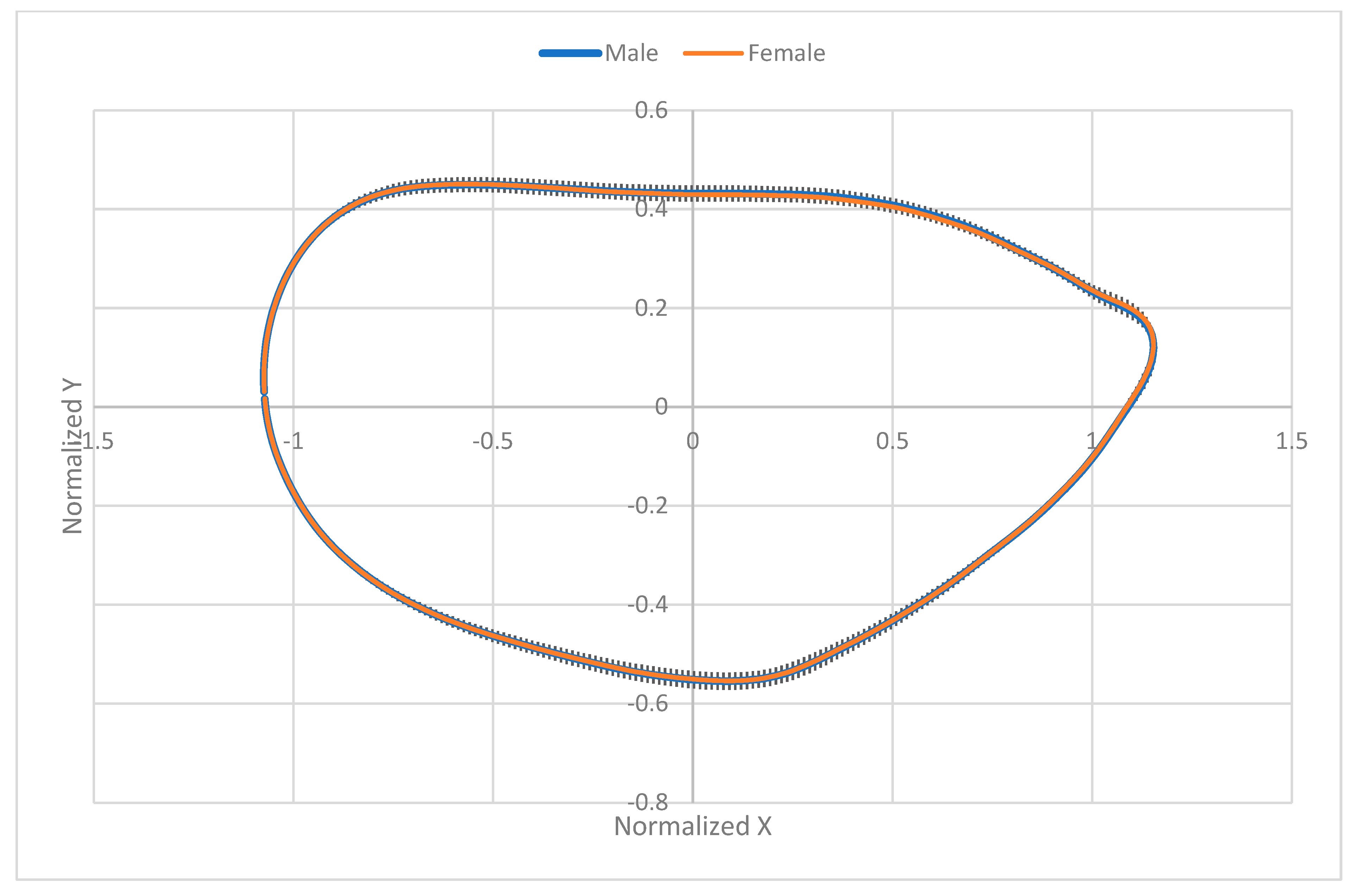
3.5. Results of Elliptic Fourier Analysis
3.6. Artificial Neural Networks Analysis of EFA Shape Parameters
3.7. PCA Analysis of EFA Shape Parameters
3.8. Canonical Discriminant Analysis of EFA Shape Parameters
3.9. Random Forests Analysis of EFA Shape Parameters
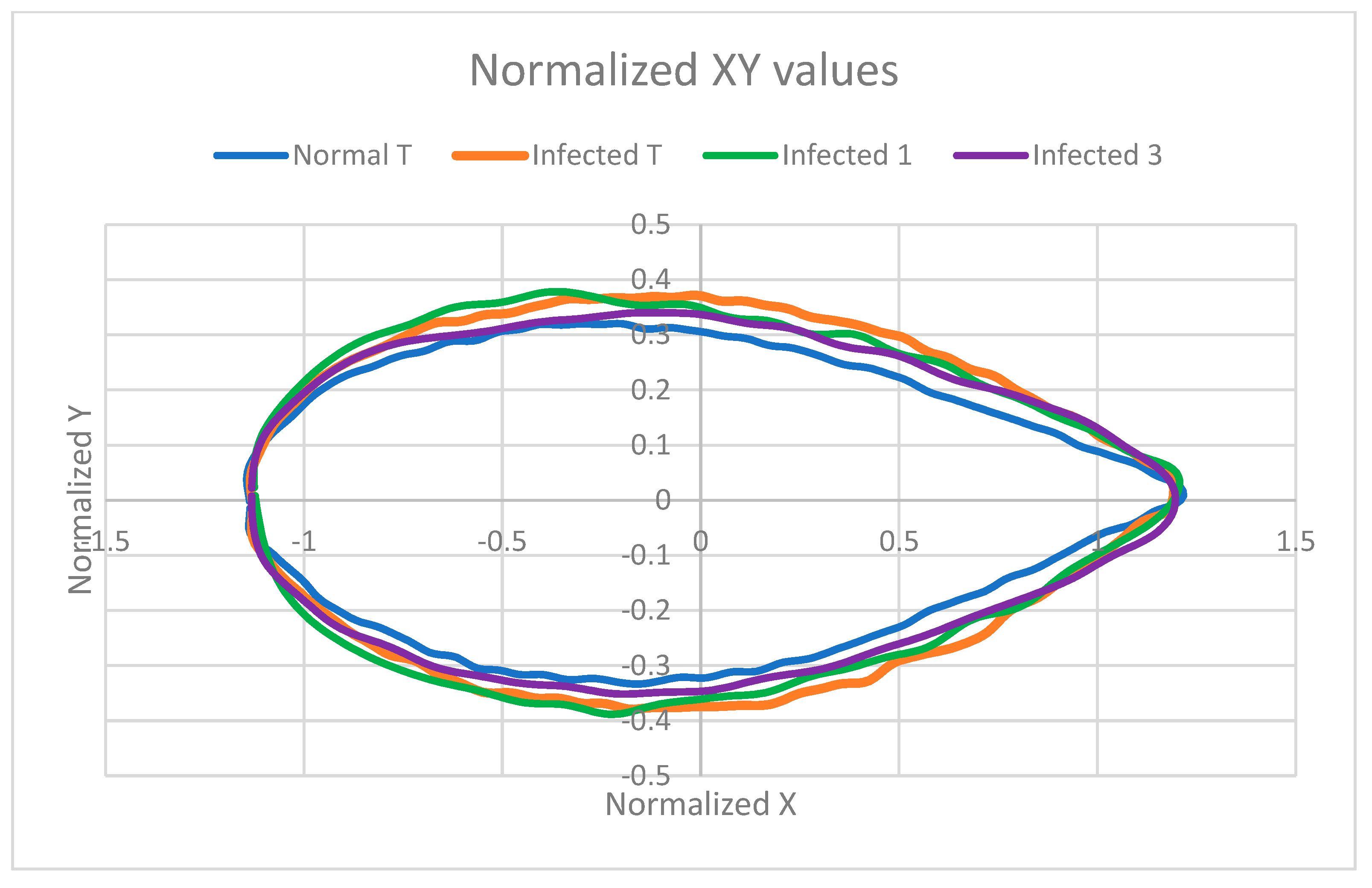
3.10. Effect of Pea Crab on Shape
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, M. Comparison of Lipid Classes and Fatty Acid Profiles of Lipids from Raw, Steamed and High Pressure Treated New Zealand Greenshell™ Mussel Meat of Different Genders. Master’s Thesis, University of Auckland, Auckland, New Zealand, 2013. [Google Scholar]
- MacDonald, G.A.; Hall, B.I.; Vlieg, P. Seasonal Changes in Proximate Composition and Glycogen Content of Greenshell™ Mussels; Mussel Industry Council Seminar, Crop & Food Research Ltd.: Nelson, New Zealand, 2000. [Google Scholar]
- Sokolowski, A.; Bawazir, A.S.; Sokolowska, E.; Wolowicz, M. Seasonal variation in the reproductive activity, physiological condition and biochemical components of the brown mussel Perna perna from the coastal waters of Yemen (Gulf of Aden). Aquat. Living Resour. 2010, 23, 177–186. [Google Scholar] [CrossRef]
- Zhou, M.; Balaban, M.O.; Gupta, S.; Fletcher, G. Comparison of lipid classes and fatty acid profiles of lipids from raw, steamed and high-pressure-treated New Zealand Greenshell™ mussel of different genders. J. Shellfish. Res. 2014, 33, 473–479. [Google Scholar] [CrossRef]
- Aquaculture New Zealand. Available online: https://www.aquaculture.org.nz/news-article/aiformussels (accessed on 9 April 2025).
- Zieritz, A.; Aldridge, D.C. Sexual, habitat-constrained and parasite-induced dimorphism in the shell of a freshwater mussel (A. anatine, Unionidae). J. Morphol. 2011, 272, 1365–1375. [Google Scholar] [CrossRef]
- Kotrla, M.B.; James, F.C. Sexual dimorphism of shell shape and growth of Villosa villosa (Wright) and Elliptio icterina (Conrad) (Bivalvia: Unioidae). J. Molloscan Stud. 1987, 53, 13–23. [Google Scholar] [CrossRef]
- Trivellini, M.M.; Van der Molen, S.; Márquez, F. Fluctuating asymmetry in the shell shape of the Atlantic Patagonian mussel, Mytilus platensis, generated by habitat-specific constraints. Hydrobiologia 2018, 822, 189–201. [Google Scholar] [CrossRef]
- Steffani, C.N.; Branch, G.M. Growth rate, condition, and shell shape of Mytilus galloprovincialis: Response to wave exposure. Mar. Ecol. Prog. Ser. 2003, 246, 197–209. [Google Scholar] [CrossRef]
- Reimer, O.; Tedengren, M. Phenotypical improvement of morphological defenses in the mussel Mytilus edulis induced by exposure to the predator Asterias rubens. Oikos 1996, 75, 383–390. [Google Scholar] [CrossRef]
- Telesca, L.; Michalek, K.; Sanders, T.; Peck, L.S.; Thyrring, J.; Harper, E.M. Blue mussel shell shape plasticity and natural environments: A quantitative approach. Sci. Rep. 2018, 8, 2865. [Google Scholar] [CrossRef]
- Hickman, R.W. Allometry and growth of the green-lipped mussel Perna canaliculus in New Zealand. Mar. Biol. 1979, 51, 311–327. [Google Scholar] [CrossRef]
- Thejasvi, A.; Shenoy, C.; Thippeswamy, S. Morphometric and length-weight relationships of the green mussel Perna viridis (Linnaeus) from a subtidal habitat of Karwar coas, Karnataka, India. Int. J. Recent Sci. Res. 2014, 5, 295–299. [Google Scholar]
- Alcicek, Z.; Balaban, M.O. Estimation of whole volume of green shelled mussels using their geometric attributes obtained from image analysis. Int. J. Food Prop. 2014, 17, 1987–1997. [Google Scholar] [CrossRef]
- Balaban, M.O.; MChombeau Cırban, D.; Gümüş, B. Prediction of the weight of Alaskan pollock using image analysis. J. Food Sci. 2010, 75, E552–E556. [Google Scholar] [CrossRef] [PubMed]
- Dommergues, E.; Dommergues, J.L.; Magniez, F.; Neige, P.; Varrechia, E.P. Geometric measurement analysis versus Fourier series analysis for shape characterization using the gastropod shell (Trivia) as an example. Math. Geol. 2003, 35, 887–894. [Google Scholar] [CrossRef]
- Balaban, M.; Odabasi, A.Z. Measuring color with machine vision. Food Technol. 2006, 60, 32–36. [Google Scholar]
- Zahn, C.; Roskies, R.R. Fourier descriptors for plane closed curves. IEEE Trans Comput. 1972, C-21, 269–281. [Google Scholar] [CrossRef]
- Costa, C.; Aguzzi, J.; Menesatti, P.; Antonucci, F.; Rimatori, V.; Mattoccia, M. Shape analysis of different poulations of clams in relation to their geographical structure. J. Zool. 2008, 276, 71–80. [Google Scholar] [CrossRef]
- Palmer, M.; Pons, G.X.; Linde, M. Discriminating between geographical groups of a Mediterranean commercial clam (Chamelea gallina L. Veneridae) by shape analysis. Fish. Res. 2004, 67, 93–98. [Google Scholar] [CrossRef]
- Rufino, M.M.; Gaspar, M.B.; Pereira, A.M.; Vasconcelos, P. Use of shape to distinguish Chamelea gallina and Chamelea striatula (Bivalvia: Veneridae): Linear and geometric morphometric methods. J. Morphol. 2006, 267, 1433–1440. [Google Scholar] [CrossRef]
- Costa, C.; Menesatti, P.; Aguzzi, J.; D’Andrea, S.; Antonucci, F.; Rimatori, V.; Pallottino, F.; Mattocia, M. External shape differences between Sympatric populations of commercial clams Tapes decussatus and T. philippinarum. Food Bioprocess Technol. 2010, 3, 43–48. [Google Scholar] [CrossRef]
- Marquez, F.; Van der Molen, S. Intraspecific shell shape variation in the razor clam Ensis macha along the Patagonian coast. J. Molluscan Stud. 2011, 77, 123–130. [Google Scholar] [CrossRef]
- Preston, S.J.; Harrison, A.; Lundy, M.; Roberts, D.; Beddoe, N.; Rogowski, D. Square pegs in round holes—The implications of shell shape variation on the translocation of adult Margaritifera margaritifera (L.). Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 568–573. [Google Scholar] [CrossRef]
- Luzuriaga, D.; Balaban, M.O.; Yeralan, S. Analysis of visual quality attributes of white shrimp by machine vision. J. Food Sci. 1997, 62, 113–118. [Google Scholar] [CrossRef]
- Leyva-Valencia, I.; Alvarez-Castaneda, S.T.; Lluch-Cota, D.B.; Onzalez-Pelaez, S.; Perez-Valencia, S.; Vadopalas, B.; Ramirez-Perez, S.; Cruz-Hernandez, P. Shell shape differences between two Panopea species and phenotypic variation among P. globose at different sites using two geometric morphometric approaches. Malacologia 2012, 55, 1–13. [Google Scholar] [CrossRef]
- Ninomiya, S.; Ohsawa, R.; Yoshida, M. Evaluation of buckwheat and tartary buckwheat kernel shape by elliptic Fourier method. Curr. Adv. Buckwheat Res. 1995, 389–396. [Google Scholar]
- MacLeod, N. (Nanjing University, Nanjing, China). Personal communication, 2020.
- Gumus, B.; Balaban, M.O. Prediction of the weight of aquacultured rainbow trout (Oncorhynchus mykiss) by image analysis. J. Aquatic Food Prod. Technol. 2010, 19, 227–237. [Google Scholar] [CrossRef]
- Hickman, R.W. Incidence of pea crab and a trematode in cultivated and natural green-lipped mussels. N. Z. J. Mar. Freshw. Res. 1978, 12, 211–215. [Google Scholar] [CrossRef]
- Trottier, O.J. Biology and Impact of the New Zealand Pea Crab (Nepinnotheres novaezelandiae) in Aquacultured Green Lipped Mussels (Perna canaliculus). Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 2014. [Google Scholar]

| Camera Settings | Value |
|---|---|
| Exposure Mode | Manual |
| Shutter Speed | 1.6 s |
| Aperture | f/6.3 |
| Exposure Compensation | 0 EV |
| ISO sensitivity | 200 |
| White Balance | Preset manual |
| Image size (pixels) | 2144 × 1424 |
| Average Length (cm) | Width (cm) | Height (cm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Female | Male | Overall | Female | Male | Overall | Female | Male | |
| Average | 10.01 | 10.03 | 10.00 | 4.47 | 4.47 | 4.48 | 2.91 | 2.90 | 2.92 |
| St Dev | 0.63 | 0.61 | 0.66 | 0.26 | 0.25 | 0.27 | 0.15 | 0.15 | 0.15 |
| Min | 7.93 | 8.22 | 7.93 | 3.69 | 3.69 | 3.69 | 2.35 | 2.43 | 2.35 |
| Max | 11.59 | 11.55 | 11.59 | 5.11 | 5.11 | 5.07 | 3.3 | 3.22 | 3.3 |
| Height vs. Length | Weight vs. Area | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Y = A + B X | ||||
| A | 1.195 ± 0.247 | 1.186 ± 0.187 | 0.793 ± 5.654 | −5.269 ± 4.541 |
| B | 0.170 ± 0.025 | 0.173 ± 0.019 | 1.705 ± 0.162 | 1.881 ± 0.13 |
| R2 | 0.463 | 0.564 | 0.666 | 0.757 |
| Y = A XB | ||||
| A | 0.745 ± 1.214 | 0.742 ± 1.155 | 1.722 ± 1.393 | 1.105 ± 1.306 |
| B | 0.589 ± 0.084 | 0.595 ± 0.063 | 1.000 ± 0.094 | 1.125 ± 0.075 |
| R2 | 0.469 | 0.575 | 0.673 | 0.769 |
| Y = Co + C1X + C2X2 | ||||
| C0 | −0.334 | −0.426 | −4.189 | −21.362 |
| C1 | 0.479 | 0.503 | 1.999 | 2.846 |
| C2 | −0.016 | −0.017 | −0.004 | −0.014 |
| R2 | 0.466 | 0.569 | 0.667 | 0.759 |
| View Area (Square cm) | Percent Green: a* < −5 | Critical EFA Harmonic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Female | Male | Overall | Female | Male | Overall | Female | Male | |
| Average | 34.68 | 34.70 | 34.66 | 20.36 | 20.91 | 19.91 | 9.77 | 9.74 | 9.79 |
| St Dev | 3.93 | 3.77 | 4.07 | 8.30 | 8.12 | 8.43 | 1.36 | 1.27 | 1.43 |
| Min | 23.05 | 24.29 | 23.05 | 0 | 1.44 | 0 | 6 | 6 | 7 |
| Max | 43.34 | 43.09 | 43.34 | 52.44 | 40.36 | 52.44 | 17 | 14 | 17 |
| Average L* | Average a* | Average b* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Female | Male | Overall | Female | Male | Overall | Female | Male | |
| Average | 45.79 | 45.64 | 45.92 | 0.97 | 0.76 | 1.15 | 14.99 | 14.72 | 15.22 |
| St Dev | 3.62 | 3.65 | 3.60 | 2.59 | 2.39 | 2.74 | 3.92 | 3.84 | 3.98 |
| Min | 36.86 | 36.94 | 36.86 | −6.99 | −4.77 | −6.99 | 4.61 | 4.61 | 6.53 |
| Max | 57.09 | 56.74 | 57.09 | 11.5 | 6.91 | 11.5 | 29.04 | 28.74 | 29.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaban, M.O.; Fletcher, G.C.; Zhou, M. Visual Characterization of Male and Female Greenshell™ Mussels (Perna canaliculus) from New Zealand Using Image-Based Shape and Color Analysis. Fishes 2025, 10, 325. https://doi.org/10.3390/fishes10070325
Balaban MO, Fletcher GC, Zhou M. Visual Characterization of Male and Female Greenshell™ Mussels (Perna canaliculus) from New Zealand Using Image-Based Shape and Color Analysis. Fishes. 2025; 10(7):325. https://doi.org/10.3390/fishes10070325
Chicago/Turabian StyleBalaban, Murat O., Graham C. Fletcher, and Meng Zhou. 2025. "Visual Characterization of Male and Female Greenshell™ Mussels (Perna canaliculus) from New Zealand Using Image-Based Shape and Color Analysis" Fishes 10, no. 7: 325. https://doi.org/10.3390/fishes10070325
APA StyleBalaban, M. O., Fletcher, G. C., & Zhou, M. (2025). Visual Characterization of Male and Female Greenshell™ Mussels (Perna canaliculus) from New Zealand Using Image-Based Shape and Color Analysis. Fishes, 10(7), 325. https://doi.org/10.3390/fishes10070325







