Estimates on Age, Growth, Sex Composition, and Mortality of Silurus lanzhouensis (Chen, 1977) in the Upper Yellow River, China
Abstract
1. Introduction
2. Materials and Methods
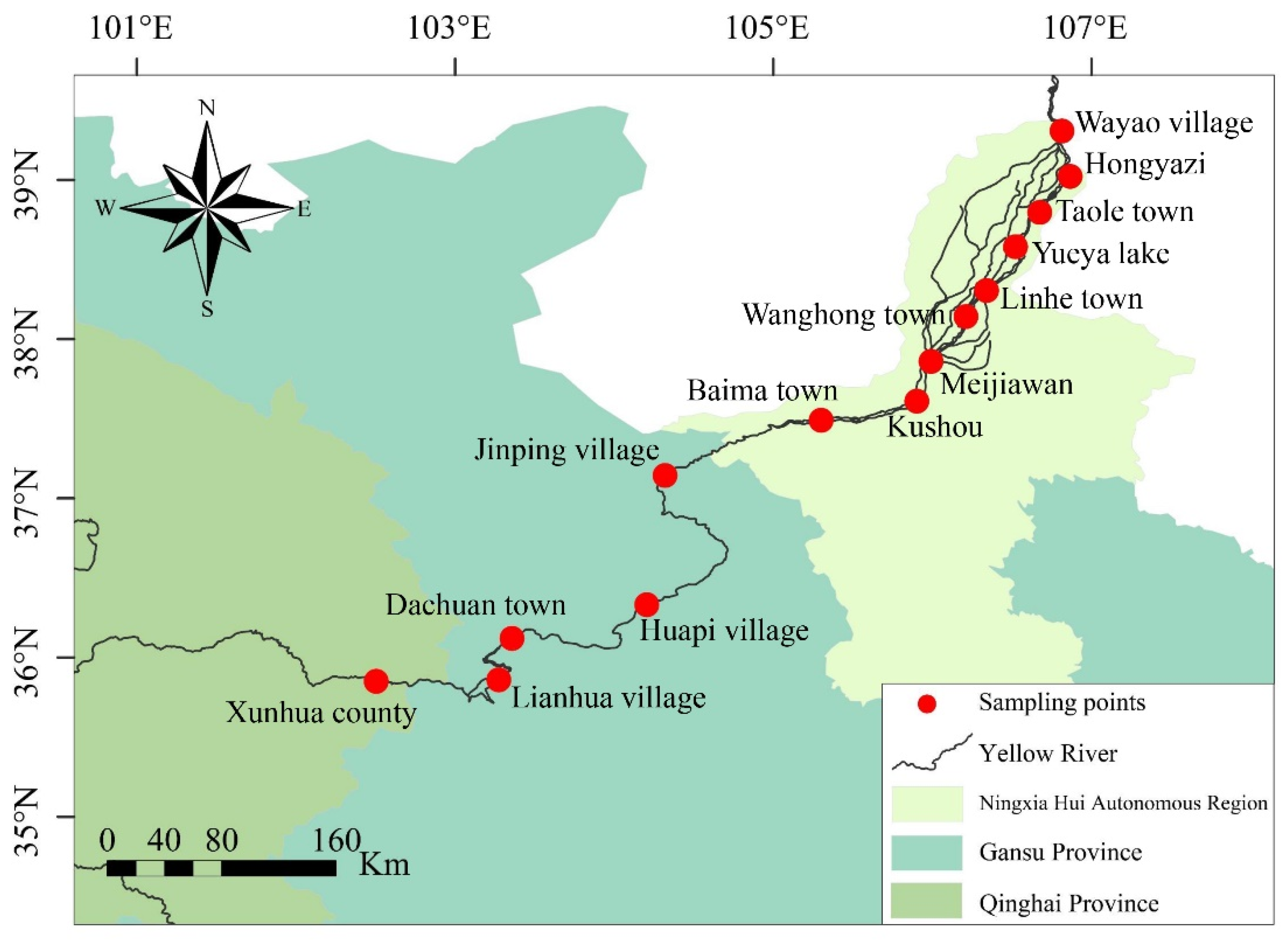
2.1. Study Area and Sample Collection
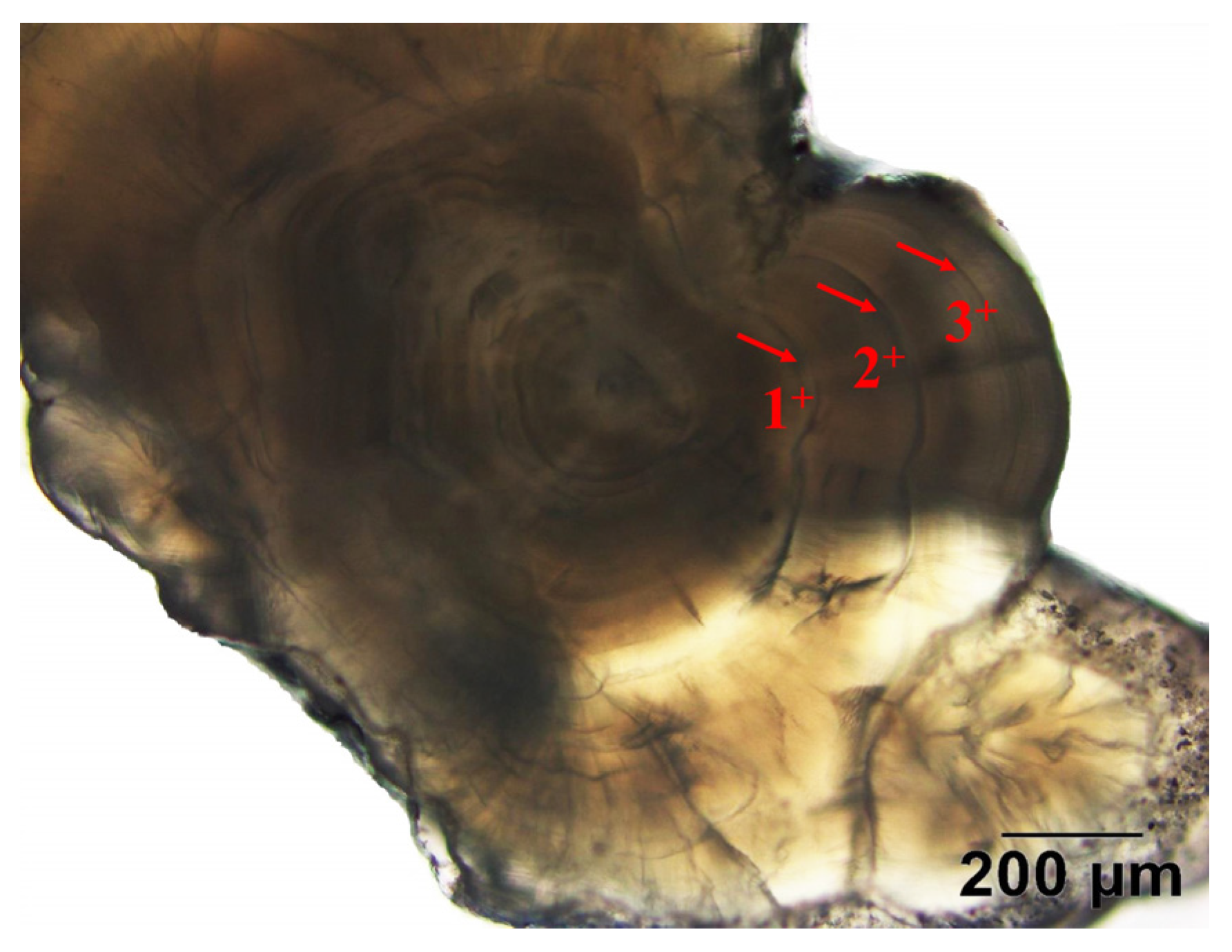
2.2. Age Estimation
2.3. The Relationship Between Total Length and Body Weight
2.4. Estimation of Growth Equation Parameters
2.5. Total Mortality, Fishing Mortality and Exploitation Rate
2.6. Statistical Analyses
3. Results
3.1. Population Structure
3.2. Age Distribution
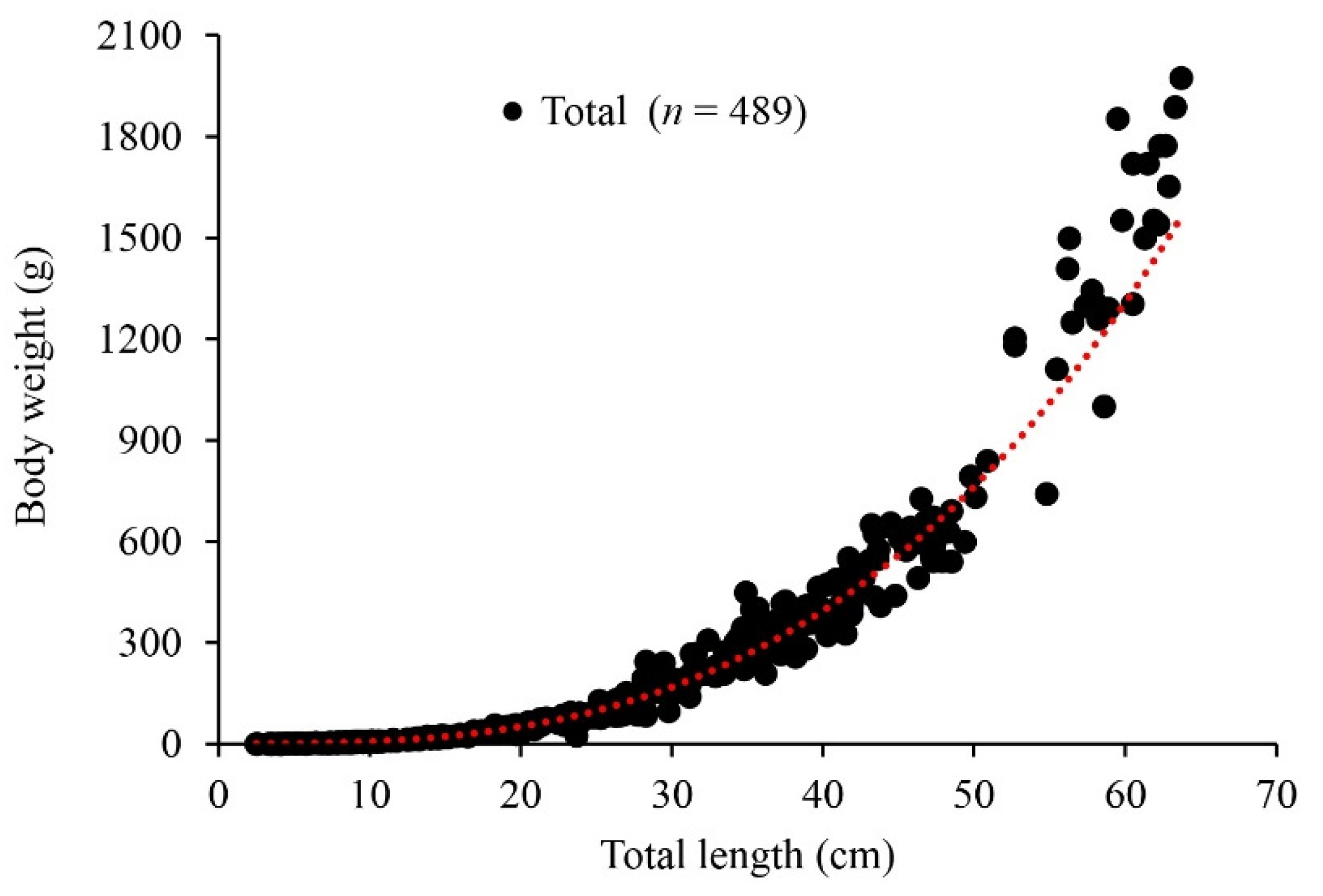
3.3. Length–Weight Relationship
3.4. Growth Equation
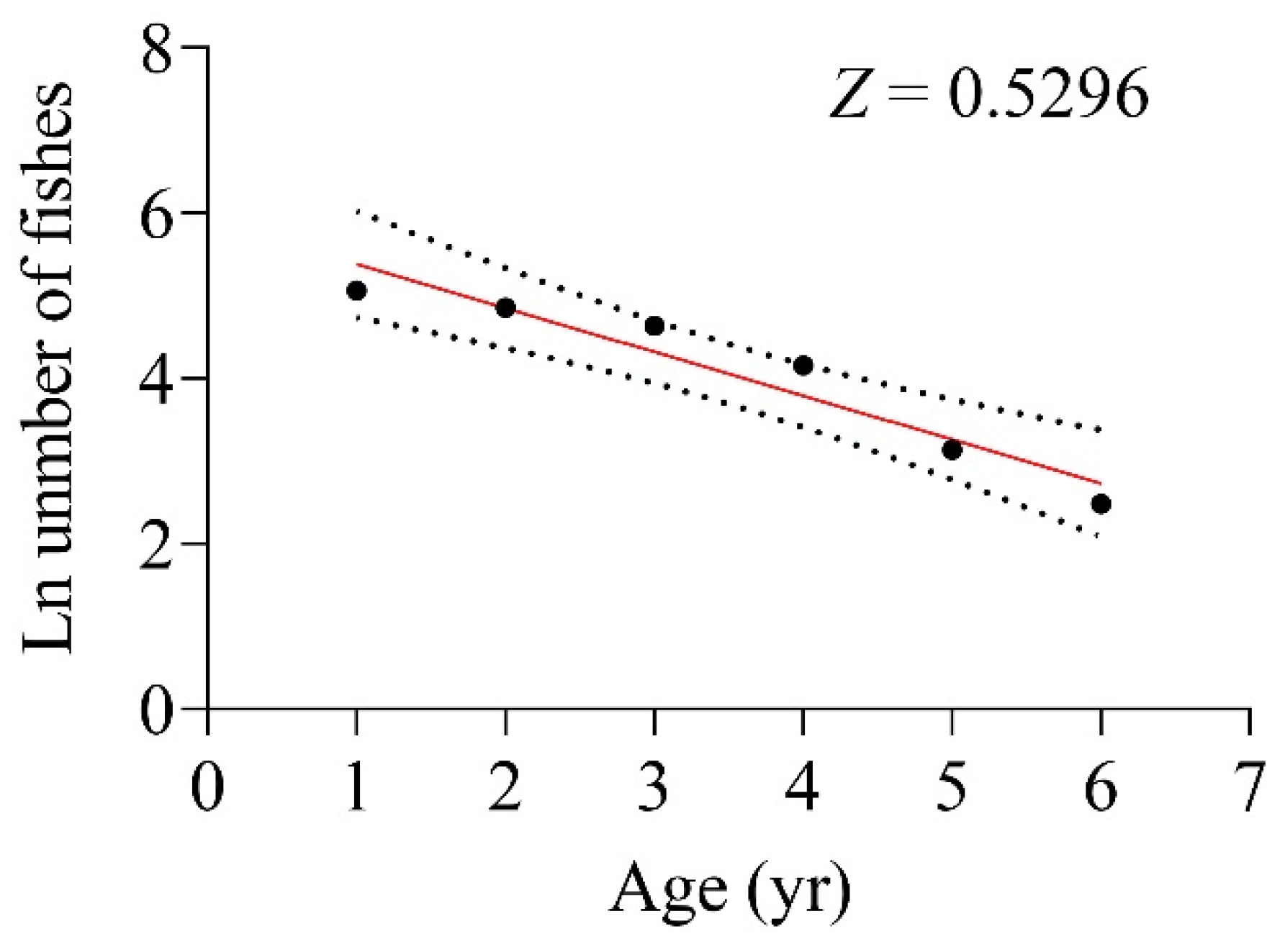
3.5. Mortality and Exploitation Rate
4. Discussion
| Species | Investigation Area | Sexual | Growth Parameters | Source | |||
|---|---|---|---|---|---|---|---|
| b | L∞ (cm) | K (yr−1) | φ | ||||
| Silurus meridionalis | Mid-upper Yangtze River, China | ♀ and ♂ | 2.9203 | 130.95 | 0.2019 | 3.5393 | [45] |
| Silurus aristotelis | Lake Pamvotis, Greece | ♀ | 3.0160 | 36.12 | 0.3700 | 2.6837 | [47] |
| ♂ | 3.2130 | ||||||
| Silurus glanis | Yili River, China | ♀ and ♂ | 2.8602 | 191.10 | 0.0870 | 3.5020 | [46] |
| Silurus glanis | Lower Kama Reservoir, Russia | ♀ and ♂ | 191.83 | 0.1050 | 3.5870 | [48] | |
| Silurus glanis | Menzelet Reservoir, Turkey | ♀ | 3.1295 | 260.00 | 0.0640 | 3.6361 | [50] |
| ♂ | 2.9133 | 303.20 | 0.0510 | 3.6710 | |||
| Silurus lanzhouensis | Upper Yellow River, China | ♀ | 3.0449 | 120.30 | 0.1267 | 3.2633 | This study |
| ♂ | 3.0970 | 119.10 | 0.1280 | 3.2590 | |||
| ♀ and ♂ | 2.9562 | 119.30 | 0.1278 | 3.2598 | |||
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salman, K.; Afzal, K.M.; Shahista, K. Estimation of age and growth in different stocks of striped snakehead, Channa striata (Bloch, 1793) inhabiting the three rivers of Gangetic river system. Indian J. Fish. 2022, 69, 27–36. [Google Scholar]
- Longo, F.; Malara, D.; Stipa, M.G.; Consoli, P.; Romeo, T.; Sanfilippo, M.; Abbate, F.; Andaloro, F.; Battaglia, P. Age, growth and otolith microstructure of the spotted Lanternfish Myctophum punctatum Rafinesque 1810. J. Mar. Sci. Eng. 2021, 8, 801. [Google Scholar] [CrossRef]
- Li, P.L.; Liu, J.C.; Lu, W.Q.; Sun, S.Y.; Wang, J.L. Age, growth, reproduction and mortality of Xenocypris argentea (Günther, 1868) in the lower reaches of the Tangwang River, China. PeerJ 2024, 12, e16673. [Google Scholar] [CrossRef]
- Li, L.; Ji, H.; Yang, Y.H. Current status and historical changes of Silurus lanzhouensis resources in the Shaanxi section of the Yellow River. Hebei Fish. 2017, 2, 29–33. (In Chinese) [Google Scholar]
- Wang, J.; Chen, L.; Tang, W.J.; Heino, J.N.; Jiang, X.M. Effects of dam construction and fish invasion on the species, functional and phylogenetic diversity of fish assemblages in the Yellow River Basin. J. Environ. Manag. 2021, 293, 112863. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.L.; Tian, S.M.; Chen, R.X.; Cao, Y.T.; Zhang, Y.; Han, B. Effect of global climate change on the sustainability of cold-water fish habitat in the alpine region: A case study on the Gymnocypris eckloni in the source region of the Yellow River. J. Environ. Manag. 2024, 367, 121926. [Google Scholar] [CrossRef]
- Miao, J.D.; Zhang, X.M.; Zhao, Y.; Wei, T.X.; Yang, Z.; Li, P.; Zhang, Y.G.; Chen, Y.X.; Wang, Y.S. Evolution patterns and spatial sources of water and sediment discharge over the last 70 years in the Yellow River, China: A case study in the Ningxia Reach. Sci. Total Environ. 2022, 838, 155952. [Google Scholar] [CrossRef]
- Zhou, H.; Niu, L.; Li, H.F.; Zhang, N.C. Path of hydropower development and ecological protection in the upper reaches of the Yellow River. Haihe Water Resour. 2023, 6, 12–18. (In Chinese) [Google Scholar]
- Li, Z.S. The Historia Piscium of Yellow River; China Ocean University Press: Qingdao, China, 2017; pp. 260–261. (In Chinese) [Google Scholar]
- Lian, Z.Q.; Wu, X.D.; Xiao, W.; Sai, Q.Y.; Gun, S.B. Complete sequence and characterization of the Silurus lanzhouensis (Siluriformes: Siluridae) mitochondrial genome. Mitochondrial DNA A 2015, 27, 2483–2484. [Google Scholar] [CrossRef]
- Wang, T.; Li, Z.; Yu, Z.X.; Wang, Z.W.; Lian, Z.Q.; Du, W.X.; Zhao, X.; Wang, M.T.; Miao, C.; Ding, M.; et al. Production of YY males through self-fertilization of an occasional hermaphrodite in Lanzhou catfish (Silurus lanzhouensis). Aquaculture 2021, 539, 736622. [Google Scholar] [CrossRef]
- Editorial Department of Fishery Science & Technology Information. Aquatic organisms such as finless porpoises were selected as “Top Ten River Business Cards”. Fish. Sci. Technol. Inf. 2014, 41, 216. [Google Scholar]
- Lian, Z.Q.; Chen, Q.F.; Wang, Y.; Sai, Q.Y.; Xiao, W.; Liu, Y.B.; Tian, Y.H.; Yu, Z.X.; Wu, X.D. Microencapsulated forage for Silurus lanzhouensis larvae and juvenile aquaculture. Fujian J. Agric. Sci. 2020, 3, 254–259. (In Chinese) [Google Scholar]
- Xiao, W.; Lian, Z.Q.; Wu, J.P.; Wu, X.D.; Yu, Z.X.; Sai, Q.Y. De novo RNA sequencing for identification of growth-related genes in Silurus lanzhouensis muscle tissues. Fish. Sci. 2022, 88, 565–580. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y. China Species Red List; Higher Education Press: Beijing, China, 2009; Volume 2. (In Chinese) [Google Scholar]
- Dong, C.J.; Wang, Y.J.; Li, X.J. Research on construction status and countermeasures of National Aquatic Germplasm Resource Reserves in Provinces and Autonomous Regions along the Yellow River. Chin. J. Fish. 2024, 5, 101–136. (In Chinese) [Google Scholar]
- Li, L.L.; Xiao, W.; Xing, L.M.; Sai, Q.Y.; Liu, Y.B.; Tian, Y.H.; Wang, Y.; Yu, Z.X.; Liu, Z.; Lian, Z.Q. Study on morphological characteristics and embryonic development of the gynogenesis Silurus lanzhouensis. Chin. J. Cell. Biol. 2020, 5, 820–828. (In Chinese) [Google Scholar]
- Wu, X.D.; Lian, Z.Q.; Hou, Y.X.; Li, L.; Xiao, W. Genetic structure analyses between wild population and artificial breeding ones of Silurus lanzhouensis. Freshw. Fish. 2011, 3, 34–38. (In Chinese) [Google Scholar]
- Liu, T.; Li, M.M.; Lai, Z.L.; Liu, Y.B.; Liu, K.; Xiao, W.; Sai, Q.Y.; Tian, Y.H.; Yang, L.Q.; Ruan, C.L.; et al. Cloning of Dmrta1 gene and its temporal and spatial expression in tissue of Silurus lanzhouensis. Acta Agric. Boreali-Sin. 2023, 6, 228–238. (In Chinese) [Google Scholar]
- Yang, Y.; Zhang, Y.; Li, F.; Wen, S.; Wang, L.; Lan, G.; Zhou, J.; Li, Y.; Zhu, Z. Comparative genomic analysis unveils potential factors contributing to the endangerment of Silurus lanzhouensis. Fishes 2023, 8, 613. [Google Scholar] [CrossRef]
- Niu, J.S.; Wang, T.; Li, Z.; Wang, Z.W.; Ding, M.; Wang, M.T.; Lian, Z.Q.; Mei, J.; Wang, Y.; Zhou, L.; et al. Efficient breeding and growth advantage of all-male population in Lanzhou catfish (Silurus lanzhouensis). Aquaculture 2024, 578, 740023. [Google Scholar] [CrossRef]
- Li, X.Q.; Zheng, J.; Wan, Z.K.; Zeng, R.K.; Ding, Y.W.; Song, Y.X.; Zhao, C.X.; Feng, X.; Ni, D.F.; Fu, M.; et al. Population strucure and growthcharacteristics of Gymnodiplychus pachycheilus in the middle reach of Yalong River. Acta Ecol. Sin. 2023, 16, 6833–6850. (In Chinese) [Google Scholar]
- Wang, J.L.; Liu, W.; Li, P.L.; Tang, F.J.; Lu, W.Q. Estimation of Coregonus ussuriensis age, growth, and maturation in China’s Amur River. PeerJ 2022, 10, e12817. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. Fish population dynamics in tropical waters: A manual for use with programmable calculator. ICLARM Stud. Rev. 1984, 8, 325. [Google Scholar]
- Von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws II). Hum. Biol. 1938, 2, 181–213. [Google Scholar]
- Chen, Y.; Jackson, D.A.; Harvey, H.H. A comparison of Von Bertalanffy and polynomial functions in the modeling fish growth data. Can. J. Fish. Aquat. Sci. 1992, 49, 1228–1235. [Google Scholar] [CrossRef]
- Pauly, D.; Moreau, J.; Abad, N. Comparison of age-structured and length-converted catch curves of brown trout Salmo trutta in two French rivers. Fish. Res. 1995, 22, 197–204. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Chapman and Hall: London, UK, 1957; p. 533. [Google Scholar]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. J. Conserv. Inter. Explor. Marit. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Ralston, S. Mortality rates of snappers and groupers. In Tropical Snappers and Grouper: Biology and Fisheries Management; Polovina, J.J., Ralston, S., Eds.; Westview: Boulder, CO, USA, 1987; pp. 375–404. [Google Scholar]
- Zhan, B.Y.; Lou, D.C.; Zhong, J.S. An assessment of the filefish population and ratenal exploitation of the resource. J. Fish. China 1986, 10, 409–418. (In Chinese) [Google Scholar]
- Gray, C.A.; Barnes, L.M.; Robbins, W.D.; Meulen, D.E.V.D.; Ochwada-Doyle, F.A.; Kendall, B.W. Length- and age-based demographics of exploited populations of stout whiting, Sillago robusta stead, 1908. J. Appl. Ichthyol. 2017, 33, 1073–1082. [Google Scholar] [CrossRef]
- Ma, B.S.; Nie, Y.Y.; Wei, K.J.; Xu, B.; Zhu, X.Y.; Xu, J. Estimates on age, growth, and mortality of Gymnocypris firmispinatus (Cyprinidae: Schizothoracinae) in the Anning River, China. J. Oceanol. Limnol. 2019, 2, 736–744. [Google Scholar] [CrossRef]
- Kunishima, T.; Higuchi, S.; Kawabata, Y.; Furumitsu, K.; Nakamura, I.; Yamaguchi, A.; Tachihara, K.; Tokeshi, M.; Arakaki, S. Age, growth, and reproductive biology of the blackfin seabass Lateolabrax latus, a major predator in rocky coastal ecosystems of southwestern Japan. Reg. Stud. Mar. Sci. 2021, 41, 101597. [Google Scholar] [CrossRef]
- Alonzo, S.; Mangel, M. The elfects of size-selective fisheries on the stock dynamics of and sperm limitation in sex-changing fish. Fish. B-Noaa. 2003, 1, 1–13. [Google Scholar]
- Smith, G.H.; Murie, D.J.; Parkyn, D.C. Effects of sex-specific fishing mortality on sex ratio and population dynamics of Gulf of Mexico greater amberjack. Fish. Res. 2018, 208, 219–228. [Google Scholar] [CrossRef]
- Lou, Z.Y.; Qin, Y.; Wang, T.; Zhou, R. Reproductive biology and spawning habitats of Gymnodiptychus pachycheilus. Fish. Sci. 2012, 1, 32–36. (In Chinese) [Google Scholar]
- Li, P.L.; Liu, J.C.; Wang, T.; Wang, J.L. Estimates of the age, growth, and mortality of Triplophysa scleroptera (Herzenstein, 1888) in the upper reaches of the Yellow River, China. Fishes 2023, 8, 457. [Google Scholar] [CrossRef]
- Xiong, S.H.; Liu, J.C.; Li, P.L.; Liu, Y.B.; Liu, K.; Wang, Y.J.; Wang, J.L. Estimates on age, growth, and mortality of Leuciscus chuanchicus (Kessler 1876) in the Ningxia section of the upper reaches of the Yellow River, China. PeerJ 2024, 12, e17351. [Google Scholar] [CrossRef] [PubMed]
- Li, P.L.; Liu, J.C.; Liu, Y.B.; Wang, T.; Liu, K.; Wang, J.L. A comparative study on the age, growth, and mortality of Gobio huanghensis (Luo, Le & Chen, 1977) in the Gansu and Ningxia sections of the upper Yellow River, China. BMC Ecol. Evol. 2024, 24, 30. [Google Scholar]
- Li, X.Q.; Chen, Y.F. Age structure, growth and mortality estimates of an endemic Ptychobarbus dipogon (Regan, 1905) (Cyprinidae: Schizothoracinae) in the Lhasa River, Tibet. Environ. Biol. Fish. 2009, 86, 97–105. [Google Scholar] [CrossRef]
- De Santana, H.S.; Minte-Vera, C.V. Age and growth of Prochilodus lineatus in a spatially structured population: Is there concordance between otoliths and scales? Environ. Biol. Fish. 2017, 3, 223–235. [Google Scholar] [CrossRef]
- Xiong, S.H.; Wang, J.L.; Li, P.L.; Wang, T.; Liu, J.C. Estimates of age, growth, and mortality of Triplophysa pseudoscleroptera in the upstream of the Yellow River, China. Fish. Sci. 2025, 91, 237–247. [Google Scholar] [CrossRef]
- Campana, S.E.; Chouinard, G.A.; Hanson, J.M.; Fréchet, A.; Brattey, J. Otolith elemental fingerprints as biological tracers of fish stocks. Fish. Res. 2000, 46, 343–357. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wu, G.X.; Yang, D.G.; Liu, L.H. Age and growth of the Silurus meridionalis in the mid-upper reaches of the Yangtze River. Freshw. Fish. 1990, 6, 3–7. (In Chinese) [Google Scholar]
- Ren, B. Studies on certain biological characteristics of the Silurus glanis in the Ili River. Chin. J. Fish. 2012, 2, 51–55. (In Chinese) [Google Scholar]
- Leonardos, I.D.; Tsikliras, A.C.; Batzakas, I.; Ntakis, A.; Liousia, V. Life-history characteristics of the endangered Aristotle’s catfish (Silurus aristotelis Garman, 1890), Lake Pamvotis, north-western Greece. J. Appl. Ichthyol. 2009, 25, 746–751. [Google Scholar] [CrossRef]
- Severov, Y.A. Size-age structure, growth rate, and fishery of European catfish Silurus glanis in the lower Kama Reservoir. J. Ichthyol. 2020, 1, 118–121. [Google Scholar] [CrossRef]
- Flura, M.Z.; Rahman, B.S.; Rahman, M.A.; Ashraful, M.; Alam, M.; Pramanik, M.H. Length-weight relationship and GSI of hilsa, Tenualosa ilisha (hamilton, 1822) fishes in Meghna river. Bangl. Int. J. Nat. Soc. Sci. 2015, 3, 82–88. [Google Scholar]
- Alp, A.; Kara, C.; Üçkardeş, F.; Carol, J.; García-Berthou, E. Age and growth of the European catfish (Silurus glanis) in a Turkish Reservoir and comparison with introduced populations. Rev. Fish. Biol. Fish. 2011, 21, 283–294. [Google Scholar] [CrossRef]
- Smoliński, S.; Berg, F. Varying relationships between fish length and scale size under changing environmental conditions-multidecadal perspective in Atlantic herring. Ecol. Indic. 2022, 134, 108494. [Google Scholar] [CrossRef]
- Gulland, J.A. Fish Stock Assessment: A Manual of Basic Methods; Fao/Wiley Series on Food and Agriculture; Wiley: Hoboken, NJ, USA, 1983; Volume I, pp. 1–223. [Google Scholar]
- Zhao, Y.; Shao, W. Silurus lanzhouensis. The IUCN Red List of Threatened Species 2024. 2024. e.T212958890A212958893. Available online: https://www.iucnredlist.org/ (accessed on 17 June 2025).



| Sex | Age (yr) | n | L (cm) | W (g) | ||
|---|---|---|---|---|---|---|
| Min–Max | Mean ± S.D. | Min–Max | Mean ± S.D. | |||
| Unknown | 1 | 147 | 2.5–15.8 | 8.80 ± 3.15 | 0.11–22.77 | 6.32 ± 6.04 |
| Female | 1 | 5 | 13.8–16.0 | 14.86 ± 0.82 | 19.17–27.76 | 22.74 ± 3.67 |
| 2 | 59 | 16.9–29.8 | 22.13 ± 3.38 | 28.15–170.98 | 67.06 ± 30.93 | |
| 3 | 60 | 25.8–42.0 | 34.59 ± 3.64 | 120.72–448.58 | 276.47 ± 77.65 | |
| 4 | 45 | 36.9–50.9 | 43.08 ± 3.46 | 342.97–838.5 | 496.37 ± 117.70 | |
| 5 | 16 | 43.2–59.8 | 53.81 ± 5.48 | 575.32–1552.08 | 1085.38 ± 300.81 | |
| 6 | 7 | 59.5–63.7 | 61.71 ± 1.42 | 1303.83–1974.15 | 1624.71 ± 225.81 | |
| Male | 1 | 6 | 14.5–16.8 | 15.53 ± 0.95 | 17.88–28.65 | 21.69 ± 3.82 |
| 2 | 70 | 17.3–28.4 | 23.24 ± 3.48 | 23.89–175.82 | 72.78 ± 27.32 | |
| 3 | 43 | 28.1–39.7 | 33.51 ± 3.35 | 139.30–423.62 | 263.80 ± 78.08 | |
| 4 | 19 | 34.7–47.4 | 42.96 ± 3.66 | 320.89–726.80 | 493.66 ± 129.23 | |
| 5 | 7 | 48.5–58.2 | 55.20 ± 3.57 | 540.61–1498.62 | 1126.89 ± 349.33 | |
| 6 | 5 | 60.5–63.3 | 62.06 ± 1.09 | 1719.87–1887.51 | 1773.42 ± 69.10 | |
| Total | 1 | 158 | 2.5–16.8 | 9.25 ± 3.46 | 0.11–28.65 | 7.42 ± 7.15 |
| 2 | 129 | 16.9–29.8 | 22.74 ± 3.47 | 23.89–175.82 | 70.17 ± 29.05 | |
| 3 | 103 | 25.8–42.0 | 34.14 ± 3.55 | 120.72–448.58 | 271.18 ± 77.70 | |
| 4 | 64 | 34.7–50.9 | 43.05 ± 3.49 | 320.89–838.50 | 495.57 ± 120.20 | |
| 5 | 23 | 43.2–59.8 | 54.23 ± 4.94 | 540.61–1552.08 | 1098.01 ± 308.80 | |
| 6 | 12 | 59.5–63.7 | 61.86 ± 1.25 | 1303.83–1974.15 | 1687.10 ± 188.31 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Liu, J.; Xiong, S.; Wang, T.; Wang, Y.; Wang, J. Estimates on Age, Growth, Sex Composition, and Mortality of Silurus lanzhouensis (Chen, 1977) in the Upper Yellow River, China. Fishes 2025, 10, 322. https://doi.org/10.3390/fishes10070322
Li P, Liu J, Xiong S, Wang T, Wang Y, Wang J. Estimates on Age, Growth, Sex Composition, and Mortality of Silurus lanzhouensis (Chen, 1977) in the Upper Yellow River, China. Fishes. 2025; 10(7):322. https://doi.org/10.3390/fishes10070322
Chicago/Turabian StyleLi, Peilun, Jiacheng Liu, Shuhan Xiong, Tai Wang, Yongjie Wang, and Jilong Wang. 2025. "Estimates on Age, Growth, Sex Composition, and Mortality of Silurus lanzhouensis (Chen, 1977) in the Upper Yellow River, China" Fishes 10, no. 7: 322. https://doi.org/10.3390/fishes10070322
APA StyleLi, P., Liu, J., Xiong, S., Wang, T., Wang, Y., & Wang, J. (2025). Estimates on Age, Growth, Sex Composition, and Mortality of Silurus lanzhouensis (Chen, 1977) in the Upper Yellow River, China. Fishes, 10(7), 322. https://doi.org/10.3390/fishes10070322






