Abstract
Plastic pollution poses a massive problem to the environment, particularly seas and oceans. Microplastics (MPs) ingestion by marine species can generate many adverse effects, including causing oxidative stress. This study evaluated the effects of anthropic activity-related MP presence in two coastal fish species—Serranus scriba (more related to rocky bottoms) and Lithognathus mormyrus (more related to sandy bottoms)—in two areas of Mallorca Island (Western Mediterranean) with varying anthropic pressures with similar mixed rocky/sandy bottoms. A total of eight fish samples per species and per area (total n = 32), as well as three water samples (500 mL each) and three sediment samples per area, were collected and analyzed. The results showed that despite plastic presence in both areas, the area with higher tourism affluence was also the most polluted. Fourier transform infrared spectroscopy analysis confirmed that the majority of recovered polymers were polyethylene and polypropylene. The pattern of MPs presence was reflected in the biomarker analysis, which showed higher values of antioxidants, namely catalase (CAT) and superoxide dismutase (SOD); detoxification, namely glutathione s-transferase (GST); and inflammation, namely myeloperoxidase (MPO)—enzymes in the gastrointestinal tract of fish from the more polluted area. However, no statistical differences were found for malondialdehyde (MDA) as a marker of lipid peroxidation. As for differences between species, S. scriba presented a higher presence of MPs and measured biomarkers than in L. Mormyrus, suggesting higher exposure. In conclusion, these results showed that increased anthropic activity is associated with a higher presence of MPs which, in turn, induces an adaptative response in exposed fish. Moreover, species living in the same area could be differentially affected by MPs, which is probably associated with different behavioural and feeding habits.
Key Contribution:
This work highlights the effects of anthropic affluence over plastic pollution, as well as the potential effects of microplastic ingestion on two types of local fish Serranus scriba and Lithognathus mormyrus in the Mediterranean.
1. Introduction
Microplastics (MPs), defined as plastic particles which are smaller than 5 mm [1], are now found virtually everywhere. Present in expected places such as soil [2] or the oceans [3], and the unexpected, such as human blood [4], human placenta [5], rainwater [6] or Mount Everest [7], their pervasiveness is indisputable. Despite this worldwide ubiquity, marine environments continue to be the most vulnerable to this danger. A total of 413.8 Mt of plastic were produced globally in 2023 [8], of which approximately half was projected to be waste [9]. Furthermore, 4–23 MMT of plastic is estimated to enter the oceans each year [10]. These plastics can enter as primary MPs (pellets, microbeads, etc.), or macroplastics which break apart and, because they do not biodegrade, remain in the ocean as secondary MPs [11]. Moreover, MPs persist in all environments of the oceans, both in sediment and the water column, and can enter the food chain, being consumed by species of all taxa. These MPs can be consumed directly due to their availability in the water column and sediment, or indirectly through the ingestion of plastic-containing prey, with many possible entry points into the food chain—from copepods to whales [12,13,14,15]. Because of the use of the entire water column and feeding strategies—filtering, suction, ingesting water or sediment alongside the prey—marine organisms are extremely vulnerable to plastic ingestion, which can cause intestinal blockage, and other physical damage. Additionally, plastics have additives in their composition which give them certain desired properties of the product, e.g., durability, malleability, etc. These additives, such as plasticizers, colourants, and flame retardants, are often toxic substances which can leach from the plastic and affect species in many ways, including the disruption of hormonal pathways, provoke negative effects in biomolecules and generate oxidative stress [16,17,18]. Oxidative stress occurs when there is an imbalance between the production and effective detoxification of reactive oxygen species (ROS) [19]. These ROS are generated as a by-product of the elimination of more toxic substances, but if accumulated, they can cause major disruptions to the organism. Thus, antioxidant and detoxifying enzymes such as catalase (CAT) and superoxide dismutase (SOD) act to neutralize ROS and help restore body equilibrium, whereas glutathione s-transferase (GST) is a detoxifying enzyme that reduces the toxicity of pollutants and leads to their excretion [20]. If this equilibrium is not restored, the organism begins to oxidize and can cause a situation of oxidative stress. Lipids, particularly, due to their structure are especially susceptible to oxidation, leading to the creation of products such as malondialdehyde (MDA) which is an indicator of lipid peroxidation [21]. Moreover, the presence of plastics or other harmful substances can lead to inflammation and the consequent production of myeloperoxidase (MPO), which is an enzyme present in plasma after an infection or an inflammatory response. Thus, MPO is a good bioindicator of immune system activity and inflammation, in addition to the elimination of external substances, like ingested MP [22].
The painted comber (Figure 1A), Serranus scriba (Linnaeus, 1758), from the family Serranidae, is a subtropical marine demersal fish [23]. This sequential hermaphroditic species is found widespread in Eastern Atlantic waters along the Bay of Biscay to Mauritania including the Canary, Azores and Madeira islands and in the Mediterranean and Black Sea [24]. This littoral benthic species generally lives in shallow bottoms, up to 150 m depth, although it is generally found at around 30 m depth [24] and generally prefers rocky substrate or areas with seagrass Posidonia oceanica or Cymodocea nodosa [25]. This species has been included in the FAO catalogues of species of interest to fisheries in the Eastern Central Atlantic and the Mediterranean and the Black Sea [26]. S. scriba is a carnivorous fish and an active predator of various benthic organisms [27], particularly crustaceans [23,28]. Studies in the Mediterranean show that this species fed preferentially on natantia and reptantia decapods, but exhibited a wide dietary spectrum, including Posidonia tissues, amphipods, and other animal items [23,29,30].
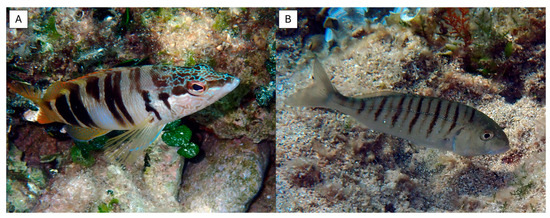
Figure 1.
Representative images of the sampled fish: (A) the painted comber Serranus scriba; (B) the striped seabream Lithognathus mormyrus.
The striped seabream (Figure 1B), Lithongathus mormyrus (Linneaus, 1758), is a demersal fish which belongs to the family Sparidae. This species inhabits a wide array of sea bottom types, particularly sandy and seagrass beds, from 0 to 150 m [31], although it is mainly found from 10 to 30 m. It is found in the Atlantic, from the Bay of Biscay to the Cape of Good Hope, and around the Canaries and Cape Verde. It is also present in the Mediterranean, Black, Azov and Red seas. In the Western Indian Ocean, it is found from Southern Mozambique to the Cape of Good Hope [31,32]. L. mormyrus is a protandrous hermaphrodite, which spawns from May to September [33]. As for its diet, the striped seabream is thought to be a carnivore species which feeds mostly on benthic invertebrates, particularly Polychaeta Sedentaria and Bivalvia, as well as Polychaeta Errantia and Amphipoda, although its diet varies throughout the life cycle, becoming a more opportunistic feeder with age [34].
The Mediterranean Sea is an exceptionally biodiverse sea, with temperate and subtropical species and a high number of endemism, forming a unique mixture and a very high species richness [35]. However, due to its specific conditions, it is especially vulnerable to plastic contamination [36]. Mallorca, situated in the Balearic Archipelago in the Western Mediterranean Sea, due to the currents, the geomorphology of the island and the tourism affluence and subsequent pollution, is a “resident area” for plastics, as characterized by Mansui et al., 2015 and Fagiano et al., 2023 [37,38]. This high plastic affluence can, and does, affect the species living in the ocean, leading to high plastic ingestion recordings [20,39,40]. S. scriba and L. mormyrus are two very common species in Balearic waters, with similar habitats. Additionally, they are two of the most important recreational fishing targets in the Mediterranean [23,34], and particularly in the island of Mallorca [30]. Thus, the study of these species, and particularly their affectation to a widespread problematic such as plastic pollution, could be relevant to local populations.
The aim of this study was to assess plastic pollution affection on two recreational fishing-relevant species—S. scriba and L. mormyrus—in two areas with more and less anthropic influence off the island of Mallorca. This effect is gauged using biomarkers to evaluate the possible oxidative stress caused by MP ingestion.
2. Materials and Methods
2.1. Study Area
The study was carried out in the south-western coast of the island of Mallorca in the Balearic Islands, Spain (Figure 2). Two areas with similar conditions were selected, combining both rocky and sand substrates, but with different anthropic influence. Zone 1, the more affected area, was established on Magaluf Beach. This is one of the most touristic beaches in Mallorca, and is surrounded by hotels, restaurants, etc., and receives millions of tourists each year [41]. Zone 2, the more pristine area, is an area between Cala Bella Dona and Cala Falcó, on the same coast. This area, due to its difficulty to access, in addition to the lack of hotels, restaurants and few houses near it, is a much less affected area.
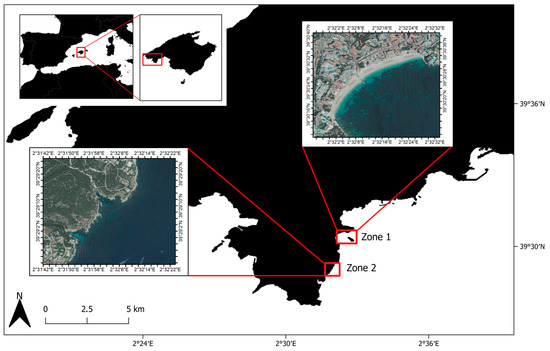
Figure 2.
Map of the sampled zones: Zone 1 (Magaluf Beach) and Zone 2 (between Cala Bella Dona and Cala Falcó). The map was created using the open-source Quantum Geographic System (QGIS) version 3.40.6 (Bratislava, Slovakia). Satellite images for each zone are included, with corresponding coordinates shown along the axes.
2.2. Sampling
The sampling was carried out from September to November 2023 in both areas. Fish were caught using a hook and worms as bait. The sampling was carried out from the coast, eliminating the need for a boat. A total of 8 fish of both species were caught in each of the areas, resulting in a total of 16 fish of each species for both areas, 32 fish samples in total. The fish were anesthetized with tricaine methanesulfonate (MS-222) (0.1 g/L of seawater) to reduce stress. Subsequently, the total length (TL; ±0.1 cm) of each specimen was recorded, and then the fish were eviscerated. The entire digestive tract was saved and frozen, stored at −20 °C for MP analysis. Liver and spleen tissue were also extracted and frozen at −80 °C for further biomarker analysis. The experimental protocol was approved by the Animal Experimentation Ethics Committee of the University of the Balearic Islands (Reference CEEA 96/05/18).
Abiotic samples of water and sediment were collected from both areas by freedivers. Three water samples were collected in each area randomly (500 mL) and stored at room temperature to be processed. Sediment samples (50 mL) were also extracted at random with an auger inserted at a 0°–45° angle to minimize water entering and loss of surface sediment. No concerns about sediment mixing were necessary as it was to be used to analyze MP. The samples were stored in clean glass and prelabelled containers. The flowchart of the sample processing procedure is shown in Figure 3.
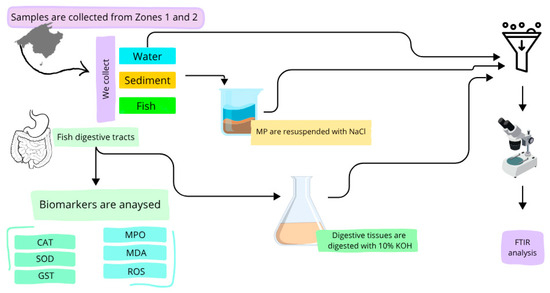
Figure 3.
Flowchart of the sample processing procedure, including both microplastic identification and biomarker analysis steps. CAT (Catalase), SOD (Superoxide dismutase), GST (Glutathione s-transferase), MPO (Myeloperoxidase), MDA (Malondialdehyde), ROS (Reactive oxygen species), FTIR (Fourier Transform Infrared Spectroscopy).
2.3. MP Processing
Digestive tracts were thawed, weighed and set in Erlenmeyer flasks and digested with 10% potassium hydroxide (KOH), adding 20 mL of the solution per every gram of the digestive tissue. The flasks were covered with aluminum foil to avoid airborne contamination and were digested for 24 h. Once chemically digested, the tracts were filtered through polycarbonate membrane filters (FILTER-LAB, pore size 10.0 μm, diameter 47 mm, Prat Dumas, Couze-et-Saint-Front, France) and set to dry.
Water samples were filtered individually with a vacuum filter and with polycarbonate membrane filters. The filters were then placed in Petri dishes and saved for future observation. As for the sediment analysis, 250 g of sediment was mixed with previously filtered high-density saline solution (1 L H2O + 120 g NaCl, 1.2 g·cm−3 NaCl). The density of said solution permits the flotation of MP particles. The mix was stirred for 2 min, and the sediment was then allowed to settle for 1 h [42]. After, the saline solution with floating MPs was filtered with polycarbonate membrane filters with the same methodology as water samples and filters were also saved for future observation.
To prevent contamination during field and laboratory analyses, all instruments were washed thrice with pure water (Milli-Q® A10 Direct Water Purification System, Merck KGaA, Darmstadt, Germany). In addition, cotton lab coats and nitrile gloves were worn throughout experimental procedures, and blank controls using distilled water were also visually inspected under the stereomicroscope to detect any possible airborne plastic contamination. Moreover, all saline solutions used were previously filtered to remove the possible microparticles present in water and salt.
After drying, filters were observed under the stereomicroscope (Euromex Microscopen bv NZ 1903-S, Duiven, Nederland) for MPs identification. Each MP was quantified and classified as fragment or fibre, and by colour.
To confirm the composition of MP particles found in fish, water and sediment samples, 40% of the potential plastic particles (60 items) samples were separated and analyzed using micro-attenuated total reflection micro-Fourier transform infrared spectroscopy (µ-ATR-FTIR) (Bruker Optics GmbH & Co. KG, Ettlingen, Germany). Due to the small size of the MPs, analysis was carried out with the Hyperion ATR microscope. FTIR absorption spectra were recorded as an average of 250 scans in the mid-infrared range of 400–4000 cm−1. The obtained spectrum was compared with commercial and in-house spectral databases, and a minimum of 700 hit quality index was necessary to accept a confirmed polymer [43].
2.4. Biomarker Assessment
The determination of possible effects of MP ingestion in both fish species was measured through several biomarkers in digestive tissue. CAT and SOD were determined as antioxidant defences, and GST as an enzyme implicated in detoxification processes were determined. MDA, indicating oxidative damage to lipids and MPO a biomarker of potential inflammation, were also evaluated. Gut samples were homogenized under ice-cold conditions in 10 volumes (w/v) of 100 mM Tris–HCl buffer pH 7.5 using a small sample dispersing system (Ultra-Turrax® T10 Disperser, IKA, Staufen, Germany) and centrifuged (9000× g, for 10 min, 4 °C; Sigma 3K30, Osterode am Harz, Germany) [19]. After centrifugation, supernatants were collected and used for all the biochemical analyses.
CAT activity (mK/mg protein) was determined according to Aebi (1984) at 240nm [44] SOD activity (pKat/mg protein) was measured as described by Flohé & Ötting (1984), at 550 nm [45]. GST activity (nKat/mg protein) was determined at 340 nm using the technique of Habig et al. (1974) [46]. The activity of myeloperoxidase (MPO) activity was assessed as described by Capeillere-Blandin (1998) [47]. ROS production was measured using 2,7 diclorofluoresceinadiacetate (DCFH-DA) as indicator. SOD, CAT, GST, and MPO activities were determined using a Shimadzu DC 5000-Pro spectrophotometer (Kyoto, Japan) at 25 °C. MDA levels (nM/mg protein) were quantified by a colorimetric assay kit (Merck, Madrid, Spain), following the manufacturer’s instructions. ROS was ascertained through the fluorescence reading with the microplate spectrophotometer (Bio Tek Instruments, Inc., Agilent Technologies, Madrid, Spain) [48].
All results were referred to the total protein content of the samples determined with the colorimetric Bradford method (Biorad® Protein Assay, Alcobendas, Spain) using bovine serum albumin as a standard.
2.5. Statistical Analysis
Statistical analysis was carried out with the statistical programme Rstudio (Version 4.3.1). Kolmogorov–Smirnov and Levene tests were used to evaluate normality of distribution and variance of data. Then, statistical significance was determined through two-way analysis of variance (ANOVA). When data did not fit the normality and variance requirements for ANOVA, a non-parametric Kruskal–Wallis test was performed. Results are expressed as the mean ± standard error of the mean (SEM), and a p-value < 0.05 was considered statistically significant. Bivariate correlations between the MP levels and the different biomarkers were also analyzed through Pearson correlation.
3. Results
3.1. Fish
A total of 32 fish were caught, 8 for each species–area combination. In Zone 1, S. scriba had an average weight of 51.4 ± 12.0 g and length of 16.1 ± 2.1 cm, whilst in Zone 2, the average weight and length was 57.6 ± 14.8 g and 17.1 ± 1.1 cm, respectively. As for L. mormyrus, in Zone 1, it presented an average weight of 20.4 ± 7.6 g and 11.6 ± 1.7 cm of length. In Zone 2, the weight averaged at 21.0 ± 5.7 g and length 13.1 ± 1.4 cm. No significant differences were found between size and weight in the studied areas.
3.2. MP Observation and Quantification
Observed MP were characterized by type (fibre or fragment) and colours (Figure 4). In the digestive tract of the fishes, a total of 91 MPs were found. In S. scriba digestive tracts in Zone 1, an average of 2.4 ± 2.3 MP/fish, whilst in Zone 2, the average was of 1.75 ± 1.4. For L. mormyrus, however, the average in Zone 1 was 4.5 ± 3.1 MP/fish and in Zone 2, 1.25 ± 0.9.
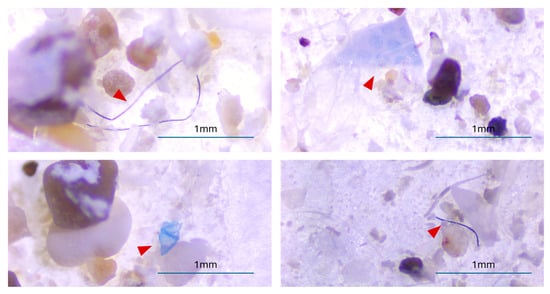
Figure 4.
Representative images of the MP(Microplastic) found in both digestive tracts of S. scriba and L. mormyrus. Scale bar = 1 mm. Red arrows indicate the identified MP particles.
As for MP type, most of the retrieved plastics were fibres: with a percentage of approximately 70–30 for fibre fragments for both fish in both zones (Figure 5). The colour was predominantly blue for fibres (75%), as well as for fragments (84%). Size did not vary greatly between zones: for fibres, Zone 1 average MP size was 1.22 ± 0.87 mm, whilst the Zone 2 MP’s average size was 1.27 ± 0.624 mm. As for fragments, in Zone 1, the average was 1.00 ± 0.89 mm, and in Zone 2, 0.10 ± 0.11 mm. No differences were found between MP abundance and area (p = 0.246) or species (p = 0.566).
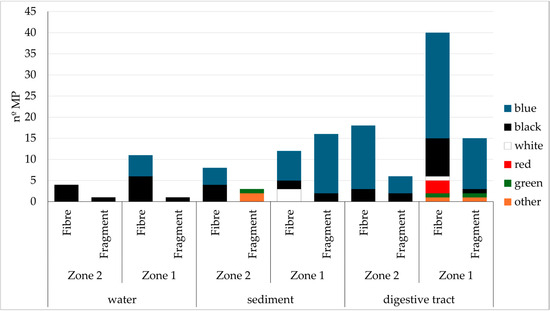
Figure 5.
Characterization of microplastics (shape and colour) found in water, sediment and digestive tracts for Zone 1 and Zone 2. Colours represent different microplastic types.
For water samples, a total of 17 MPs were found, 88% of which were fibres. This equates to 11.33 MP items per litre of water. Zone 1 had a higher quantity of plastics (12) compared to Zone 2 (5). As for colours, black was dominant both in fibres (66.6%), as well as in fragments (100%). The only other colour found was blue. The average size in the water column was 2.92 ± 0.66 mm for Zone 1 and for Zone 2, the average size was 1.37 ± 1.1 mm.
As for sediment samples, a total of 41 MPs were retrieved, of which 30 corresponded to Zone 1, and 11 to Zone 2. There was a higher proportion of fragments vs. fibres in Zone 1, where over 50% of MPs were fragments, whilst in Zone 2, over 70% were fibres. The majority of MPs were blue (70%), followed by black (19%) and the remaining 11% composed by various colours such as red, transparent, green and white.
The results showed that 94% of all analyzed particles were confirmed as plastics. The remaining particles had a plastic base but were coated with varnishes or paints that hindered proper identification. In sediment samples, 71% of the plastics were identified as high-density polyethylene (HDPE), 12% as copolyimide and 6% each as polyvinyl chloride (PVC), polypropylene (PP) and low-density polyethylene (LDPE). In water samples, the most common polymers were PP (38%), followed by HDPE (24%), LDPE (24%), polystyrene (10%) and polyester (5%). For particles found in digestive tracts, the composition was mainly PP (33%), HDPE (32%), LDPE (12%) and polyester (9%), with the remaining 17% consisting of cellulose acetate, copolyester, polystyrene and other polymers.
3.3. Biomarker Results
The results indicated that in both species, CAT, SOD, GST and MPO activity was significantly higher for both species in Zone 1 than in Zone 2 (p < 0.01, p = 0.042, p < 0.01 and p = 0.032, respectively). MDA and ROS did not present differences between zones (p = 0.994 and p = 0.291). As for differences between species, S. scriba presented higher values of CAT (p < 0.01), SOD (p < 0.01), GST (p < 0.01), MPO (p < 0.01) and MDA (p < 0.01), whilst no differences were observed in ROS production (p = 0.059) (Figure 6).
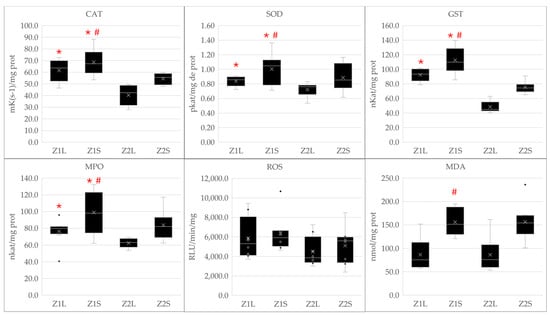
Figure 6.
Boxplot graph showing biomarker activity (CAT, SOD, GST, MPO, ROS and MDA) comparing L. mormyrus and S. scriba in Zone 1 (Z1L and Z1S, respectively) and in Zone 2 (Z2L and Z2S, respectively). Statistical significance is represented with ‘*’ to indicate significant differences between zones (Zone 1 vs. Zone 2) within the same species, and with ‘#’ to indicate significant differences between species (S. scriba vs. L. mormyrus) within the same zone.
4. Discussion
Anthropogenic activity poses an inevitable pressure on ecosystems and their associated fauna. Among these pressures, plastic pollution stands out as a major threat to marine species, where plastic particles are available throughout the water column and feeding and breathing strategies enable the entry of these particles into the organism. The Mediterranean Sea is particularly vulnerable to plastic pollution and has been recognized as one of the most affected regions by marine litter worldwide [49]. This is particularly evident in tourist-heavy areas, where the affluence of visitors searching for the three S’s (sea, sand and sun) cause an increase in waste generation [50]. The island of Mallorca, due to its particular oceanographic currents, geomorphology and high tourism pressure, is one of the most affected by marine litter [38,49]. In 2023 alone, over 12 million tourists visited Mallorca [51]. This high inflow of travellers signifies an increase in plastic waste, particularly in those areas most visited and exploited. Such an area is Magaluf, which is one of the most popular areas in the island. This type of tourism is particularly prone to plastic waste, as it is centred around partying and alcohol, which increases the use of disposable containers (bottles, cups, food packaging, etc.) and often leads to improper disposal of these plastics [52].
Plastic ingestion can negatively affect marine species both physically—through intestinal blockages—and chemically—through toxicological effects. Such impacts have been documented in species including Seriola dumerili [20], Dicentrarchus labrax [53], Sparus aurata [54] and Mullus surmuletus and Engraulis encrasicolus [55].
The present study identified the presence of MP in the marine environment and explored the possible toxicological effects in two fish species: S. scriba and L. mormyrus in two different areas: one with high anthropogenic impact and another with very limited human pressures. The effect of tourism on plastic prevalence is evident in the results, where plastic pollution is higher, both in water and sediment, in Magaluf (Zone 1) compared to the non-affected area (Zone 2). Despite the fact that MP presence is greatly influenced by hydrodynamic factors, local human activity can amplify accumulation in specific areas [56,57]. Similar patterns have been reported in studies such as Santucci et al. (2024), who found higher levels of meso- and MPs in more anthropized zones [58]. Additionally, a local study revealed that during the summer months the majority of marine waste originates from tourism and recreational use of the coast, while in winter, discharge from inland sources becomes more significant [59]. In many coastal areas waste is delivered to the ocean from run-offs [60,61]. Although Mallorca lacks rivers, it has seasonal torrents systems that transport waste to coastal areas during rainfall. Specifically, the Magaluf torrent discharges directly into the beach, adding debris to the waste already deposited by beachgoers.
Most of the MPs identified in our samples were fibres, which aligns with previous literature reporting fibres as the dominant form across various marine compartments [37]. Sediment samples contained the highest number of plastic fragments, suggesting that heavier plastics are more likely to sink and accumulate on the seafloor [62]. As for the colour, MP were predominantly blue and black, which is also common in the literature [16,60] and may originate from sources such as tyre wear, synthetic clothing and plastic bottles. The polymer composition identified via FTIR—predominantly polyethylene and polypropylene—closely aligns with findings from other Mediterranean studies, indicating a consistent regional signature in both water, sediment and fish samples [63]. This predominance of PE and PP is likely a reflection of their status as the most widely produced and versatile commodity plastics used in packaging, single-use items, ropes and containers [36].
Regarding the fish species, although no statistically significant differences in total plastic ingestion were found, individuals from Zone 1 exhibited higher MP loads in their digestive tracts, indicating increased environmental availability. L. mormyrus is a benthic feeder that actively searches for prey within sediment layers [34,64]. It feeds by indiscriminately ingesting sediment and then filtering it within the buccal cavity [65]. Given that sediment harbours high MP concentrations [37,66], this feeding strategy likely leads to greater MP ingestion in polluted environments—consistent with our findings. This is also reflected in the biomarker results, which show elevated oxidative stress, inflammation and detoxification responses in L. mormyrus from the more contaminated site (Magaluf beach). CAT, SOD and GST had significantly higher values for L. mormyrus in the polluted Zone 1. These findings are similar to other studies, where MP presence has been found to alter ROS production, leading to an increase in antioxidant and detoxification responses [67,68], thus causing higher values of biomarkers such as CAT, SOD and GST. Moreover, MPs have also been found to cause inflammation, which can be confirmed by the higher values of MPO in Zone 1, a pro-inflammatory enzyme located in neutrophils (Figure 5) [69]. These results indicate the effects of pollutants, and, in particular, MP ingestion, which can cause significant damages to the species consuming it.
Furthermore, S. scriba ingested a lower quantity of MP than L. mormyrus in Zone 1, likely due to differences in feeding strategies. Painted combers are known to predate from behind rocks or lurk under boulders, designated as the “sit and wait” predation mode. Once the prey is identified, these fish shift to the “burst chase” prey pursuit mode [70]. This species also combines a suction action in swimming to catch the prey; therefore this suction could ingest MP from the water column which, despite less abundant than in sediment, are still present [71]. However, despite the lower quantity of MP ingestion when compared to L. mormyrus, the biomarker activity for S. scriba is higher for all biomarkers. Thus, CAT, SOD, GST, and MPO were higher for S. scriba in the more anthropic Zone 1. Additionally, CAT, SOD, GST, MPO, and MDA were higher for S. scriba than for L. mormyrus even within the same area. The enzymatic or immune response to pollutants can vary greatly between species [16,72]. This might be due to a more efficient detoxifying or cleaning mechanisms, or higher metabolism which consumes more oxygen, and consequently could lead to a greater production of ROS [73]. Additionally, the physiognomy of intestinal tracts could also factor in, as it has been suggested that a higher number of folds and internal cavities could favour the accumulation of MPs [74], allowing them to remain toxic in the system for a longer amount of time, potentially releasing more additives due to digestive secretions and processes. This leads to differences in oxidative stress, and enzymatic and immune response biomarkers with the same or similar exposure. This study thus adds to the expanding current literature studying the effect of MPs in fish through biomarkers, which has been a growing trend in recent years [16,67,75].
5. Conclusions
In conclusion, this study confirms the presence of MPs in two benthic fish species of the Mediterranean Sea, Serranus scriba and Lithognathus mormyrus, across areas with contrasting levels of anthropogenic activity. A clear link was observed between higher tourist pressure and increased concentrations of MPs in both the water column and sediment, along with elevated biomarker responses in fish. The results suggest a relationship between MP exposure and physiological stress responses, particularly oxidative stress and inflammation, in the digestive tract. Moreover, our findings highlight species-specific responses to MP pollution, likely influenced by differences in feeding behaviour, physiology and metabolism. However, these types of studies are limited as the data is collected at a single point in time. Furthermore, chemical analysis could be performed to identify which pollutants are leaching from the plastics. Future studies should aim to expand the number of species and sampling sites to better understand the ecological impact of MPs across different trophic levels and habitats. Additionally, long-term monitoring and experimental approaches under both field and controlled conditions could help elucidate the chronic effects of MP exposure and the potential for bioaccumulation and trophic transfer within marine food webs.
Author Contributions
Conceptualization, M.C., L.G., S.T., S.P. and A.C.-S.; methodology, A.C.-S., M.C., M.M.Q.-L., M.d.M.R.-T., L.G., S.T., S.P. and A.C.-S.; formal analysis, A.C.-S., J.A.S. and A.S.; investigation, A.C.-S., J.A.S., M.C., M.M.Q.-L., M.d.M.R.-T., L.G., S.T., S.P. and A.S.; writing—original draft preparation, A.C.-S., J.A.S., M.C. and A.S.; writing—review and editing, A.C.-S., J.A.S., M.C., M.M.Q.-L., M.d.M.R.-T., L.G., S.T., S.P. and A.S.; project administration, A.S.; funding acquisition, S.P. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Programme of Promotion of Biomedical Research and Health Sciences, Instituto de Salud Carlos III (CIBEROBN CB12/03/30038). Additional funding was provided within the framework of the Biodibal project, through the agreements of the University of the Balearic Islands and Red Eléctrica de España. A.C. was supported by an FPI Fellowship co-financed by the Balearic Government and the European Social Fund Plus (EFS+) as part of the EFS 2021–2027 operational programme. (FPI 027/2023).
Institutional Review Board Statement
The experimental procedures were approved by the Animal Experimentation Ethics Committee of the University of the Balearic Islands (Reference: CEEA 96/05/18).
Data Availability Statement
Researchers wishing to access the data used in this study can make a request to the corresponding author: antoni.sureda@uib.es.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CAT | Catalase |
| EROD | Ethoxyresorufin-O-deethylase |
| GST | Glutathione s-transferase |
| HDPE | High-density polyethylene |
| LDPE | Low-density polyethylene |
| MDA | Malondialdehyde |
| MP | Microplastic |
| MPO | Myeloperoxidase |
| PP | Polypropylene |
| PVC | Polyvinyl chloride |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
References
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Huang, X.P.; Xiang, L.; Wang, Y.Z.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H. Source, Migration and Toxicology of Microplastics in Soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef] [PubMed]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic Pollution in the Marine Environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.V.L.; Liddle, C.R.; Atherall, C.A.; Chapman, E.; Watkins, M.; Calaminus, S.D.J.; Rotchell, J.M. Microplastics in Human Blood: Polymer Types, Concentrations and Characterisation Using μFTIR. Environ. Int. 2024, 188, 108751. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Wei, L.; Yue, Q.; Chen, G.; Wang, J. Microplastics in Rainwater/Stormwater Environments: Influencing Factors, Sources, Transport, Fate, and Removal Techniques. TrAC 2023, 165, 117147. [Google Scholar] [CrossRef]
- Napper, I.E.; Davies, B.F.R.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching New Heights in Plastic Pollution—Preliminary Findings of Microplastics on Mount Everest. One Earth 2020, 3, 621–630. [Google Scholar] [CrossRef]
- Plastics Europe. Plastcs—The Fast Facts 2024. Available online: https://plasticseurope.org/es/knowledge-hub/plastics-the-fast-facts-2024/ (accessed on 12 June 2025).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Zhu, X.; Rochman, C.M.; Hardesty, B.D.; Wilcox, C. Plastics in the Deep Sea—A Global Estimate of the Ocean Floor Reservoir. Deep. Sea Res. Part I Deep.-Sea Res. 2024, 206, 104266. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; Macleod, M. Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Renzi, M.; Specchiulli, A.; Blašković, A.; Manzo, C.; Mancinelli, G.; Cilenti, L. Marine Litter in Stomach Content of Small Pelagic Fishes from the Adriatic Sea: Sardines (Sardina pilchardus) and Anchovies (Engraulis encrasicolus). Environ. Sci. Pollut. Res. 2019, 26, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef]
- Filimon, A.; Ciucă, A.M.; Harcotă, G.E.; Stoica, E. Preliminary study on microplastic contamination in Black Sea cetaceans: Gastrointestinal analysis of Phocoena phocoena relicta and Tursiops truncatus ponticus. Animals 2024, 14, 886. [Google Scholar] [CrossRef]
- Subaramaniyam, U.; Allimuthu, R.S.; Vappu, S.; Ramalingam, D.; Balan, R.; Paital, B.; Panda, N.; Rath, P.K.; Ramalingam, N.; Sahoo, D.K. Effects of microplastics, pesticides and nano-materials on fish health, oxidative stress and antioxidant defense mechanism. Front. Physiol. 2023, 14, 1217666. [Google Scholar] [CrossRef]
- Sridharan, S.; Kumar, M.; Saha, M.; Kirkham, M.B.; Singh, L.; Bolan, N.S. The polymers and their additives in particulate plastics: What makes them hazardous to the fauna? Sci. Total Environ. 2022, 824, 153828. [Google Scholar] [CrossRef]
- Junaid, M.; Siddiqui, J.A.; Liu, S.; Lan, R.; Abbas, Z.; Chen, G.; Wang, J. Adverse multigeneration combined impacts of micro(nano)plastics and emerging pollutants in the aquatic environment. Sci. Total Environ. 2023, 882, 163679. [Google Scholar] [CrossRef]
- Sureda, A.; Box, A.; Enseñat, M.; Alou, E.; Tauler, P.; Deudero, S.; Pons, A. Enzymatic Antioxidant Response of a Labrid Fish (Coris julis) Liver to Environmental Caulerpenyne. Comp. Biochem. Physiol. C Toxicol. Pharmacol. Comp. Biochem. Physiol. C 2006, 144, 191–196. [Google Scholar] [CrossRef]
- Solomando, A.; Cohen-Sánchez, A.; Box, A.; Montero, I.; Pinya, S.; Sureda, A. Microplastic Presence in the Pelagic Fish, Seriola dumerili, from Balearic Islands (Western Mediterranean), and Assessment of Oxidative Stress and Detoxification Biomarkers in Liver. Environ. Res. 2022, 212, 113369. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress-Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Compa, M.; Valencia, J.M.; Sureda, A.; Deudero, S. Assessment of the Effect of Long-Term Exposure to Microplastics and Depuration Period in Sparus aurata Linnaeus, 1758: Liver and Blood Biomarkers. Sci. Total Environ. 2021, 786, 147479. [Google Scholar] [CrossRef] [PubMed]
- Kutsyn, D.N.; Tamoikin, I.Y.; Samotoy, Y.V.; Donchik, P.I. Age, Growth, and Maturity of Painted Comber Serranus Scriba (Serranidae) from the Crimea Region, the Black Sea. J. Ichthyol. 2023, 63, 902–910. [Google Scholar] [CrossRef]
- Tuset, V.M.; García-Díaz, M.M.; González, J.A.; Lorente, M.J.; Lozano, I.J. Reproduction and Growth of the Painted Comber Serranus Scriba (Serranidae) of the Marine Reserve of Lanzarote Island (Central-Eastern Atlantic). Estuar. Coast. Shelf Sci. 2005, 64, 335–346. [Google Scholar] [CrossRef]
- Zorica, B.; Sinovčić, G.; Pallaoro, A.; Keč, Č. Reproductive Biology and Length-Weight Relationship of Painted Comber, Serranus scriba (Linnaeus, 1758), in the Trogir Bay Area (Middle-Eastern Adriatic). J. Appl. Ichthyol. 2006, 22, 260–263. [Google Scholar] [CrossRef]
- Bauchot, M.L. Serranidae. In Faune des Poissons D’eaux Douces et Saumâtres de L’afrique de L’ouest = The Fresh and Brackish Water Fishes of West Africa; Faune Tropicale; ORSTOM: Paris, France, 1992; Volume 28, pp. 664–667. [Google Scholar]
- Šantić, M.; Apostolska, B. Diet Composition of Painted Comber Searranus scriba (Linnaeus 1758) in the Eastern-Central Adriatic Sea. Ribarstvo Croat. J. Fish 2024, 82, 121–128. [Google Scholar] [CrossRef]
- Makri, V. An Estimation of the Diet of the Species Serranus scriba (Linnaeus, 1758) in the Area of Nisiopi, in South-West Lesvos. J. Environ. Sci. Eng. 2016, 5, 593–600. [Google Scholar] [CrossRef]
- Zupo, V.; Stübing, D. Diet of Fish Populations in Posidonia Oceanica Meadows off the Island of Ischia (Gulf of Naples, Italy). Nat. Sci. 2010, 02, 1274–1286. [Google Scholar] [CrossRef][Green Version]
- Morales-Nin, B.; Moranta, J.; García, C.; Tugores, M.P.; Grau, A.M.; Riera, F.; Cerdà, M. The Recreational Fishery off Majorca Island (Western Mediterranean): Some Implications for Coastal Resource Management. ICES J. Mar. Sci. 2005, 62, 727–739. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pajuelo, J.G.; Méndez-Villamil, M.; Coca, J.; Ramos, A.G. Age, Growth, Reproduction and Mortality of the Striped Seabream, Lithognathus mormyrus (Pisces, off the Canary Islands (Central-East Atlantic. J. Appl. Ichthyol. 2002, 18, 204–209. [Google Scholar] [CrossRef]
- HammamI, I.; BaHrI-Sfar, L.; Kaoueche, M.; Grenouillet, G.; Lek, S.; Kara, M.; Hassine, O.B. Morphological Characterization of Striped Seabream (Lithognathus mormyrus, Sparidae) in Some Mediterranean Lagoons. Cybium Int. J. Ichthyol. 2013, 37, 127–129. [Google Scholar]
- Boufersaoui, S.; Kassar, A.; Mokrane, Z.; Elleboode, R.; Mahé, K. Age and Growth of the Striped Seabream, Lithognathus mormyrus (Actinopterygii: Perciformes: Sparidae), in the Central Coast of Algeria, Mediterranean Sea. Acta Ichthyol. Piscat. 2018, 48, 319–328. [Google Scholar] [CrossRef]
- Kallianiotis, A.; Torre, M.; Argyri, A. Age, Growth, Mortality, Reproduction and Feeding Habits of the Striped Seabream, Lithognathus mormyrus (Pisces: Sparidae) in the Coastal Waters of the Thracian Sea, Greece. Sci. Mar. 2005, 69, 391–404. [Google Scholar] [CrossRef]
- Templado, J. Future Trends of Mediterranean Biodiversity. In The Mediterranean Sea; Springer: Dordrecht, The Netherlands, 2014; pp. 479–498. [Google Scholar]
- Sharma, S.; Sharma, V.; Chatterjee, S. Microplastics in the Mediterranean Sea: Sources, Pollution Intensity, Sea Health, and Regulatory Policies. Front. Mar. Sci. 2021, 8, 634934. [Google Scholar] [CrossRef]
- Fagiano, V.; Compa, M.; Alomar, C.; Rios-Fuster, B.; Morató, M.; Capó, X.; Deudero, S. Breaking the Paradigm: Marine Sediments Hold Two-Fold Microplastics than Sea Surface Waters and Are Dominated by Fibers. Sci. Total Environ. 2023, 858, 159722. [Google Scholar] [CrossRef]
- Mansui, J.; Molcard, A.; Ourmières, Y. Modelling the Transport and Accumulation of Floating Marine Debris in the Mediterranean Basin. Mar. Pollut. Bull. 2015, 91, 249–257. [Google Scholar] [CrossRef]
- Nadal, M.A.; Alomar, C.; Deudero, S. High Levels of Microplastic Ingestion by the Semipelagic Fish Bogue Boops boops (L.) around the Balearic Islands. Environ. Pollut. 2016, 214, 517–523. [Google Scholar] [CrossRef]
- Savoca, M.S.; McInturf, A.G.; Hazen, E.L. Plastic ingestion by marine fish is widespread and increasing. Glob. Change Biol. 2021, 27, 2188–2199. [Google Scholar] [CrossRef]
- Abril Sellarés, M.; Azpelicueta Criado, M.D.C.; Sánchez Fernández, M.D. ¿Vale Todo En Turismo? Residentes Frente a Turistas. Estudio Comparativo Entre El Barrio de La Barceloneta, Barcelona y La Localidad de Magaluf, Calvia. In Proceedings of the Libro de Ponencias de las VIII Jornadas de Investigación en Turismo: Impulso al Desarrollo Económico a Través del Turismo, Bertrange, Luxemburg, 15–17 June 2015; Dialnet: Los Angeles, CA, USA, 2015; Volume 1, pp. 493–510. [Google Scholar]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic Contamination of River Beds Significantly Reduced by Catchment-Wide Flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High Quantities of Microplastic in Arctic Deep-Sea Sediments from the HAUSGARTEN Observatory. Environ. Sci. Technol. 2017, 51, 11000–11010. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Flohé, L.; Ötting, F. Superoxide Dismutase Assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Capeillère-Blandin, C. Oxidation of Guaiacol by Myeloperoxidase: A Two-Electron-Oxidized Guaiacol Transient Species as a Mediator of NADPH Oxidation. Biochem. J. 1998, 336, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Capó, X.; Tejada, S.; Box, A.; Deudero, S.; Sureda, A. Oxidative Status Assessment of the Endemic Bivalve Pinna Nobilis Affected by the Oil Spill from the Sinking of the Don Pedro. Mar. Environ. Res. 2015, 110, 19–24. [Google Scholar] [CrossRef]
- Grelaud, M.; Ziveri, P. The Generation of Marine Litter in Mediterranean Island Beaches as an Effect of Tourism and Its Mitigation. Sci. Rep. 2020, 10, 20326. [Google Scholar] [CrossRef]
- Drius, M.; Bongiorni, L.; Depellegrin, D.; Menegon, S.; Pugnetti, A.; Stifter, S. Tackling Challenges for Mediterranean Sustainable Coastal Tourism: An Ecosystem Service Perspective. Sci. Total Environ. 2019, 652, 1302–1317. [Google Scholar] [CrossRef]
- Ministerio de Modelos Económicos, Tecnología y Empleo; Consejería de Turismo de las Islas Baleares. Anuario de Turismo 2023. Available online: https://www.caib.es/sites/estadistiquesdelturisme/es/anuarios_de_turismo-22816/ (accessed on 12 June 2025).
- Nuevo, A.; Martínez, G. Turismo Sostenible versus Depredación Turística. Islas Baleares, España. Rev. Geog. Venez. 2021, 62, 394–409. [Google Scholar] [CrossRef]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary Administration of PVC and PE Microplastics Produces Histological Damage, Oxidative Stress and Immunoregulation in European Sea Bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Álvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-Term Exposure to Microplastics Induces Oxidative Stress and a pro-Inflammatory Response in the Gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 2020, 266, 115295. [Google Scholar] [CrossRef]
- Capó, X.; Morató, M.; Alomar, C.; Rios-Fuster, B.; Valls, M.; Compa, M.; Deudero, S. A Biomarker Approach as Responses of Bioindicator Commercial Fish Species to Microplastic Ingestion: Assessing Tissue and Biochemical Relationships. Biology 2022, 11, 1634. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in Wild Fish from North East Atlantic Ocean and Its Potential for Causing Neurotoxic Effects, Lipid Oxidative Damage, and Human Health Risks Associated with Ingestion Exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- Franco-Herrera, A.; Polania-Zenner, P.I.; Otálora-Rincón, C.D.; Tigreros-Benavides, P.C. Distribución Espacial y Temporal de Microplásticos Flotantes En Aguas Del Caribe Central Colombiano. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2022, 46, 406–425. [Google Scholar] [CrossRef]
- Santucci, L.; Fernández-Severini, M.D.; Rimondino, G.N.; Colombo, C.V.; Prieto, G.; Forero-López, A.D.; Carol, E.S. Assessment of Meso- and Microplastics Distribution in Coastal Sediments and Waters at the Middle Estuary of the Rio De La Plata, Argentina (SW Atlantic Ocean). Sci. Total Environ. 2024, 914, 170026. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ribes, L.; Basterretxea, G.; Palmer, M.; Tintoré, J. Origin and Abundance of Beach Debris in the Balearic Islands. Sci. Mar. 2007, 71, 305–314. [Google Scholar] [CrossRef]
- Patidar, K.; Ambade, B.; Younis, A.M.; Alluhayb, A.H. Characteristics, Fate, and Sources of Microplastics Contaminant in Surface Water and Sediments of River Water. Phys. Chem. Earth (Pt A B C) 2024, 134, 103596. [Google Scholar] [CrossRef]
- Xia, F.; Tan, Q.; Qin, H.; Wang, D.; Cai, Y.; Zhang, J. Sequestration and Export of Microplastics in Urban River Sediments. Environ. Int. 2023, 181, 108265. [Google Scholar] [CrossRef]
- Chubarenko, I.; Bagaev, A.; Zobkov, M.; Esiukova, E. On Some Physical and Dynamical Properties of Microplastic Particles in Marine Environment. Mar. Pollut. Bull. 2016, 108, 105–112. [Google Scholar] [CrossRef]
- Papadimitriu, M.; Allinson, G. Microplastics in the Mediterranean marine environment: A combined bibliometric and systematic analysis to identify current trends and challenges. Micropl. Nanopl. 2022, 2, 8. [Google Scholar] [CrossRef]
- Ben Hadj Hamida, N.; Ben Abdallah-Ben Hadj Hamida, O.; Jarboui, O.; Missaoui, H. Diet Composition and Feeding Habits of Lithognathus mormyrus (Sparidae) from the Gulf of Gabes (Central Mediterranean). J. Mar. Biol. Assoc. UK 2016, 96, 1491–1498. [Google Scholar] [CrossRef]
- Jaerisch, J.; Dieter Zander, C.; Giere, O. Feeding Behaviour and Feeding Ecology of Two Substrate Teleosts, Mullus surmuletus (Mullidae) and Lithognathus mormyrus (Sparidae), in the Mediterranean Sea. Bull. Fish Biol. 2010, 12, 27–39. [Google Scholar]
- Expósito, N.; Rovira, J.; Sierra, J.; Folch, J.; Schuhmacher, M. Microplastics Levels, Size, Morphology and Composition in Marine Water, Sediments and Sand Beaches. Case Study of Tarragona Coast (Western Mediterranean). Sci. Total Environ. 2021, 786, 147453. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yu, Y.B.; Choi, J.H. Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Neurotoxicity in Fish Exposed to Microplastics: A Review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Capó, X.; Company, J.J.; Alomar, C.; Compa, M.; Sureda, A.; Grau, A.; Hansjosten, B.; López-Vázquez, J.; Quintana, J.B.; Rodil, R.; et al. Long-Term Exposure to Virgin and Seawater Exposed Microplastic Enriched-Diet Causes Liver Oxidative Stress and Inflammation in Gilthead Seabream Sparus aurata, Linnaeus 1758. Sci. Total Environ. 2021, 767, 144976. [Google Scholar] [CrossRef]
- Lokovšek, A.; Orlando-Bonaca, M.; Trkov, D.; Lipej, L. An Insight into the Feeding Ecology of Serranus scriba, a Shallow Water Mesopredator in the Northern Adriatic Sea, with a Non-Destructive Method. Fishes 2022, 7, 210. [Google Scholar] [CrossRef]
- Vandewalle, P.; Casinos, A.; Viladiu, C.; Osse, J.W.M. Suction Feeding Strategies of Two Species of Mediterranean Serranidae (Serranus cabrilla and Serranus scriba). Neth. J. Zool. 1999, 49, 81–95. [Google Scholar] [CrossRef]
- Reichert, J.; Arnold, A.L.; Hoogenboom, M.O.; Schubert, P.; Wilke, T. Impacts of Microplastics on Growth and Health of Hermatypic Corals Are Species-Specific. Environ. Pollut. 2019, 254, 113074. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and Diseases: Role in Metabolism and Energy Supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Amini-Birami, F.; Keshavarzi, B.; Esmaeili, H.R.; Moore, F.; Busquets, R.; Saemi-Komsari, M.; Zarei, M.; Zarandian, A. Microplastics in Aquatic Species of Anzali Wetland: An Important Freshwater Biodiversity Hotspot in Iran. Environ. Pollut. 2023, 330, 121762. [Google Scholar] [CrossRef]
- Atamanalp, M.; Kokturk, M.; Kırıcı, M.; Ucar, A.; Kırıcı, M.; Parlak, V.; Aydın, A.; Alak, G. Interaction of Microplastic Presence and Oxidative Stress in Freshwater Fish: A Regional Scale Research, East Anatolia of Türkiye (Erzurum & Erzincan & Bingöl). Sustainability 2022, 14, 12009. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).