Sardine-Based Diet Mitigates Growth Depression at Low Temperatures in Juvenile Meagre (Argyrosomus regius, Asso 1801)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Rearing and Experimental Design
- Group A: 20.35 cm ± 0.207 total length, 106.30 g ± 3.12 wet weight;
- Group B: 20.6 cm ± 0.27 total length, 108.25 g ± 4.38 wet weight;
- Group C: 20.65 cm ± 0.19 total length, 107.14 g ± 3.04 wet weight.
- Group A (diet S—sardines). All sardines (Sardina pilchardus, Walbaum, 1792) were from a single haul, individually quick-frozen (IQF) on the same day. Before feeding, sardines were thawed and cut into appropriately sized pieces;
- Group B (diet P—commercial dry pellets). Commercial pellets, which were declared for meagre farming;
- Group C (diet MP—moisturized commercial pellets). Commercial pellets, sourced identically to diet P, were sprayed with 10% water by weight to achieve 15% moisture, and soaked for 30 min before feeding.
2.2. Determination of Somatic and Growth Indices
2.2.1. Morphometric Measurements
2.2.2. Fulton’s Condition Factor (K)
2.2.3. Somatic Indices
- Hepatosomatic index (HSI):
- Viscerosomatic index (VSI):
2.2.4. Growth Parameters
- Thermal growth coefficient, TGC:
- W1 = initial mean weight (g);
- W2 = final mean weight (g);
- T = daily seawater temperature (°C);
- t = duration of the experiment (days).
2.2.5. Feed Consumption Parameters:
- Daily feeding ratio, DFR:where AF is the dry matter of the administered feed per group:
- Total feed intake per fish (TFI):
- Gross energy intake per fish (GEI):
2.3. Analytical Methods
2.3.1. Diet Composition Analysis:
- Lipid content: lipids were extracted according to the method of Folch et al. [33]. The total lipid content was then gravimetrically determined after solvent evaporation using a Soxhlet apparatus (Ser 158, Velp Scientifica Srl. Usmate, Italy);
- Protein content: protein content was determined using the Kjeldahl method; total nitrogen content was measured and multiplied by a conversion factor of 6.25 to estimate protein levels;
- Water content: water content of the feed was determined by oven-drying of minced feed at 105 °C until a constant weight was achieved, followed by reweighing;
- Ash content: ash content was determined by combusting a 1 g homogenized feed sample in a muffle furnace until a stable weight was obtained;
- Carbohydrate content: carbohydrate content was calculated as the following remaining fraction:100 − (% lipid content + % protein content + % water content + % ash content);
- Gross energy, GE: gross energy was calculated based on the determined macronutrient content using the energy conversion factors provided by Steffens [34]: 23.9 kJ/g for protein, 39.8 kJ/g for lipids, and 17.6 kJ/g for carbohydrates.
2.3.2. Fatty Acid Composition Analysis:
2.4. Statistical Analysis
3. Results
3.1. Environmental Conditions and Diet Composition
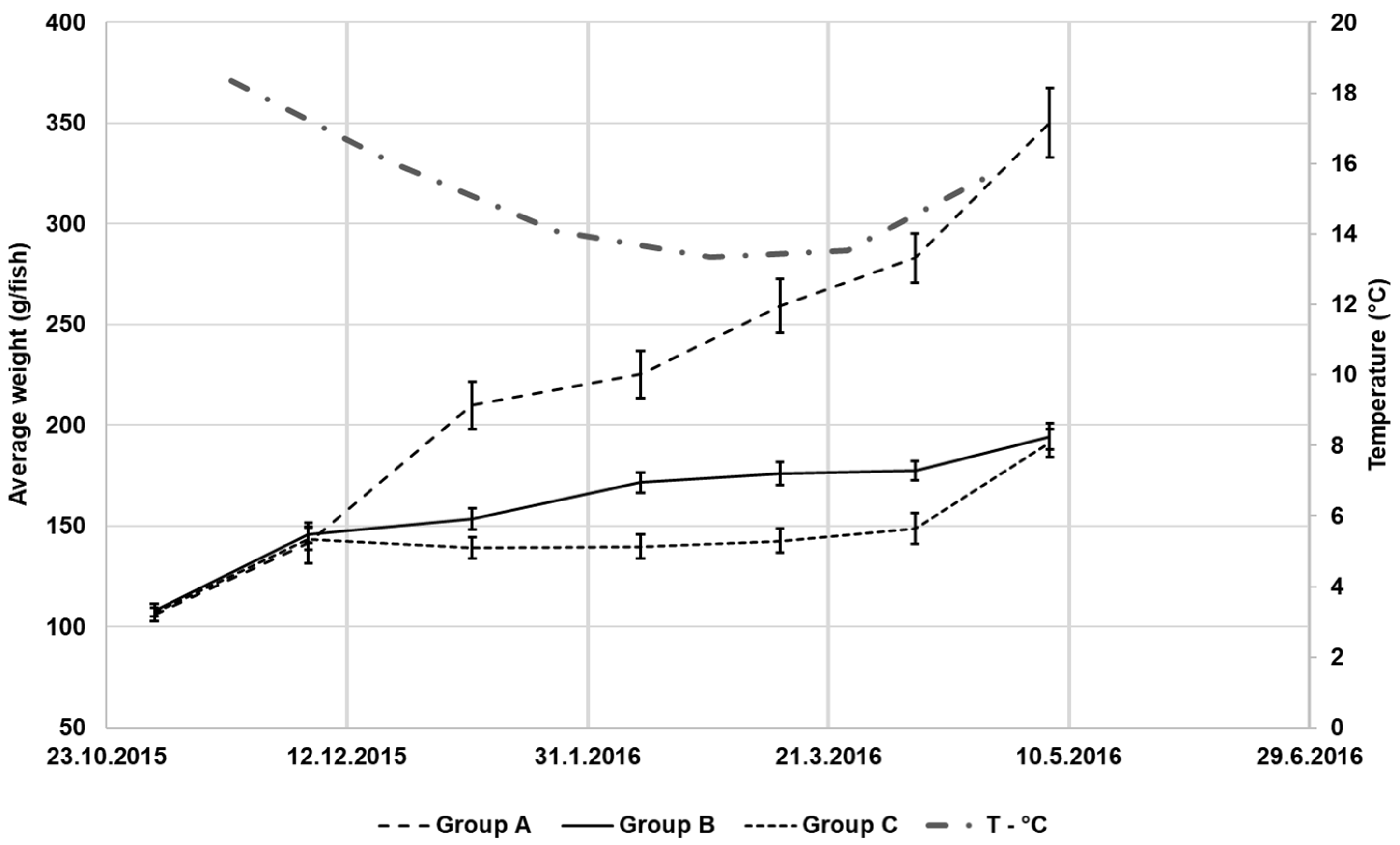
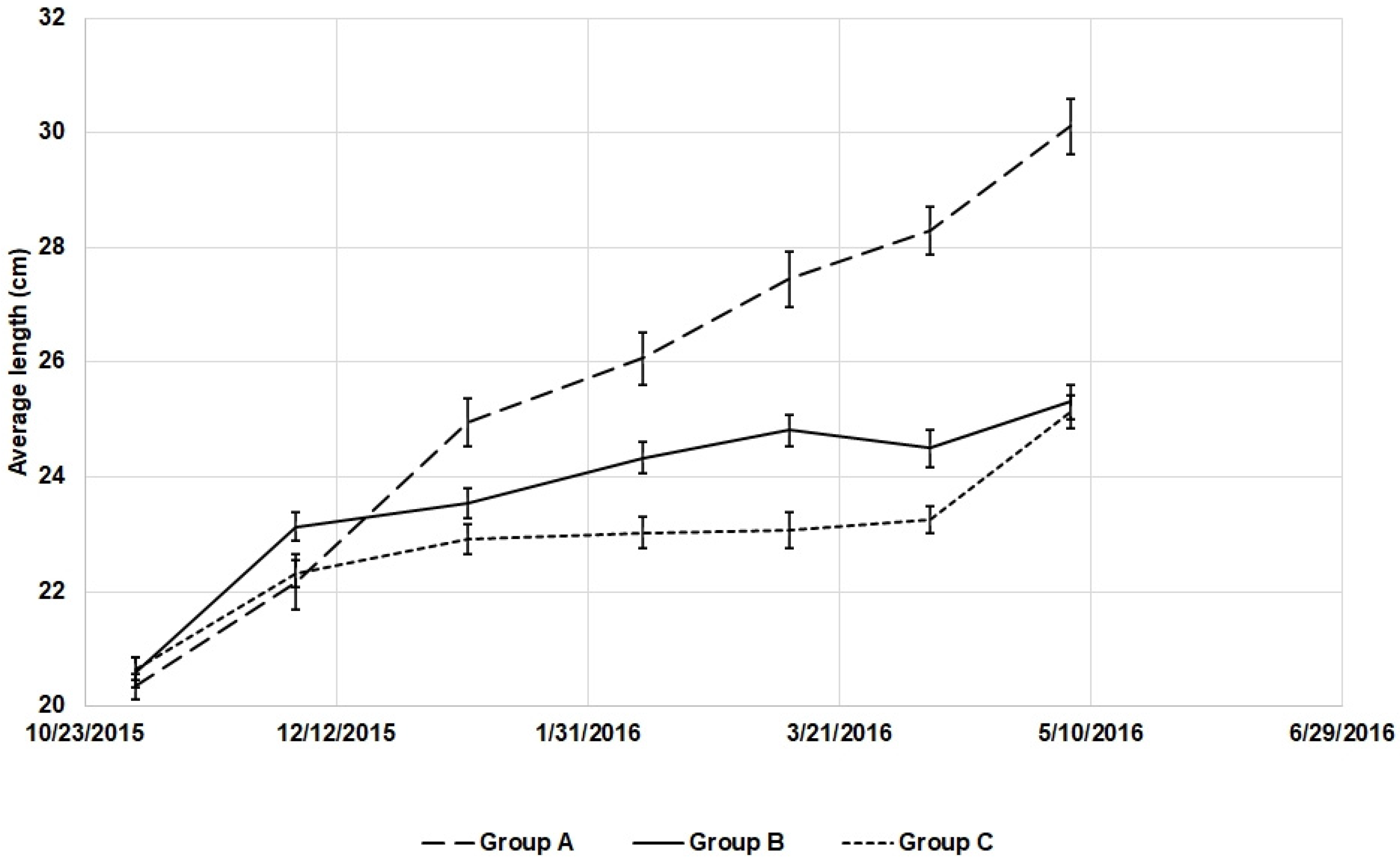
3.2. Growth and Somatic Indices
3.3. Feed Consumption
3.4. Fatty Acid Profiles
4. Discussion
- Optimizing macronutrient ratios: determining the ideal high protein and lipid levels, coupled with minimized carbohydrates, that mimic the sardine diet’s success in cold-water meagre such as increasing the proportion of n-3 PUFA to above 35% [48] and/or increasing dietary tryptophan to mitigate stress [49];
- Enhancing essential fatty acid bioavailability: investigating how to achieve the superior EPA and DHA deposition seen with sardines, potentially through specific lipid sources, processing methods, or functional additives in pellets such as Fucus vesiculosus, shown to promote feed efficiency [50];
- Improving pellet functionality: formulating pellets that retain high palatability and are easily digestible even when fish appetite and metabolism are reduced in cold temperatures;
- Improving sustainability: investigate alternative protein and lipid sources such as plant-based proteins and animal by-products suggested by Matias et al. [51], which replicate the sardine’s beneficial profile without relying on wild fisheries.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiménez, M.T.; Pastor, E.; Grau, A.; Alconchel, J.I.; Cárdenas, S. Revisión sobre el cultivo de esciénidos en el mundo, con especial atención a la corvina (Argyrosomus regius). Bol. Inst. Esp. Oceanogr. 2005, 21, 169–176. [Google Scholar]
- Pastor, G.E.; Grau, J.A.; Massutí, P.E.; Sánchez, L.R.Y.A. Preliminary results on growth of meagre, Argyrosomus regius (Asso, 1801) in sea cages and indoor tanks. Aquaculture Europe Seafarming, today and tommorow. EAS/Spec. Publ. 2002, 32, 422–423. [Google Scholar]
- Poli, B.M.; Parisi, G.; Zampacavallo, G.; Iurzan, F.; Mecatti, M.; Lupi, P.; Bonelli, A. Preliminary results on quality and quality changes in reared meagre (Argyrosomus regius): Body and fillet traits and freshness changes in refrigerated commercial-size fish. Aquac. Int. 2003, 11, 301–311. [Google Scholar] [CrossRef]
- Grigorakis, K.; Fountoulaki, E.; Vasilaki, A.; Mittakos, I.; Nathanailides, C. Lipid quality and filleting yield of reared meagre (Argyrosomus regius). Int. J. Food Sci. Technol. 2011, 46, 711–716. [Google Scholar] [CrossRef]
- FAO. Global Aquaculture Production Quantity (1950–2021). 2023. Available online: https://www.fao.org/fishery/statistics-query/en/aquaculture/aquaculture_quantity (accessed on 20 November 2023).
- Brett, J.R. Environmental factors and growth. In Fish Physiology; Hoar, D.J., Randall, J.R., Brett, J.R., Eds.; Academic Press: New York, NY, USA, 1979; pp. 599–667. [Google Scholar]
- Ibarz, A.; Padrós, F.; Galardo, M.Á.; Fernández-Borràs, J.; Blasco, J.; Tort, L. Low-temperature challenges to gilthead sea bream culture: Review of cold-induced alterations and “Winter Syndrome”. Rev. Fish Biol. Fish. 2010, 20, 539–556. [Google Scholar] [CrossRef]
- Bowyer, J.N.; Qin, J.G.; Stone, D.A.J. Protein, lipid and energy requirements of cultured marine fish in cold, temperate and warm water. Rev. Aquac. 2013, 5, 10–32. [Google Scholar] [CrossRef]
- Lavié, A.; Rodríguez-Rúa, A.; Ruiz-Jarabo, I.; Vargas-Chacoff, L.; Mancera, J.M.; Cárdenas, S. Influencia de la densidad de cultivo y la temperatura sobre el crecimiento y el metabolismo en alevines de corvina, (Argyrosomus regius, Asso, 1801). In Proceedings of the IV Jornadas de Acuicultura del Litoral Suratlántico, Cartaya, Spain, 16–17 April 2008. [Google Scholar]
- Gandra, M.; Winkler, A.C.; Afonso, P.; Abecasis, D. Long-distance migrations and seasonal movements of meagre (Argyrosomus regius), a large coastal predator, along the Iberian Peninsula coast. Mov. Ecol. 2024, 12, 35. [Google Scholar] [CrossRef]
- Calderón, J.A.; Esteban, J.C.; Carrascosa, M.A.; Ruiz, P.L.; Valera, F. Rearing and growth in captivity of a lot of meagre reproducers (Argyrosomus regius). In Proceedings of the Actas VI Congreso Nacional de Acuicultura, Cartagena, Murcia, Spain, 9–11 July 1997; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1997; pp. 365–370. [Google Scholar]
- FAO. Argyrosomus regius. Cultured Aquatic Species Information Programme. Text by Stipa, P.; Angelini, M. Fisheries and Aquaculture Division [online]. Rome. 2022. Available online: https://www.fao.org/fishery/en/culturedspecies/argyrosomus_regius/en (accessed on 29 November 2022).
- Kružić, N.; Mustać, B.; Župan, I.; Čolak, S. Meagre (Argyrosomus regius, Asso, 1801) Aquaculture in Croatia. Croat. J. Fish. 2016, 74, 14–19. [Google Scholar] [CrossRef]
- Gustinelli, A.; Čolak, S.; Quaglio, F.; Sirri, R.; Kolega, M.; Mejdandžić, D.; Caffara, M.; Barić, R.; Fioravanti, M.L. Histological assessment of systemic granulomatosis progression in meagre Argyrosomus regius during cage ongrowing phase. Dis. Aquat. Org. 2021, 145, 165–172. [Google Scholar] [CrossRef]
- Piccolo, G.; Bovera, F.; De Riu, N.; Marono, S.; Salati, F.; Cappuccinelli, R.; Moniello, G. Effect of two different protein/fat ratios of the diet on meagre (Argyrosomus regius) traits. Ital. J. Anim. Sci. 2008, 7, 363–371. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Panagiotidou, M.; Papaioannou, N.; Pavlidis, M.; Nengas, I.; Mylonas, C.C. Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 2010, 301, 65–70. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Panagiotidou, M.; Divanach, P. Effect of protein and lipid dietary levels on the growth of juvenile meagre (Argyrosomus regius). Aquac. Int. 2012, 20, 91–98. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Espert, J.; Moya, J.; Cerdá, M.J.; Tomás-Vidal, A. Growth and nutrient efficiency of meagre (Argyrosomus regius, Asso, 1801) fed extruded diets with different protein and lipid levels. Int. J. Aquac. Fish. Sci. 2011, 3, 195–203. [Google Scholar]
- Fountoulaki, E.; Grigorakis, K.; Kounna, C.; Rigos, G.; Papandroulakis, N.; Diakogeorgakis, J.; Kokou, F. Growth performance and product quality of meagre (Argyrosomus regius) fed diets of different protein/lipid levels at industrial scale. Ital. J. Anim. Sci. 2017, 16, 685–694. [Google Scholar] [CrossRef]
- Velazco-Vargas, J.; Martínez-Llorens, S.; Cerda, M.J.; Tomás-Vidal, A. Evaluation of soybean meal as protein source for Argyrosomus regius (Asso, 1801) (Sciaenidae). Int. J. Fish. Aquac. 2013, 5, 35–44. [Google Scholar]
- Emre, Y.; Kurtoğlu, A.; Emre, N.; Güroy, B.; Güroy, D. Effect of replacing dietary fish oil with soybean oil on growth performance, fatty acid composition and haematological parameters of juvenile meagre, Argyrosomus regius. Aquac. Res. 2015, 47, 2256–2265. [Google Scholar] [CrossRef]
- Ribeiro, L.; Moura, J.; Santos, M.; Colen, R.; Rodrigues, V.; Bandarra, N.; Soares, F.; Ramalho, P.; Barata, M.; Moura, P.; et al. Effect of vegetable-based diets on growth, intestinal morphology, activity of intestinal enzymes and haematological stress indicators in meagre (Argyrosomus regius). Aquaculture 2015, 447, 116–128. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Páscoa, I.; Gonçalves, O.; Mancera, J.M. Yearly growth and metabolic changes in earthen pond-cultured meagre Argyrosomus regius. Sci. Mar. 2014, 78, 193–202. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Papadakis, A.E.; Divanach, P. Effects of dietary water on growth of dentex Dentex dentex. Fish. Sci. 2005, 71, 1243–1248. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Chatzifotis, S.; Divanach, P.; Kentouri, M. Weaning of greater amberjack (Seriola dumerilli Risso, 1810) juveniles from moist to dry pellet. Aquac. Int. 2008, 16, 13–25. [Google Scholar] [CrossRef]
- Pryzybla, C.; Fievet, J.; Callier, M.; Blancheton, J.P. Effect of dietary water content on European sea bass (Dicentrarchus labrax) growth and disease resisitance. Aquat. Living Resour. 2014, 27, 73–81. [Google Scholar] [CrossRef]
- Cardenete, G.; Skalli, A.; Hidalgo, M.C.; Abellán, E.; Massuti, S. Feeding Dentex dentex with dry diets: Growth response and diet utilization. In Feeding Tomorrow’s Fish; Tacon, A.G.J., Basurco, B., Eds.; CIHEAM Cahiers Options Méditerranéennes: Zaragoza, Spain, 1997; Volume 22, pp. 141–151. [Google Scholar]
- Mazzola, A.; Favaloro, E.; Sarà, G. Cultivation of the Mediterranean amberjack, Seriola dumerili (Risso, 1810), in submerged cages in the Western Mediterranean Sea. Aquaculture 2000, 181, 257–268. [Google Scholar] [CrossRef]
- Duncan, N.J.; Estévez, A.; Fernández-Palacios, H.; Gairin, I.; Hernández-Cruz, C.M.; Roo, J.; Schuchardt, D.; Vallés, R. Aquaculture production of meagre (Argyrosomus regius): Hatchery techniques, ongrowing and market. In Advances in Aquaculture Hatchery Technology; Alan, G., Burnell, G., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 519–541. [Google Scholar]
- Abdelhamid, A.M.; Fedekar, F.; Madkour, F.F.; Abou Elregal, M.A.; Radwan, A.M. Effect of feeding regimen on growth performance, feed utilization, body composition and economic efficiency by Argyrosomus regius (Asso, 1801). J. Arab. Aquac. Soc. 2013, 8, 53–56. [Google Scholar]
- Bagenal, T.B.; Tesch, F.W. Age and Growth in Methods for Assessment of Fish Production in Fresh Waters; Blackwell Scientific Publications: Oxford, UK, 1978; pp. 101–136. [Google Scholar]
- Association of Official Analytical Chemists. Association of Official Analytical Chemists (AOAC) Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Steffens, W. Principles of Fish Nutrition; Ellis Horwood: Chichester, UK, 1989; 397p. [Google Scholar]
- ISO 12966-2; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters-Part 2: Preparation of Methyl Esters of Fatty Acids. International Standards Organization (ISO): Geneva, Switzerland, 2017.
- ISO 12966-4; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters-Part 4: Determination by Capillary Gas Chromatography. International Standards Organization (ISO): Geneva, Switzerland, 2015.
- Estévez, A.; Treviño, L.; Kotzamanis, Y.; Karacosta, I.; Tort, L.; Gisbert, E. Effects of different levels of plant proteins on the ongrowing of meagre (Argyrosomus regius) juveniles at low temperatures. Aquac. Nutr. 2011, 17, 572–582. [Google Scholar] [CrossRef]
- Hardy, R.W.; Barrows, F.T. Diet Formulation and Manufacture. In Fish Nutrition; Halver, J.E., Hardy, R.W., Eds.; Academic Press: London, UK, 2002; pp. 505–600. [Google Scholar]
- Kokou, F.; Henry, M.; Alexis, M. Growth performance, feed utilization and non-specific immune response of gilthead sea bream (Sparus aurata L.) fed graded levels of a bioprocessed soybean meal. Aquaculture 2012, 364, 74–81. [Google Scholar] [CrossRef]
- Mongile, F.; Mandrioli, L.; Mazzoni, M.; Pirini, M.; Zaccaroni, A.; Sirri, R.; Parma, L.; Gatta, P.P.; Sarli, G.; Bonaldo, A. Dietary Inclusion of Mussel Meal Enhances Performance and Improves Feed and Protein Utilization in Common Sole (Solea solea, Linnaeus, 1758) Juveniles. J. Appl. Ichtyol. 2015, 31, 1077–1085. [Google Scholar] [CrossRef]
- Boujard, T.; Gélineau, A.; Covès, D.; Corraze, G.; Dutto, G.; Gasset, E.; Kaushik, S. Regulation of feed intake, growth, nutrient and energy utilisation in European sea bass (Dicentrarchus labrax) fed high fat diets. Aquaculture 2004, 231, 529–545. [Google Scholar] [CrossRef]
- Velazco-Vargas, J.; Tomás-Vidal, A.; Hamdan, M.; Moyano López, F.J.; Jover Cerda, M.; Martínez-Llorens, S. Influence of digestible protein levels on growth and feed utilization of juvenile meagre Argyrosomus regius. Aquac. Nutr. 2014, 20, 520–531. [Google Scholar] [CrossRef]
- Gil, M.M.; Palmer, M.; Hernández, M.D.; Grau, A.; Durán, J.; García, B.G.; Jover, M.; Pastor, E. Rearing diet may determine fish restocking success: A case study of hatchery-reared juvenile meagre, Argyrosomus regius. Sci. Mar. 2015, 79, 431–441. [Google Scholar] [CrossRef]
- Kounna, C.; Fountoulaki, E.; Miliou, H.; Chatzifotis, S. Water temperature effects on growth performance, proximate body and tissue composition, morphometric characteristics and gastrointestinal evacuation processes of juvenile meagre, Argyrosomus regius (Asso, 1801). Aquaculture 2021, 540, 736683. [Google Scholar] [CrossRef]
- Quéro, J.C.; Vayne, J.J. Le maigre, Argyrosomus regius (Asso 1801) (Pisces, Perciformes, Sciaenidae) du Golfe de Gascogne et des eaux plus septentrionales. Rev. Trav. Péches Marit. 1987, 49, 35–66. [Google Scholar]
- Mongile, F.; Bonaldo, A.; Fontanillas, R.; Mariani, L.; Badiani, A.; Bonvini, E.; Parma, L. Effects of Dietary Lipid Level on Growth and Feed Utilisation of Gilthead Seabream (Sparus aurata L.) Reared at Mediterranean Summer Temperature. Ital. J. Anim. Sci. 2014, 13, 2999. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Matulić, D.; Blažina, M.; Pritišanac, E.; Čolak, S.; Bavčević, L.; Barić, R.; Križanac, S.; Vitlov, B.; Šuran, J.; Strunjak Perović, I.; et al. Growth, Fatty Acid Profile and Malondialdehyde Concentration of Meagre Argyrosomus regius Fed Diets with Different Lipid Content. Appl. Sci. 2024, 14, 4842. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Soares, M.C.; Barata, M.; Couto, A.; Teixeira, B.; Ribeiro, L.; Pousão-Ferreira, P.; Mendes, R.; Saavedra, M. Effect of Tryptophan Dietary Content on Meagre, Argyrosomus regius, Juveniles Stress and Behavioral Response. Animals 2023, 13, 3762. [Google Scholar] [CrossRef]
- Monteiro, M.; Sousa, C.; Coutinho, F.; Castro, C.; Fontinha, F.; Guerreiro, I.; Pousão, P.; Matos, E.; Díaz-Rosales, P.; Oliva-Teles, A.; et al. Functional Feeds to TackleMeagre (Argyrosomus regius) Stress: Physiological Responses under Acute Stressful Handling Conditions. Mar. Drugs 2021, 19, 598. [Google Scholar] [CrossRef]
- Matias, A.C.; Quental-Ferreira, H.; Dias, J.; Saavedra, M.; Bandarra, N.M.; Araújo, R.L.; Gamboa, M.; Soares, F.; Pousão-Ferreira, P. Evaluation of Alternative Dietary Ingredients as a Sustainable and Ecological Solution for Meagre (Argyrosomus regius) Production in Earthen Ponds. Fishes 2024, 9, 517. [Google Scholar] [CrossRef]
| Diet S | Diet P | Diet MP | |
|---|---|---|---|
| Moisture | 72.6 ± 0.15 | 6.367 ± 0.10 | 15.01 ± 025 |
| Crude protein | 17.27 ± 0.14 | 46.97 ± 0.27 | 42.66 ± 0.39 |
| Crude lipid | 6.65 ± 0.21 | 16.05 ± 0.05 | 14.58 ± 0.18 |
| Carbohydrates | 0.21 ± 0.01 | 21.24 ± 1.02 | 18.74 ± 1.13 |
| Ash | 3.36 ± 0.06 | 9.27 ± 0.30 | 8.42 ± 0.13 |
| Diet S | Diet P | Diet MP | F | p | |
|---|---|---|---|---|---|
| Crude protein | 62.8 ± 0.90 a | 50.23 ± 0.29 b | 50.49 ± 0.25 b | 157.56 | 0.000006 |
| Crude lipid | 24.17 ± 1.11 a | 17.17 ± 0.16 b | 17.23 ± 0.21 b | 42.67 | 0.000284 |
| Carbohydrate | 0.76 ± 0.04 a | 22.68 ± 0.05 b | 22.36 ± 0.21 b | 4051.42 | 0.000000 |
| Ash | 12.23 ± 0.48 a | 9.90 ± 0.54 b | 9.90 ± 0.0.76 b | 10.05 | 0.012137 |
| GE (kJ/g feed) | 24.51 ± 0.26 a | 22.56 ± 0.11 b | 22.59 ± 0.03 b | 47.243 | 0.000212 |
| GP/GE (g/MJ) | 25.6 ± 0.6 a | 22.3 ± 0.2 b | 22.4 ± 0.1 b | 29.202 | 0.000809 |
| Group A | Group B | Group C | F | p | |
|---|---|---|---|---|---|
| Initial weight (g) | 106.30 ± 3.12 a | 108.25 ± 4.38 a | 107.14 ± 3.04 a | 0.07 | 0.929 |
| Final weight (g) | 346.13 ± 17.39 a | 194.44 ± 7.45 b | 188.93 ± 6.22 b | 67.8 | <1 × 10−5 |
| Init. lengths (cm) | 20.35 ± 0.207 a | 20.6 ± 0.27 a | 20.65 ± 0.19 a | 0.56 | 0.573 |
| Final length (cm) | 30.01 ± 0.49 a | 25.31 ± 0.33 b | 25.07 ± 0.28 b | 42.17 | <1 × 10−5 |
| Initial K | 1.18 ± 0.01 a | 1.17 ± 0.01 a | 1.17 ± 0.01 a | 1.2 | 0.309 |
| Final K | 1.22 ± 0.01 a | 1.14 ± 0.11 b | 1.16 ± 0.01 b | 9.41 | 0.0001 |
| Initial HSI | 2.83 ± 0.14 a | 2.81 ± 0.17 a | 2.93 ± 0.14 a | 0.16 | 0.849 |
| Final HSI | 1.85 ± 0.17 a | 2.03 ± 0.09 a | 2.17 ± 0.12 a | 1.55 | 0.230 |
| Initial VSI | 8.34 ± 0.51 a | 8.38 ± 0.33 a | 8.73 ± 0.31 a | 0.272 | 0.764 |
| Final VSI | 6.70 ± 0.31 a | 6.05 ± 0.20 b | 6.92 ± 0.14 a | 3.996 | 0.030 |
| TGC | 0.808 ± 0.003 a | 0.359 ± 0.003 b | 0.358 ± 0.021 b | 386.5 | 0.0002 |
| DFR (ratio) | 0.583 ± 0.11 a | 0.465 ± 0.07 b | 0.478 ± 0.08 b | 3.654 | 0.029 |
| TFI (g/fish) | 227.8 | 139.6 | 117.0 | ||
| GEI (kJ/fish) | 5584.3 | 3150.0 | 2642.1 |
| Diet S | Diet P | Group A | Group B | Group C | |
|---|---|---|---|---|---|
| C12:0 | 0.2 | 0.1 | 0.0 | 0.0 | 0.1 |
| C14:0 | 12.3 | 3.3 | 7.3 | 2.8 | 3.2 |
| C15:0 | 1.5 | 0.3 | 0.94 | 0.45 | 0.5 |
| C16:0 | 29.2 | 13.5 | 23.2 | 18.7 | 19.8 |
| C16:1n7t | 0.5 | 0.2 | 0.0 | 0.0 | 0.41 |
| C16:1n7c | 7.4 | 3.5 | 7.3 | 4.28 | 4.38 |
| C17:0 | 1.6 | 0.4 | 0.94 | 0.6 | 0.69 |
| C17:1 | 0.3 | 0.2 | 0.49 | 0.3 | 0.29 |
| C18:0 | 7.8 | 3.6 | 6.1 | 4.9 | 4.81 |
| C18:1n9c | 9.8 | 30.4 | 17.69 | 32.1 | 33.78 |
| C18:1n7 | 3.4 | 2.8 | 3.77 | 3.24 | 3.23 |
| C18:2n6t | 0.2 | 0.1 | 0.2 | 0.0 | 0.1 |
| C18:2n6c | 0.2 | 13.9 | 5.5 | 13.6 | 11.3 |
| C18:3n6 | 0.1 | 0.1 | 0.01 | 0.34 | 0.0 |
| C18:3n3 | 1.3 | 3.9 | 1.55 | 2.21 | 1.51 |
| C18:4n3 | 1.6 | 1.1 | 1.2 | 0.4 | 0.22 |
| C20:0 | 1.4 | 0.5 | 0.71 | 0.5 | 0.48 |
| C20:1n9 | 1.4 | 2.4 | 1.09 | 2.12 | 2.98 |
| C20:2n6 | 0.4 | 0.3 | 0.37 | 0.33 | 0.31 |
| C21:0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| C20:3n6 | 0 | 0.1 | 0.21 | 0.1 | 0.0 |
| C20:4n6 (ARA) | 0 | 0.6 | 0.8 | 0.99 | 0.28 |
| C20:3n3 | 0.1 | 0.1 | 0.18 | 0.31 | 0.0 |
| C20:4n3 | 0.6 | 0.5 | 0.64 | 0.49 | 0.23 |
| C20:5n3 (EPA) | 4.9 | 5.8 | 5.23 | 2.31 | 2.24 |
| C22:0 | 0.3 | 0.2 | 0.0 | 0.0 | 0.3 |
| C22:1n11 | 0.3 | 2 | 0.74 | 1.9 | 2.12 |
| C22:1n9 | 0.3 | 0.4 | 0.12 | 0.69 | 0.72 |
| C22:5n3 | 0.3 | 0.3 | 1.65 | 0.19 | 0.79 |
| C24:0 | 0.7 | 1.1 | 0.0 | 0.0 | 0.4 |
| C22:6n3 (DHA) | 8.9 | 7.8 | 10.6 | 4.42 | 3.99 |
| C24:1n9 | 1.3 | 0.4 | 1.2 | 0.82 | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bavčević, L.; Čolak, S.; Barić, R.; Petrović, S.; Klanjscek, T. Sardine-Based Diet Mitigates Growth Depression at Low Temperatures in Juvenile Meagre (Argyrosomus regius, Asso 1801). Fishes 2025, 10, 314. https://doi.org/10.3390/fishes10070314
Bavčević L, Čolak S, Barić R, Petrović S, Klanjscek T. Sardine-Based Diet Mitigates Growth Depression at Low Temperatures in Juvenile Meagre (Argyrosomus regius, Asso 1801). Fishes. 2025; 10(7):314. https://doi.org/10.3390/fishes10070314
Chicago/Turabian StyleBavčević, Lav, Slavica Čolak, Renata Barić, Siniša Petrović, and Tin Klanjscek. 2025. "Sardine-Based Diet Mitigates Growth Depression at Low Temperatures in Juvenile Meagre (Argyrosomus regius, Asso 1801)" Fishes 10, no. 7: 314. https://doi.org/10.3390/fishes10070314
APA StyleBavčević, L., Čolak, S., Barić, R., Petrović, S., & Klanjscek, T. (2025). Sardine-Based Diet Mitigates Growth Depression at Low Temperatures in Juvenile Meagre (Argyrosomus regius, Asso 1801). Fishes, 10(7), 314. https://doi.org/10.3390/fishes10070314






