Abstract
An 8-week feeding trial was conducted to assess the effects of different dietary lipid levels on the growth performance, feed utilization, and body composition of juvenile kelp grouper Epinephelus moara. Six diets with varying lipid levels of 2.82%, 5.30%, 7.83%, 11.76%, 14.19%, and 16.32% (designated as CL1 to CL6) were carefully formulated. A cohort of 324 juvenile fish (initial body weight of 5.87 ± 0.09 g fish−1) were randomly divided into six groups with three replicates in each group. The results showed that weight gain (WG) significantly improved as the dietary lipid level increased to 7.83%, followed by a decline with further increases. Fish fed the diet with 7.83% lipids also exhibited the highest feed efficiency (FE) and protein efficiency ratio. Although daily nitrogen intake and daily energy intake varied, no significant differences in nitrogen retention and energy retention were detected among the groups. With the increase in dietary lipid levels, daily lipid intake and daily lipid gain significantly increased, but lipid retention showed a consistent decline. Additionally, the viscerosomatic index and intraperitoneal fat ratio and the lipid content of the whole body, dorsal muscle, and liver increased significantly with increasing dietary lipid levels. The hepatic glycogen content inversely decreased with the elevation in dietary lipid levels. The lowest total cholesterol content in the serum was detected in the 16.32% dietary lipid treatment. According to the second-order polynomial regression analysis of WG and FE, a dietary lipid level of 6.56–9.31% was optimal for the growth performance and feed efficiency of juvenile kelp grouper.
Key Contribution:
This study contributes to investigating the effects of dietary lipid levels on growth performance, feed utilization, and body composition of juvenile kelp grouper. The results demonstrated that appropriate lipid levels could improve the growth and feed utilization of kelp grouper. Excessive dietary lipid intake can lead to the accumulation of lipids in the whole body, muscles, and visceral cavity. The research outcomes are expected to possess significant commercial potential for the development of formula feed tailored for kelp grouper.
1. Introduction
Dietary lipids are well known for providing essential fatty acids, phospholipids, and sterols [1]. They also facilitate the transport of fat-soluble vitamins and maintain cell membrane fluidity [2]. As critical nutrients and energy sources, lipids play a vital role in the growth, development, and reproduction of fish [3]. Generally, fish, particularly marine carnivorous species, preferentially utilize lipids rather than carbohydrates as their main energy source [4]. Compared to proteins, lipids are regarded as relatively cost-effective feed ingredients [3]. Numerous studies have reported that appropriate levels of dietary lipids can improve fish growth performance and enhance nutrient availability [2,5,6,7,8]. The supplementation of lipids to the diet also contributes to the effective utilization of dietary protein via the protein-sparing effect in fish and reduces nitrogen excretion [9,10,11,12]. However, an insufficient content of dietary lipids can lead to growth retardation in fish [8,13,14]. In contrast, excessive lipid intake not only leads to unbalanced nutrient absorption and utilization but also increases lipid accumulation, impairs liver function, and reduces carcass quality [7]. This ultimately has a negative impact on fish health and survival [8,15]. In conclusion, determining appropriate lipid requirements is crucial for developing balanced nutrition and environment-friendly feed for aquatic animals.
The kelp grouper Epinephelus moara is a carnivorous coral reef fish and a protogynous hermaphrodite species with mostly females in a group [16]. It is widely distributed across the Indo-northwestern Pacific Oceans, including all of the Chinese seas [17,18]. Noted for its rapid growth (with a maximum body length of 120 cm), strong adaptability, palatable taste, and significant economic and ecological value, E. moara is recognized as an important species for commercial mariculture and serves as a representative species for artificial release in China [16,17,19,20]. Additionally, E. moara has been used in research focused on germplasm preservation [20], genetic analyses [17,19], hybridization [21], and developmental biology [22]. Research on E. moara also encompasses sperm cryopreservation, embryo preservation, and cell culture techniques [16,23]. However, the current understanding of the nutritional requirements in E. moara remains limited. Su et al. [24] established its optimal dietary protein range at 54.61–56.22%, but its lipid requirements remain poorly characterized. A preliminary evaluation by Peng et al. [25] under suboptimal protein–lipid combinations (35–45% protein with 9–15% lipids) suggested that juvenile E. moara achieves acceptable growth at 45% protein and 12% lipids. However, the above research neither defined the optimal dietary lipid range nor elucidated the effects of varying lipid levels on growth performance and feed efficiency under optimal protein conditions, especially when fish oil serves as the sole lipid source. Establishing optimal lipid parameters is particularly crucial for this emerging aquaculture species, as lipids not only impact fish health, energy supply, protein utilization, nutrient provision (such as essential fatty acids), aquaculture costs, and environmental protection but also influence the sustainability of the aquaculture industry. Inadequate understanding of lipid requirements in E. moara poses significant challenges to its breeding practices, reproduction strategies, and artificial release initiatives, highlighting the urgent need for comprehensive nutritional research. Therefore, the objective of the present study aims to assess the effects of increasing dietary lipid levels from approximately 2.82% to 16.32% on the growth performance, feed utilization, and body composition of juvenile kelp grouper to determine optimal lipid inclusion levels in their diet.
2. Materials and Methods
2.1. Experimental Diets
Six experimental diets, detailed in Table 1, were formulated with various crude lipid levels of 2.82%, 5.30%, 7.83%, 11.76%, 14.19%, and 16.32% (denoted as CL1, CL2, CL3, CL4, CL5, and CL6). Fish oil was used as the only lipid source. White fish meal, casein, and corn starch served as sources of protein and carbohydrates, respectively. Prior to diet preparation, all solid ingredients were finely pulverized and passed through a 120 μm mesh. The powdered raw materials were then accurately weighed according to the formulation and thoroughly mixed in a mixer for 20 min. Subsequently, the weighed fish oil and an appropriate amount of double-distilled water were combined in the mixer. Each of the experimental diets was then individually processed into 1.5 mm granules using a twin-screw extruder (G-250; Machine factory of South China University of Technology, Guangzhou, China) and dried in a convection oven at 45 °C. Once dried, the granules were sealed in plastic bags and stored in a −20 °C refrigerator. The fatty acid compositions of the diets are presented in Table 2.

Table 1.
Formulation and composition of experimental diets (% as dry matter basis).

Table 2.
Fatty acid composition of the experimental diets (% total fatty acids).
2.2. Culture Experiment
Juvenile kelp grouper were procured from a commercial hatchery and subsequently transported to the nutrition laboratory of Zhejiang Ocean University (Zhoushan, China). Prior to the experiment, the fish were acclimated to laboratory conditions for a fortnight by being fed a 9.11% lipid diet [6]. At the start of the feeding trial, 324 juveniles (weighing 5.87 ± 0.09 g·fish−1) were randomly selected after a 24 h fasting period and evenly distributed into 18 cylindrical tanks (350 L each). Each tank housed 18 fish that were fed different diets twice daily for 8 weeks. During the feeding period, fish were hand-fed until apparent satiation at 09:00 and 17:00 under a natural photoperiod. The tanks were supplied with sand-filtered seawater at a flow rate of 1.0 L·min−1, and a roots blower was used to ensure that the dissolved oxygen exceeded 6 mg·L−1. The salinity, pH, and ammonia nitrogen were measured (twice daily) using a YSI Proplus instrument (YSI, Yellow Springs, OH, USA). Salinity and water temperature were maintained at 25.1 ± 0.9 g·L−1 and 25.5 ± 1.3 °C, respectively. The pH value ranged from 7.8 to 8.0; ammonia nitrogen levels were monitored below 0.05 mg·L−1.
2.3. Sample Collection and Chemical Analysis
Before the trial, 20 fish with an initial body weight of 5.87 g·fish−1 were euthanized for sampling, then placed in pre-marked sealed bags and stored in a −80 °C refrigerator for subsequent body composition and energy determination. Following the feeding trial, all surviving fish were fasted for 24 h and then anesthetized with 150 mg·L−1 of MS-222. The number and weight of the fish in each tank were recorded. Subsequently, 6 fish were randomly sampled from each tank to measure their body weight and length. Blood samples were collected from the caudal vein using a hypodermic syringe and subsequently centrifuged at a rate of 4000 g for 10 min at 4 °C (centrifuge model CT15RE, Hitachi, Tokyo, Japan). The serum samples were immediately removed and stored at −80 °C for later measurement of biochemical parameters. Additionally, the viscera, liver, intraperitoneal fat, and dorsal muscle were excised, weighed, and stored at −80 °C for the calculation of morphological parameters and analysis of tissue proximate composition. Three fish from each tank were randomly selected and stored at −80 °C for whole-body composition analysis.
Chemical composition analyses of the experimental diets, as well as the whole body, dorsal muscle, and liver, were conducted according to AOAC [26]. Moisture was assessed by freeze-drying at −110 °C using a lyophilizer (LL1500, Thermo Fisher Scientific, Waltham, MA, USA). Crude protein (N × 6.25) was determined using an automatic Kjeldahl system (K355/K437 Buchi, Flawil, Switzerland) based on the Kieldahl method. Ash content was evaluated by incineration at 550 °C for 12 h in a muffle furnace. A Soxhlet device (E816, Buchi, Flawil, Switzerland) was utilized to determine crude lipids via petroleum ether extraction. Gross energy content was analyzed using an adiabatic bomb calorimeter (HWR-15E, Shangli, Shanghai, China). The fatty acid profile of the samples was determined by gas chromatography (GC7890B, Agilent Technologies Inc., Palo Alto, CA, USA).
Liver glycogen content was measured according to the method outlined by Dalrymple and Hamm [27]. Serum glucose concentration was determined by the oxidase–peroxidase method [28], while serum triacylglycerol (TG) and total cholesterol (CHO) were measured via a colorimetric method [29]. High-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) concentrations were measured based on methods described by Gordon et al. [30] and Okada et al. [31], respectively. All parameters were assessed using commercial kits (Nanjing Jianchen Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocols, and absorbance was measured with a microplate reader (iMark, Bio-Rad, Hercules, CA, USA).
2.4. Calculation and Statistical Analysis
Weight gain (WG, %) = 100 × (final body weight − initial body weight)/initial body weight;
Specific growth rate (SGR, %·day−1) = 100 × (ln (final body weight) − ln (initial body weight))/days;
Feed conversion ratio (FCR) = dry feed consumed/wet weight gain;
Feed efficiency (FE, %) = 100× (wet weight gain/dry feed intake);
Protein efficiency ratio (PER) = wet weight gain/protein intake;
Daily feed intake (DFI, g·100 g·fish−1·day−1) = 100 × feed offered/average total weight/days;
Intraperitoneal fat ratio (IPF, %) = 100 × (intraperitoneal fat weight/whole body weight);
Hepatosomatic index (HSI, %) = 100 × (hepatosomatic weight/whole body weight);
Viscerosomatic index (VSI, %) = 100 × (viscera weight/whole body weight)
Condition factor (CF) = 100 × (live weight/length3);
Average body weight (ABW) = (initial body weight + final body weight)/2;
Daily nitrogen intake (DNI, g·kg−1·day−1) = feed intake nitrogen/ABW × days;
Daily nitrogen gain (DNG, g·k−1·day−1) = (final body weight × final body nitrogen − initial body weight × initial body nitrogen)/ABW × days;
Nitrogen retention (NR, %) = 100 × DNG/DNI;
Daily lipid intake (DLI, g·kg−1·day−1) = feed intake lipid/ABW × days;
Daily lipid gain (DLG, g·k−1·day−1) = (final body weight × final body lipid − initial body weight × initial body lipid)/ABW × days;
Lipid retention (LR, %) = 100 × DLG/DLI;
Daily energy intake (DEI, 102 kJ·kg−1·day−1) = feed intake energy/ABW × days;
Daily energy gain (DEG, 102 kJ·kg−1·day−1) = (final body weight × final body energy − initial body weight × initial body energy)/ABW × days
Energy retention (ER, %) = 100 × DEG/DEI.
Statistical analysis was conducted using SPSS 24.0 (IBM, Chicago, IL, USA). After assessing homogeneity and normality, all data were subjected to one-way analysis of variance (ANOVA). Duncan’s multiple range test was employed to evaluate the significance of differences among replicates. A significance level of 5% (p < 0.05) was established. Data were presented as the mean ± SD.
3. Results
3.1. Growth Performance and Feed Utilization
Table 3 presents the growth performance and feed utilization of the juvenile kelp grouper fed different dietary lipid levels. During the feeding trial, all experimental diets were properly accepted by the juvenile kelp grouper. No mortalities were ascribed to the dietary lipid treatments. Before reaching 7.83% lipids, the values of WG and SGR exhibited a significant increase with the increase in dietary lipid levels, after which both metrics declined with further increases in dietary lipids (p < 0.05). In addition, although there were no statistically significant differences, FE and PER demonstrated similar trends to those observed in WG. The highest values of FE (103.42) and PER (1.96) were recorded in the CL3 diet (7.83% lipid). Regression analysis based on a second-order polynomial model indicated that the diets containing 9.31% (Figure 1) and 6.56% (Figure 2) lipids yielded the maximum weight gain % and optimal feed efficiency, respectively, in the juvenile kelp grouper. While no significant difference in DFI was noted, a relatively high DFI was observed in the high-lipid-level group.

Table 3.
Growth performance and feed utilization of juvenile kelp grouper fed diets containing different lipid levels.
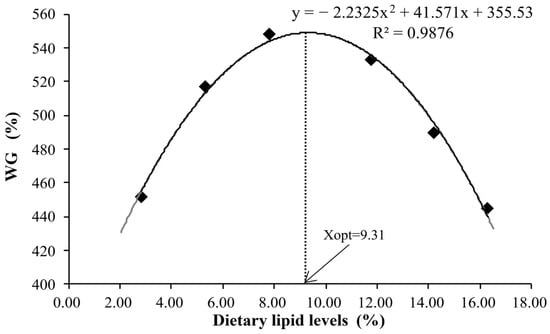
Figure 1.
Relationship of the weight gain (WG) with dietary lipid levels of juvenile kelp grouper.
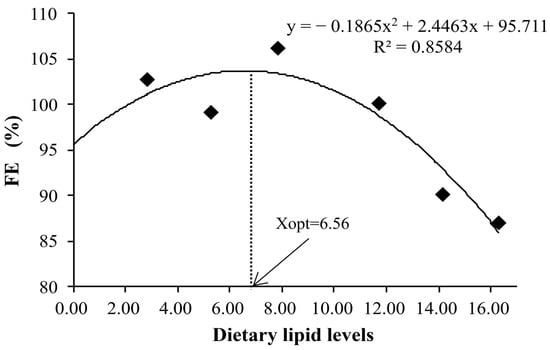
Figure 2.
Relationship of the feed efficiency (FE) with dietary lipid levels of juvenile kelp grouper.
3.2. Morphological Parameters
The morphometric parameters of the juvenile kelp grouper are shown in Table 4. The values VSI and IPF increased significantly with the increase in dietary lipid levels (p < 0.05). Fish fed the CL5 diet exhibited a significantly higher HSI compared to those fed the CL1, CL2, CL4, and CL6 diets (p < 0.05). Juvenile kelp grouper receiving a diet with 14.19% lipids demonstrated a significantly higher CF than those fed diets with 2.82% and 5.30% lipids (p < 0.05).

Table 4.
Morphometrical parameters of juvenile kelp grouper fed diets containing different lipid levels.
3.3. Retention and Deposition of Energy, Nitrogen, and Lipids
As shown in Table 5, DNI, DEI, and DEG significantly increased with higher lipid levels (p < 0.05). Fish fed the CL2, CL3, and CL4 diets exhibited significantly higher DNG compared to those fed the CL1 diet (p < 0.05). The values of NR and ER initially increased and then decreased as dietary lipid levels rose, with no significant differences among the groups (p > 0.05). The highest NR value (34.61) was recorded in fish fed a diet containing 7.83% lipids. An increase in dietary lipid levels also led to significant enhancements in DLI and DLG, whereas LR values decreased significantly (p < 0.05).

Table 5.
Nitrogen, energy, and lipid utilization of juvenile kelp grouper fed diets containing different lipid levels.
3.4. Whole Body and Tissue Composition
The moisture content of both the whole body and liver exhibited significant reductions as dietary lipid levels increased (p < 0.05; Table 6). Concurrently, an elevation in dietary lipid levels led to a significant increase in the crude lipid content within the whole body, dorsal muscle, and liver (p < 0.05). Conversely, the hepatic glycogen content significantly decreased with the escalation in dietary lipid levels (p < 0.05). Fish fed the CL3 diet obtained significantly lower liver protein contents than those fed the other treatments (p < 0.05). Fish fed the CL1 and CL2 diets had significantly higher whole body ash contents than those fed the CL4 and CL6 diets (p < 0.05).

Table 6.
Body and tissue proximate composition of juvenile kelp grouper fed diets containing different lipid levels.
3.5. Hematological Parameters
As depicted in Table 7, the serum glucose concentrations demonstrated a decreasing trend with the increase in dietary lipid levels (p > 0.05). Conversely, the serum T-CHO concentrations rose significantly with the rise in dietary lipid levels (p < 0.05). No statistically significant differences were observed in the serum TG contents across the various treatments (p > 0.05). Notably, the concentrations of HDL-C and LDL-C in the CL6 group were significantly higher than those in the other groups (p < 0.05).

Table 7.
Hematological parameters of juvenile kelp grouper fed diets containing different lipid levels.
4. Discussion
Following an 8-week culture period, the different dietary lipid levels significantly affected the growth performance of the juvenile kelp grouper. The weight gain (WG) showed a general increasing trend with the increase in the dietary lipid level up to 7.83% (CL3) and then decreased with the further increase in the dietary lipid level. Similar responses were documented in other carnivorous fish, such as meagre Argyrosomus regius [32], giant croaker Nibea japonica [7], hybrid pufferfish Takifugu obscurus × T. rubripes [33], lumpfish Cyclopterus lumpus [34], and yellow catfish Pelteobagrus fulvidraco [12]. In the present study, the change in the feed efficiency (FE) was consistent with the response of the growth performance. Specially, fish fed the CL3 diet with 7.83% lipids had the highest FE value (103.42%). Based on the WG and FE findings, a dietary lipid level ranging from 6.56% to 9.31% can enable juvenile kelp grouper to achieve good growth performance and efficient feed utilization. This level is lower than the lipid requirements reported in other carnivorous fish, such as 8.50% for spinefoot rabbitfish Siganus rivulatus [35], 9.00% for N. japonica [36], 8.22% for N. japonica 8.22% [7], and 8.50% for tench Tinca tinca L. [14], as well as some members of the Epinephelinae family species, i.e., 9.00% for malabar grouper E. malabaricus [37] and 9.11% for red spotted grouper E. akaara [6]. Nevertheless, several previous studies showed relatively higher lipid requirements in some species of the Epinephelinae family. For instance, a diet containing 150 to 154 g·kg−1 lipids (from fish oil) and 470 g·kg−1 crude protein was optimal for E. bruneus (initial weight of 6.38 g·fish−1) [38]. Furthermore, there are various reports about lipid requirements even for the same species. For example, Li et al. [39] reported that a diet with 15.99% lipids (anchovy oil) and 57% crude protein was ideal for orange spotted grouper E. coioides (initial weight 71 mg·fish−1). However, Luo et al. [40] recommend that a dietary lipid requirement of 10% (derived from fish oil) was optimal for maximizing the growth of 10 to 25 g E. coioides. In addition to species variations, these differences could be ascribed to the deviations in feed formulation, lipid sources, or fish sizes, etc.
In general, fish adapt their feed intake to fulfill their energy requirements, a behavior documented in various studies [4,41]. However, when there is a deficiency of an essential nutrient within their diet, fish compensate by increasing their feed consumption to meet their nutritional demands [40,42]. Some researchers suggest that feed intake appears to prefer to regulate protein intake rather than energy intake [5,6,32]. In this study, DFI decreased slightly with the increase in dietary lipid levels before the kelp grouper obtained 7.83% lipids required for maximum growth. Similar phenomena were observed in studies of N. japonica [7], largemouth bass Micropterus salmoides [4], and spotted knifejaw Oplegnathus punctatus [8]. On the other hand, DFI increased obviously with further increases in dietary lipid levels (CL3–CL6), along with daily lipid intake (DLI) and daily nitrogen intake (DNI), and the corresponding daily energy intake (DEI) also increased significantly. Notably, the highest DFI value was recorded in the group with the highest dietary lipid level. Studies on European sea bass Dicentrarchus labrax [43] and Senegalese sole Solea senegalensis [44] showed that high-lipid diets also resulted in increased feed intake, possibly indicative of a hyperphagic phenomenon akin to that observed in humans [45,46]. Interestingly, while DNI increased with dietary lipid enhancement, nitrogen retention (NR) first increased and then decreased, leading to no significant differences in daily nitrogen gain (DNG) among the treatments, except for the CL1 diet. Conversely, daily lipid gain (DLG) significantly increased, while lipid retention (LR) decreased with increasing dietary lipid levels, with LR exceeding 100% at lipid levels below 7.83%. These observations suggest that the increase in feed intake among fish fed a low-lipid diet is insufficient to meet their lipid requirements, and the fish may need to convert a considerable amount of dietary carbohydrates into lipids. Consistent with previous reports on E. coioides [40] and N. japonica [7], LR began to decline only after these species met the dietary lipid levels needed for maximum growth. Furthermore, at a high lipid level, the LR value of the kelp grouper ranged from 62.47 to 73.9%, exceeding those of N. japonica (31.89–52.13%) [7] and O. punctatus (38.31–55.14%) [8].
Substantial studies have demonstrated that insufficient dietary lipids can lead to inferior growth and even death of cultured fish [3,6,8,47]. Consistent with these findings, the kelp grouper fed the diet with the lowest lipid content (2.82%) exhibited significantly poorer growth performance compared to those fed the diet containing 7.83% lipids. n-3 Long-chain polyunsaturated fatty acids (n-3 LC-PUFAs), primarily including EPA and DHA, are essential for the survival and growth of marine fish [8,15,33,48]. There is evidence that marine fish can exhibit excellent growth performance when the n-3 LC-PUFA content exceeds 0.9% in their diets [3,48,49,50]. In this trial, the CL1 diet (2.82% lipids) contained only approximately 0.67% n-3 LC-PUFAs. In a sense, an inadequate dietary n-3 LC-PUFAs in the low-lipid diet might be one of the reasons for the poor growth performance of this group. A similar result was also observed in O. punctatus [8]. Furthermore, the diets in this study were formulated with varying levels of corn starch (ranging from 0% to 31.75%) and cellulose (0.24–17.16%) to balance the energy and lipid contents. The results showed that the ER of the fish fed the CL1 diet with the highest level of corn starch (31.75%) and the lowest lipid content was obviously lower compared to that of the fish fed the CL3 diet. According to NRC [3], lipids are more efficiently utilized than carbohydrates in fish, particularly carnivorous fish that prefer to use lipids as their main energy source. Wilson [51] suggests that the inclusion of digestible carbohydrates is generally considered to be below 20% in marine and carnivorous fish diets. Therefore, the excessive corn starch content in the CL1 diet might be another reason for the poor growth performance of the juvenile kelp grouper. Similar results were also reported in N. japonica [7], E. akaara [1], and triangular bream megalobrama terminalis [42].
In this study, the DFI of the group with the highest dietary lipid content was clearly higher than that of the other treatment groups. Correspondingly, the fish provided with the diet containing the highest level of dietary lipids demonstrated significantly increased DNI, DLI, and DEI when contrasted with those receiving the other diets. However, the PER, NR, and WG of the fish fed the diet with the highest lipid level (CL6) were obviously lower compared to those fed the CL3 diet. Most studies attributed the reduction in feed intake caused by high lipid levels to excessive dietary energy intake, which limits the fish’s ability to digest and absorb a large amount of lipids, leading to growth retardation [4,7,52]. Therefore, high lipid contents interfere with digestion and absorption and affect the metabolic response of other nutrients, resulting in the reduction in NR, LR, and ER in the CL6 diet. Furthermore, Sargent et al. [48] suggested that the growth reduction may be due to inhibition of de novo fatty acid synthesis and reduced ability to digest and absorb fatty acids under excessive dietary lipid levels. Excessive dietary lipids can lead to increased lipid deposition in various fish tissues, which will further affect product quality, storage stability, and, ultimately, commercial value [2,7]. In this study, both VSI and IPF showed a significant increasing trend with the increase in dietary lipid levels and DFI. Parallel observations have been reported in N. japonica [7], marble goby Oxyeleotris marmorata [53], and M. salmoides [4]. These results suggest that an excessive lipid content in the diet leads to lipid deposition in the visceral cavity, resulting in a lower commercial value of the product [2,52]. Moreover, with the increase in DLG, the lipid content in the whole body, dorsal muscle, and liver showed a significant increase, with the highest value obtained in the fish fed the diet containing 16.32% lipids (CL6). These observations suggest that a diet rich in lipids causes excessive body fat deposition, a phenomenon that is undesirable in the context of edible fish. Similar responses were observed in O. marmorata [53] and turbot Psetta maxima [54]. In addition, there is a negative correlation between the moisture and lipid contents of the whole body, dorsal muscle, and liver. This relationship was also found in other fish species, such as grass carp Ctenopharyngodon idella [5], E. coioides [40], and silver barb Puntius gonionotous [55].
Several studies have demonstrated that an excessive intake of dietary carbohydrates elevates serum glucose levels and promotes glycogen accumulation in the liver of various fish species [42,47]. Similarly, the serum glucose content showed an upward trend with the increase in the dietary starch content in this study. The fish fed the diet with the highest corn starch and lowest dietary lipid levels had the highest concentrations of serum glucose and liver glycogen. A similar result was also reported in E. akaara [47]. Interestingly, the fish fed the CL1 diet achieved the highest LR value, which may indicate that the high carbohydrate content in the diet was converted into lipids. On the other hand, the fish fed the diet with a lipid level of 7.83% had the highest values of FE, PER, NR, and WG among all the groups. These results suggest that an appropriate dietary lipid level can effectively promote the retention of dietary protein, thereby improving PER and the growth of juvenile kelp grouper. Similar results were reported in O. punctatus [8] and A. regius [32].
Cholesterol has been well established as a precursor for numerous biologically active compounds essential for the growth and health of aquaculture species [5,8,47]. Low serum cholesterol levels have usually been associated with stunted growth in fish [56,57]. It was reported that cholesterol can be transported from peripheral tissues to the liver by high-density lipoprotein (HDL), while low-density lipoprotein (LDL) plays a role in transporting cholesterol from the liver to body tissues [11,58]. In this study, the serum T-CHO concentration was the lowest in the group with the lowest dietary lipids, which coincided with the fish experiencing growth retardation. Similar results were observed in O. punctatus [8]. As dietary lipid levels increased, serum T-CHO concentrations significantly rose, peaking in the group fed the CL6 diet. Concurrently, serum HDL-C and LDL-C levels increased significantly with the increase in dietary lipid levels. It was also found that the plasma T-CHO contents increased with the increase in dietary lipid levels in C. Idella [5] and N. albiflora [47], indicating more active endogenous lipid transport in response to elevated dietary lipid levels.
5. Conclusions
Under the experimental conditions of this study, an 8-week feeding trial revealed that dietary lipid levels significantly influenced the growth performance and feed utilization of juvenile kelp grouper. The fish fed a diet containing 7.83% lipids exhibited the highest values of FE, PER, NR, and WG among all groups, indicating that an appropriate lipid content enhances protein utilization and promotes growth in juvenile kelp grouper. However, the diet with a lipid level of 2.82% was insufficient to meet the lipid and n-3 LC-PUFA requirements of this species, leading to the conversion of excess dietary carbohydrates into lipids. On the other hand, a high lipid content in the diet made the endogenous lipid transportation active, resulting in the accumulation of lipids in the whole body, muscles, and visceral cavities, thus reducing the commercial value of the edible fish. Regression analysis based on a second-order polynomial model indicated that diets containing 9.31% and 6.56% lipids yielded the maximum weight gain % and optimal feed efficiency, respectively, in the juvenile kelp grouper. It is recommended that the appropriate lipid addition level in the diet of kelp grouper is 6.56–9.31%. These findings provide a strong scientific basis for the development of the kelp grouper farming industry.
Author Contributions
Conceptualization: F.D., Y.S., Q.C., T.H. and J.W.; data curation, T.L., F.D., H.S., P.Z. and Q.C.; formal analysis, T.L., H.S. and P.Z.; funding acquisition, T.H. and J.W.; investigation, T.L., F.D., H.S., P.Z. and Q.C.; methodology, T.L., F.D., Y.S., H.S., P.Z., Q.C., T.H. and J.W.; project administration, Y.S., T.H. and J.W.; supervision, Y.S., T.H. and J.W.; writing—original draft, T.L. and H.S.; writing—review and editing, T.L., Y.S. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ten-thousand Talents Plan of Zhejiang Province (No. 2022R52021), Major scientific and technological research projects of Zhoushan (2023C13017), the Zhejiang Province “three agricultural six-party” science and technology collaboration program (2024SNJF059), and The Key Research and Development Program of Zhejiang Province (2021C04016).
Institutional Review Board Statement
All experiments involving fish in this study were meticulously conducted in accordance with the Management Rule of Laboratory Animals (Chinese Order No. 676 of the State Council, revised on 1 March 2017). These experiments were executed under the vigilant supervision and inspection of the Experimental Animal Welfare Ethics Committee of Zhejiang Ocean University (under permit No. 2025052; approval date: 14 April 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the first and corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, L.G.; Lu, Q.; Luo, S.Y.; Zhan, W.; Chen, R.Y.; Lou, B.; Xu, D.D. Effect of dietary lipid on growth performance, body composition, plasma biochemical parameters and liver fatty acids content of juvenile yellow drum Nibea albiflora. Aquac. Rep. 2016, 4, 10–16. [Google Scholar] [CrossRef][Green Version]
- Lee, C.H.; Kim, H.S.; Lee, K.W.; Han, G.S.; Byun, S.G.; Lim, H.J.; Lee, D.Y.; Choi, J. Effects of dietary lipid level on growth performance, feed utilization, fatty composition and antioxidant parameters of juvenile walleye pollock, Gadus chalcogrammus. Aquac. Rep. 2021, 19, 100631. [Google Scholar] [CrossRef]
- NRC. Lipids. In Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011; pp. 102–134. [Google Scholar]
- Guo, J.L.; Zhou, Y.L.; Zhao, H.; Chen, W.Y.; Chen, Y.J.; Lin, S.M. Effect of dietary lipid level on growth, lipid metabolism and oxidative status of largemouth bass, Micropterus salmoides. Aquaculture 2019, 506, 399–400. [Google Scholar] [CrossRef]
- Du, Z.Y.; Liu, Y.J.; Tian, L.X.; Wang, J.T.; Wang, Y.; Liang, G.Y. Effect of dietary lipid level on growth, feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2005, 11, 139–146. [Google Scholar] [CrossRef]
- Jiang, Y.D.; Wang, J.T.; Han, T.; Li, X.Y.; Hu, S.X. Effect of dietary lipid level on growth performance, feed utilization and body composition by juvenile red spotted grouper (Epinephelus akaara). Aquac. Int. 2015, 23, 99–110. [Google Scholar] [CrossRef]
- Han, T.; Li, X.Y.; Wang, J.T.; Hu, S.X.; Jiang, Y.D.; Zhong, X.D. Effect of dietary lipid level on growth, feed utilization and body composition of juvenile giant croaker Nibea japonica. Aquaculture 2014, 434, 145–150. [Google Scholar] [CrossRef]
- Wang, J.T.; Liu, T.; Zheng, P.Q.; Xu, H.Y.; Su, H.; Han, T.; Yang, Y.X. Effect of dietary lipid levels on growth performance, body composition, and feed utilization of juvenile spotted knifejaw Oplegnathus punctatus. Aquac. Rep. 2021, 21, 100797. [Google Scholar] [CrossRef]
- Yigit, M.; Yardim, O.; Koshio, S. The protein sparing effects of high lipid levels in diets for rainbow trout (Oncorhynchus mykiss, W. 1792) with special reference to reduction of total nitrogen excretion. Isr. J. Aquac. Bamidgeh 2002, 54, 79–88. [Google Scholar] [CrossRef]
- Perez-Velazquez, M.; Minjarez, C.; González-Félix, M. Effect of dietary lipid level on growth performance, feed utilization and body composition of totoaba, Totoaba macdonaldi (Gilbert, 1890). Aquac. Res. 2016, 48, 2607–2617. [Google Scholar] [CrossRef]
- Wang, J.T.; Han, T.; Li, X.Y.; Yang, Y.X.; Yang, M.; Hu, S.X.; Jiang, Y.D.; Harpaz, S. Effects of dietary protein and lipid levels with different protein-to-energy ratios on growth performance, feed utilization and body composition of juvenile red-spoted grouper, Epinephelus akaara. Aquac. Nutr. 2017, 23, 994–1002. [Google Scholar] [CrossRef]
- Fei, S.Z.; Chen, Z.; Duan, Y.H.; Liu, H.K.; Jin, J.Y.; Yang, Y.X.; Han, D. Growth, reproduction, fatty acid profiles and offspring performance of broodstock yellow catfish Pelteobagrus fulvidraco fed diets with different lipid levels. Aquaculture 2024, 580, 740273. [Google Scholar] [CrossRef]
- Shiau, S.Y.; Lan, C.W. Optimum dietary protein level and protein to energy ratio for growth of grouper (Epinephelus malabaricus). Aquaculture 1996, 145, 259–266. [Google Scholar] [CrossRef]
- Royuela, M.S.; Casado, M.; Celada, J.D.; Carral, J.M.; Rodríguez, G.A. Effect of dietary lipid level on survival, growth performance and body composition of juvenile tench (Tinca tinca L.) fed practical diets. Aquaculture 2015, 439, 14–19. [Google Scholar] [CrossRef]
- López, L.; Luisa Torres, A.; Durazo, E.; Drawbridge, M.; Bureau, D. Effects of lipid on growth and feed utilization of white seabass (Atractoscion nobilis) fingerlings. Aquaculture 2006, 253, 557–563. [Google Scholar] [CrossRef]
- Tian, Y.S.; Chen, Z.F.; Tang, J.; Duan, H.M.; Zhai, J.M.; Li, B.; Ma, W.H.; Liu, J.C.; Hou, Y.X.; Sun, Z.X. Effects of cryopreservation at various temperatures on the survival of kelp grouper (Epinephelus moara) embryos from fertilization with cryopreserved sperm. Cryobiology 2017, 75, 37–44. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, H.Y.; Xu, H.; Lin, H.R.; Chen, S.L. A Chromosomal-scale Reference Genome of the Kelp Grouper Epinephelus moara. Mar. Biotechnol. 2020, 19, 1322–1332. [Google Scholar] [CrossRef]
- Chen, Z.F.; Tian, Y.S.; Mai, W.H.; Zhai, J.M. Gene expression changes in response to low temperatures in embryos of the kelp grouper, Epinephelus moara. Cryobiol. Int. J. Low Temp. Biol. Med. 2020, 97, 159–167. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.L.; Ding, S.X.; Liu, Z.Q. Epinephelus moara: A valid species of the family Epinephelidae (Pisces: Perciformes). J. Fish Biol. 2013, 82, 1684–1699. [Google Scholar] [CrossRef]
- Guo, M.; Wang, S.; Su, Y.; Zhou, Y.; Wang, J. Molecular cytogenetic analyses of Epinephelus bruneus and Epinephelus moara (Perciformes, Epinephelidae). Peerj 2014, 2, 412. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, Q.Y.; Chen, C.; Zhai, J.M.; Wu, L.M.; Ma, W.H.; Song, Z.X.; Sun, F.F. Embryonic and morphological development in larva, juvenile, and young stages of F1 by Epinephelus moara ♀ × E. Septemfasciatus ♂. China J. Fish. Sci. 2012, 19, 821–832. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, T.T.; Chao, C.; Shi, Z.H.; Li, Y.L.; Yu, H.H.; Ren, B.H.; Xu, W.T. Effects of temperature on the embryonic development and larval activity of Epinephelus moara. Prog. Fish. Sci. 2016, 37, 28–33. (In Chinese) [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, S.L.; Sha, Z.X.; Wu, Y.H.; Yu, Y.; Wang, N.; Xiu, W.S. Establishment characterization of a new cell line from heart of Kelp Bass (Epinephelus moara). China J. Agric. Biotechol. 2015, 23, 1394–1400. (In Chinese) [Google Scholar] [CrossRef]
- Su, H.; Han, T.; Li, X.Y.; Zheng, P.Q.; Wang, Y.B.; Wang, J.T. Dietary protein requirement of juvenile kelp grouper (Epinephelus moara). Aquac. Res. 2019, 50, 3783–3792. [Google Scholar] [CrossRef]
- Peng, S.M.; Zhang, C.J.; Gao, Q.X.; Shi, Z.H.; Chen, C.; Wang, J.G. Growth performance and metabolic response of juvenile grouper Epinephelus moara (Temminck & Schlegel, 1842) fed low dietary protein and high lipid levels. J. Appl. Ichthyol. 2017, 33, 790–796. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC International: Arlington, VA, USA, 1995; pp. 1141–1154. [Google Scholar]
- Dalrymple, R.H.; Hamm, R. Amethod for the extraction of glycogen and metabolites from a single muscle sample. Int. J. Food Sci. Technol. 1973, 8, 439–444. [Google Scholar] [CrossRef]
- Barham, D.; Trinder, P. An improved color reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef]
- Coz-Rakovac, R.; Hacmanjek, M.; Popovic, N.T.; Lipej, Z.; Sostaric, B. Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the northern Adriatic Sea. Vet. Res. Commun. 2005, 29, 677–687. [Google Scholar] [CrossRef]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease: The Framingham Study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Okada, M.; Matsui, H.; Ito, Y.; Fujiwara, A.; Inano, K. Low-density lipoprotein cholesterol can be chemically measured: A new superior method. J. Lab. Clin. Med. 1998, 132, 195–201. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Panagiotidou, M.; Papaioannou, N.; Pavlidis, M.; Nengas, I.; Mylonas, C.C. Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 2010, 307, 65–70. [Google Scholar] [CrossRef]
- Yoo, G.Y.; Park, I.S.; Lee, S. Effects of graded dietary lipid levels on growth performance fatty acid profile, and hematological characteristics of hybrid pufferfish (Takifugu obscurus × T. rubripes) juveniles. Aquac. Rep. 2022, 24, 101120. [Google Scholar] [CrossRef]
- Berge, G.M.; Zhou, W.W.; Johansen, L.H.; Chikwati, E.; Kortner, T.M.; Lein, I.; Krogdahl, Å. Effects of dietary lipid level on growth, digestive physiology and disease resistance in lumpfish (Cyclopterus lumpus). Aquaculture 2023, 566, 739209. [Google Scholar] [CrossRef]
- Ghanawi, J.; Roy, L.; Davis, D.; Saoud, I. Effects of dietary lipid levels on growth performance of marbled spinefoot rabbitfish Siganus rivulatus. Aquaculture 2011, 310, 395–400. [Google Scholar] [CrossRef]
- Chai, X.J.; Ji, W.X.; Han, H.; Dai, Y.X.; Wang, Y. Growth, feed utilization, body composition and swimming performance of giant croaker, Nibea japonica. Temmick and Schlegel, fed at different dietary protein and lipid levels. Aquac. Nutr. 2013, 19, 928–935. [Google Scholar] [CrossRef]
- Lin, Y.H.; Shiau, S.Y. Dietary lipid requirement of grouper, Epinephelus malabaricus, and effects on immune responses. Aquaculture 2003, 225, 243–250. [Google Scholar] [CrossRef]
- Yoshii, K.; Takakuwa, F.; Nguyen, H.P.; Masumoto, T.; Fukada, H. Effect of dietary lipid level on growth performance and feed utilization of juvenile kelp grouper Epinephelus bruneus. Fish. Sci. 2010, 76, 139–145. [Google Scholar] [CrossRef]
- Li, S.; Mai, K.; Xu, W.; Yuan, Y.; Zhang, Y.; Zhou, H.; Ai, Q. Effects of dietary lipid level on growth, fatty acid composition, digestive enzymes and expression of some lipid metabolism related genes of orange-spotted grouper larvae (Epinephelus coioides H.). Aquac. Res. 2016, 47, 2481–2495. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.J.; Mai, K.S.; Tian, L.X.; Liu, D.H.; Tan, X.Y.; Lin, H.Z. Effect of dietary lipid level on growth performance, feed utilization and body composition of grouper Epinephelus coioides juveniles fed isonitrogenous diets in floating netcages. Aquac. Int. 2005, 13, 257–269. [Google Scholar] [CrossRef]
- Coutinho, F.; Peres, H.; Guerreiro, I.; Pousão-Ferreira, P.; Oliva-Teles, A. Dietary protein requirement of sharpsnout sea bream (Diplodus puntazzo, cetti 1777) juveniles. Aquaculture 2012, 356–357, 391–397. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.T.; Han, T.; Yang, Y.X.; Li, X.Y.; Tian, H.L.; Zheng, P.Q. Dietary protein requirement of juvenile triangular bream Megalobrama terminalis (richardson,1846). J. Appl. Ichthyol. 2017, 33, 971–977. [Google Scholar] [CrossRef]
- Peres, H.; Oliva-Teles, A. Effect of dietary lipid level on growth performance and feed utilization by European sea bass juveniles (Dicentrarchus labrax). Aquaculture 1999, 179, 325–334. [Google Scholar] [CrossRef]
- Borges, P.; Oliveira, B.; Casal, S.; Dias, J.; Conceição, L.; Valente, L.M.P. Dietary lipid level affects growth performance and nutrient utilisation of senegalese sole (Solea senegalensis) juveniles. Br. J. Nutr. 2009, 102, 1007. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Kurth, C.; Holden-Wiltse, J.; Saari, J. Food preferences in human obesity: Carbohydrates versus fats. Appetite 1992, 18, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Pérez, H.E.; Chambers, A.P.; Sandoval, D.A.; Stefater, M.A.; Woods, S.C.; Benoit, S.C.; Seeley, R.J. The effect of vertical sleeve gastrectomy on food choice in rats. Int. J. Obes. 2013, 37, 288–295. [Google Scholar] [CrossRef]
- Wang, J.T.; Jiang, Y.D.; Li, X.Y.; Han, T.; Yang, Y.X.; Hu, S.X.; Yang, M. Dietary protein requirement of juvenile red spotted grouper (Epinephelus akaara). Aquaculture 2016, 450, 289–294. [Google Scholar] [CrossRef]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The lipids. In Fish Nutrition; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 182–246. [Google Scholar]
- Whalen, K.S.; Brown, J.A.; Parrish, C.C.; Lall, S.P.; Goddard, J.S. Effect of dietary n-3 HUFA on growth and body composition of juvenile yellowtail founder (Pleuronectes ferrugineus). Bull. Aquacult. Assoc. Can. 1998, 2, 21–22. [Google Scholar]
- Skalli, A.; Robin, J.H. Requirement of n-3 long chain polyunsaturated fatty acids for European sea bass (Dicentrarchus labrax) juveniles: Growth and fatty acid composition. Aquaculture 2004, 240, 399–415. [Google Scholar] [CrossRef]
- Wilson, R.P. Utilization of dietary carbohydrate by fish. Aquaculture 1994, 124, 67–80. [Google Scholar] [CrossRef]
- Wang, J.T.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Du, Z.Y.; Wang, Y.; Yang, H.J. Effect of dietary lipid level on growth performance, lipid deposition, hepatic lipogenesis in juvenile cobia (Rachycentron canadum). Aquaculture 2005, 249, 439–447. [Google Scholar] [CrossRef]
- Yong, A.S.K.; Ooi, S.Y.; Shapawi, R.; Biswas, A.K.; Kenji, T. Effects of Dietary Lipid Increments on Growth Performance, Feed Utilization, Carcass Composition and Intraperitoneal Fat of Marble Goby, Oxyeleotris marmorata, Juveniles. Turk. J. Fish. Aquat. Sci. 2015, 15, 653–660. [Google Scholar] [CrossRef]
- Sevgili, H.; Kurtoğlu, A.; Oikawa, M.; Öztürk, E.; Dedebali, N.; Emre, N.; Pak, F. High dietary lipids elevate carbon loss without sparing protein in adequate proteinfed juvenile turbot (Psetta maxima). Aquac. Int. 2014, 22, 797–810. [Google Scholar] [CrossRef]
- Nayak, M.; Saha, A.; Pradhan, A.; Samanta, M.; Mohanty, T.K.; Giri, S.S. Influence of dietary lipid levels on growth, nutrient utilization, tissue fatty acid composition and desaturase gene expression in silver barb (Puntius gonionotous) fingerlings. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 226, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bae, H.J.; Yoon, B.W.; Ho Kim, P.; Kim, D.E.; Roh, J.K. Low concentration of serum total cholesterol is associated with multifocal signal loss lesions on gradient-echo magnetic resonance imaging: Analysis of risk factors for multifocal signal loss lesions. Stroke 2002, 33, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.F.; Ai, Q.H.; Mai, K.S.; Xu, W.; Zhou, H.H.; Liu, Z.G. Feed intake, growth performance and cholesterol metabolism in juvenile turbot (Scophthalmus maximus L.) fed defatted fish meal diets with graded levels of cholesterol. Aquaculture 2014, 428–429, 290–296. [Google Scholar] [CrossRef]
- Luo, L.; Xue, M.; Vachot, C.; Geurden, I.; Kaushik, S. Dietary medium chain fatty acids from coconut oil have little effects on postprandial plasma metabolite profiles in rainbow trout (Oncorhynchus mykiss). Aquaculture 2014, 420–421, 24–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).