Comparative Analyses of IGF-Induced Liver Transcriptomes Reveal Genes and Signaling Pathways Associated with Ovarian Growth and Development in Golden Pompano (Trachinotus ovatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Handling and Treatment
2.3. RNA Sequencing and Transcriptome Analysis
2.4. Screening of Differentially Expressed Genes
2.5. Functional Enrichment Analysis
2.6. Gene Expression Validation Using Real-Time Quantitative PCR
3. Results
3.1. Overview of Transcriptome Sequencing
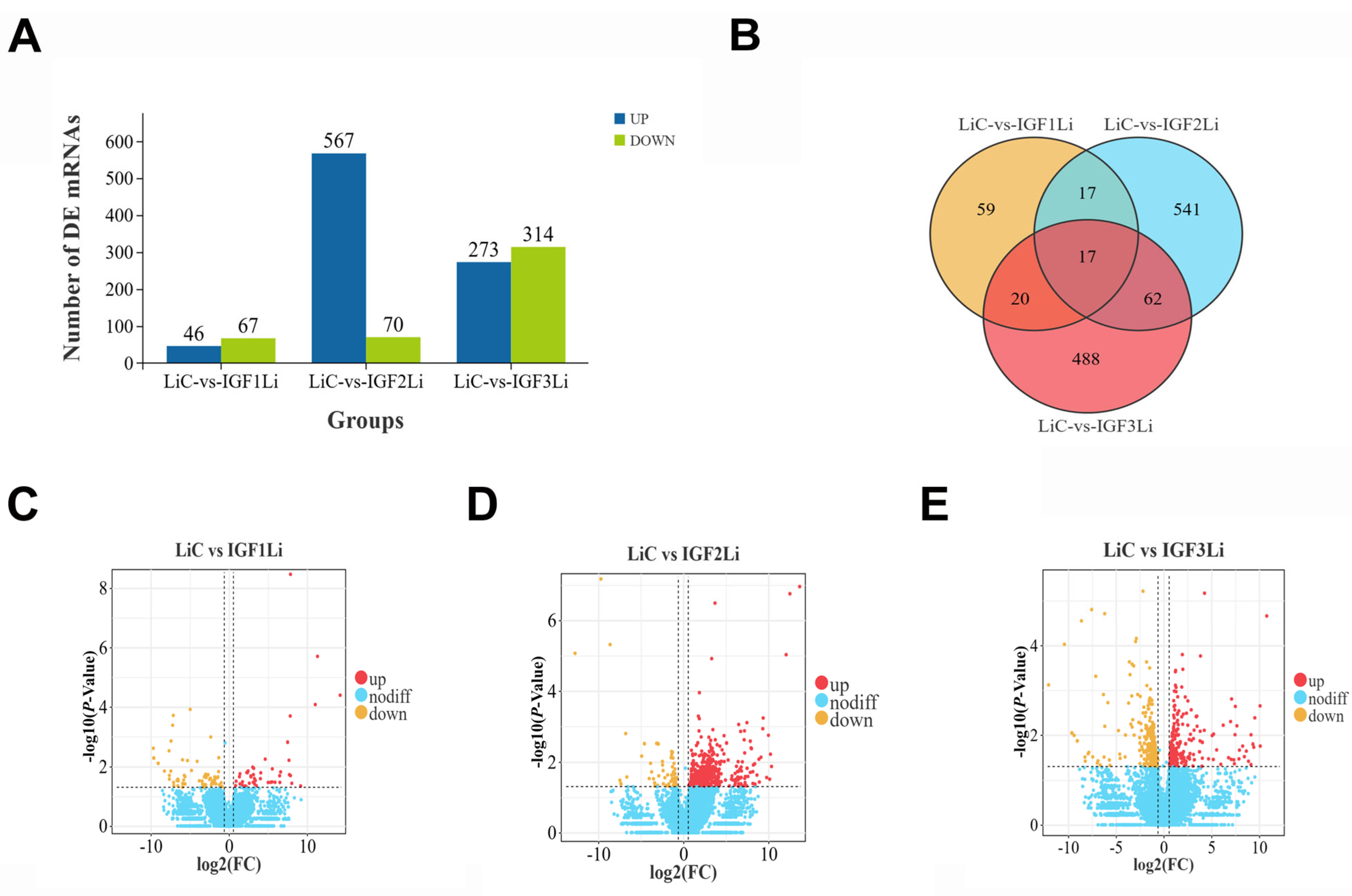
3.2. DEG Profiles Following IGF1, IGF2, and IGF3 Treatment
3.3. DEGs Associated with Metabolism and Energy Storage
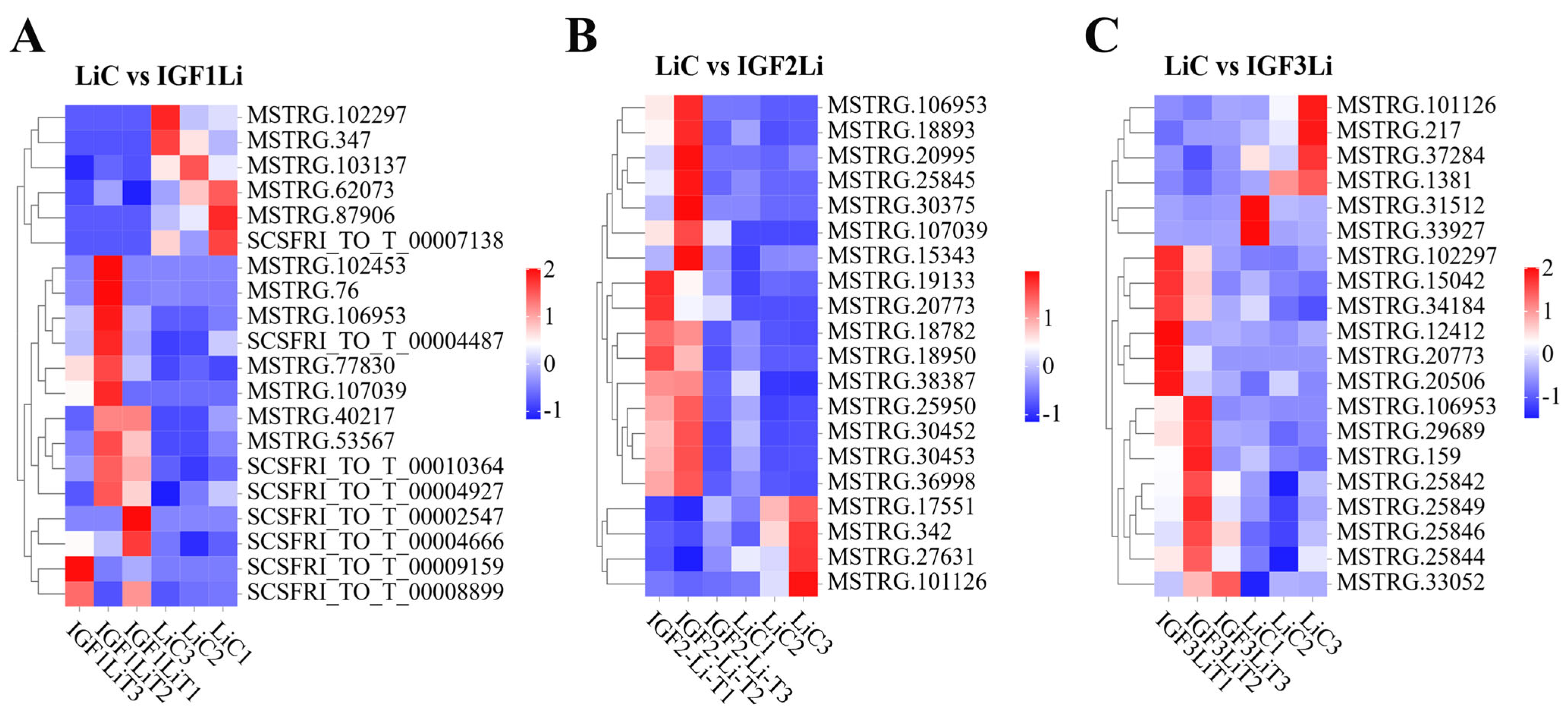
3.4. DEGs Associated with Growth and Development Following IGF Induction
3.5. DEGs Associated with Steroid Biosynthesis Following IGF Stimulation
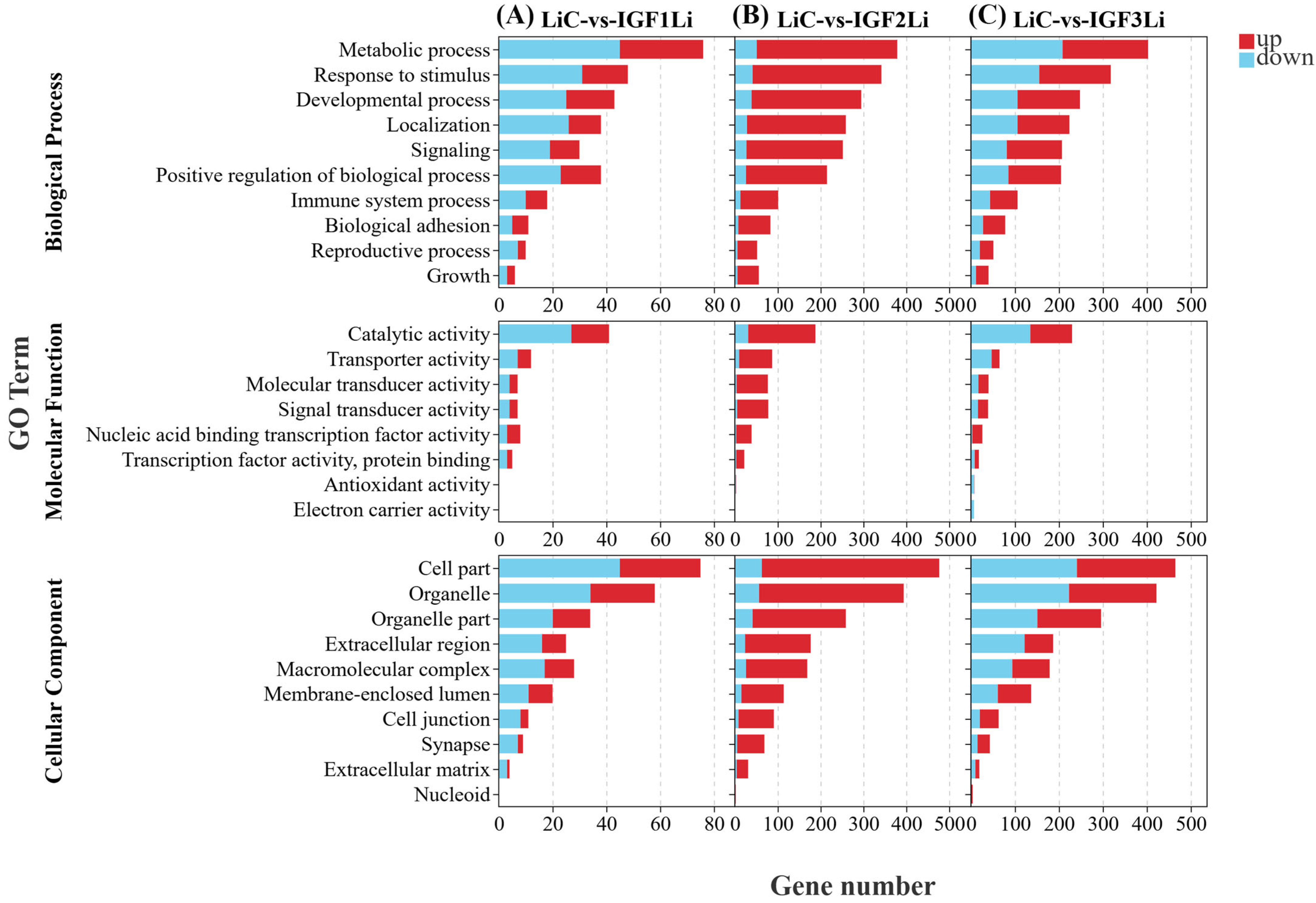
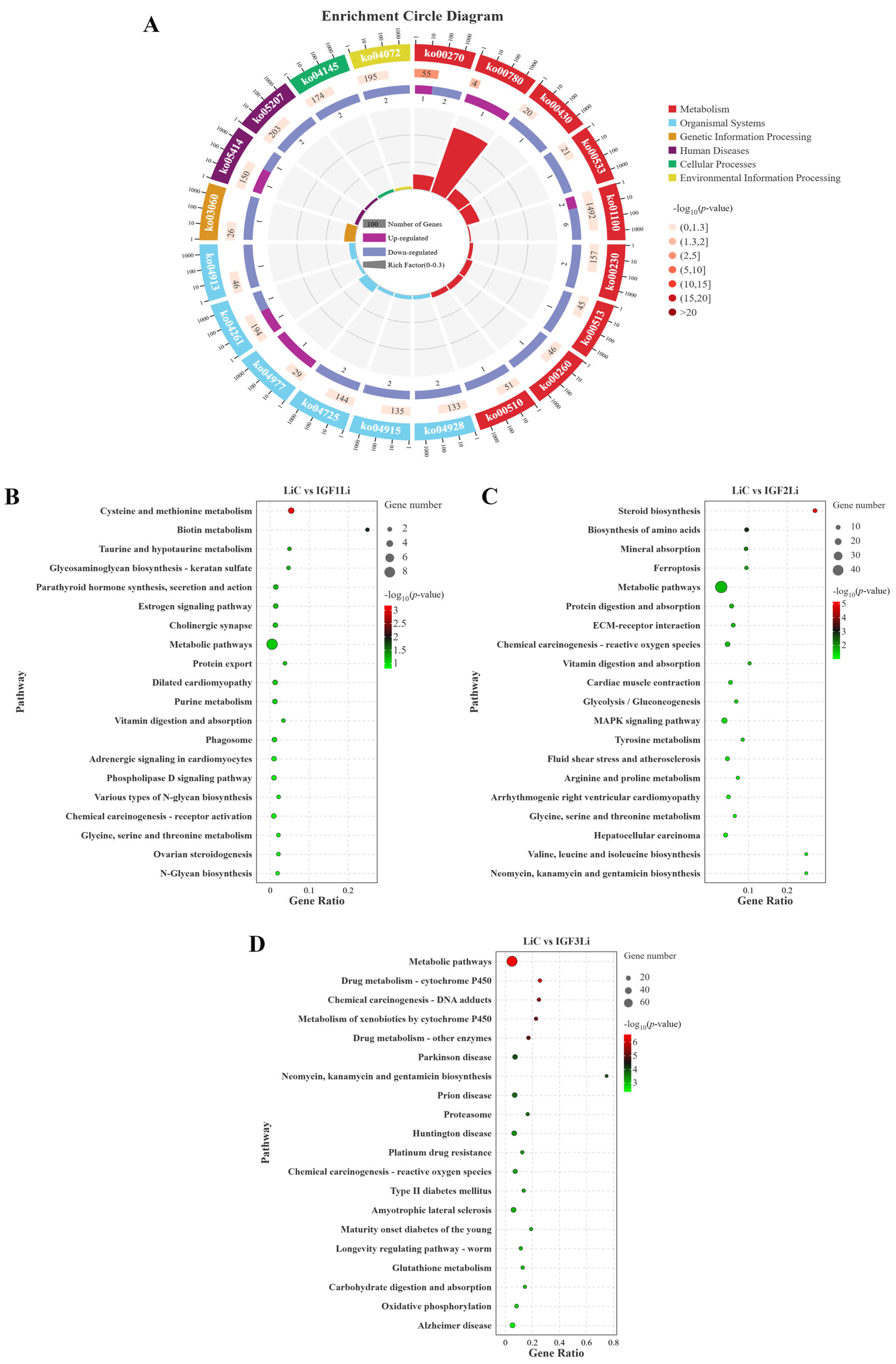
3.6. Functional Annotation and Pathway Analysis of DEGs
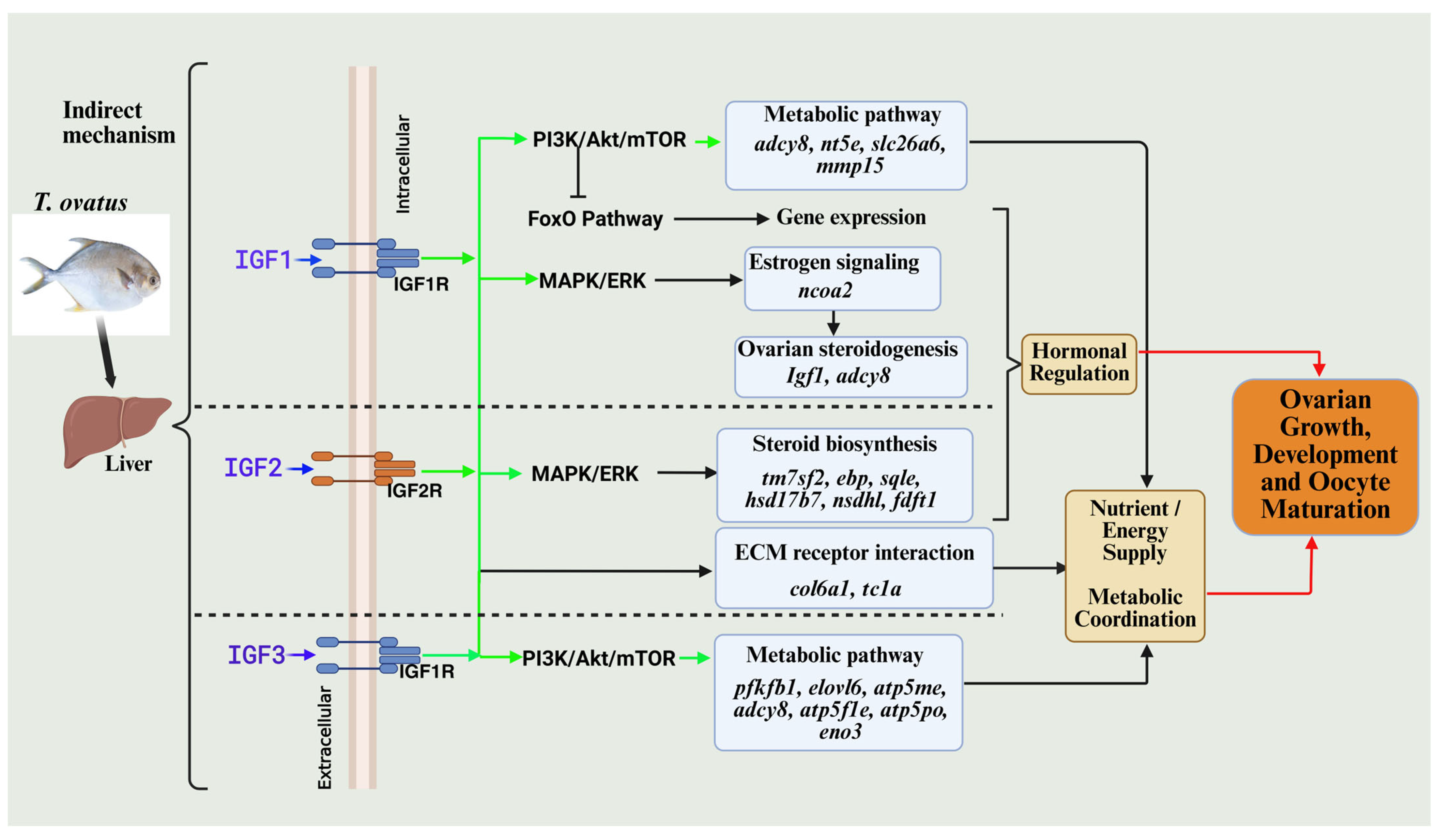
3.7. Liver-Derived Gene Expression Changes Induced by IGFs and Their Hypothesized Impact on Ovarian Function
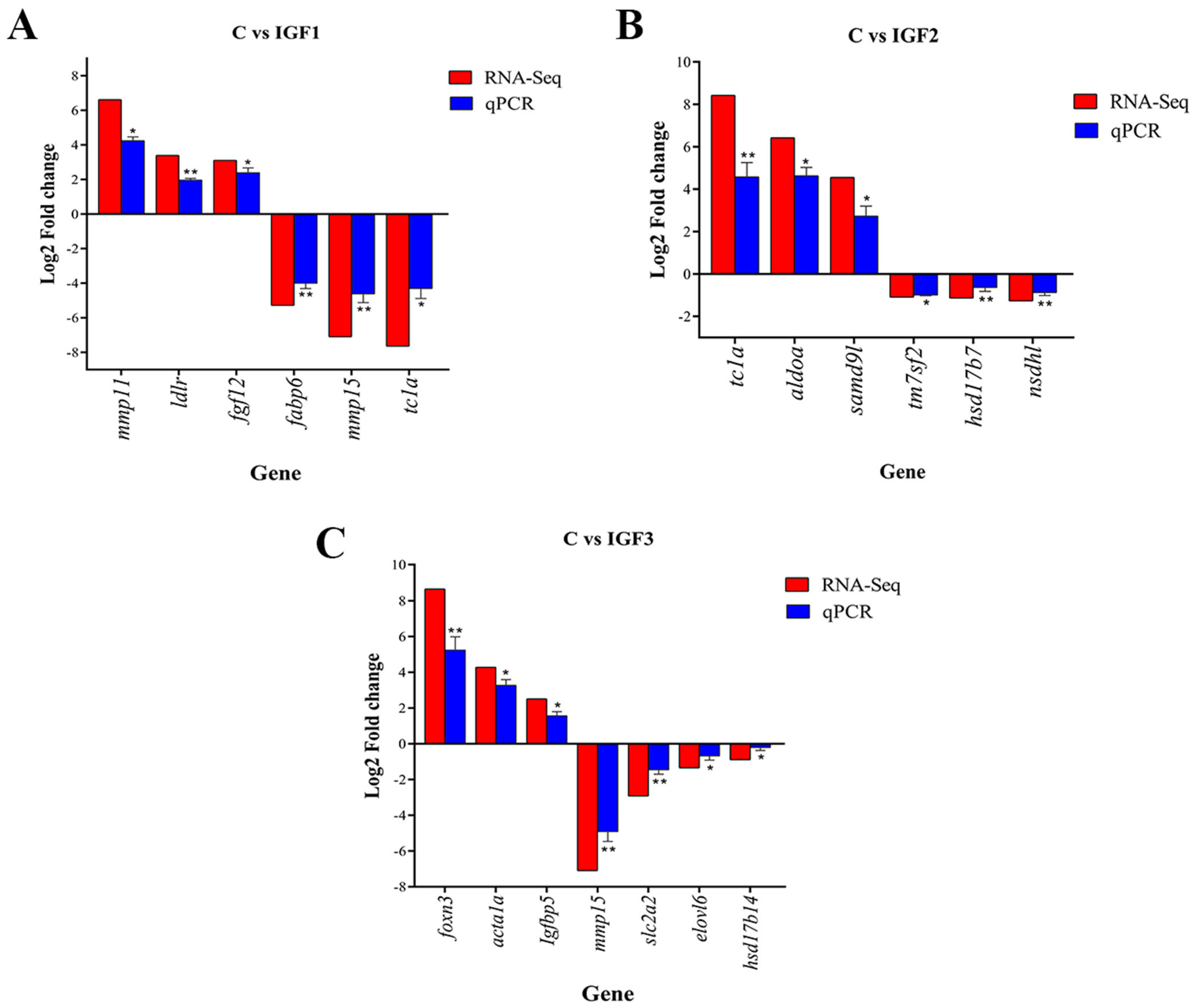
3.8. Validation of DE mRNAs by RT-qPCR
4. Discussion
4.1. IGFs Regulate Nutrient Metabolism and Energy Storage Through the Liver
4.2. IGFs Affect the Growth and Development of T. ovatus
4.3. IGFs Influence Steroid Biosynthesis in the Liver
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Codina, M.; Montserrat, N.; De La Serrana, D.G.; Rallière, C.; Gabillard, J.C.; Navarro, I.; Rescan, P.; Gutiérrez, J. Role of Insulin and IGFs in Fish Muscle Development and Quality. Arch. Fur Tierz. 2006, 49, 33–38. [Google Scholar]
- Pierce, A.L.; Breves, J.P.; Moriyama, S.; Hirano, T.; Grau, E.G. Differential Regulation of Igf1 and Igf2 MRNA Levels in Tilapia Hepatocytes: Effects of Insulin and Cortisol on GH Sensitivity. J. Endocrinol. 2011, 211, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hack, N.L.; Strobel, J.S.; Journey, M.L.; Beckman, B.R.; Lema, S.C. Response of the Insulin-like Growth Factor-1 (Igf1) System to Nutritional Status and Growth Rate Variation in Olive Rockfish (Sebastes serranoides). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 224, 42–52. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Kang, T.; Li, M.; Wang, D.; Cheng, C.H.K. Igf3: A Novel Player in Fish Reproduction. Biol. Reprod. 2021, 104, 1194–1204. [Google Scholar] [CrossRef]
- Li, J.; Niu, C.; Cheng, C.H.K. Igf3 Serves as a Mediator of Luteinizing Hormone in Zebrafish Ovulation. Biol. Reprod. 2018, 99, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Rizky, D.; Mahardini, A.; Byun, J.; Takemura, A. General and Comparative Endocrinology Molecular Cloning of Insulin-like Growth Factor 3 (Igf3) and Its Expression in the Tissues of a Female Damsel Fish, Chrysiptera cyanea, in Relation to Seasonal and Food-Manipulated Reproduction. Gen. Comp. Endocrinol. 2020, 295, 113479. [Google Scholar] [CrossRef]
- Beckman, B.R. Perspectives on Concordant and Discordant Relations between Insulin-like Growth Factor 1 (IGF1) and Growth in Fishes. Gen. Comp. Endocrinol. 2011, 170, 233–252. [Google Scholar] [CrossRef]
- Ndandala, C.B.; Dai, M.; Farouk, U.; Li, X.; Liu, J.; Huang, H.; Li, G.; Chen, H. Current Research and Future Perspectives of GH and IGFs Family Genes in Somatic Growth and Reproduction of Teleost Fish. Aquac. Rep. 2022, 26, 101289. [Google Scholar] [CrossRef]
- Reinecke, M. Insulin-like Growth Factors and Fish Reproduction. Biol. Reprod. 2010, 82, 656–661. [Google Scholar] [CrossRef]
- Ferńndez, I.; Darias, M.; Andree, K.B.; Mazurais, D.; Zambonino-Infante, J.L.; Gisbert, E. Coordinated Gene Expression during Gilthead Sea Bream Skeletogenesis and Its Disruption by Nutritional Hypervitaminosis A. BMC Dev. Biol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, D.; Chu, L.; Liu, Y.; Sun, X.; Li, J.; Cheng, C.H.K. IGF3 Is Essential for Ovary Differentiation in Zebrafish. Biol. Reprod. 2021, 104, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Safian, D.; Bogerd, J.; Schulz, R.W. Igf3 Activates β-Catenin Signaling to Stimulate Spermatogonial Differentiation in Zebrafish. J. Endocrinol. 2018, 238, 245–257. [Google Scholar] [CrossRef]
- Nóbrega, R.H.; De Souza Morais, R.D.V.; Crespo, D.; De Waal, P.P.; De França, L.R.; Schulz, R.W.; Bogerd, J. Fsh Stimulates Spermatogonial Proliferation and Differentiation in Zebrafish via Igf3. Endocrinology 2015, 156, 3804–3817. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, S.; Dang, Y.; Wang, M.; Ren, Y. Identification of a New Insulin-like Growth Factor 3 (Igf3) in Turbot (Scophthalmus maximus): Comparison and Expression Analysis of IGF System Genes during Gonadal Development. Fishes 2023, 8, 240. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Guo, Y.; Nong, C.; Ndandala, C.B.; Yang, H.; Huang, H.; Li, G.; Chen, H. Analysis on LncRNA and MRNA Expression Profiles of IGF3-Induced Ovarian Maturation in Spotted Scat (Scatophagus argus). Aquac. Rep. 2022, 27, 101367. [Google Scholar] [CrossRef]
- Song, F.; Wang, L.; Zhu, W.; Fu, J.; Dong, J.; Dong, Z. A Novel Igf3 Gene in Common Carp (Cyprinus carpio): Evidence for Its Role in Regulating Gonadal Development. PLoS ONE 2016, 11, e0168874. [Google Scholar] [CrossRef]
- Safian, D.; van der Kan, H.J.G.; Cresp, D.; Bogerd, J.; Schulz, R.W. Follicle-Stimulating Hormone Regulates Igfbp Gene Expression Directly or via Downstream Effectors to Modulate Igf3 Effects on Zebrafish Spermatogenesis. Front. Endocrinol. (Lausanne) 2017, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Wang, L.; Yu, Z.; Zhu, W.; Fu, J.; Dong, Z. Knockdown of Igf3 Gene by RNA Interference Causes Gonadal Development Inhibition of Common Carp (Cyprinus carpio). Aquac. Res. 2021, 52, 3006–3014. [Google Scholar] [CrossRef]
- Li, Z.; Brighton, C.; Guo, Y.; Zhou, Q.; Huang, C. Impact of IGF3 Stimulation on Spotted Scat (Scatophagus argus) Liver: Implications for a Role in Ovarian Development Impact of IGF3. Agric. Commun. 2023, 1, 100016. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, C.Y.; Wang, D.D.; Li, X.F.; Xiao, L.; Zhang, X.; You, X.; Shi, Q.; Hu, G.J.; Fang, C.; et al. Transcriptome Analysis Reveals the Molecular Mechanisms Underlying Growth Superiority in a Novel Grouper Hybrid (Epinephelus fuscogutatus ♀ × E. lanceolatus ♂). BMC Genet. 2016, 17, 24. [Google Scholar] [CrossRef]
- Niu, J.; Sun, M.; Li, Z.; Wang, Z.; Kong, M.; Wang, Y.; Song, J.; Zhang, Q.; He, Y.; Qi, J. Whole Transcriptome Analysis Provides New Insight on Immune Response Mechanism of Golden Pompano (Trachinotus ovatus) to Amyloodinium ocellatum Infestation. Aquaculture 2022, 560, 738396. [Google Scholar] [CrossRef]
- Ma, X.; Wang, G.; Wu, L.; Liu, H.; Jiang, H.; Wang, L.; Liu, Q.; Wu, Q.; Tian, X.; Li, X. Dynamic Expression and Functional Analysis of Circular RNA in the Gonads of Chinese Soft-Shelled Turtles (Pelodiscus sinensis). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 39, 100863. [Google Scholar] [CrossRef]
- Kasprzak, A. Insulin-like Growth Factor 1 (Igf-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef]
- Hayes, J.; Volkoff, H. Characterization of the Endocrine, Digestive and Morphological Adjustments of the Intestine in Response to Food Deprivation and Torpor in Cunner, Tautogolabrus adspersus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 170, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.M.; Chen, J.; Tao, Y.; Jiang, X.Y.; Zou, S.M. Molecular Cloning and Function Analysis of Insulin-like Growth Factor-Binding Protein 1a in Blunt Snout Bream (Megalobrama amblycephala). Dongwuxue. Yanjiu 2014, 35, 300–306. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.; Xie, J.; Ge, X.; Zhao, Z. Comparative Proteomic Analysis of Liver Antioxidant Mechanisms in Megalobrama amblycephala Stimulated with Dietary Emodin. Sci. Rep. 2017, 7, 40356. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, M.; Mousavi, S.E. The Mechanism of Reproduction and Hormonal Function in Finfish Species: A Review. Sci. Res. Essays 2011, 6, 3561–3570. [Google Scholar] [CrossRef]
- Ren, X.; Liu, J.; Ndandala, C.B.; Li, X.; Guo, Y.; Li, G.; Chen, H.; Wang, B. Physiological Effects and Transcriptomic Analysis of SbGnRH on the Liver in Pompano (Trachinotus ovatus). Front. Endocrinol. 2022, 13, 869021. [Google Scholar] [CrossRef]
- Liu, B.; Liang, Y.Y.; Guo, H.Y.; Liu, B.S.; Yang, J.W.; Zhang, N.; Xian, L.; Zhu, K.C.; Zhang, D.C. Genomic Structures of Insulin-like Growth Factor from Golden Pompano (Trachinotus ovatus) and Their Expression Responses to the Feed Types. Fish Physiol. Biochem. 2024, 50, 1791–1809. [Google Scholar] [CrossRef]
- Ma, Q.W.; Guo, H.Y.; Zhu, K.C.; Guo, L.; Liu, B.S.; Zhang, N.; Liu, B.; Yang, J.W.; Jiang, S.G.; Zhang, D.C. Dietary Taurine Intake Affects Growth and Taurine Synthesis Regulation in Golden Pompano, Trachinotus ovatus (Linnaeus 1758). Aquaculture 2021, 530, 735918. [Google Scholar] [CrossRef]
- Li, X.; Brighton Ndandala, C.; Zhou, Q.; Huang, C.; Li, G.; Chen, H. Molecular Cloning of Estrogen Receptor and Its Function on Vitellogenesis in Pompano (Trachinotus ovatus). Gen. Comp. Endocrinol. 2024, 346, 114403. [Google Scholar] [CrossRef] [PubMed]
- Ndandala, C.B.; Zhou, Q.; Li, Z.; Guo, Y.; Li, G. Identification of Insulin-like Growth Factor (IGF) Family Genes in the Golden Pompano, Trachinotus ovatus: Molecular Cloning, Characterization and Gene Expression. Int. J. Mol. Sci. 2024, 25, 2499. [Google Scholar] [CrossRef] [PubMed]
- Ndandala, C.B.; Guo, Y.; Ju, Z.; Fachri, M.; Mwemi, H.M.; Chen, H. Integrated LncRNA and MRNA Transcriptome Analyses of IGF1 and IGF2 Stimulated Ovaries Reveal Genes and Pathways Potentially Associated with Ovarian Development and Oocyte Maturation in Golden Pompano (Trachinotus ovatus). Animals 2025, 15, 1134. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg1, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements Daehwan HHS Public Access. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L.; Author, N.B. Differential Analysis of Gene Regulation at Transcript Resolution with RNA-Seq NIH Public Access Author Manuscript. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef]
- Lu, X.; Wei, H.; Yang, C.; Li, Q.; Li, P.; Chen, J.; Sun, Y.; Wen, H.; Jiang, M.; Wang, G. Effects of Dietary Protein Levels on Growth Performance and Liver Transcriptome Changes in Juvenile Top-Mouth Culter Erythroculter ilishaeformis. Aquac. Rep. 2021, 21, 100964. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, X.; Yao, G.; Chen, X.; Qi, M.; Zhou, Q.; Zhu, N.; Meng, Q.; Zhang, Y.; Ding, X. Transcriptomic Comparison of Liver Tissue across Different Largemouth Bass (Micropterus salmoides) Strains. Fishes 2023, 8, 558. [Google Scholar] [CrossRef]
- Kolditz, C.; Borthaire, M.; Richard, N.; Corraze, G.; Panserat, S.; Vachot, C.; Lefèvre, F.; Médale, F. Liver and Muscle Metabolic Changes Induced by Dietary Energy Content and Genetic Selection in Rainbow Trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1154–R1164. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.; Kang, M.; Wei, X.; Geng, C.; Liu, W.; Han, L.; Yuan, F.; Wang, P.; Wang, B.; et al. UPLC-QTOF/MS Metabolomics and Biochemical Assays Reveal Changes in Hepatic Nutrition and Energy Metabolism during Sexual Maturation in Female Rainbow Trout (Oncorhynchus mykiss). Biology 2022, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, Z.; Wen, B.; Gao, J.; Chen, Z. Gonad-Specific Transcriptomes Reveal Differential Expression of Gene and MiRNA Between Male and Female of the Discus Fish (Symphysodon aequifasciatus). Front. Physiol. 2020, 11, 754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Yu, Y.; Zhang, L.; Dong, L.; Gan, J.; Mao, T.; Liu, T.; Peng, J.; He, L. Comparative Analyses of Liver Transcriptomes Reveal the Effect of Exercise on Growth, Glucose Metabolism, and Oxygen Transport-Related Genes and Signaling Pathways in Grass Carp (Ctenopharyngodon idella). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 262, 111081. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Z.-L.; Li, G.-L.; Mustapha, U.F.; Ndandala, C.B.; Shi, H.-J.; Zhu, C.-H.; Chen, H.-P.; Huang, Y.; Jiang, D.-N. Ovary Transcriptomic Analysis Reveals Regulation Effects of Dietary Fish Oil on Hormone, Lipid, and Glucose Metabolism in Female Adult Spotted Scat (Scatophagus argus). Front. Mar. Sci. 2022, 9, 1480. [Google Scholar] [CrossRef]
- Li, F.; Xing, Y.; Zhang, J.; Mu, J.; Ge, J.; Zhao, M.; Liu, L.; Gong, D.; Geng, T. Goose Hepatic IGFBP2 Is Regulated by Nutritional Status and Participates in Energy Metabolism Mainly through the Cytokine−Cytokine Receptor Pathway. Animals 2023, 13, 2336. [Google Scholar] [CrossRef]
- Yu, H.; Mo, H.; An, X.; Yao, M.; Gao, J.; Yu, J.; Xiong, D.; Liu, H.; Li, Y.; Wang, L. Autocrine/Paracrine Fibroblast Growth Factor 1 (FGF1) Enhances Systemic Lipid Metabolism in Rainbow Trout Fed with a High-Fat Diet by Elevating Uridine Levels through the AMPK Signaling Pathway and Activating the Upp2 Promoter via Irf1. Aquaculture 2025, 596, 741885. [Google Scholar] [CrossRef]
- Zhu, B.; Pardeshi, L.; Chen, Y.; Ge, W. Transcriptomic Analysis for Differentially Expressed Genes in Ovarian Follicle Activation in the Zebrafish. Front. Endocrinol. (Lausanne) 2018, 9, 593. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, H.; Hou, L.; Xing, K.; Shu, H. Transcriptional Profiling of Skeletal Muscle Reveals Starvation Response and Compensatory Growth in Spinibarbus hollandi. BMC Genom. 2019, 20, 938. [Google Scholar] [CrossRef]
- Song, Z.; Xiong, H.; Meng, X.; Ma, Q.; Wei, Y.; Li, Y.; Liu, J.; Liang, M.; Xu, H. Dietary Cholesterol Supplementation Inhibits the Steroid Biosynthesis but Does Not Affect the Cholesterol Transport in Two Marine Teleosts: A Hepatic Transcriptome Study. Aquac. Nutr. 2023, 2023, 2308669. [Google Scholar] [CrossRef]
- Yu, Z.B.; Mu, C.K.; Song, W.W.; Li, R.H.; Chen, Y.E.; Wang, C.L. Screening of Genes Related to Ovarian Development in the Swimming Crab, Portunus trituberculatus, by Suppression Subtractive Hybridization. Genet. Mol. Res. 2015, 14, 18675–18686. [Google Scholar] [CrossRef]
- Murakami, Y.; Ando, M.; Futamata, R.; Horibe, T.; Ueda, K.; Kinoshita, M.; Kobayashi, T. Targeted Deletion of Ecto-5′-Nucleotidase Results in Retention of Inosine Monophosphate Content in Postmortem Muscle of Medaka (Oryzias latipes). Sci. Rep. 2022, 12, 18588. [Google Scholar] [CrossRef] [PubMed]
- Sciara, A.A.; Somoza, G.M.; Arranz, S.E. Insulin-like Growth Factor-I of Pejerrey, Odontesthes Bonariensis: CDNA Characterization, Tissue Distribution and Expression Profiles after Growth Hormone Administration. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2008, 309, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Reverter, J.M.; Anglade, I.; Martínez-Rodríguez, G.; Mazurais, D.; Muñoz-Cueto, J.A.; Carrillo, M.; Kah, O.; Zanuy, S. Characterization of Neuropeptide Y Expression in the Brain of a Perciform Fish, the Sea Bass (Dicentrarchus labrax). J. Chem. Neuroanat. 2000, 19, 197–210. [Google Scholar] [CrossRef]
- Assan, D.; Huang, Y.; Mustapha, U.F.; Addah, M.N.; Li, G.; Chen, H. Fish Feed Intake, Feeding Behavior, and the Physiological Response of Apelin to Fasting and Refeeding. Front. Endocrinol. (Lausanne) 2021, 12, 798903. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Zhou, C.; Yuan, D.; Wang, T.; Lin, F.; Chen, H.; Wu, H.; Xin, Z.; Yang, S.; Wang, Y.; et al. Characterization, Tissue Distribution and Regulation of NeuropeptideY in Schizothorax prenanti. J. Fish Biol. 2014, 85, 278–291. [Google Scholar] [CrossRef]
- Yokobori, E.; Azuma, M.; Nishiguchi, R.; Kang, K.S.; Kamijo, M.; Uchiyama, M.; Matsuda, K. Neuropeptide Y Stimulates Food Intake in the Zebrafish, Danio rerio. J. Neuroendocrinol. 2012, 24, 766–773. [Google Scholar] [CrossRef]
- Hosomi, N.; Furutani, T.; Takahashi, N.; Masumoto, T.; Fukada, H. Yellowtail Neuropeptide Y: Molecular Cloning, Tissue Distribution, and Response to Fasting. Fish. Sci. 2014, 80, 483–492. [Google Scholar] [CrossRef]
- Canosa, L.F.; Bertucci, J.I. The Effect of Environmental Stressors on Growth in Fish and Its Endocrine Control. Front. Endocrinol. (Lausanne) 2023, 14, 1109461. [Google Scholar] [CrossRef]
- Puckett, S. Analysis of the Effect of Feeding Regimen on GH/IGF Axis in Channel Catfish (Ictalurus punctatus). Undergraduate Honors Thesis, University of Arkansas, Fayetteville, AR, USA, 2012. Available online: https://scholarworks.uark.edu/baeguht/30 (accessed on 25 March 2025).
- Berishvili, G.; Baroiller, J.; Eppler, E.; Reinecke, M. General and Comparative Endocrinology Insulin-like Growth Factor-3 (IGF-3) in Male and Female Gonads of the Tilapia: Development and Regulation of Gene Expression by Growth Hormone (GH) And. Gen. Comp. Endocrinol. 2010, 167, 128–134. [Google Scholar] [CrossRef]
- Gabillard, J.C.; Kamangar, B.B.; Montserrat, N. Coordinated Regulation of the GH/IGF System Genes during Refeeding in Rainbow Trout (Oncorhynchus mykiss). J. Endocrinol. 2006, 191, 15–24. [Google Scholar] [CrossRef]
- Kajimura, S.; Hirano, T.; Visitacion, N.; Moriyama, S.; Aida, K.; Grau, E.G. Dual Mode of Cortisol Action on GH/IGF-I/IGF Binding Proteins in the Tilapia, Oreochromis mossambicus. J. Endocrinol. 2003, 178, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Guo, C.; Du, J.; Xu, Q.; Li, J.; Huang, D.; Zheng, X.; Tu, L. PPP1R14B-Mediated Phosphorylation Enhances Protein Stability of RPS6KA1 to Promote Hepatocellular Carcinoma Tumorigenesis. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119840. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, W.; Jiang, S.; Qiao, H.; Xiong, Y.; Jin, S.; Fu, H. Genome-Wide Association and Transcriptomic Analysis and the Identification of Growth-Related Genes in Macrobrachium nipponense. BMC Genom. 2024, 25, 1182. [Google Scholar] [CrossRef] [PubMed]
- Fernández Míguez, M.; Presa, P.; Puvanendran, V.; Tveiten, H.; Hansen, Ø.J.; Pérez, M. Gene Expression and Phenotypic Assessment of Egg Quality across Developmental Stages of Atlantic Cod throughout the Spawning Season. Int. J. Mol. Sci. 2024, 25, 7488. [Google Scholar] [CrossRef]
- Meng, F.; Sun, S.; Xu, X.; Yu, W.; Gan, R.; Zhang, L.; Zhang, W. Transcriptomic Analysis Provides Insights into the Growth and Maturation of Ovarian Follicles in the Ricefield Eel (Monopterus albus). Aquaculture 2022, 555, 738251. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Shi, Y.; Bai, C.; Wang, T.; Ruan, F.; Hu, C. Upregulation of MMPs in Placentas of Patients with Gestational Diabetes Mellitus: Involvement of the PI3K/Akt Pathway. Heliyon 2024, 10, e32518. [Google Scholar] [CrossRef]
- Dong, Y.; Lyu, L.; Zhang, D.; Li, J.; Wen, H.; Shi, B. Integrated LncRNA and MRNA Transcriptome Analyses in the Ovary of Cynoglossus semilaevis Reveal Genes and Pathways Potentially Involved in Reproduction. Front. Genet. 2021, 12, 671729. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Yang, C.; Fan, S.; Huang, L.; Zhou, T.; Wang, Q.; Zhao, R.; Tang, C.; Tao, M.; et al. Genomics Comparative Analyses of Hypothalamus Transcriptomes Reveal Fertility, Growth, and Immune-Related Genes and Signal Pathways in Different Ploidy Cyprinid Fish. Genomics 2021, 113, 595–605. [Google Scholar] [CrossRef]
- Xiang, X.; Huang, X.; Wang, J.; Zhang, H.; Zhou, W.; Xu, C.; Huang, Y.; Tan, Y.; Yin, Z. Transcriptome Analysis of the Ovaries of Taihe Black-Bone Silky Fowls at Different Egg-Laying Stages. Genes 2022, 13, 2066. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Z.; Wei, J.; Jiang, L.; Ma, Q.; Wu, M.; Huang, X.; Ye, S.; Li, Y.; Ma, D.; et al. Sema4d Is Required for the Development of the Hindbrain Boundary and Skeletal Muscle in Zebrafish. Biochem. Biophys. Res. Commun. 2013, 433, 213–219. [Google Scholar] [CrossRef]
- Nakada, C.; Satoh, S.; Tabata, Y.; Arai, K.; Watanabe, S. Transcriptional Repressor Foxl1 Regulates Central Nervous System Development by Suppressing Shh Expression in Zebra Fish. Mol. Cell. Biol. 2006, 26, 7246–7257. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dong, P.; Sewo, D.Y.; Su, S.; Li, J.; Feng, W.; Liao, Y.; Tang, Y. Multi-Omics Analysis Unravels the Underlying Mechanisms of the Escape Response of the Common Strain Black Carp (Cyprinus carpio Var. Baisenensis). Aquac. Int. 2024, 32, 9547–9563. [Google Scholar] [CrossRef]
- DeKryger, W.; Chroneos, Z.C. Emerging Concepts of Myosin 18A Isoform Mechanobiology in Organismal and Immune System Physiology, Development, and Function. FASEB J. 2024, 38, e23649. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Nakayama, Y.; Konishi, M. Roles of FGFs As Paracrine or Endocrine Signals in Liver Development, Health, and Disease. Front. Cell Dev. Biol. 2016, 4, 30. [Google Scholar] [CrossRef]
- Jin, C.; Yan, K.; Wang, M.; Song, W.; Kong, X.; Zhang, Z. Identification, Characterization and Functional Analysis of Fibroblast Growth Factors in Black Rockfish (Sebastes schlegelii). Int. J. Mol. Sci. 2023, 24, 3626. [Google Scholar] [CrossRef]
- Kang, B.; Jiang, D.M.; Bai, L.; He, H.; Ma, R. Molecular Characterisation and Expression Profiling of the ENO1 Gene in the Ovarian Follicle of the Sichuan White Goose. Mol. Biol. Rep. 2014, 41, 1927–1935. [Google Scholar] [CrossRef]
- Holmes, R.S. Bioinformatic Studies of Vertebrate Enolases: Multifunctional Genes and Proteins. Open Access Bioinform. 2011, 3, 43–59. [Google Scholar] [CrossRef]
- Hall-Glenn, F.; Lyons, K.M. Roles for CCN2 in Normal Physiological Processes. Cell. Mol. Life Sci. 2011, 68, 3209–3217. [Google Scholar] [CrossRef]
- Lyche, J.L.; Nourizadeh-Lillabadi, R.; Almaas, C.; Stavik, B.; Berg, V.; Skare, J.U.; Alestrøm, P.; Ropstad, E. Natural Mixtures of Persistent Organic Pollutants (POP) Increase Weight Gain, Advance Puberty, and Induce Changes in Gene Expression Associated with Steroid Hormones and Obesity in Female Zebrafish. J. Toxicol. Environ. Health Part A Curr. Issues 2010, 73, 1032–1057. [Google Scholar] [CrossRef]
- Rollins, D.A.; Coppo, M.; Rogatsky, I. Minireview: Nuclear Receptor Coregulators of the P160 Family: Insights into Inflammation and Metabolism. Mol. Endocrinol. 2015, 29, 502–517. [Google Scholar] [CrossRef]
- Liu, M.; Ding, H.; Jin, C.; Wang, M.; Li, P.; Bao, Z.; Wang, B.; Hu, J. Theoretical Analysis and Expression Profiling of 17β-Hydroxysteroid Dehydrogenase Genes in Gonadal Development and Steroidogenesis of Leopard Coral Grouper (Plectropomus leopardus). Int. J. Mol. Sci. 2024, 25, 2180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ndandala, C.B.; Fachri, M.; Shija, V.M.; Li, P.; Chen, H. Comparative Analyses of IGF-Induced Liver Transcriptomes Reveal Genes and Signaling Pathways Associated with Ovarian Growth and Development in Golden Pompano (Trachinotus ovatus). Fishes 2025, 10, 315. https://doi.org/10.3390/fishes10070315
Wang Y, Ndandala CB, Fachri M, Shija VM, Li P, Chen H. Comparative Analyses of IGF-Induced Liver Transcriptomes Reveal Genes and Signaling Pathways Associated with Ovarian Growth and Development in Golden Pompano (Trachinotus ovatus). Fishes. 2025; 10(7):315. https://doi.org/10.3390/fishes10070315
Chicago/Turabian StyleWang, Yan, Charles Brighton Ndandala, Muhammad Fachri, Vicent Michael Shija, Pengfei Li, and Huapu Chen. 2025. "Comparative Analyses of IGF-Induced Liver Transcriptomes Reveal Genes and Signaling Pathways Associated with Ovarian Growth and Development in Golden Pompano (Trachinotus ovatus)" Fishes 10, no. 7: 315. https://doi.org/10.3390/fishes10070315
APA StyleWang, Y., Ndandala, C. B., Fachri, M., Shija, V. M., Li, P., & Chen, H. (2025). Comparative Analyses of IGF-Induced Liver Transcriptomes Reveal Genes and Signaling Pathways Associated with Ovarian Growth and Development in Golden Pompano (Trachinotus ovatus). Fishes, 10(7), 315. https://doi.org/10.3390/fishes10070315






