Abstract
This study evaluated the therapeutic potential of 70% ethanol extracts from six medicinal plants (Chenopodium album, Cassia tora, Cudrania tricuspidata, Dioscorea polystachya, Lonicera japonica, Solidago virgaurea subsp. gigantea) through their antibacterial, antioxidant, cytotoxic, and immunomodulatory activities, targeting applications in aquaculture. All extracts exhibited potent antibacterial activity (MIC ≤ 10 μg/mL) against Aeromonas spp. and Photobacterium damselae subsp. damselae, but limited efficacy against Streptococcus parauberis. C. tricuspidata (CTR) and C. tora (CTO) demonstrated superior antioxidant activity (IC50 = 1292 μg/mL and IC50 = 227 μg/mL, respectively), correlating with high polyphenol content (1498 and 1409 mg GAE/g). CTR displayed significant concentration-dependent cytotoxicity (IC50 = 904.2 μg/mL), while C. album (CA) promoted cell proliferation (132.3% viability). In LPS-stimulated CHSE-214 cells, D. polystachya (DP) induced the highest IL-8 expression (207-fold), followed by Chenopodium album (CA) (194-fold IL-8, 49-fold TNF-α) and CTR (245-fold RIPK2), activating NF-κB, MAPK, and NOD-like receptor pathways critical for teleost immunity. Lonicera japonica (LJ) suppressed TNF-α (0.4-fold) and IRF1 (0.3-fold), indicating anti-inflammatory potential, while S. virgaurea subsp. gigantea (SV) showed biphasic TNF-α modulation (79-fold at 10 μg/mL, 5-fold at 100 μg/mL). These diverse bioactivities, particularly the robust immunomodulatory effects, highlight the promise of these extracts as natural therapeutic agents for fish health management in aquaculture.
Keywords:
antioxidant capacity; aquaculture health; fish pathogens; immune modulation; plant extracts Key Contribution:
Ethanol extracts from six medicinal plants exhibited potent antibacterial activity against key fish pathogens (Aeromonas spp., Photobacterium damselae subsp. damselae) and modulated immune gene expression (IL-8, TNF-α, RIPK2) in CHSE-214 cells, demonstrating their potential as natural nutritional supplements to enhance fish health and immunity in sustainable aquaculture.
1. Introduction
Aquaculture has rapidly evolved from a supplementary food source to a critical contributor to global protein production, playing a pivotal role in ensuring food security worldwide. However, the intensification of aquaculture practices has concurrently heightened the risk of infectious disease outbreaks, particularly those caused by bacterial pathogens such as Aeromonas salmonicida [1], Photobacterium damselae subsp. damselae [2] and Streptococcus parauberis [3]. These pathogens compromise fish health, leading to significant economic losses across the industry [4]. Furthermore, acute infections are often associated with reduced growth rates, impaired feed efficiency, and increased vulnerability to secondary infections, all of which negatively impact overall productivity [5]. Although antibiotics and vaccines remain the primary strategies for disease management, growing concerns over antimicrobial resistance and environmental side effects have spurred the search for safer, eco-friendly alternatives to support fish health and immunity. Among these, plant-derived natural products have emerged as promising candidates due to their broad-spectrum biological activities [6,7]. Medicinal plants are known to exhibit selective antibacterial, antioxidant, and immunomodulatory properties, with varying efficacy depending on the target pathogen and bioactive compounds [8]. Several studies have demonstrated that plant extracts can modulate innate immune responses, either by enhancing pro-inflammatory pathways or suppressing excessive inflammation, depending on their bioactive composition [9,10,11]. This growing body of evidence reflects a broader transition towards sustainable aquaculture practices that favor phytogenic compounds over synthetic chemicals, aiming to potentially reduce ecological impact while maintaining fish welfare [12].
In this study, six medicinal plants were selected based on their traditional use and documented pharmacological effects. Chenopodium album, widely recognized for its nutritional value and antiparasitic properties [13], may support host defense mechanisms. Cassia tora has hepatoprotective and antimicrobial activities [14,15], suggesting its possible role in bacterial defense. Cudrania tricuspidata has been reported to exhibit strong antioxidant and anti-inflammatory activities [16,17], potentially enhancing immune regulation in stimulated epithelial cells. Dioscorea polystachya, traditionally used in herbal medicine, demonstrates immunomodulatory potential through activation of immune cells [18]. D. polystachya ethanol extract modulated key inflammatory signaling pathways including MAPK and NF-κB and enhanced the proliferation of prebiotic bacteria such as Lactobacillus and Bifidobacterium [19]. Lonicera japonica, commonly used for its antiviral and anti-inflammatory effects [20,21], may help modulate inflammatory responses during infection. Solidago virgaurea subsp. gigantea is known for its wound-healing and anti-inflammatory actions [22], which could alleviate infection-induced tissue damage. Polyphenolic compounds (protocatechuic acid, chlorogenic acid, kaempferol-3-O-rutinoside) from S. subsp. gigantea inhibited lipid accumulation in 3T3-L1 cells, showing over 106% adipogenesis inhibition [23]. Despite their known efficacy in mammalian systems, the immunological and antimicrobial effects of these plants in aquatic species remain underexplored. Their integration into aquaculture systems warrants further investigation to validate their potential as functional feed additives or therapeutic agents.
The CHSE-214 cell line, derived from Chinook salmon, serves as an effective platform for fish immunology research. Lipopolysaccharide (LPS) was used to induce inflammatory responses in the CHSE-214 cell line, a widely adopted model for studying innate immune signaling pathways in teleost fish [24]. Key immune-related genes, such as TNF-α and IL-8, are critical mediators of inflammation in fish, while RIPK2 and IRF1 play roles in NOD-like receptor signaling and interferon responses, respectively, making them valuable markers for assessing immunomodulatory effects in aquaculture species [25,26].
This study evaluates six medicinal plant extracts for their potential as natural immunostimulants in aquaculture, focusing on antibacterial, antioxidant, and immunomodulatory effects. Antibacterial efficacy was tested against representative fish pathogens, and antioxidant potential was quantified using DPPH, ABTS, and total polyphenol assays. Cell viability was assessed using the CHSE-214 fish cell line, while immunological responses were examined by analyzing mRNA expression of immune-related genes (TNF-α, IL-8, RIPK2, IRF1) following LPS stimulation. The findings of this study are expected to offer valuable insights into the development of plant-based immunostimulants for sustainable disease management in aquaculture.
2. Materials and Methods
2.1. Preparation of Plant Extracts
Six medicinal plants—Chenopodium album (CA, Lamb’s quarters), Cassia tora (CTO, Sickle senna), Cudrania tricuspidata (CTR, Chinese mulberry), Dioscorea polystachya (DP, Chinese yam), Lonicera japonica (LJ, Japanese honeysuckle), and Solidago virgaurea subsp. gigantea (SV, Giant goldenrod)—were used in this study. Plant materials were obtained from Daehak Herb Pharmacy (Shinyong-dong, Iksan, Jeollabuk-do, Republic of Korea) in February 2024. Six medicinal plants were selected based on morphological characteristics and authenticated by Prof. Dong-Sung Lee (College of Pharmacy, Chosun University). Voucher specimens were deposited in the herbarium of the Natural Products Chemistry Laboratory, Chosun University. The specific plant parts used are detailed in Table 1. Each of the six dried medicinal plants (10 g) was extracted twice by heating with 500 mL of 70% ethanol at 80 °C for 3 h. This extraction method was based on established protocols for maximizing the recovery of bioactive compounds [27]. The resulting extracts were filtered through Whatman filter paper (No. 1), and the filtrates were concentrated using a rotary evaporator (EYELA, 155 Keyland Court Bohemia, NY 11716, USA) under reduced pressure at 40 °C. The weights of the obtained dry residue were 2.21 g (CA), 0.29 g (CTO), 3.25 g (CTR), 1.37 g (DP), 1.22 g (LJ), and 2.87 g (SV), respectively. The powdered samples were stored in airtight containers at 4 °C until analysis. Detailed information on the plant materials and extraction conditions is provided in Table 1.

Table 1.
Plant materials, extraction conditions, and yields of the six medicinal plant extracts.
2.2. Antibacterial Activity Assay
The antibacterial activity of 70% ethanol extracts from six medicinal plants was evaluated against fish pathogens including Aeromonas salmonicida, A. hydrophila, A. sobria, Photobacterium damselae subsp. damselae, Streptococcus parauberis, and Vibrio anguillarum. Bacterial strains (A. hydrophila ATCC 7966, A. salmonicida ATCC 33658, A. sobria ATCC 43979, P. damselae subsp. damselae ATCC 33539, S. parauberis KCTC 11537, V. anguillarum ATCC 19264) were obtained from the National Institute of Fisheries Science. They were maintained on tryptic soy agar (TSA, Difco, MD, USA) supplemented with 1% NaCl at 4 °C and subcultured in tryptic soy broth (TSB, Difco) at 24 °C with shaking (200 rpm) to ensure log-phase growth. For V. anguillarum was refined to align with its marine physiological requirements. TSA and TSB were supplemented with 2.5% NaCl to support optimal growth, as recommended for marine bacteria. Ethanol extracts were dissolved in 10% dimethyl sulfoxide (DMSO) to prepare a stock solution of 10 mg/mL and stored at −20 °C in the dark. The broth microdilution assay was conducted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [28]. Bacterial suspensions were standardized to a 0.5 McFarland turbidity (~1.5 × 108 CFU/mL) and diluted to 1.5 × 106 CFU/mL in Mueller–Hinton broth (MHB, Difco). Extracts were serially diluted two-fold in MHB (0.5–512 μg/mL) in 96-well plates (100 μL/well). An equal volume of bacterial inoculum (100 μL) was added to each well. Controls included 10% DMSO (negative control) and oxytetracycline (positive control, Sigma-Aldrich, St. Louis, MO, USA). Plates were incubated at 25 °C for 24 h. The minimum inhibitory concentration (MIC) was defined as the lowest concentration preventing visible bacterial growth, determined by measuring optical density at 600 nm (BioTek, Winooski, VT, USA). Experiments were performed in triplicate, and median MIC values were reported. MIC data were analyzed using one-way analysis of variance (ANOVA).
2.3. Antioxidant Activity Assays
2.3.1. DPPH Radical Scavenging Assay
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay was performed with EZ-DPPH Assay Kit (DoGenBio, Seoul, Korea) in accordance with the manufacturer’s protocol. Briefly, 100 μL of ethanol extract (200–4000 μg/mL in 10% DMSO) was mixed with 100 μL of 0.2 mM DPPH solution followed by reaction at 25 °C in the dark for 10 min. Absorbance was measured at 517 nm using a microplate reader (BioTek). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as a positive control, tested at concentrations ranging from 25 to 125 μg/mL in ethanol. The percentage of DPPH radical scavenging activity was calculated using the formula provided in the EZ-DPPH Assay Kit protocol. The inhibitory concentration 50 (IC50) value, defined as the concentration of sample or Trolox required to inhibit 50% of the DPPH radicals, was calculated following the manufacturer’s protocol. Experiments were performed in triplicate.
2.3.2. ABTS Radical Scavenging Assay
The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assay was performed as described in a previous study [29], with modifications. ABTS radical cation (ABTS•+) was generated by mixing 7 mM ABTS with 2.45 mM potassium persulfate and incubating the mixture at 25 °C in the dark for 16 h. The resulting ABTS•+ solution was diluted with distilled water to achieve an absorbance of 0.70 ± 0.02 at 734 nm. For the assay, 20 μL of ethanol extract (100–10,000 μg/mL) was mixed with 180 μL of ABTS•+ solution in a 96-well microplate and incubated at 25 °C for 30 min. Absorbance was recorded at 734 nm using a microplate reader (BioTek). Ascorbic acid served as the positive control. All experiments were performed in triplicate.
2.3.3. Total Polyphenol Content
Total polyphenol content (TPC) was determined using the Folin–Ciocalteu method with modifications [30]. Briefly, 20 μL of ethanol extract (1 mg/mL) was mixed with 100 μL of 10% (v/v) Folin–Ciocalteu reagent and 80 μL of 7.5% (w/v) sodium carbonate in a 96-well microplate. The mixture was incubated at 25 °C in the dark for 30 min. Absorbance was measured at 765 nm using a UV–Vis spectrophotometer (BioTek). Gallic acid was used as the standard, and results were expressed as milligrams of gallic acid equivalents per gram of extract (mg GAE/g extract). All experiments were performed in triplicate.
2.4. Cell Culture
The CHSE-214 cell line (Chinook salmon embryo) was obtained from Sigma-Aldrich (St. Louis, MO, USA; catalogue #ATCC-CRL1681) and cultured in Eagle’s Minimum Essential Medium (EMEM, Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA), 100 U/mL penicillin (Gibco), and 100 μg/mL streptomycin (Gibco). Cells were seeded in 75T flasks at a density of 5 × 103 cells/well and maintained at 20 °C in a humidified atmosphere with 5% CO2.
2.5. Cell Viability Assay and IC50 Determination
Cell viability was evaluated with Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) in accordance with the manufacturer’s protocol. Briefly, cells were seeded in 96-well plates at a density of 5 × 103 cells/well and incubated at 20 °C for 24 h. Subsequently, the cells were treated with ethanol extracts at concentrations of 10, 50, 100, 200, and 1000 µg/mL (dissolved in DMSO) for 24 h. Control cells were treated with 0.1% DMSO. After treatment, 10 µL of CCK-8 solution was added to each well, and the plates were incubated at 37 °C for 2 h. Absorbance was measured at 450 nm using a microplate reader (Tecan, Männedorf, Switzerland). Cell viability was calculated as a percentage using the following formula:
cell viability (%) = [(Absorbance of treated group − Absorbance of blank group)/(Absorbance of control group − Absorbance of blank group)] × 100.
To determine the IC50 values (the concentration of extract required to inhibit 50% of cell viability), dose–response data were analyzed using a four-parameter logistic regression model (4PL). The 4PL model was fitted to the data using SPSS (Version 23, IBM, Armonk, NY, USA) through the Nonlinear Regression procedure. All experiments were performed in triplicate, and data were expressed as mean values.
2.6. Immune Gene Expression Analysis
CHSE-214 cells were seeded at a density of 5 × 105 cells/mL in 6-well plates and incubated overnight at 22 °C to allow cell adherence. Subsequently, the cells were treated with ethanol extracts at concentrations of 10, 50, and 100 μg/mL for 3 h. Following this pre-treatment, lipopolysaccharide (LPS, Sigma-Aldrich) was added to each well at a final concentration of 0.1 μg/mL, and the cells were incubated for an additional 12 h at 22 °C. The LPS concentration of 0.1 μg/mL was selected based on previous studies demonstrating effective induction of inflammatory responses in CHSE-214 cells without cytotoxicity [31]. Morphological analysis was performed only for CTR due to its pronounced immunomodulatory effects in preliminary screenings. Post-treatment, cells were washed three times with phosphate-buffered saline (PBS) to remove residual compounds and lysed for total RNA extraction using the RNeasy® Mini Kit (Qiagen, Madrid, Spain), following the manufacturer’s protocol. The purity (A260/A280: 1.8–2.0) and concentration (100–500 ng/μL) of extracted RNA were assessed using 1 μL of sample on a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using the SuperScript™ IV First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA), with oligo(dT) primers, at 50 °C for 10 min, followed by inactivation at 80 °C for 10 min, per the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was performed using SYBR Green dye in 96-well plates on the QuantStudio™ 5 Real-Time PCR System (Thermo Fisher Scientific) with gene-specific primers for tumor necrosis factor-alpha (TNF-α), interleukin-8 (IL-8), receptor-interacting serine/threonine-protein kinase 2 (RIPK2), interferon regulatory factor 1 (IRF1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table 2). Primers for TNF-α, IL-8, RIPK2, and IRF1 were selected as key markers of teleost inflammatory and innate immune responses [25,26]. Primer efficiencies were determined using the standard curve method with five-point serial dilutions of cDNA, yielding efficiencies ranging from 98% to 105% for all gene-specific primers. The amplification protocol consisted of an initial denaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 10 s and annealing/extension at 60 °C for 15 s. A final extension step was carried out at 72 °C for 5 min. GAPDH was employed as the housekeeping gene to normalize the expression levels of target genes. Relative gene expression was calculated using the 2ΔΔ Ct method [32]. cDNA quality was not verified via thermal cycler PCR, though qRT-PCR results suggest reliable synthesis.

Table 2.
Primers used for quantitative real-time PCR.
2.7. Statistical Analysis
Statistical analysis was performed using SPSS software (version 21.0; SPSS Inc., Chicago, IL, USA) and GraphPad Prism (version 9.0; GraphPad Software, San Diego, CA, USA). All data are presented as means ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was used to evaluate significant differences among treatment groups. A p-value of less than 0.05 (p < 0.05) was considered statistically significant.
3. Results
3.1. Antibacterial Activity
The antibacterial activity of 70% ethanol extracts from six medicinal plants was assessed against six pathogenic bacteria in fish (A. salmonicida, A. hydrophila, A. sobria, P. damselae subsp. damselae, S. parauberis, and V. anguillarum) using the broth microdilution method. Minimum inhibitory concentration (MIC) values are presented in Table 3. All extracts exhibited strong antibacterial activity against A. salmonicida, A. hydrophila, A. sobria, A. sobria, P. damselae subsp. damselae, with MIC values ≤ 10 μg/mL. Notably, CA, CTO, LJ, and SV showed high activity against V. anguillarum (MIC ≤ 10 μg/mL), whereas DP displayed no significant activity against V. anguillarum (MIC = 10,000 μg/mL). Conversely, all extracts displayed low activity against S. parauberis (MIC = 10,000 μg/mL), indicating limited efficacy against this bacterium. These results suggest that the extracts are highly effective against Aeromonas spp. and A. sobria, P. damselae subsp. damselae, with variable activity against V. anguillarum and minimal efficacy against S. parauberis.

Table 3.
Minimum inhibitory concentrations of 70% ethanol extracts from six medicinal plants against fish pathogens.
3.2. DPPH Radical Scavenging Activity
The DPPH radical scavenging activity of 70% ethanol extracts from six medicinal plants was evaluated at concentrations ranging from 200 to 4000 μg/mL. Scavenging activities are presented in Supplementary Table S1, with IC50 values summarized in Table 4. All extracts exhibited concentration-dependent scavenging activity. At 4000 μg/mL, scavenging activities ranged from 73.1% (DP) to 85.9% (LJ), while at 200 μg/mL, activities ranged from 0.2% (CA) to 11.4% (CTO). Trolox, used as a positive control, achieved 72.1% scavenging at 100 μg/mL. CTR showed the highest antioxidant activity with the lowest IC50 (1292 μg/mL), while DP had the weakest activity (2788 μg/mL). Trolox outperformed all extracts with an IC50 of 61 μg/mL (p < 0.05).

Table 4.
DPPH and ABTS radical scavenging activities and total polyphenol content of 70% ethanol extracts from six medicinal plants.
3.3. ABTS Radical Scavenging Activity
The ABTS radical scavenging activity of 70% ethanol extracts from six medicinal plants was assessed at concentrations ranging from 100 to 10,000 μg/mL (Table 4). All extracts exhibited concentration-dependent scavenging activity (Table S2). At 10,000 μg/mL, LJ showed the highest inhibition (92.9%), followed by CTO (91.1%) and DP (90.7%), while CA had the lowest (73.9%). At 1000 μg/mL, LJ maintained high inhibition (91.9%), whereas DP dropped to 15.4%. At 100 μg/mL, CA exhibited the highest inhibition (21.7%), and CTR the lowest (7.4%). Statistical analysis revealed significant differences among the extracts at each concentration (p < 0.05). The IC50 values, representing the concentration required to inhibit 50% of ABTS radicals, are summarized in Table 4. CTO exhibited the highest potency with an IC50 value of 227 µg/mL, followed by LJ (315 µg/mL) and CTR (712 µg/mL). DP showed the lowest activity with an IC50 of 5109 µg/mL. These IC50 values align with the inhibition rate trends observed at different concentrations.
3.4. Total Polyphenol Content
The total polyphenol content (TPC) of 70% ethanol extracts from six medicinal plants was determined and expressed as milligrams of gallic acid equivalent per gram (mg GAE/g) (Table 4). CTR exhibited the highest TPC (1498 mg GAE/g), followed by LJ (1428 mg GAE/g) and CTO (1409 mg GAE/g). In contrast, DP showed the lowest TPC (1214 mg GAE/g). The TPC of CTR and LJ was significantly higher than that of DP (p < 0.05). CA and SV recorded TPC values of 1287 mg GAE/g and 1330 mg GAE/g, respectively. To complement TPC analysis, potential bioactive compounds were predicted based on literature (Supplementary Table S3), identifying compounds such as quercetin and kaempferol in CTR, chlorogenic acid and luteolin in LJ, and diosgenin in DP, which likely contribute to the observed bioactivities. Definitive chemical profiling awaits future HPLC analysis, as discussed in Section 4.
3.5. Cell Viability on CHSE-214 Cells
The cell viability of 70% ethanol extracts from six medicinal plants was evaluated on CHSE-214 cells using the CCK-8 assay at concentrations ranging from 0 to 1000 μg/mL (Table 5). CTO, CTR, DP, LJ, and SV maintained cell viability above 80% at concentrations up to 200 μg/mL, indicating low cytotoxicity. At 1000 μg/mL, CTR significantly reduced cell viability to 45.6%, and SV showed a moderate reduction to 58.3%. In contrast, CA, CTO, DP, and LJ exhibited cell viability above 85% at 1000 μg/mL, with CA showing an unusually high value of 132.3%, suggesting potential stimulatory effects on cell proliferation. The IC50 value, representing the concentration reducing cell viability by 50%, was calculated for CTR (904.2 μg/mL) using linear interpolation. IC50 values for CA, CTO, DP, LJ, and SV could not be determined, as cell viability remained above 50% at 1000 μg/mL. These findings indicate that CTR exhibits concentration-dependent cytotoxicity, while other extracts are relatively non-cytotoxic to CHSE-214 cells.

Table 5.
Cell viability of 70% ethanol extracts from six medicinal plants on CHSE-214 cells.
3.6. Morphological Changes in CHSE-214 Cells
To investigate the cellular effects of CTR ethanol extract, morphological changes in LPS-stimulated CHSE-214 cells were examined. Treatment with CTR at 100 μg/mL for 12 h induced significant morphological alterations, including changes in cell shape and increased aggregation, compared to untreated control cells (Figure 1). These observations are consistent with the pro-inflammatory effects of CTR, as evidenced by the significant upregulation of immune-related genes, such as RIPK2 and IRF1, in subsequent gene expression analyses (see Section 3.6). Morphological analysis was focused on CTR due to its pronounced immunomodulatory activity observed in preliminary experiments.
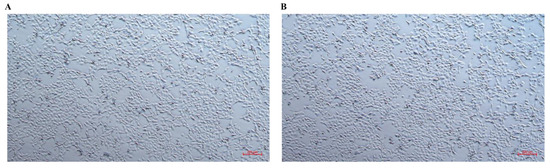
Figure 1.
Morphological changes in CHSE-214 cells following treatment with Cudrania tricuspidata ethanol extract. (A) Untreated control cells. (B) Cells stimulated with LPS and subsequently treated with C. tricuspidata ethanol extract at 100 μg/mL for 12 h. Images were captured using an inverted light microscope at ×200 magnification.
3.7. Relative mRNA Gene Expression in CHSE-214 Cells
The immunomodulatory effects of 70% ethanol extracts from six medicinal plants were investigated by quantifying relative mRNA expression of TNF-α, IL-8, RIPK2, and IRF1 in LPS-stimulated CHSE-214 cells (Figure 2). The CA markedly increased IL-8 (194-fold at 50 μg/mL) and TNF-α (49-fold at 100 μg/mL), with RIPK2 and IRF1 elevated to 45-fold and 11-fold, respectively, at 50 μg/mL (Figure 2A). CTO enhanced IL-8 (160-fold) and TNF-α (48-fold) at 10 μg/mL (Figure 2B). CTR significantly enhanced LPS-induced expression, with IL-8 increasing 74-fold at 50 μg/mL, RIPK2 245-fold at 100 μg/mL, and IRF1 24-fold at 100 μg/mL, indicating pro-inflammatory effects. TNF-α expression increased 3.7-fold at 100 μg/mL compared to the LPS-induced 22-fold (Figure 2C). DP induced the highest IL-8 expression (207-fold at 10 μg/mL), with TNF-α (30-fold) and RIPK2 (34-fold) also significantly upregulated at 10 μg/mL (Figure 2D). LJ consistently suppressed LPS-induced expression, reducing TNF-α to 0.4-fold, IL-8 to 2-fold, RIPK2 to 2.4-fold, and IRF1 to 0.3-fold at 100 μg/mL, demonstrating anti-inflammatory activity (Figure 2E). SV showed concentration-dependent effects, with TNF-α suppressed to 5-fold at 100 μg/mL but increased to 79-fold at 10 μg/mL, and IL-8 and RIPK2 elevated to 110-fold and 59-fold, respectively, at higher concentrations (Figure 2F). Primer efficiency graphs and melt curves were not included due to study constraints, but efficiencies (98–105%, Table 2) confirm reliable qRT-PCR results.

Figure 2.
Effects of ethanol extracts from six medicinal plants on the relative mRNA expression levels of TNF-α, IL-8, RIPK2, and IRF1 in CHSE-214 cells stimulated with LPS. (A) Chenopodium album (CA), (B) Cassia tora (CTO), (C) Cudrania tricuspidata (CTR) (D) Dioscorea polystachya (DP), (E) Lonicera japonica (LJ), (F) Solidago virgaurea subsp. gigantea (SV). Statistical differences were obtained using one-way ANOVA followed by Tukey’s multiple comparison test with * p < 0.05; ** p < 0.01.
4. Discussion
This study assessed the antibacterial, antioxidant, cytotoxic, and immunomodulatory properties of 70% ethanol extracts from six medicinal plants, revealing a spectrum of bioactivities with potential therapeutic applications. All tested ethanol extracts displayed strong antibacterial activity (MIC ≤ 10 µg/mL) against A. salmonicida, A. hydrophila, A. sobria, and A. sobria, P. damselae subsp. damselae. This high efficacy suggests that the bioactive compounds in these plants, such as polyphenols, flavonoids, or terpenoids, may target critical bacterial structures or metabolic pathways in these species. CTR is known to contain prenylated flavonoids, which have been reported to disrupt bacterial cell membranes and inhibit efflux pumps, thereby enhancing antibacterial activity [35]. Similarly, CA and CTO are rich in phenolic compounds, which may contribute to their broad-spectrum activity against Aeromonas spp. and P. damselae by generating oxidative stress or inhibiting key enzymes [36]. The consistent efficacy across these four pathogens highlights the potential of these extracts as natural alternatives to synthetic antibiotics in aquaculture, where Aeromonas spp. and A. sobria, P. damselae subsp. damselae are significant contributors to fish morbidity and mortality. In contrast, the antibacterial activity against V. anguillarum varied among the extracts. CA, CTO, LJ, and SV exhibited strong inhibitory effects (MIC ≤ 10 µg/mL), while CTR and DP displayed negligible activity. This variability may be attributed to differences in the chemical composition of the extracts. LJ contains chlorogenic acid and luteolin, which are known to interfere with bacterial quorum sensing and biofilm formation, potentially explaining its potent activity against V. anguillarum [37]. The poor performance of DP against V. anguillarum suggests a lack of specific bioactive compounds targeting this pathogen’s unique resistance mechanisms, such as outer membrane porins or efflux systems. Also, TSA supplemented with 1% NaCl was used for all tested pathogens, including V. anguillarum. However, as a marine bacterium, V. anguillarum typically requires higher salinity (2–3% NaCl) to support optimal growth and metabolic activity [38]. This suboptimal salinity may have affected the observed MIC values for V. anguillarum, particularly for extracts such as CTR and DP, potentially underestimating their antibacterial efficacy. Future studies should employ media with salinity levels of 2–3% NaCl to align with the physiological requirements of V. anguillarum, ensuring more accurate assessments of antibacterial activity in aquaculture-relevant conditions. All tested ethanol extracts showed weak activity against S. parauberis. S. parauberis, a Gram-positive bacterium, possesses a thick peptidoglycan layer that may confer resistance to the bioactive compounds in these extracts, which appear more effective against Gram-negative bacteria like Aeromonas spp. and A. sobria, P. damselae subsp. damselae. This observation aligns with previous studies indicating that plant-derived compounds often exhibit stronger activity against Gram-negative bacteria due to differences in cell wall structure and permeability [39]. No prior in vitro studies exist on the antibacterial effects from plants against S. parauberis, limiting comparative analysis of our findings. The potent antibacterial activity of these extracts against key fish pathogens, contrasted by their limited efficacy against S. parauberis, highlights their potential and limitations as natural antimicrobials, paving the way for exploring their complementary bioactivities, such as antioxidant and cytotoxic properties.
The antioxidant activities of the six medicinal plant extracts varied significantly across DPPH and ABTS assays, reflecting differences in their chemical compositions and assay-specific reaction mechanisms. The differences in the results of DPPH and ABTS assays by extract are due to the reaction mechanism of each assay and the solubility characteristics of the compounds in the extract. In the DPPH assay, CTR exhibited the highest antioxidant capacity with the lowest IC50 (1292 μg/mL), likely due to its high total polyphenol content (TPC, 1498 mg GAE/g) and the presence of prenylated flavonoids or xanthones, which are effective hydrogen atom donors [39]. In contrast, CTO showed superior performance in the ABTS assay (IC50 = 227 μg/mL), suggesting that compounds such as quercetin or kaempferol, known for their electron transfer capabilities, are more soluble in the aqueous conditions of the ABTS assay. The discrepancy between DPPH and ABTS results highlights the influence of assay conditions on antioxidant performance, with DPPH favoring hydrophobic compounds and ABTS favoring hydrophilic ones. A strong positive correlation between TPC and antioxidant activity was observed for CTR, LJ, and CTO, which exhibited TPC values of 1498, 1428, and 1409 mg GAE/g, respectively. However, DP, with the lowest TPC (1214 mg GAE/g), showed unexpectedly high ABTS scavenging activity (90.7% at 10,000 μg/mL), suggesting contributions from non-phenolic compounds such as saponins or alkaloids, which warrant further investigation. Previous studies on D. polystachya have reported the presence of diosgenin, a steroidal saponin with antioxidant properties, which may explain this anomaly [40]. In general, methanol extracts tend to show higher TPC than ethanol extracts due to their high polarity. It has been reported that methanol/HCl mixed solvent of D. alata extracts more effectively extracted anthocyanins and total phenol content than other solvents [19]. The high antioxidant activities of CTR and LJ may be attributed to their physiological characteristics. For instance, C. tricuspidata root bark is known to accumulate flavonoids as secondary metabolites in response to environmental stress, contributing to its high TPC [17]. Ethanol extract of C. tora showed better protection than methanol and water extract against free radical induced DNA damage and contained quercetin, kaempferol, and epicatechin [15]. Similarly, L. japonica contains chlorogenic acid and luteolin, which are potent radical scavengers [41]. These findings underscore the importance of plant-specific secondary metabolites in determining antioxidant potential.
The TPC of the extracts was quantified using gallic acid as the standard, expressed as milligrams of gallic acid equivalents per gram (mg GAE/g). While gallic acid is a widely accepted standard in aquaculture and natural product research, catechin, a flavonoid, is also commonly employed and may better represent the phenolic profiles of certain plant extracts, particularly those rich in flavonoids such as CTR and CTO. The reliance on a single standard, such as gallic acid, may limit the ability to fully capture the diversity of phenolic compounds contributing to antioxidant capacity. Incorporating catechin as an additional standard in future studies could provide a more comprehensive assessment of TPC, enhance comparability with other studies, and validate the antioxidant potential of these extracts for aquaculture applications.
The cell viability of 70% ethanol extracts from six medicinal plants revealed that differential cytotoxicity profiles among extracts. CTR demonstrated significant concentration-dependent cytotoxicity, as evidenced by its IC50 value of 904.2 μg/mL. CTR is known to contain bioactive compounds such as flavonoids, xanthones, and prenylated flavonoids, which have been associated with both cytotoxic and therapeutic effects in various cell models [42]. Consistent with our findings, catecholic xanthones isolated from C. tricuspidata have previously demonstrated marked cytotoxic effects against a range of human cancer cell lines, including HT-29, HL-60, SK-OV3, AGS, and A549, primarily through the induction of apoptosis. This supports the notion that specific phytochemicals from CTR exert broad-spectrum bioactivities, encompassing both pro-apoptotic and potential therapeutic properties in diverse cellular systems [43]. CTR was the only extract with a calculable IC50 within the tested range further emphasizes its distinct bioactivity compared to the other extracts, which showed no significant cytotoxicity even at the highest tested concentration. In contrast, CA, CTO, DP, and LJ maintained high cell viability (>85%) at 1000 μg/mL, indicating low cytotoxicity and potential safety for CHSE-214 cells. Notably, CA exhibited an unusually high cell viability of 132.29% at 1000 μg/mL, suggesting a possible stimulatory effect on cell proliferation. This phenomenon could be attributed to mitogenic compounds, such as polysaccharides or other growth-promoting phytochemicals, which are known to enhance cellular metabolic activity and promote cell division, potentially through the activation of signaling pathways such as MEK/ERK and PI3K/Akt [44]. SV showed a moderate decrease in cell viability to 58.3% at 1000 μg/mL, suggesting mild cytotoxicity possibly due to phenolic compounds or terpenoids [45]. The cell viability assay incubated CHSE-214 cells at 37 °C for 2 h post-CCK-8 addition, a 17 °C rise from 20 °C (Section 2.5). This may have induced thermal stress, potentially affecting viability, especially for CA (132.3% viability). Future assays should use 20–22 °C to minimize stress. While our ethanolic extract showed no notable cytotoxicity in CHSE-214 cells, methanol extracts of the same species have shown moderate cytotoxic effects in human cancer cell lines, with compounds such as ent-germacra-4(15),5,10(14)-trien-1β-ol and 3,5-di-O-caffeoyl quinic acid exhibiting ED50 values ranging from 1.52 to 18.57 μg/mL [46]. The CCK-8 assay measures cell viability via dehydrogenase activity, reflecting metabolic activity. CHSE-214 cells have a doubling time of approximately 48–72 h [24], which may influence viability results, particularly for CA, suggesting potential metabolic stimulation. The varied cytotoxicity profiles, with CTR’s pronounced toxicity and CA’s proliferative effects, highlight their therapeutic potential, prompting further exploration of their immunomodulatory effects on inflammatory pathways in CHSE-214 cells.
The immunomodulatory effects of six plant ethanol extracts on LPS-stimulated CHSE-214 cells revealed distinct pro- and anti-inflammatory profiles, driven by their unique bioactive compounds. DP induced the most significant upregulation of IL-8 (207-fold at 10 µg/mL), followed by CA (194-fold at 50 µg/mL) and CTO (160-fold at 10 µg/mL). These pronounced pro-inflammatory effects likely stem from the activation of NF-κB and MAPK pathways, which regulate IL-8, a key chemokine for neutrophil recruitment in teleost fish [47]. DP’s diosgenin, a steroidal saponin, has been reported to enhance pro-inflammatory cytokine production via TLR4-NF-κB signaling in macrophage models [48], suggesting a similar mechanism in CHSE-214 cells. Similarly, CA’s quinoid compounds may amplify IL-8 expression by inducing reactive oxygen species (ROS), which activate MAPK pathways [47]. CTR exhibited a remarkable induction of RIPK2 (245-fold at 100 µg/mL), indicating its interaction with NOD-like receptor signaling, a conserved pathway in teleost innate immunity [26]. The high RIPK2 expression may contribute to IL-8 upregulation by enhancing NF-κB activation downstream of NOD1/NOD2, highlighting a synergistic signaling network. This pronounced pro-inflammatory response was further evidenced by morphological changes in LPS-stimulated CHSE-214 cells, where CTR treatment induced altered cell shape and increased aggregation, suggesting heightened inflammatory activation. The teleost-specific nature of NOD signaling in CHSE-214 cells is particularly noteworthy. Unlike mammals, where NOD1/NOD2 signaling is tightly regulated to prevent excessive inflammation, teleost fish exhibit heightened sensitivity to peptidoglycan ligands due to structural variations in NOD receptors [49]. In contrast, LJ consistently suppressed LPS-induced expression of all four genes, reducing TNF-α to 0.4-fold and IRF1 to 0.3-fold at 100 µg/mL. This potent anti-inflammatory activity aligns with LJ’s chlorogenic acid and luteolin, which inhibit NF-κB and STAT1 signaling [50,51,52]. The suppression of IRF1, a transcription factor critical for interferon responses, suggests LJ’s potential to mitigate excessive antiviral immunity in fish. CA extract significantly upregulates IRF1 (11-fold at 50 µg/mL) and TNF-α (49-fold at 100 µg/mL). IRF1, a transcription factor, regulates type I interferon responses and pro-inflammatory genes, including TNF-α, by binding to interferon-stimulated response elements (ISRE) or synergizing with NF-κB via STAT1 activation [25]. The concurrent upregulation of IRF1 and TNF-α by CA indicates a complex signaling network, likely driven by quinoid compounds, which are known to generate ROS to stimulate NF-κB and STAT1 pathways [53,54]. CA’s IRF1-TNF-α induction suggests its potential as an immunostimulant for aquaculture against bacterial and viral pathogens. SV displayed a biphasic response, suppressing TNF-α at high concentrations (5-fold at 100 µg/mL) while amplifying it at low concentrations (79-fold at 10 µg/mL). This dual role may stem from the concentration-dependent activity of SV’s flavonoids and terpenoids. At low concentrations, flavonoids likely activate MAPK pathways to promote NF-κB-driven TNF-α expression, whereas at high concentrations, terpenoids may induce negative feedback (e.g., IκBα upregulation) or exert antioxidant effects to dampen ROS-mediated inflammation [45]. Further studies are needed to elucidate the specific compounds and receptors involved in this response. Although chemical profiling via HPLC or GC-MS was not performed due to resource constraints, the literature suggests that CTR’s bioactivity likely stems from prenylated flavonoids (e.g., quercetin, kaempferol), which disrupt bacterial membranes and enhance NF-κB-driven IL-8 expression [35]. LJ’s anti-inflammatory effects are attributed to chlorogenic acid and luteolin, known to inhibit NF-κB and STAT1 signaling [50,51,52]. CA’s quinoid compounds may amplify IL-8 and TNF-α via ROS-mediated MAPK activation [47]. These inferred compounds align with the observed antioxidant (CTR: IC50 = 1292 μg/mL, DPPH) and immunomodulatory effects (DP: 207-fold IL-8 upregulation). The in vitro CHSE-214 model revealed distinct immunomodulatory profiles, with DP, CA, and CTR inducing robust pro-inflammatory responses (IL-8, RIPK2) via NF-κB and NOD-like receptor pathways, critical for teleost immunity [26]. LJ’s suppression of TNF-α (0.4-fold) and IRF1 (0.3-fold) suggests its potential to mitigate excessive inflammation, a key concern in intensive aquaculture [12]. Compared to Allium sativum (50–100-fold IL-8 induction) [9], our extracts exhibit superior immunostimulatory potency, underscoring their value as feed additives. The absence of in vivo validation limits the direct applicability to aquaculture. However, the CHSE-214 model effectively screens immunostimulants, as demonstrated in studies on Syzygium nervosum [10]. The extracts’ low cytotoxicity (except CTR, IC50 = 904.2 μg/mL) and high cell viability (CA: 132.3% at 1000 μg/mL) support their safety for potential dietary incorporation. Future in vivo studies using species like Oncorhynchus mykiss will evaluate their efficacy as feed additives, focusing on growth performance, disease resistance, and immune gene expression.
Limitations: The lack of HPLC/GC-MS analysis precludes identification of specific bioactive compounds. This study relied on TPC and literature-based inferences, a common approach in preliminary screenings [19]. Future research will employ HPLC to quantify key compounds (e.g., quercetin, chlorogenic acid). The in vitro focus limits extrapolation to in vivo conditions, necessitating feeding trials to validate immunostimulatory effects in aquaculture settings. These limitations do not detract from the study’s contribution to identifying phytogenic candidates for sustainable fish health management.
5. Conclusions
In conclusion, 70% ethanol extracts from six medicinal plants demonstrated potent antibacterial activity against Aeromonas spp. and A. sobria, P. damselae subsp. damselae, with limited efficacy against S. parauberis. All extracts exhibited concentration-dependent DPPH and ABTS radical scavenging activities, with CTR and CTO showing the highest potency and elevated polyphenol content. CTR displayed significant cytotoxicity on CHSE-214 cells, while CA promoted cell proliferation. DP, CA, and CTR induced strong pro-inflammatory responses, notably upregulating IL-8 and RIPK2, whereas LJ exhibited anti-inflammatory effects. These plant extracts show potential as feed additives to support fish health, pending in vivo validation. Also, the strong immunomodulatory effects highlight their potential as natural therapeutic agents for sustainable fish health management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10070313/s1, Table S1: DPPH radical scavenging activity (%) of 70% ethanol extracts from six medicinal plants; Table S2: ABTS radical scavenging activity (%) of 70% ethanol extracts from six medicinal plants.; Table S3: Predicted bioactive compounds in 70% ethanol extracts based on literature.
Author Contributions
Conceptualization, S.-J.W.; methodology, S.-J.W. and N.-Y.K.; software, S.-J.W. and N.-Y.K.; validation, S.-S.K. and D.-S.L.; formal analysis, E.-J.J.; investigation, E.-J.J.; resources, D.-S.L.; data curation, D.-S.L.; writing—original draft preparation, S.-J.W. and S.-S.K.; writing—review and editing, S.-J.W. and S.-S.K.; visualization, S.-S.K.; supervision, N.-Y.K.; project administration, N.-Y.K.; funding acquisition, N.-Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Fisheries Science, Ministry of Oceans and Fisheries, Korea (grant number: R2025052).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| CA | Chenopodium album |
| CTO | Cassia tora |
| CTR | Cudrania tricuspidata |
| DP | Di-oscorea polystachya |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| IC50 | Half-maximal inhibitory concentration |
| IL-8 | Interleukin-8 |
| IRF1 | Interferon regulatory factor 1 |
| LJ | Lonicera japonica |
| RIPK2 | Receptor-interacting serine/threonine-protein kinase 2 |
| MIC | Minimum inhibitory concentration |
| SV | Solidago virgaurea subsp. gigantea |
| TNF-α | Tumor necrosis factor-alpha |
| TPC | Total polyphenol content |
References
- Menanteau-Ledouble, S.; Kumar, G.; Saleh, M.; El-Matbouli, M. Aeromonas salmonicida: Updates on an old acquaintance. Dis. Aquat. Org. 2016, 120, 49–68. [Google Scholar] [CrossRef]
- Gouife, M.; Chen, S.; Huang, K.; Nawaz, M.; Jin, S.; Ma, R.; Xie, J. Photobacterium damselae subsp. damselae in mariculture. Aquac. Int. 2022, 30, 1453–1480. [Google Scholar] [CrossRef]
- Haines, A.N.; Gauthier, D.T.; Nebergall, E.E.; Cole, S.D.; Nguyen, K.M.; Rhodes, M.W.; Vogelbein, W.K. First report of Streptococcus parauberis in wild finfish from North America. Vet. Microbiol. 2013, 166, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Miranda, J.J.; Castillo-Pérez, L.J.; Ponce-Hernández, A.; Carranza-Álvarez, C. Summary of economic losses due to bacterial pathogens in aquaculture industry. In Bacterial Fish Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 399–417. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Wang, W.; Sun, J.; Liu, C.; Xue, Z. Application of immunostimulants in aquaculture: Current knowledge and future perspectives. Aquac. Res. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Vijayaram, S.; Sun, Y.Z.; Zuorro, A.; Ghafarifarsani, H.; Van Doan, H.; Hoseinifar, S.H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol. 2022, 130, 294–308. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Soliman, A.A.; Gewaily, M.S.; Amer, A.A.; Shukry, M.; Khalil, R.H.; Shehata, A.I. Dietary effects of Saccharomyces cerevisiae and Allium sativum on growth, antioxidant status, hepatic and intestinal histoarchitecture, expression of growth- and immune-related genes, and resistance of Oreochromis niloticus to Aeromonas sobria. Fish Shellfish Immunol. 2024, 148, 109493. [Google Scholar] [CrossRef]
- Le Xuan, C.; Linh, N.V.; Wannavijit, S.; Outama, P.; Fontana, C.M.; Meepowpan, P.; Van Doan, H. Influences of makiang (Syzygium nervosum) seed powder on growth performance, immunological response, antioxidant and immune related gene expression in juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 2024, 588, 740943. [Google Scholar] [CrossRef]
- Noorbakhsh, M.F.; Ghaemi, M.; Gholamhosseini, A.; Heidari, A.A. Effects of dietary supplement of basil extract on biochemical and immunological parameters and growth performance in Oncorhynchus mykiss. Aquac. Nutr. 2024, 2024, 5388049. [Google Scholar] [CrossRef]
- Reverter, M.; Tapissier-Bontemps, N.; Sarter, S.; Sasal, P.; Caruso, D. Moving towards more sustainable aquaculture practices: A meta-analysis on the potential of plant-enriched diets to improve fish growth, immunity and disease resistance. Rev. Aquac. 2021, 13, 537–555. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Ojeswi, B.K.; Sharma, B.; Srivastava, M.M. Chenopodium album prevents progression of cell growth and enhances cell toxicity in human breast cancer cell lines. Oxid. Med. Cell. Longev. 2009, 2, 160–165. [Google Scholar] [CrossRef]
- Antonisamy, P.; Dhanasekaran, M.; Kim, H.R.; Jo, S.G.; Agastian, P.; Kwon, K.B. Anti-inflammatory and analgesic activity of ononitol monohydrate isolated from Cassia tora L. in animal models. Saudi J. Biol. Sci. 2017, 24, 1933–1938. [Google Scholar] [CrossRef]
- Kumar, R.S.; Narasingappa, R.B.; Joshi, C.G.; Girish, T.K.; Rao, U.J.P.; Danagoudar, A. Evaluation of Cassia tora Linn. against oxidative stress-induced DNA and cell membrane damage. J. Pharm. Bioallied Sci. 2017, 9, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.R.; Lee, S.; Lee, S.; Do, S.G.; Shin, E.; Lee, C.H. Maturity stage-specific metabolite profiling of Cudrania tricuspidata and its correlation with antioxidant activity. Ind. Crops Prod. 2015, 70, 322–331. [Google Scholar] [CrossRef]
- Ko, W.; Kim, N.; Lee, H.; Woo, E.R.; Kim, Y.C.; Oh, H.; Lee, D.S. Anti-inflammatory effects of compounds from Cudrania tricuspidata in HaCaT human keratinocytes. Int. J. Mol. Sci. 2021, 22, 7472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, S.; Tao, S.; Hou, G.; Zhao, F.; Tan, S.; Meng, Q. Dioscorea spp.: Bioactive compounds and potential for the treatment of inflammatory and metabolic diseases. Molecules 2023, 28, 2878. [Google Scholar] [CrossRef]
- Park, S.Y.; Truong, V.L.; Jeon, S.G.; Choe, S.Y.; Rarison, R.H.; Yoon, B.H.; Park, J.W.; Jeong, H.J.; Jeong, W.S. Anti-inflammatory and prebiotic potential of ethanol extracts and mucilage polysaccharides from Korean yams (Dioscorea polystachya and Dioscorea bulbifera). Foods 2025, 14, 173. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential application of Lonicera japonica extracts in animal production: From the perspective of intestinal health. Front. Microbiol. 2021, 12, 719877. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Won, C.K.; Park, K.I.; Kim, E.; Heo, J.D.; Kim, H.W.; Ahn, M.; et al. Potential antioxidant and anti-inflammatory effects of Lonicera japonica and Citri reticulatae pericarpium polyphenolic extract (LCPE). Antioxidants 2023, 12, 1582. [Google Scholar] [CrossRef]
- Malićanin, M.; Karabegović, I.; Đorđević, N.; Mančić, S.; Stojanović, S.S.; Brković, D.; Danilović, B. Influence of the extraction method on the biological potential of Solidago virgaurea L. essential oil and hydrolates. Plants 2024, 13, 2187. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Kim, H.Y.; Zuo, G.; Lee, E.H.; Kang, S.K.; Lim, S.S. Constituents from Solidago virgaurea var. gigantea and their inhibitory effect on lipid accumulation. Fitoterapia 2020, 146, 104683. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, J.; Chen, X.; Liu, M.; Ren, G.; Lu, T.; Shao, Y.; Xu, L. Autophagy induced by infectious pancreatic necrosis virus promotes its multiplication in the Chinook salmon embryo cell line CHSE-214. Fish Shellfish Immunol. 2020, 97, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, L.; Brandsma, J.H.; Wang, Y.; Hakim, M.S.; Zhou, X.; Pan, Q. Convergent transcription of interferon-stimulated genes by TNF-α and IFN-α augments antiviral activity against HCV and HEV. Sci. Rep. 2016, 6, 25482. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.Y.; Wang, K.L.; Li, L.; Li, B.; Wu, X.Y.; Zhang, Z.W.; Li, N.; Liu, L.H.; Nie, P.; Chen, S.N. Transcription of NOD1 and NOD2 and their interaction with CARD9 and RIPK2 in IFN signaling in a perciform fish, the Chinese perch, Siniperca chuatsi. Front. Immunol. 2024, 15, 1374368. [Google Scholar] [CrossRef]
- Tranquilino-Rodríguez, E.; Martínez-Flores, H.E. Ultrasound-assisted extraction of phenolic compounds from Moringa oleifera leaves by response surface methodology. Bioact. Compd. Health Dis. 2023, 6, 325–337. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI Standard M07; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Holen, E.; Austgulen, M.H.; Espe, M. RNA form baker’s yeast cultured with and without lipopolysaccharide (LPS) modulates gene transcription in an intestinal epithelial cell model, RTgutGC from rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2021, 119, 397–408. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Xu, L.M.; Ren, G.M.; Shao, Y.Z.; Liu, Q.; Teng, C.B.; Lu, T.Y. Comparative transcriptome analysis of rainbow trout gonadal cells (RTG-2) infected with U and J genogroup infectious hematopoietic necrosis virus. Front. Microbiol. 2023, 13, 1109606. [Google Scholar] [CrossRef]
- Della Torre, C.; Monti, M.; Focardi, S.; Corsi, I. Time-dependent modulation of cyp1a gene transcription and EROD activity by musk xylene in PLHC-1 and RTG-2 fish cell lines. Toxicol. Vitr. 2011, 25, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Kang, N.S.; Park, K.H. Isolation of antibacterial prenylated flavonoids from Cudrania tricuspidata. Appl. Biol. Chem. 2004, 47, 270–273. [Google Scholar]
- Chamkhi, I.; Charfi, S.; El Hachlafi, N.; Mechchate, H.; Guaouguaou, F.E.; El Omari, N.; Bakrim, S.; Balahbib, A.; Zengin, G.; Bouyahya, A. Genetic diversity, antimicrobial, nutritional, and phytochemical properties of Chenopodium album: A comprehensive review. Food Res. Int. 2022, 154, 110979. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.C.; Liu, N.Y.; Wang, C.J.; Luo, Y.; Liu, J.X. Lonicera japonica protects Pelodiscus sinensis by inhibiting the biofilm formation of Aeromonas hydrophila. Appl. Microbiol. Biotechnol. 2024, 108, 67. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Nazir, R.; Kumar, V.; Gupta, S.; Dwivedi, P.; Pandey, D.K.; Dey, A. Biotechnological strategies for the sustainable production of diosgenin from Dioscorea spp. Appl. Microbiol. Biotechnol. 2021, 105, 569–585. [Google Scholar] [CrossRef]
- Guan, R.; Guo, F.; Guo, R.; Wang, S.; Sun, X.; Zhao, Q.; Zhang, Y.; Lin, J. Integrated metabolic profiling and transcriptome analysis of Lonicera japonica flowers for chlorogenic acid, luteolin and endogenous hormone syntheses. Gene 2023, 888, 147739. [Google Scholar] [CrossRef]
- Kim, D.C.; Yoon, C.S.; Quang, T.H.; Ko, W.; Kim, J.S.; Oh, H.; Kim, Y.C. Prenylated flavonoids from Cudrania tricuspidata suppress lipopolysaccharide-induced neuroinflammatory activities in BV2 microglial cells. Int. J. Mol. Sci. 2016, 17, 255. [Google Scholar] [CrossRef]
- Lee, B.W.; Lee, J.H.; Lee, S.T.; Lee, H.S.; Lee, W.S.; Jeong, T.S.; Park, K.H. Antioxidant and cytotoxic activities of xanthones from Cudrania tricuspidata. Bioorg. Med. Chem. Lett. 2005, 15, 5548–5552. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Chen, S.H.; Chen, C.H.; Chou, P.Y.; Yang, C.C.; Lin, F.H. Polysaccharide extracted from Bletilla striata promotes proliferation and migration of human tenocytes. Polymers 2020, 12, 2567. [Google Scholar] [CrossRef]
- Budzianowski, J.; Thiem, B.; Kikowska, M. Solidago virgaurea L.—Chemical composition, traditional and medicinal use, pharmaceutical properties, potential applications, and biotechnological studies—A review. In Medicinal Plants; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Sustainable Development and Biodiversity; Springer: Cham, Switzerland, 2021; Volume 28, pp. 569–602. [Google Scholar] [CrossRef]
- Choi, S.Z.; Choi, S.U.; Lee, K.R. Pytochemical constituents of the aerial parts from Solidago virga-aurea var. gigantea. Arch. Pharm. Res. 2004, 27, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.P.; Liu, C.; Chiang, F.Y.; Wang, L.F.; Lee, K.W.; Chen, W.T.; Liang, C.H. IL-8 promotes inflammatory mediators and stimulates activation of p38 MAPK/ERK-NF-κB pathway and reduction of JNK in HNSCC. Oncotarget 2017, 8, 56375. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, H.J.; Zhang, X.W. Total saponin fraction of Dioscorea nipponica makino improves gouty arthritis symptoms in rats via M1/M2 polarization of monocytes and macrophages mediated by arachidonic acid signaling. Chin. J. Integr. Med. 2023, 29, 1007–1017. [Google Scholar] [CrossRef]
- Gao, F.Y.; Pang, J.C.; Lu, M.X.; Yang, X.L.; Zhu, H.P.; Ke, X.L.; Liu, Z.G.; Cao, J.M.; Wang, M. Molecular characterization, expression and functional analysis of NOD1, NOD2 and NLRC3 in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2018, 73, 207–219. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Z.; Jiang, C.; Wang, X.; Huang, L. Exploiting genes and functional diversity of chlorogenic acid and luteolin biosyntheses in Lonicera japonica and their substitutes. Gene 2014, 534, 408–416. [Google Scholar] [CrossRef]
- Lou, L.; Liu, Y.; Zhou, J.; Wei, Y.; Deng, J.; Dong, B.; Chai, L. Chlorogenic acid and luteolin synergistically inhibit the proliferation of interleukin-1 β-induced fibroblast-like synoviocytes through regulating the activation of NF-κB and JAK/STAT-signaling pathways. Immunopharmacol. Immunotoxicol. 2015, 37, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Feng, Z.; Wang, L.; Ma, X.; Wang, L.; Liu, K.; Geng, X.; Peng, C. Chlorogenic acid alleviates hepatic ischemia–reperfusion injury by inhibiting oxidative stress, inflammation, and mitochondria-mediated apoptosis in vivo and in vitro. Inflammation 2023, 46, 1061–1076. [Google Scholar] [CrossRef]
- Fahmideh, H.; Shapourian, H.; Moltafeti, R.; Tavakol, C.; Forghaniesfidvajani, R.; Zalpoor, H.; Nabi-Afjadi, M. The role of natural products as inhibitors of JAK/STAT signaling pathways in glioblastoma treatment. Oxid. Med. Cell. Longev. 2022, 1, 7838583. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.S.; Shaheen, M.S.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J. Saudi Chem. Soc. 2014, 18, 356–363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).