1. Introduction
In the present study, we examine the possibility of pharmacologically inducing cognitive impairments in
Gnathonemus petersii (
G. petersii) fish and evaluate the effect of pharmacological modulation on object recognition memory. This study follows our previous work, where we showed that ketamine elicited analogs of positive schizophrenia symptoms in
G. petersii [
1].
G. petersii is a species of weakly electric fish that uses pulse-type electric signaling for communication (electrocommunication) and spatial orientation (electrolocation). Pulse-type signaling consists of a single electric organ discharge (EOD) followed by a variably long inter-pulse interval (IPI). The electrocommunication, electrolocation, and their interlink, along with remarkable intelligence, create a unique combination of abilities, making
G. petersii a promising species for modeling schizophrenia and other psychiatric disorders that affect communication and cognition [
1,
2,
3,
4,
5]. Although our previous study showed that the NMDA receptor antagonist ketamine elicits positive schizophrenia symptoms, cognitive deficits have not yet been evaluated.
Schizophrenia is a devastating psychiatric disorder characterized by three major symptom classes, namely, positive symptoms, negative symptoms, and cognitive deficits [
6]. Positive symptoms refer to newly acquired behaviors, including delusions, hallucinations, and thought disorders. Negative symptoms refer to impairments or losses in normal behavior and include deficits in social interaction, emotional expression, and motivation; the core negative symptoms modeled in animals are social withdrawal and anhedonia [
7,
8,
9]. Specifically, ketamine administration has been shown to mimic negative symptoms such as reduced social interaction and motivation [
7,
8,
10,
11].
Cognitive deficits are a substantial part of the symptoms of schizophrenia spectrum disorders. In human patients, cognitive deficits include impairments in attention, working memory, learning, executive functions, and the verbal component of cognition [
4,
12]. Importantly, impairment of the verbal component represents one of the core symptoms that differentiate schizophrenia from other psychiatric disorders [
4]. It plays a crucial role in verbal hallucinations, disorganized speech, delusions, and the deterioration of higher cognitive functions, with speech being a common denominator. While disruptions in attention, working memory, and executive functions have been extensively studied in traditional model species, such as laboratory rodents and zebrafish, modeling the verbal component of cognition is not possible in these species [
1,
9,
11,
13,
14]. In contrast,
G. petersii fish use their communication system—electric signaling—for spatial orientation, and, similarly to humans, they use language for both communication and verbal thinking. This similarity between
G. petersii and humans suggests that
G. petersii can be used to study the verbal component of cognition, thereby filling a substantial gap in current schizophrenia modeling [
4]. Among the verbal component, cognitive deficits in schizophrenia are particularly evident in impairments of learning and memory, as observed in traditional model species [
4,
12,
15]. This is the first study examining the impact of pharmacological manipulation on
G. petersii’s cognition within the context of schizophrenia modeling. We chose an established behavioral paradigm, the novel object recognition test (NORT), which is frequently used to assess learning and memory in traditional model species, such as laboratory rodents and zebrafish [
16,
17,
18,
19]. The NORT measures an animal’s ability to discriminate between a familiar and a novel object after a defined interval. A shortened exploration time around the novel object is considered a marker of cognitive impairment, as it suggests the object is not recognized as novel. Prolonged exploration of the familiar object and impaired spatial learning are also indicative of cognitive deficits [
4]. Conversely, increased attention to novel objects suggests cognitive improvement [
20,
21]. Acute administration of sub-anesthetic doses of NMDA receptor antagonists, such as ketamine, can replicate (and model) schizophrenia-like symptoms in humans or exacerbate existing schizophrenia symptoms [
22]. However, typical antipsychotics, like haloperidol, do not effectively block the ketamine-induced psychomimetic effects in either healthy individuals or schizophrenia patients. In contrast, notable cognitive improvement and enhanced motor functions have been observed in schizophrenia patients treated with the atypical antipsychotic agent clozapine [
15,
23]. These findings support the assumption that NMDA receptor dysfunction is a core deficit underlying schizophrenia [
22,
24,
25,
26,
27]. NMDA receptor antagonists can either impair or consolidate recognition memory [
28,
29,
30,
31,
32]. Focusing on memory impairments, studies in rodents have shown that NMDA antagonists can degrade nonspatial, hippocampus-dependent memory by impairing both short- and long-term retention in object recognition [
12,
29,
30,
32]. Similar recognition deficits have also been observed in zebrafish [
12,
33]. In the present study, we extend these experiments to examine the pharmacological modulation of the glutamatergic system in
G. petersii and its manifestation in analogs of cognitive symptoms in schizophrenia. To investigate NMDA-receptor dysfunctions in animal models, various substances affecting processes in glutamatergic pathways were used. In particular, systemic administrations of the non-competitive NMDA receptor antagonist MK-801 (dizocilpine), ketamine, and memantine are the most frequently used substances in basic and preclinical animal research [
22,
25,
29]. Although MK-801 is highly used in rodent and fish models [
18,
22,
34,
35,
36], ketamine was used in our study as it is one of the most frequently used substances to model psychosis and cognitive deficits in human research. Moreover, although MK-801 is commonly used in preclinical research, ketamine is the only NMDA receptor antagonist that has been extensively tested in clinical settings and is primarily used to model psychosis and cognitive disruption in humans [
22,
25,
37].
Our study aimed to (1) evaluate the behavioral response to the novel object recognition task (NORT) in G. petersii; (2) verify whether ketamine-induced pharmacological modulation of the glutamatergic system would elicit changes analogical to those observed in rodent and zebrafish models; (3) extend previous NORT models with EOD analysis; and (4) assess the predictive validity of the entire model by combining the psychotogenic effects of ketamine with antipsychotics (haloperidol and clozapine). We hypothesized that individuals exposed to ketamine would spend less time exploring the novel object compared to control fish. Furthermore, we expected that clozapine would normalize the cognitive symptoms induced by ketamine, since clozapine works on both positive, negative, and cognitive symptoms of schizophrenia in humans.
2. Materials and Methods
2.1. Animal and Housing
The experimental design followed our previous studies on modeling schizophrenia in fish [
1,
2]. A total of 72
G. petersii fish were obtained from the local commercial distributor VIVARIUM Melnik (Melnik, Czech Republic). The fish were juvenile, with an average weight of 15.1 ± 5.6 g. They were housed in groups of 15–20 fish in a 350 L tank filled with filtered system water maintained at 24–27 °C, under a 12:12 h light-to-dark cycle. The fish were fed with chironomid larvae twice daily ad libitum. All fish used in this study were experimentally naïve.
2.2. Behavioral Testing and Video-Tracking
The novel object recognition test was used to assess
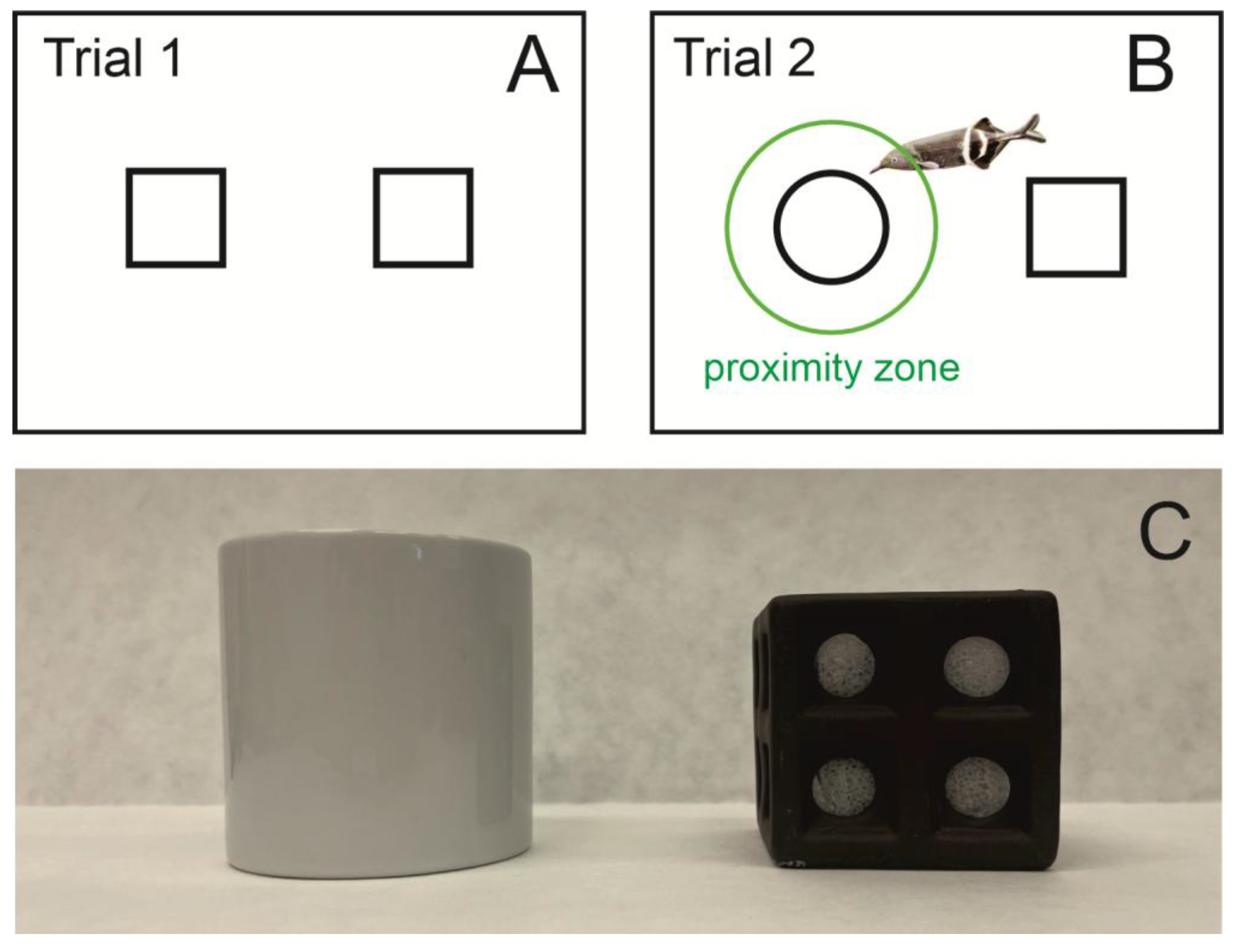
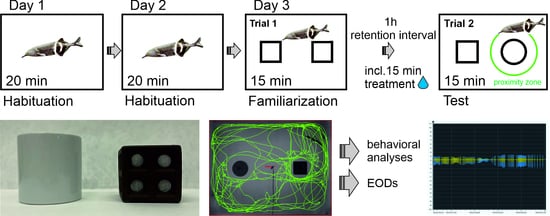
G. petersii behavior following a 15 min pre-treatment with drugs. The test tank was a 90 L rectangular tank (60 × 50 × 30 cm) filled with 30 L of water. A brown ceramic cube with holes, with a circumference of 26 cm (side length 6.5 cm), was used as the familiar object, while a white round ceramic container with a circumference of 21 cm and a height of 8 cm was used as the novel object (
Figure 1C).
Fish behavior was video-recorded using a top-view camera to measure the distance moved (m) and the time spent (sec) exploring the familiar and novel objects (proximity zones). Experimental manipulation involved the acute administration of ketamine, as well as the antipsychotics haloperidol and clozapine (see pharmacological manipulation for details), following the pharmacological doses developed in our previous experiments and published research [
1,
2,
3,
4]. Fish were individually introduced into the test arena and video-recorded for 12 min. Prior to each experiment, the experimental tank was drained and refilled with clean water under constant conditions. Water conductivity was 286 ± 50 µS, temperature was 24.83 ± 0.4 °C, and pH was 7.7 ± 0.13. The tank was illuminated with red light at an intensity of 9 lux on the water surface to reduce stress and simulate the fish’s natural habitat lighting conditions.
The experimental design for the novel object recognition task consisted of two habituation phases (Day 1 and Day 2), followed by the familiarization phase and the test phase (Day 3). The learning task was preceded by a 20 min habituation session individually over two consecutive days to minimize the stress of being placed in a novel tank (habituation phase). After habituation, fish were housed individually in 125 L holding tanks, allowing visual access to their shoal mates while enabling individual identification.
On the third day, the familiarization phase took place, during which fish were exposed to two identical objects for 15 min to familiarize themselves with the objects. After the familiarization phase, fish were removed from the testing environment for an hour (retention interval). The testing phase followed, where one of the familiar objects was replaced with a novel object, and the fish were allowed to explore both objects for 15 min to assess their recognition of the novel object [
17].
Video recordings were captured with a 1.3 MPx infrared recording camera (IDS Imaging Development Systems GmbH, Obersulm, Germany), suitable for recording in the dark, and were analyzed using LoliTrack version 4 (Loligo Systems, Viborg, Denmark). Fish from all experimental groups underwent the same experimental procedures. The testing phase was evaluated to determine whether the fish demonstrated a preference for the novel object over the familiar one.
Several parameters characterizing fish behavior during both the habituation and test phases were measured to evaluate the effects of psychotics and antipsychotics on the response to a novel object in fish. Specifically, the total time spent (sec), active time spent (sec), and distance moved (cm) in proximity to the familiar and novel objects, referred to as proximity zones, were analyzed. The proximity zones were defined as a radius 1.5 times the width of the fish’s stretched-out pectoral fins from the object, corresponding to the standard 1.5 times the width of a rat’s body.
The total time spent was measured as the overall time the fish spent exploring both the familiar and novel objects (referred to as time spent). Active time was measured as the duration during which the fish were actively moving within the proximity zones (referred to as active time). The distance moved was recorded as the distance (cm) the fish moved within the proximity zones (referred to as distance moved). These parameters were measured in the trial with two identical objects (Trial 1) and with the novel object (Trial 2) in both drug-treated and control conditions. Additionally, in all treatments, the number of EODs (electric organ discharges) emitted by the fish was recorded (see EOD signal acquisition for details).
2.3. Ethical Note
All applicable international, national, and institutional guidelines for the care and use of animals were followed. The conditions were validated by the Commission of the Ministry of Agriculture, and the study was approved by the Ethics Committee of Charles University (registration number: MZE-30994/2024-13143).
2.4. Pharmacological Manipulations
Six experimental groups of G. petersii fish were evaluated with 12 specimens as 12 individually treated replicates per group. The fish were exposed to the following treatments: ketamine at a concentration of 30 mg/L, haloperidol (24.6 mg/L), clozapine (70 µg/L), water (control), a combination of ketamine plus clozapine (ketamine 30 mg/L + clozapine 70 µg/L), and a combination of ketamine plus haloperidol (ketamine 30 mg/L + haloperidol 24.6 mg/L). Drug exposure was administered by submerging individual fish in a 2 L plastic beaker for 15 min prior to testing. In the case of combined treatment, the fish were immersed in a mixed solution of ketamine and clozapine/haloperidol for 15 min in the same environment.
The control group was exposed to drug-free water for the same duration to establish a baseline for fish behavior and to control for potential confounding factors that may affect the results. The dosages of substances used were based on our previous studies modeling schizophrenia in fish and were extrapolated from previously published research on ketamine effects in zebrafish and
G. petersii [
1,
2,
3,
4]. Pharmacological drugs used in this study (ketamine and haloperidol) were obtained from Sigma-Aldrich (
www.sigmaaldrich.com, accessed on 8 May 2025; St. Louis, MO, USA), clozapine was isolated and purified at the University of Chemistry and Technology, Prague (structure elucidation by NMR spectroscopy and LC-HRMS, purity > 99% HPLC).
2.5. EOD Signal Acquisition
The EOD signals were recorded using a specialized data acquisition system modified for EOD signal detection. The system consisted of three hardware layers, namely, sensor electrodes, an instrumental voltage amplifier, and the data acquisition unit. The first hardware layer consisted of Ag sensor electrodes originally designed for human electroencephalographic recording. Four sensor electrodes were placed in the corners of the experimental tank, 2 cm below the water surface, and a fifth reference electrode was positioned in the center of the tank, also 2 cm below the water surface. These four electrodes formed two bipolar channels on each side of the aquarium, with the difference between the two electrodes on each side being amplified and interpreted as one channel. The second hardware layer was an analogue amplifier, with the Ag electrodes connected to the amplifier, which had an amplification factor of 10. The third hardware layer was the data acquisition system. The output of the amplifier was connected to a National Instruments DAQ system (model: USB-6003 data acquisition unit, Austin, TX, USA). The signal from the amplifier was sampled at a rate of 50,000 samples per second with 16-bit resolution. Data were displayed and stored on a PC via the National Instruments application for data acquisition. The acquisition system was synchronized with the infrared recording camera using a common clock cycle, which triggered both the EOD signal acquisition and the digital image acquisition.
2.6. EOD Signal Processing
The acquired signal from each electrode was pre-processed and processed in MATLAB 2023b. First, the DC (direct current) signal component was removed by approximating the signal’s isoline through median filtering. This median-filtered signal was then subtracted from the original signal to eliminate baseline drift. Next, the signal was squared to enhance the peak amplitude, ensuring that the EOD detection algorithm accurately identified the maximum peak of the waveform due to the influence of EOD polarity, which was affected by the orientation of the fish relative to the electrodes. Single EODs were detected using a 5 mV threshold with a minimum distance of 1 ms between peaks. The threshold for EOD detection was set based on the estimated noise level from experimental recordings. The EOD detection algorithm was applied to both active bipolar channels, and only the EOD with the higher amplitude from both channels was considered for further analysis.
2.7. Statistical Analyses
The statistical analyses were performed using the mixed models within the SAS software package (SAS Institute Inc., Cary, NC, USA, version 9.4,
www.sas.com, access date 8 May 2025). Mixed models are a generalization of standard models, with the generalization indicating that the data are permitted to exhibit correlation and nonconstant variability. This method is used to cope with repeated measures experiments using the random factors. Therefore, in the present study, factors were applied to account for the repeated measures introduced by individual fish. Generalized linear mixed model with a Poisson distribution (SAS function PROC GLIMMIX) was fitted to analyze the dependent variable ‘number of peaks’, while models with a normal distribution (SAS function PROC MIXED) were applied to analyze the dependent variables, ‘time spent in zone’, ‘active time’, and ‘distance moved’. All variables were square root transformed to meet normality requirements before the analyses. The significance of the explanatory variables—trial (Trial 1 with identical objects and Trial 2 with the novel object) and treatment (drug treatment)—as well as their interactions, was assessed using F-tests. A
p-value of less than 0.05 was considered statistically significant. Least-squares means (henceforth referred to and presented in bar charts as the ‘adjusted mean’ of model predictions) were subsequently computed for the particular classes of class variables. Differences between the classes were determined with a
t-test, and the Tukey–Kramer adjustment was utilized for multiple comparisons. The degree of freedom was calculated using the Kenward–Roger method [
38].
3. Results
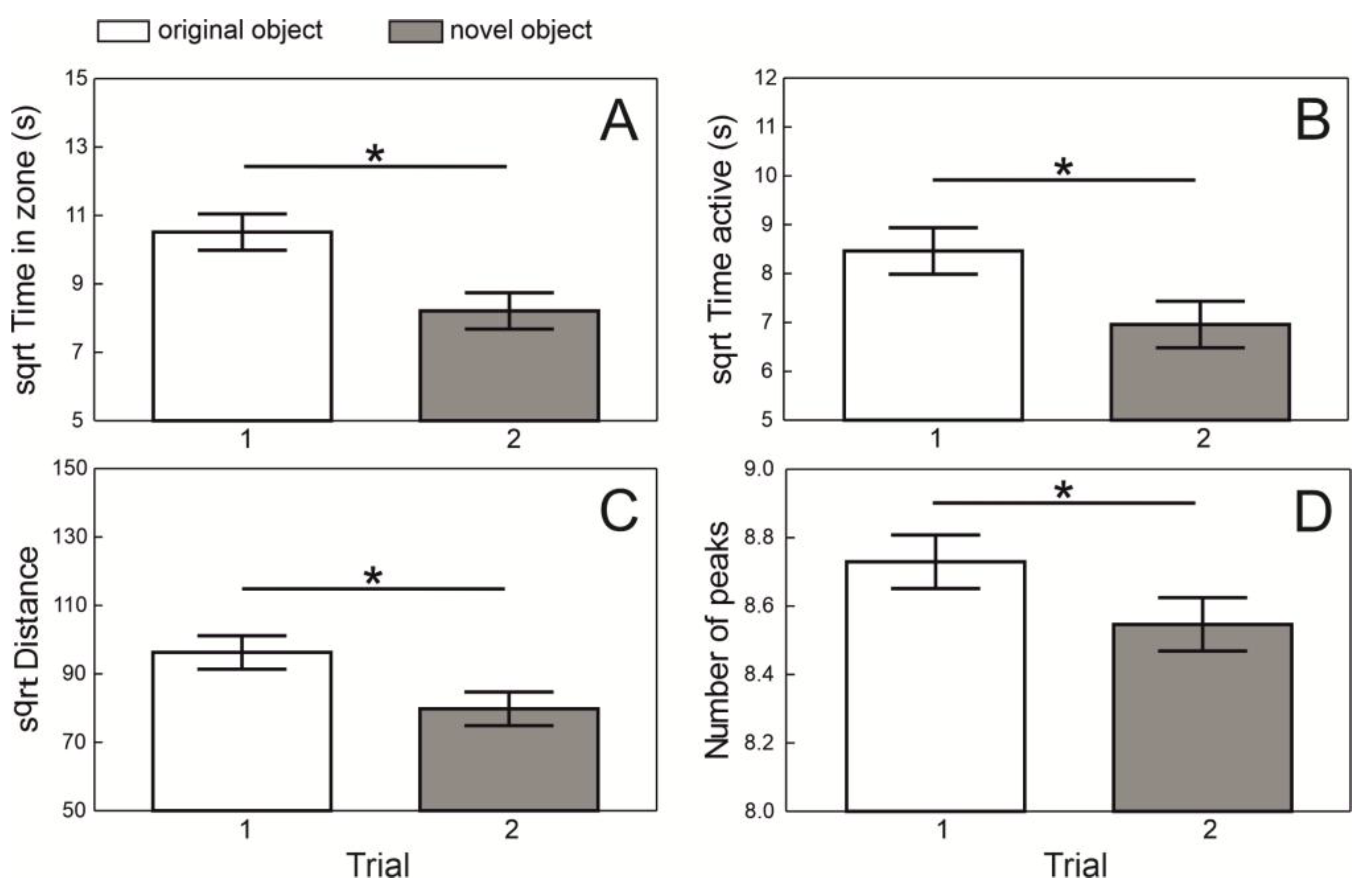
Fish behavior in the experimental arena generally differed between trials with identical objects (i.e., Trial 1) and those with one novel object (i.e., Trial 2). During Trial 1, fish spent more time near the identical objects (F
1,124 = 16.83,
p < 0.0001;
Figure 2A), were more active there (F
1,123 = 13.35,
p < 0.0004;
Figure 2B), moved a longer distance (F
1,123 = 15.72,
p < 0.0001;
Figure 2C), and expressed more EODs (F
1,60 = 2814.48,
p < 0.0001;
Figure 2D) compared to Trial 2. All these effects were related to the avoidance of the novel object.
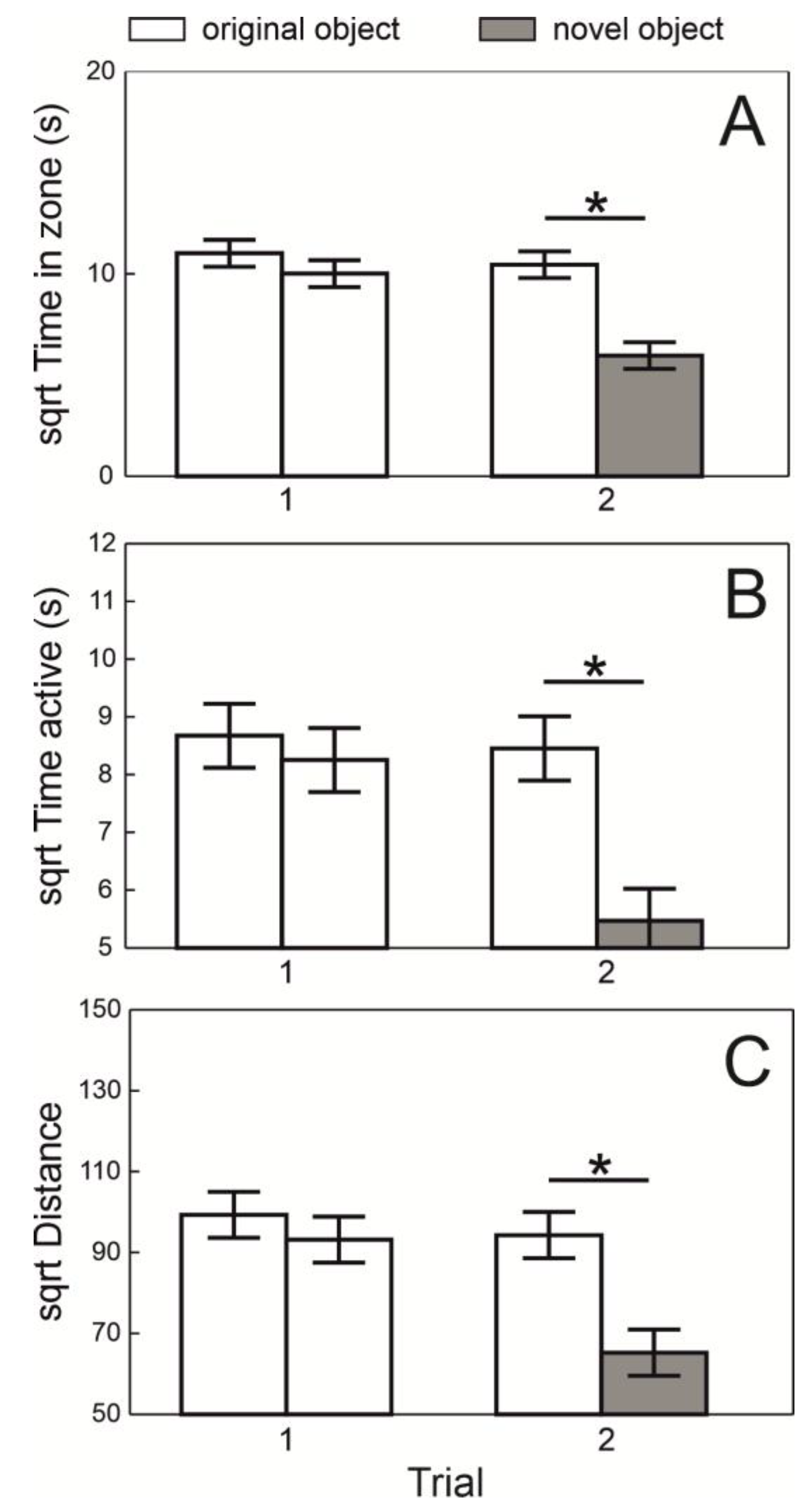
There were no differences in the time spent near the two identical objects, nor in the active time or distance moved within these zones during Trial 1. However, during Trial 2, the fish spent significantly less time near the novel object compared to the original one (F
2,121 = 16.99,
p < 0.0001;
Figure 3A), spent less active time near it (F
2,121 = 13.63,
p < 0.0001;
Figure 3B), and moved a shorter distance near the novel object (F
2,121 = 12.97,
p < 0.0001;
Figure 3C).
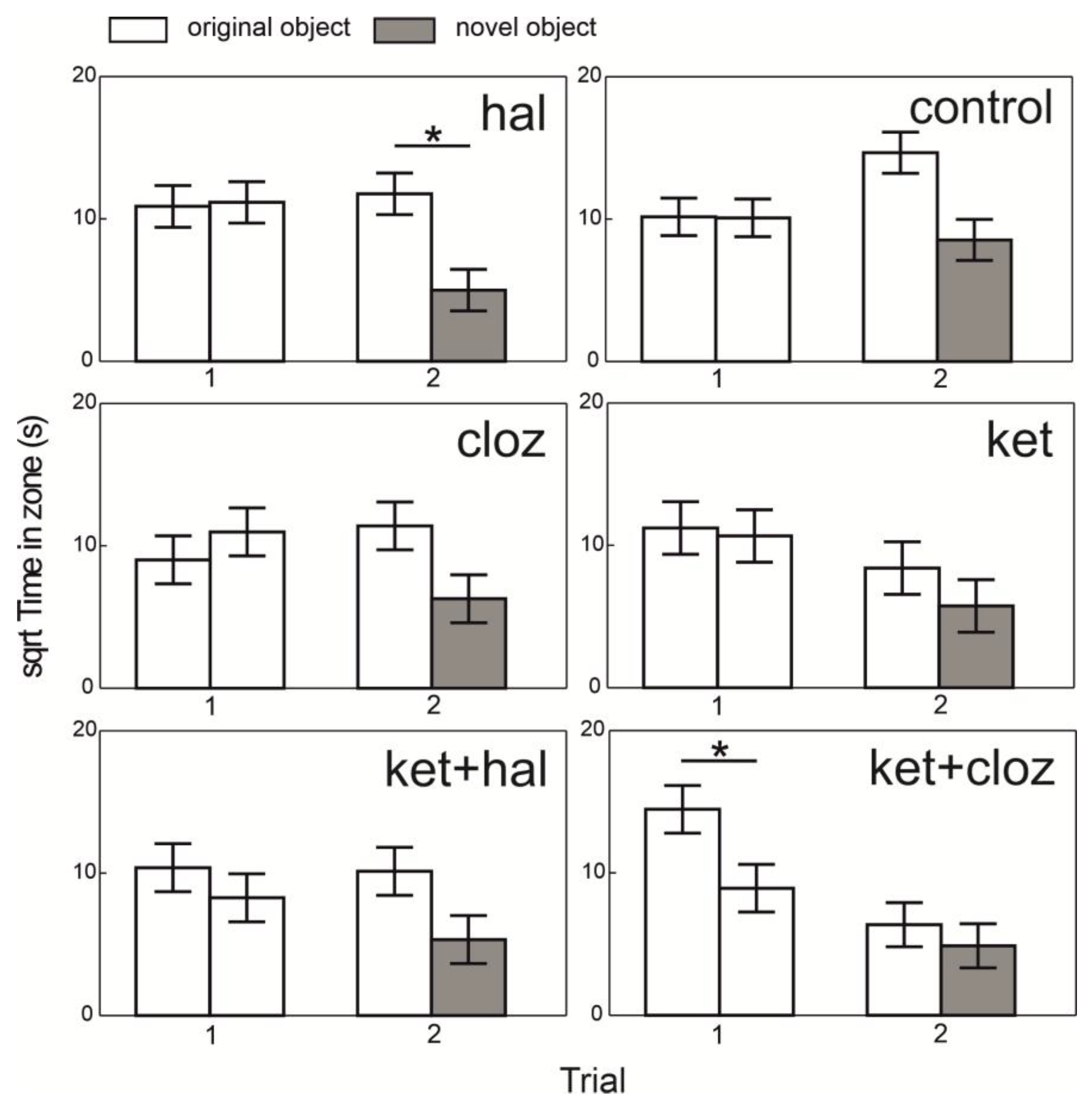
The time spent in proximity to the novel object during Trial 2 differed across the treatments (F
20,140 = 1.80,
p < 0.0257). The only significant difference was observed in the haloperidol treatment (
Figure 4), where the fish spent less time near the novel object (
Figure 4). In the control group and all other treatments (clozapine, ketamine, ketamine plus haloperidol, and ketamine plus clozapine), no significant differences were found in the time spent near the novel versus original object (
Figure 4). The results indicate that, except for the ketamine plus clozapine combination, fish spent more time near the familiar object (
Figure 4).
A significant effect of treatment was not detected for activity or distance moved; however, a lower number of EODs were observed in Trial 2 in the presence of the novel object, in both control and experimental conditions. Although the effect of treatment was significant for the number of EODs (F
10,60 = 117.16,
p < 0.0001), it was not possible to distinguish between signals produced near the original or novel object. All treatments followed the general trend, with fish showing more EODs during the familiarization phase (Trial 1) than during the test phase (Trial 2), in both drug administration and control conditions (
Figure 5).
4. Discussion
The most important finding of our study is that the NMDA antagonist ketamine induces cognitive deficits in
G. petersii fish, previously observed in standard rodent and zebrafish models. Although a direct relationship between the number of EODs and behavioral responses to ketamine and antipsychotic drug administration was not detected, the administration of the atypical antipsychotic clozapine disrupted the perception of the original object, where one of the objects was preferred. However, in the novel object trial, the time spent on the original and new object was attenuated to the same level. In the present study, control fish showed a preference for the familiar object over the novel one in the NORT paradigm under current conditions. This finding suggests that the fear of novelty, in this case, outbalanced the animal’s natural motivation to explore. This phenomenon was also previously described in laboratory rats [
39] and requires detailed examination in
G. petersii.
Although no significant differences were observed in the exploration of the two identical objects during the training phase, the fish spent less time, moved shorter distances, and emitted fewer EODs during the testing phase. This was further confirmed by the exploration of individual objects, where fish spent less time, were less active, and moved shorter distances in proximity to the novel object. Our observation that
G. petersii avoided the novel object and approached the familiar one may indicate anxiety or altered exploratory behavior [
39]. The novel object is thought to induce an approach-avoidance conflict, where animals remain alert and either approach the object closely or from a distance, depending on the perceived risk [
39]. The reason for novel object avoidance can result from the way
G. petersii perceives the objects and evaluates the perceived risk, as due to its high encephalization,
G. petersii is assumed to have higher intelligence than other fish species [
40]. This pattern may also be based on the type of object used for testing, which may be impacted by the shape, color, structure, and texture of the objects [
41].
Our finding that fish administered ketamine spent less time near the novel object aligns with previous studies showing that NMDA antagonists disrupt learning and memory, as demonstrated by impairments in novel object recognition in animal models [
12,
29,
30,
32,
33]. For example, ref. [
29] showed that administration of MK-801 impaired memory formation in adult female Wistar rats. In a mouse model, ref. [
42] found that mice chronically treated with ketamine display combined impairments in novelty exploration and recognition, and [
30] found that administration of MK-801 at doses of 0.1 or 0.2 mg kg
−1 decreased novel object exploration time in adult male mice. In fish models, ref. [
12] demonstrated that zebrafish larvae exposed to MK-801 failed to form or maintain memory of the novel object after 1 h of recovery, and [
33] discovered impairments in recognition memory and preference for exploring novel objects after MK-801 administration.
Our results are consistent with our expectation that ketamine-induced pharmacological modulation of the glutamatergic system would elicit changes similar to those observed in rodent and fish models. The fact that the fish did not increase their attention to the novel object could indicate increased anxiety and/or impaired attention to novelty. Ketamine has been reported to exhibit anxiolytic effects in some contexts [
43,
44,
45], which could lead to increased exploration of novel objects. However, such behavior was not observed in our study. There are likely two reasons for these discrepancies, as follows:
First, the novel object was of a lighter color than the familiar one, which may have repelled the fish from exploring it.
G. petersii are nocturnal and inhabit turbid waters, so the color of the object could impact their motivation to explore. Object preference in
G. petersii varies based on object characteristics [
41], similar to the variation in rodents’ motivation to explore different novel objects in the NORT [
46]. Second, the doses of psychedelic and antipsychotic drugs may have been suboptimal for some individuals, potentially leading to uneven results, as
G. petersii are known for their inter-individual differences in behavioral traits, such as boldness [
47]. Moreover, ketamine administration in fish can lead to dose-dependent alterations in locomotor activity, which can be one of the most problematic aspects of such experiments. Lower doses of ketamine may induce hyperactivity or increased swimming behavior, while higher doses can lead to decreased activity or immobilization [
48,
49]. An important question, therefore, is whether the exploration of the novel object is associated with the effects of treatment on locomotor activity. In our study, no significant effect of any treatment on locomotor activity was observed between the control and ketamine-treated groups. Thus, it can be assumed that the reported drug effects on object exploration are independent of effects on locomotor activity.
In our model, the deficits in memory and learning may be demonstrated not only by impaired spatial learning and exploration of an object, but also by reduced active signaling during spatial learning and/or increased electrical activity in the presence of the novel object. To extend previous animal models of NORT, an EOD analysis was performed in this study. The analysis of EODs revealed that the number of EODs decreased in Trial 2 with the novel object, with no significant differences between the control and drug administration groups. The reduction in EODs is consistent with our previous observation, where EODs were significantly reduced by 40 mg L
−1 of ketamine [
1]. While internally driven electric activity correlates with complex behavior, in our study, EOD signaling did not show a clear connection with drug administration. Therefore, the effects of variable doses of ketamine should be further investigated in future studies.
The ketamine administered in combination with clozapine resulted in a greater preference for one of the original objects by the fish. This may be due to the combination inducing changes in exploratory behavior and cognitive suppression, which could interfere with specific cognitive or perceptual functions. As a result, the fish may have difficulty discriminating between objects, leading to random or absent preferences or altered exploratory behavior. Another possible explanation could be altered laterality [
50]. However, this effect was attenuated in the trial involving a novel object, where fish administered ketamine and clozapine spent equal time exploring both the original and novel objects. Our results show that ketamine administration led to a slight, but not significant, decline in exploration time compared to the trial where fish were exposed to a familiar object. However, when administered in combination with ketamine and clozapine, this pattern was not observed. The predictive validity of animal models of schizophrenia is based on their potential to normalize cognitive symptoms, mirroring the clinical effects seen in patients [
4]. The administration of ketamine in combination with haloperidol did not affect the disruption, either in terms of normalization or further impairment. However, the cognitive deficits induced by ketamine were attenuated by the atypical antipsychotic clozapine. This observation is partially consistent with previous results showing that clozapine attenuates sub-chronic PCP-induced impairments in rodent and human models [
36,
37,
51,
52]. In zebrafish, the potential of haloperidol and clozapine to treat cognitive impairments was explored by [
9]. Their findings indicate that only atypical antipsychotics (sulpiride and olanzapine), but not typical (haloperidol) antipsychotics, could reverse the MK-801-induced social and cognitive deficits. Atypical antipsychotics, in contrast to conventional antipsychotics, induce neuronal plasticity and synaptic remodeling, not only in the striatum but also in other brain areas such as the prefrontal cortex and hippocampus. This mechanism may help normalize glutamatergic dysfunction and structural abnormalities, addressing core pathophysiological substrates of schizophrenia [
37,
53]. Thus, our results contribute to findings in humans, where atypical antipsychotics have been shown to have superiority in suppressing cognitive dysfunctions [
37,
53]. They align with the assumption that second-generation antipsychotics (atypical antipsychotics) are more effective at treating negative and cognitive symptoms of schizophrenia [
37], further corroborating
G. petersii as a suitable model for schizophrenia research.
The NORT is widely used to evaluate object recognition memory; however, it is important to consider the natural behavioral tendencies of model animals, as the results can be influenced by their innate preferences or aversions to novel objects [
19,
39,
54]. In rodents, it is suggested that they have an innate preference for exploring novel objects. However, in fish, behavior may be influenced differently, and exploration of novel objects could be conditioned more by the type and color of the object. The natural response of fish to a novel object may be avoidance rather than increased attention, as anxiety can outweigh the motivation to explore. Fish tend to preferentially explore objects that offer shelter rather than those with interesting shapes or colors. In future studies, objects with more appealing colors, materials, and shapes could be used, which might intensify the response to novelty. The study could also be expanded to include different doses of drugs as well as a variety of atypical antipsychotic drugs. The doses of the substances used could be more precisely tailored based on the size and behavior of the tested fish. Such an improved experimental design would provide a more detailed understanding of the underlying mechanisms influencing cognitive deficits in schizophrenia.
Probable reasons for discrepancies between the aims and results are primarily of two kinds. First, the novel object was of a lighter color than the familiar one, possibly repelling fish from the exploration.
G. petersii fish are nocturnal and live in turbid waters, therefore, the color of the object may have an impact on the motivation to explore.
G. petersii fish object preference varies based on the characteristics of the object [
55], which is complementary to the rodent’s variance in motivation to explore distinct novel objects in the NORT [
46]. In the future, objects with more appealing colors, materials, and shapes may be chosen. Second, the doses of psychedelic and antipsychotic drugs may be suboptimal for part of the population, creating uneven results.
G. petersii fish are known for their inter-individual differences in behavioral traits, such as boldness [
47]. In the future, doses of used substances may be modified more precisely based on the size and behavior of the tested fish.