Assessing Diadromous Fish Populations in the Lima River, Northwest Iberian Peninsula
Abstract
1. Introduction
2. Materials and Methods
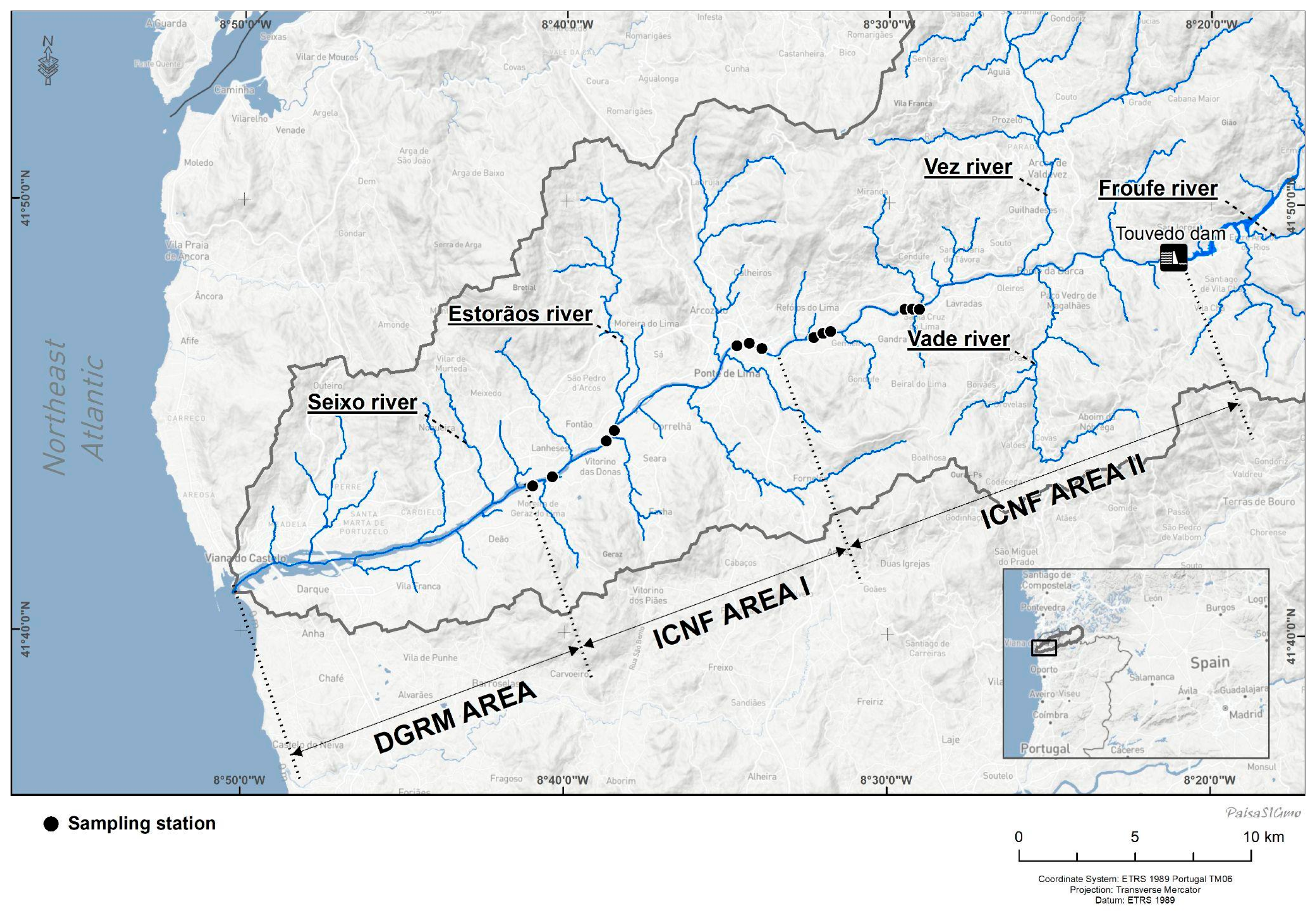
2.1. Study Area
2.2. Commercial Fishing in the Lima River
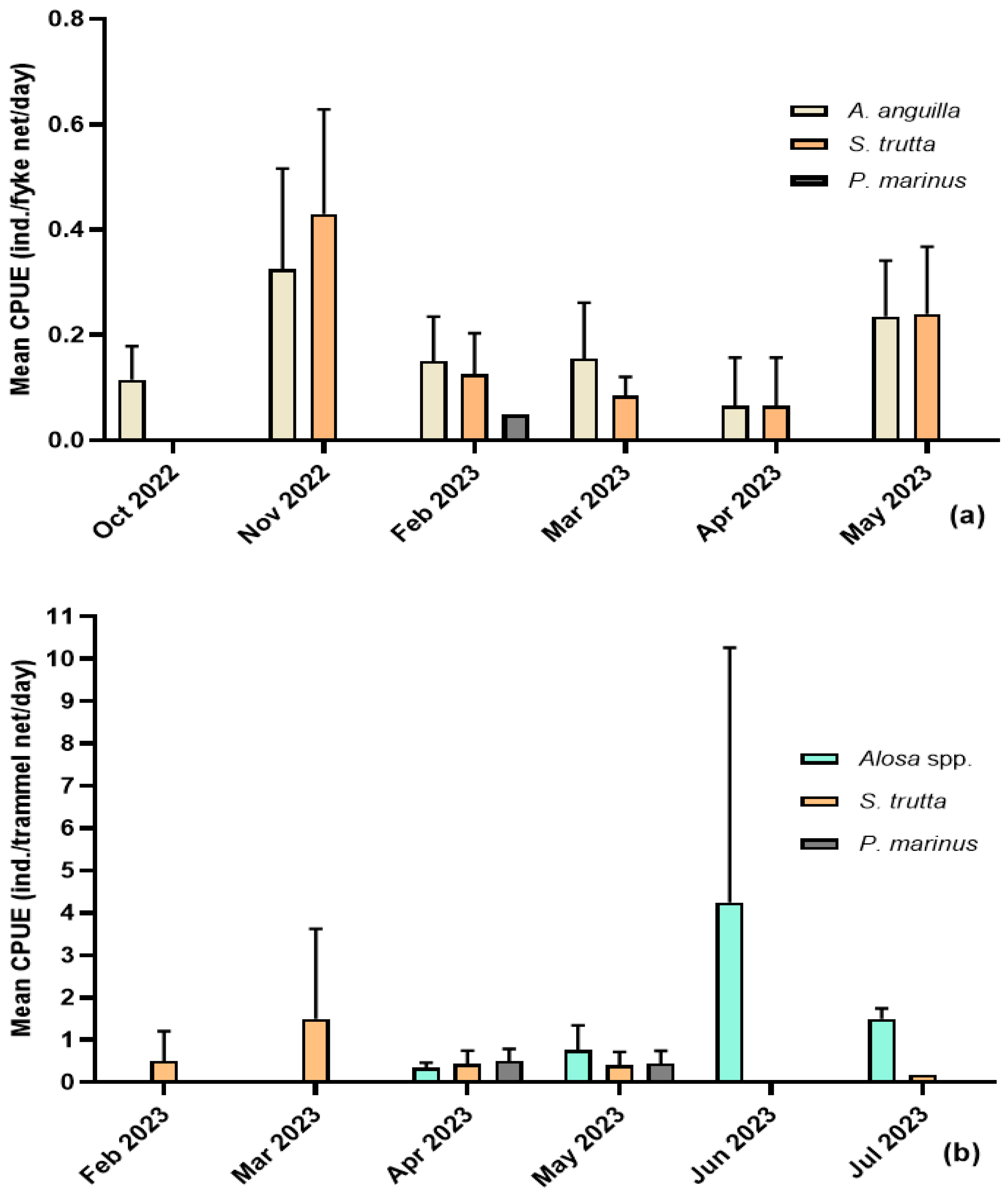
2.3. Field Sampling and Laboratory Procedures
2.4. Data Analysis
3. Results
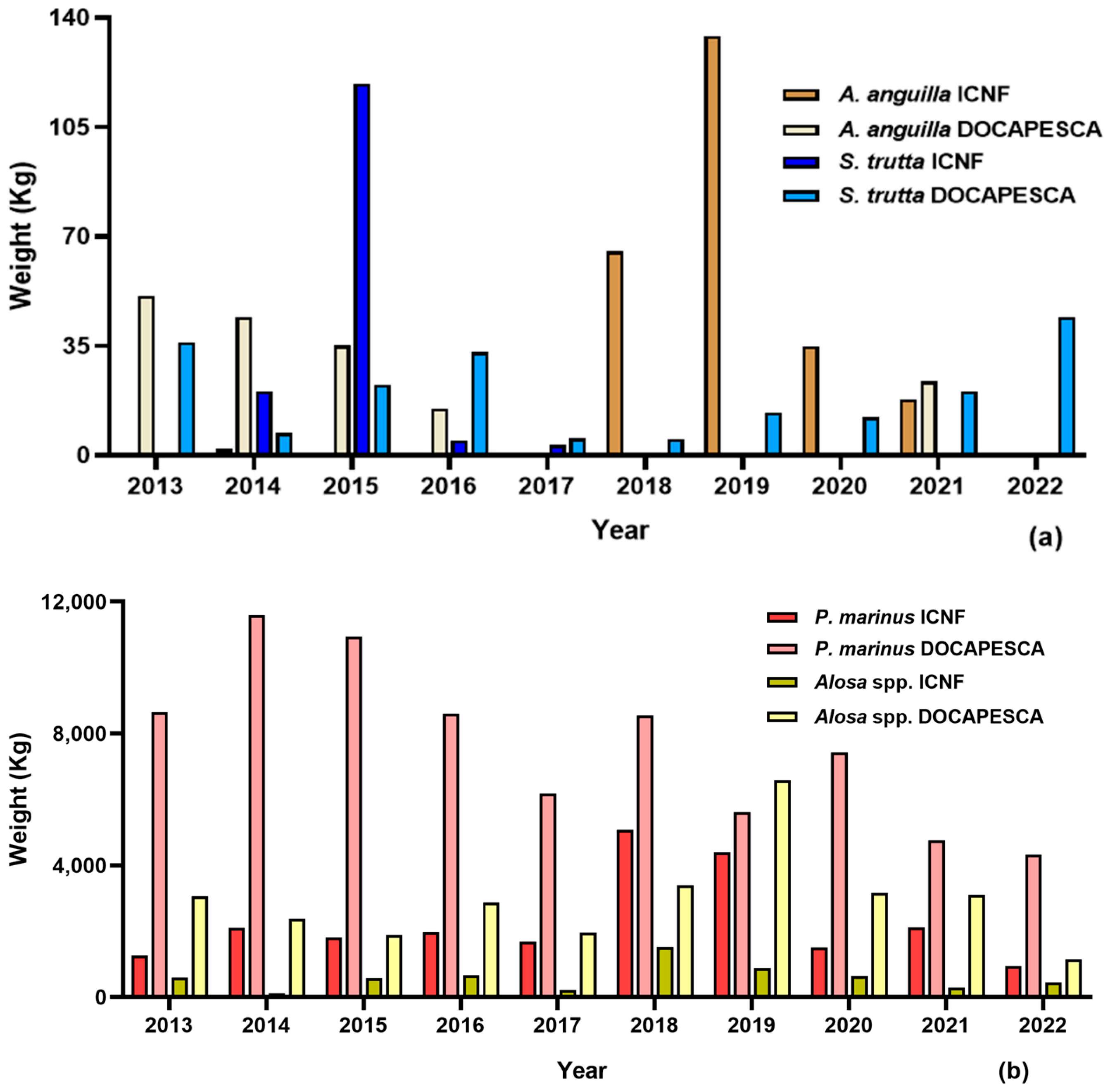
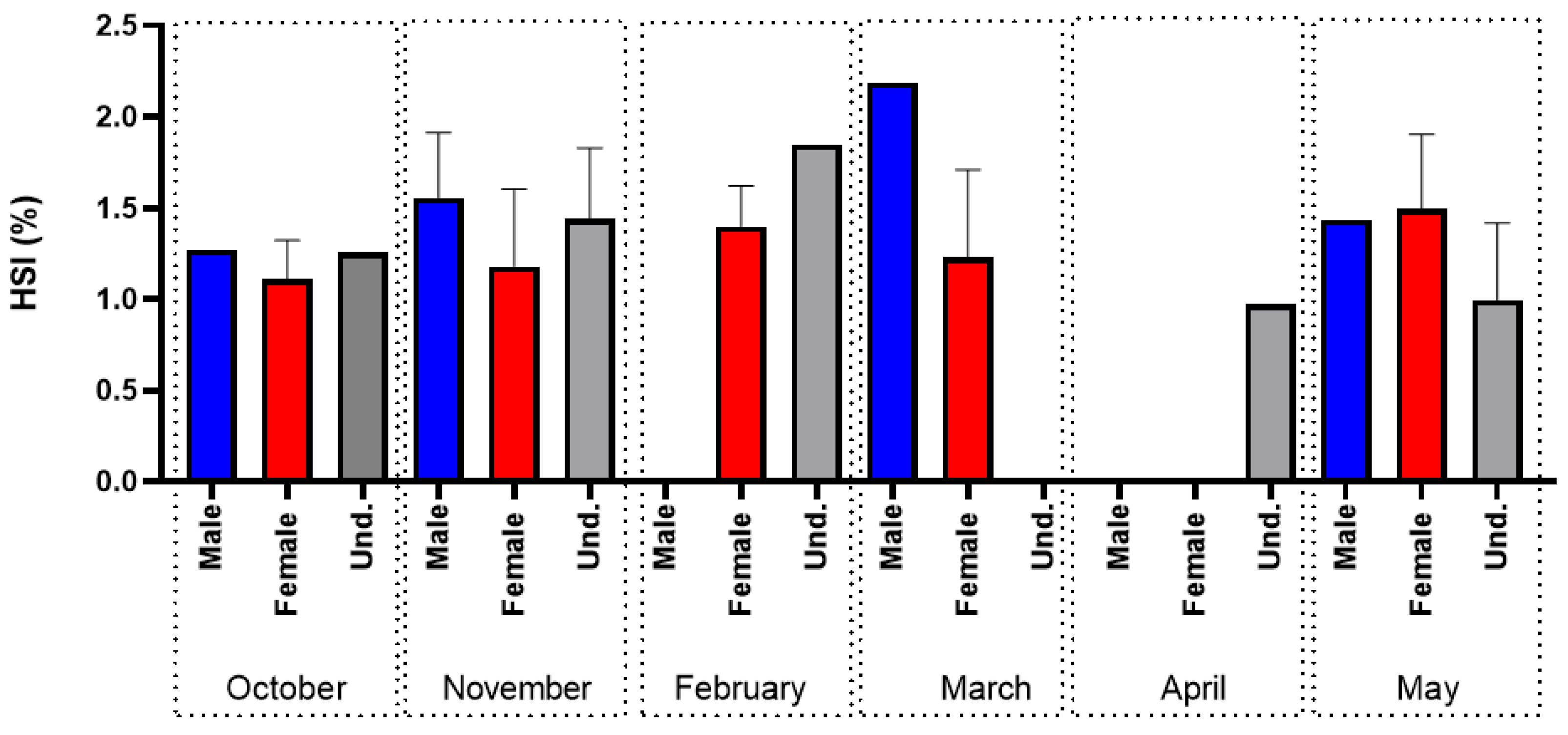
3.1. Sea Lamprey (Petromyzon marinus)
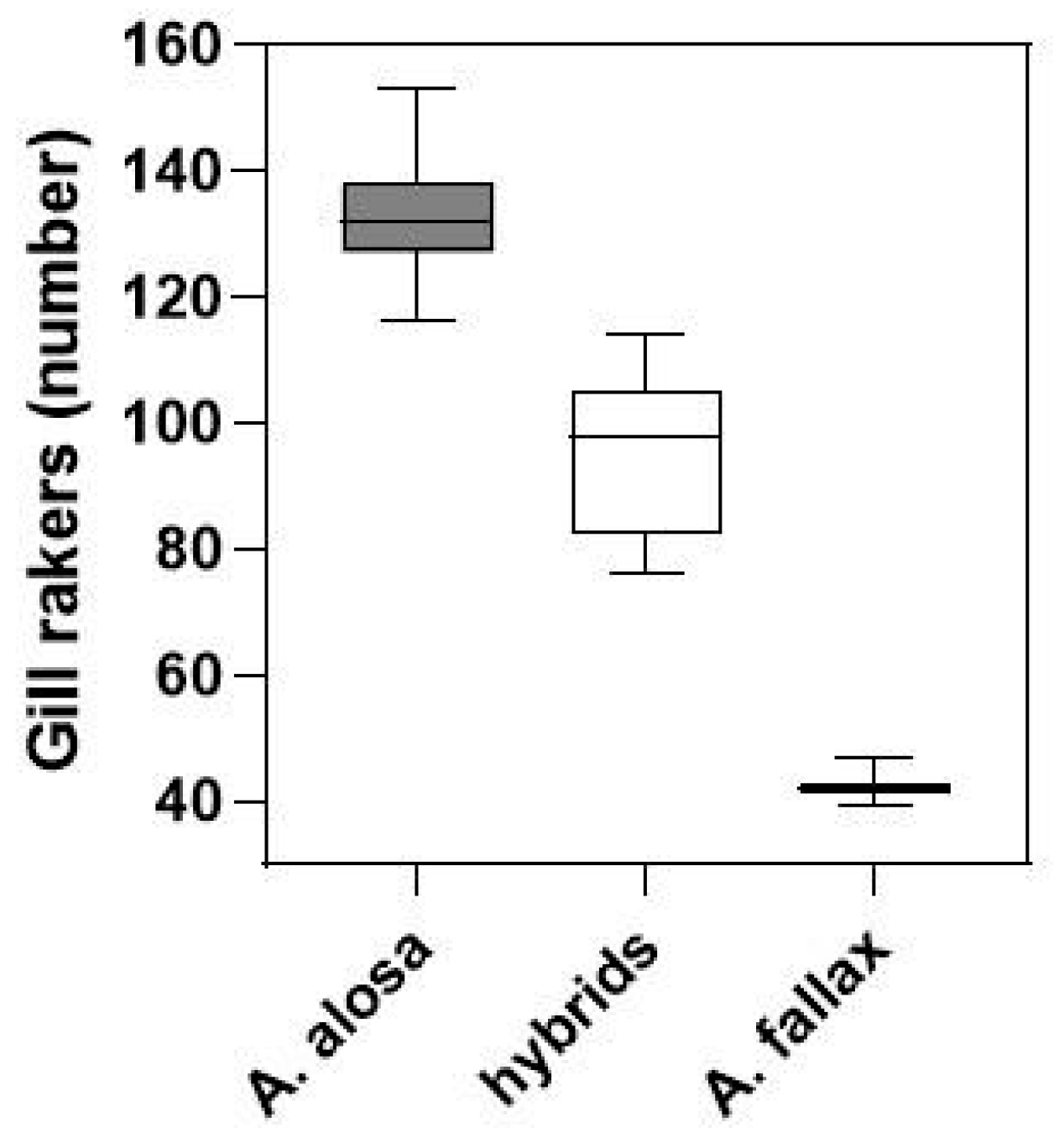
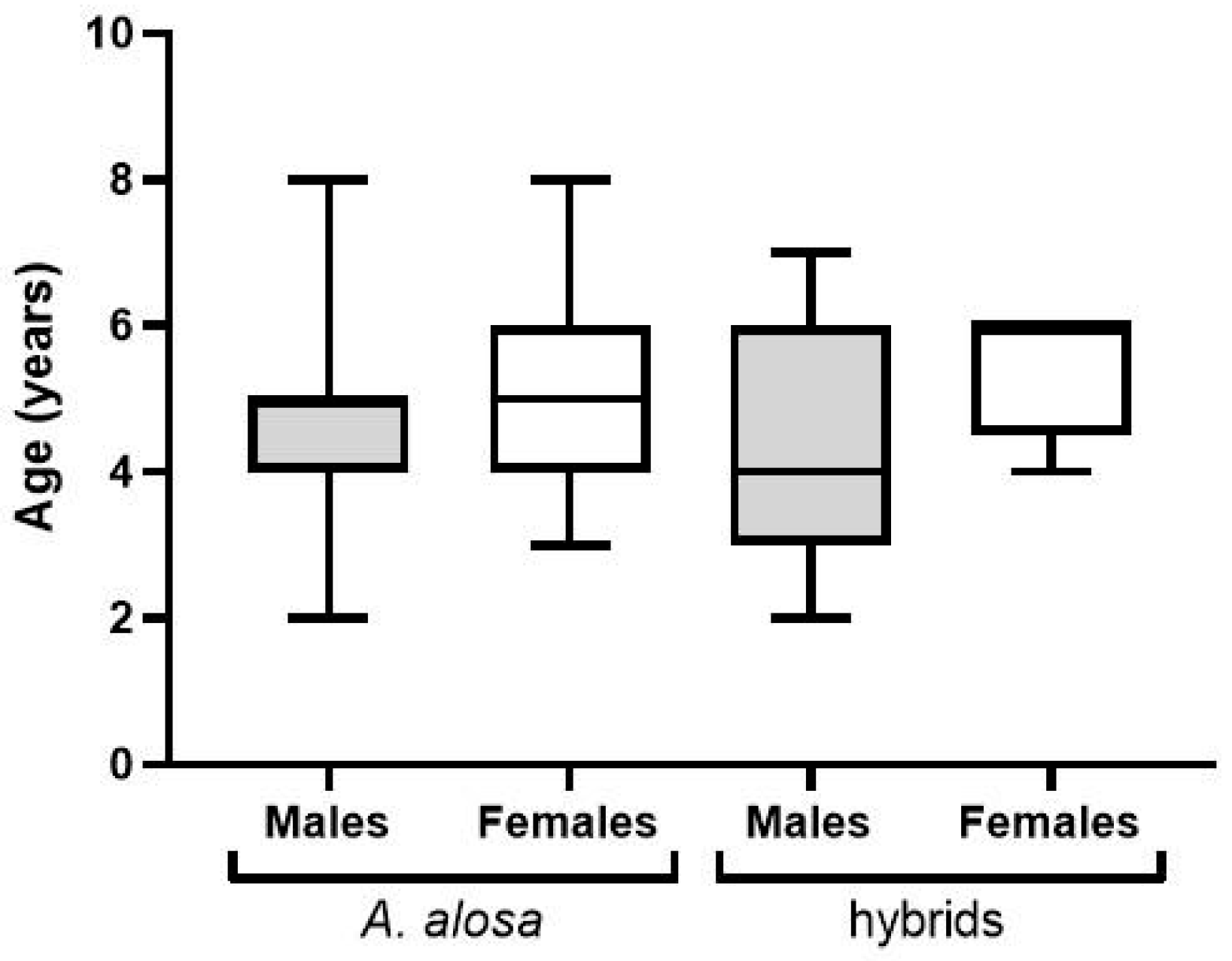
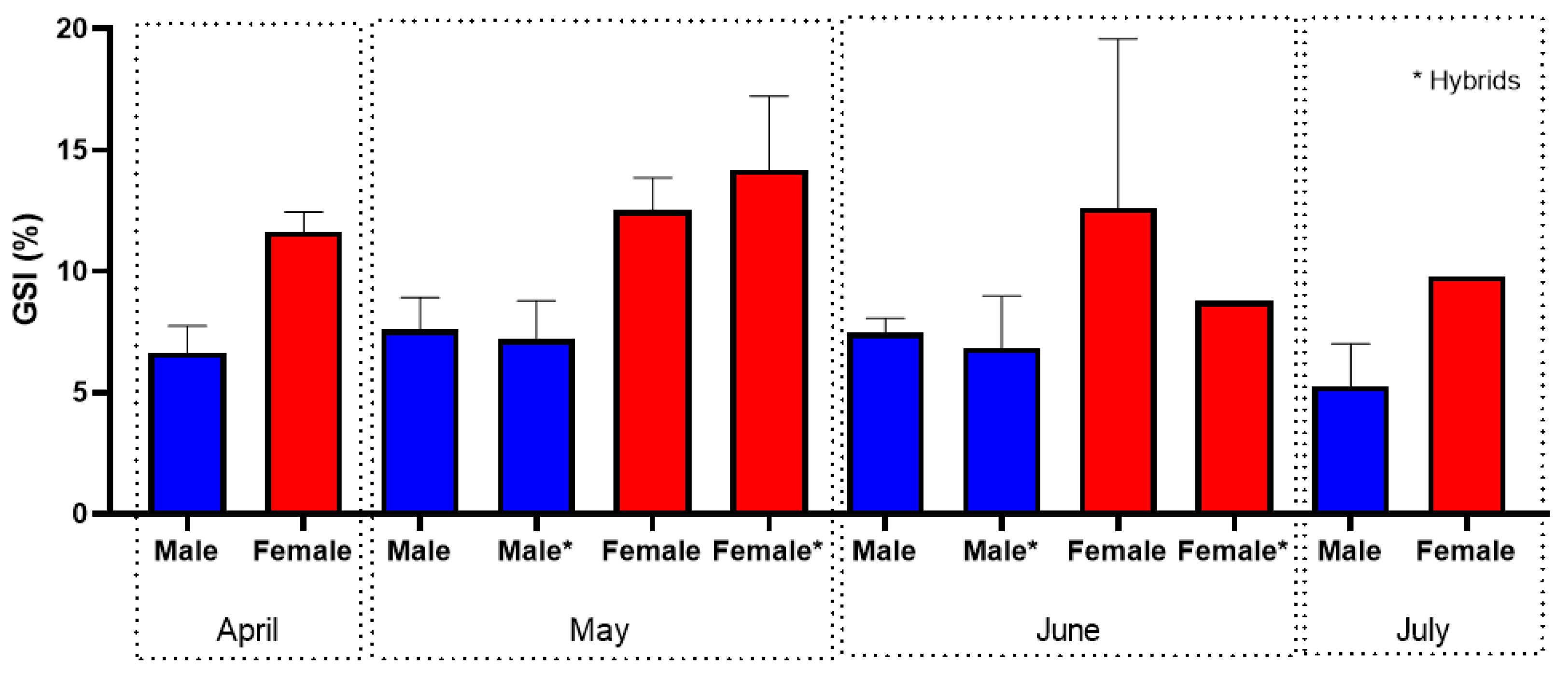
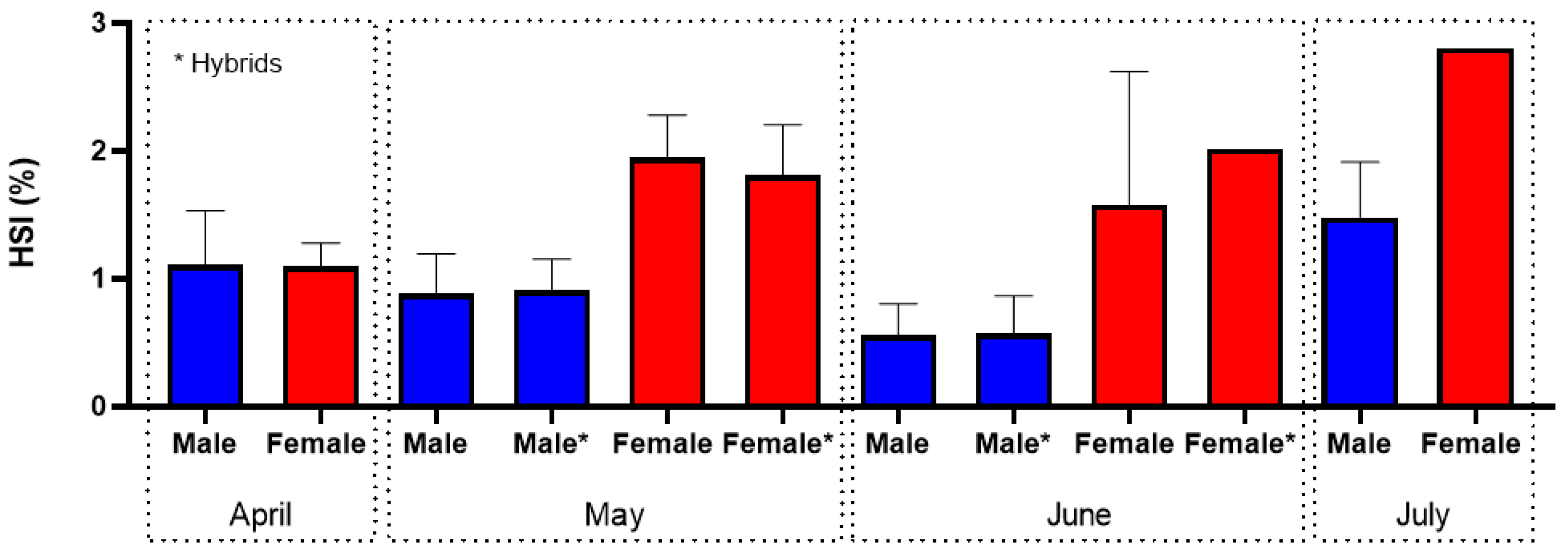
3.2. Shads (Allis Shad Alosa alosa; Twaite Shad Alosa fallax)
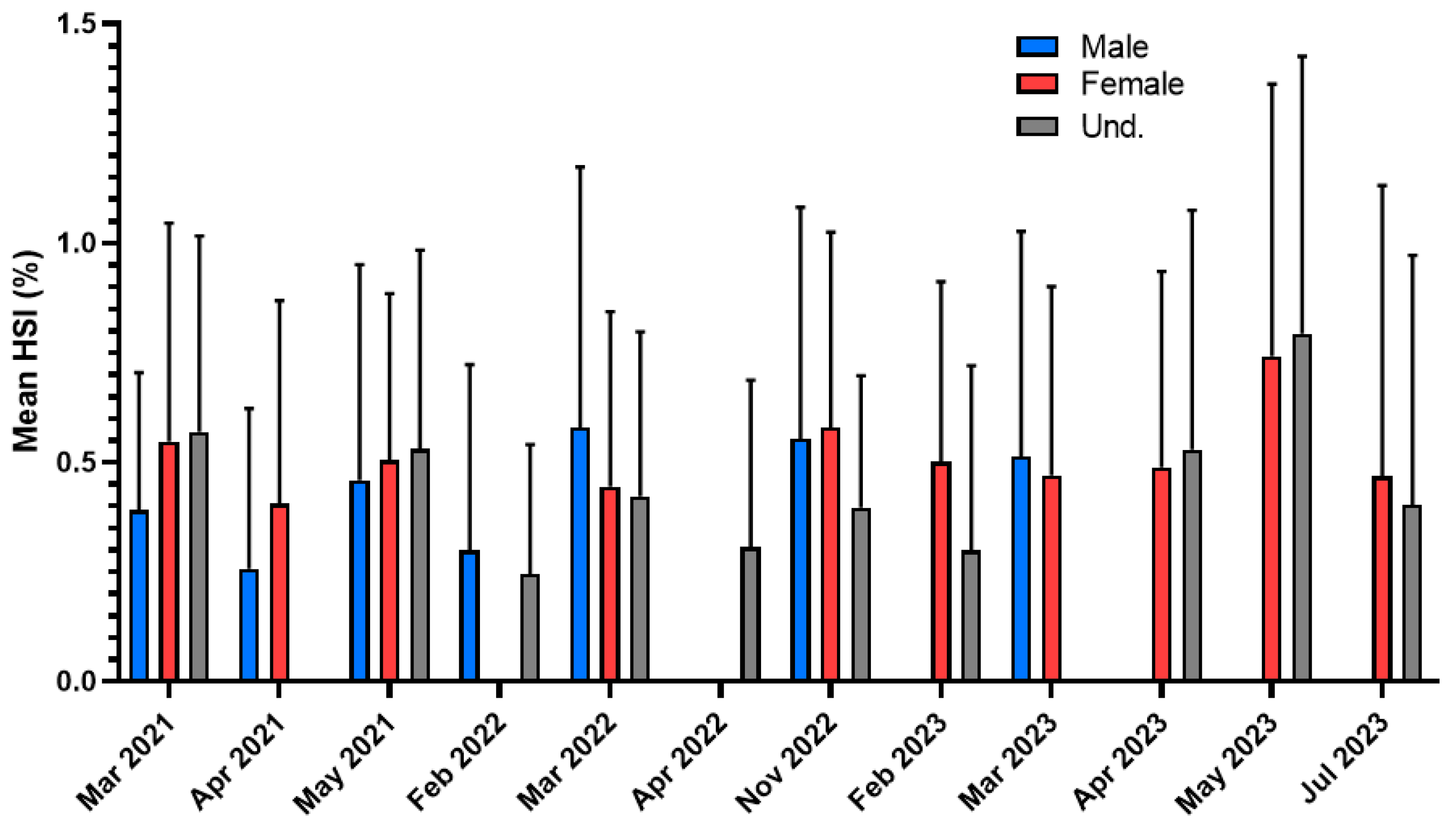
3.3. Trout (Salmo trutta)
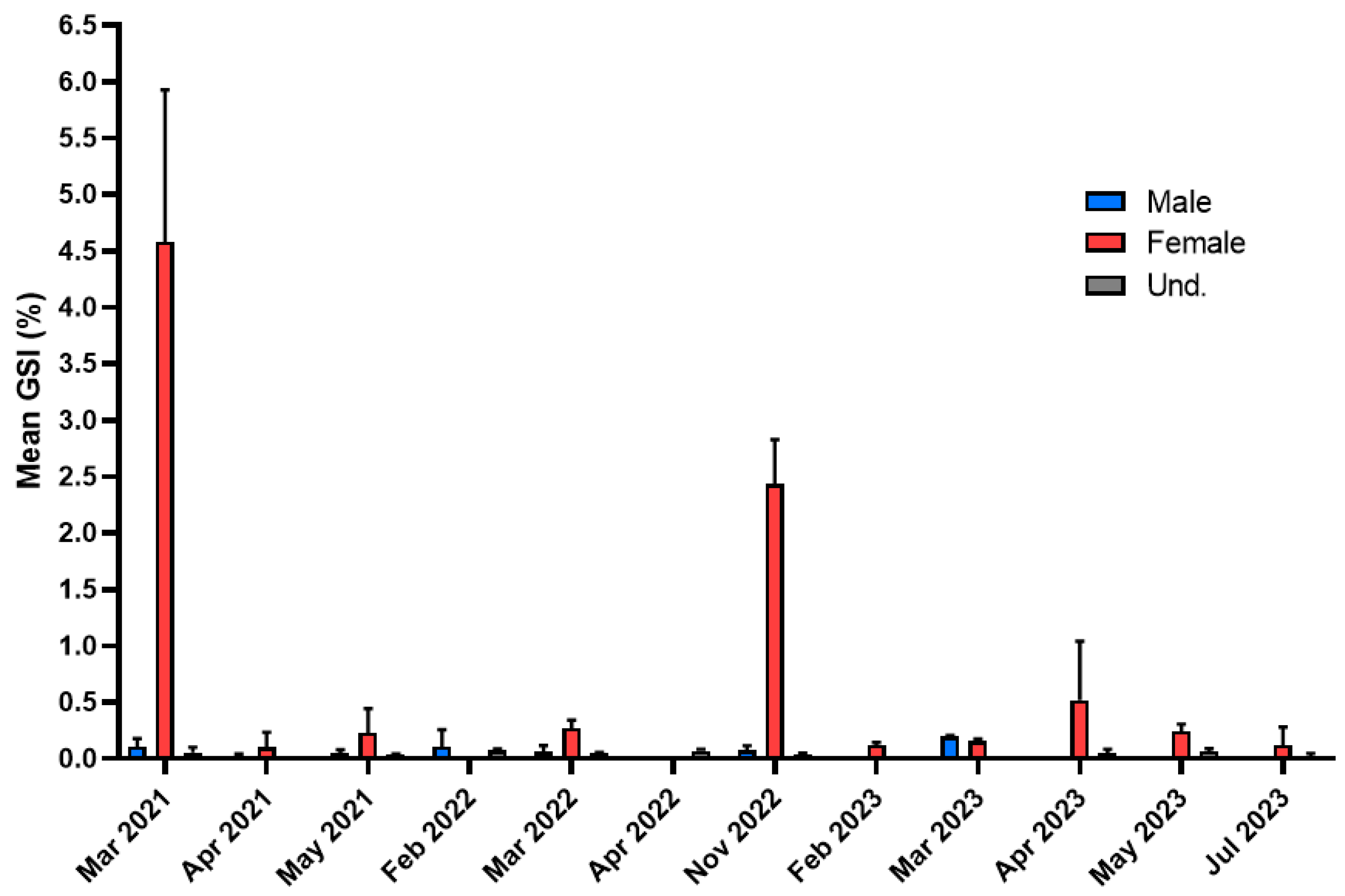
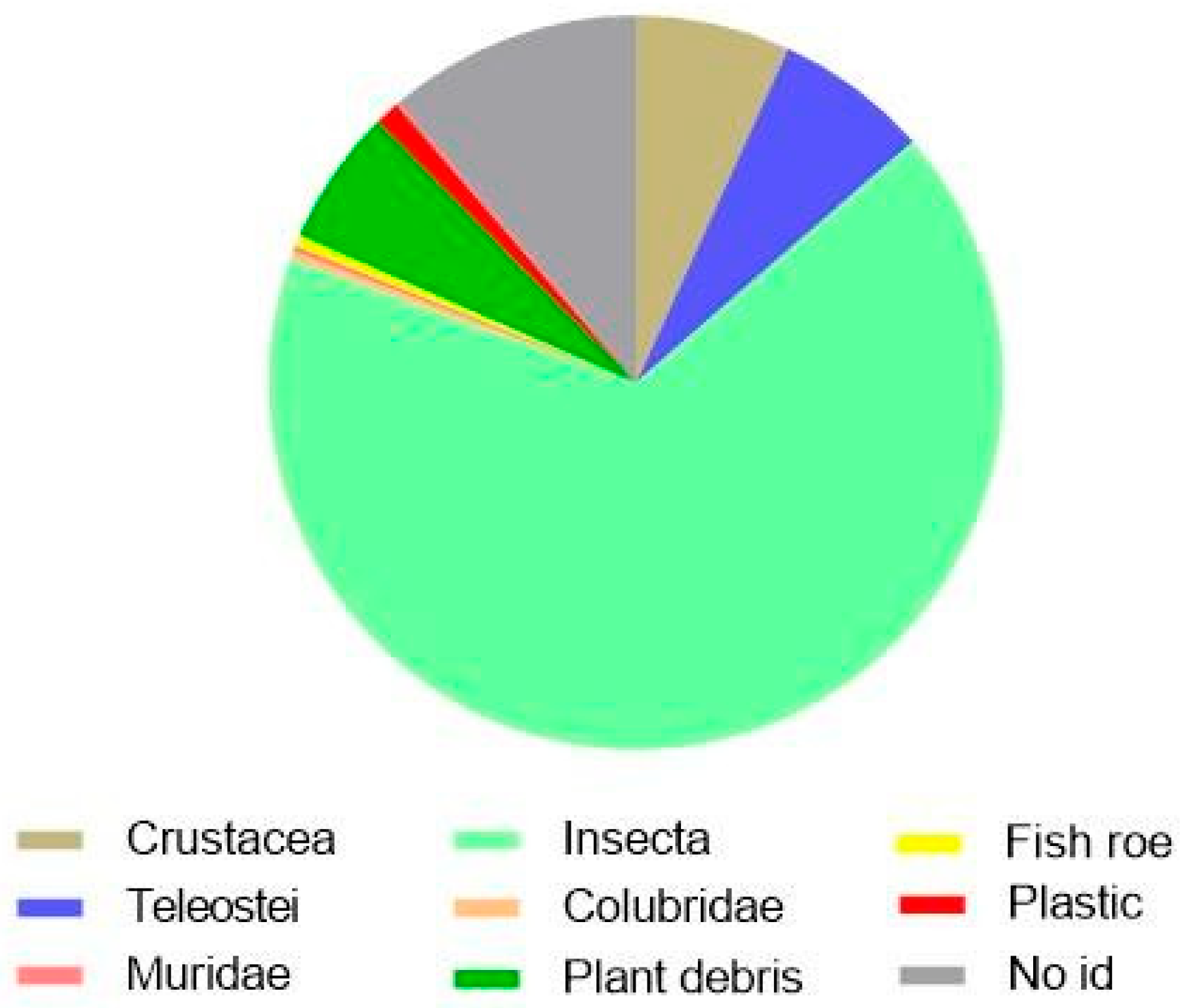
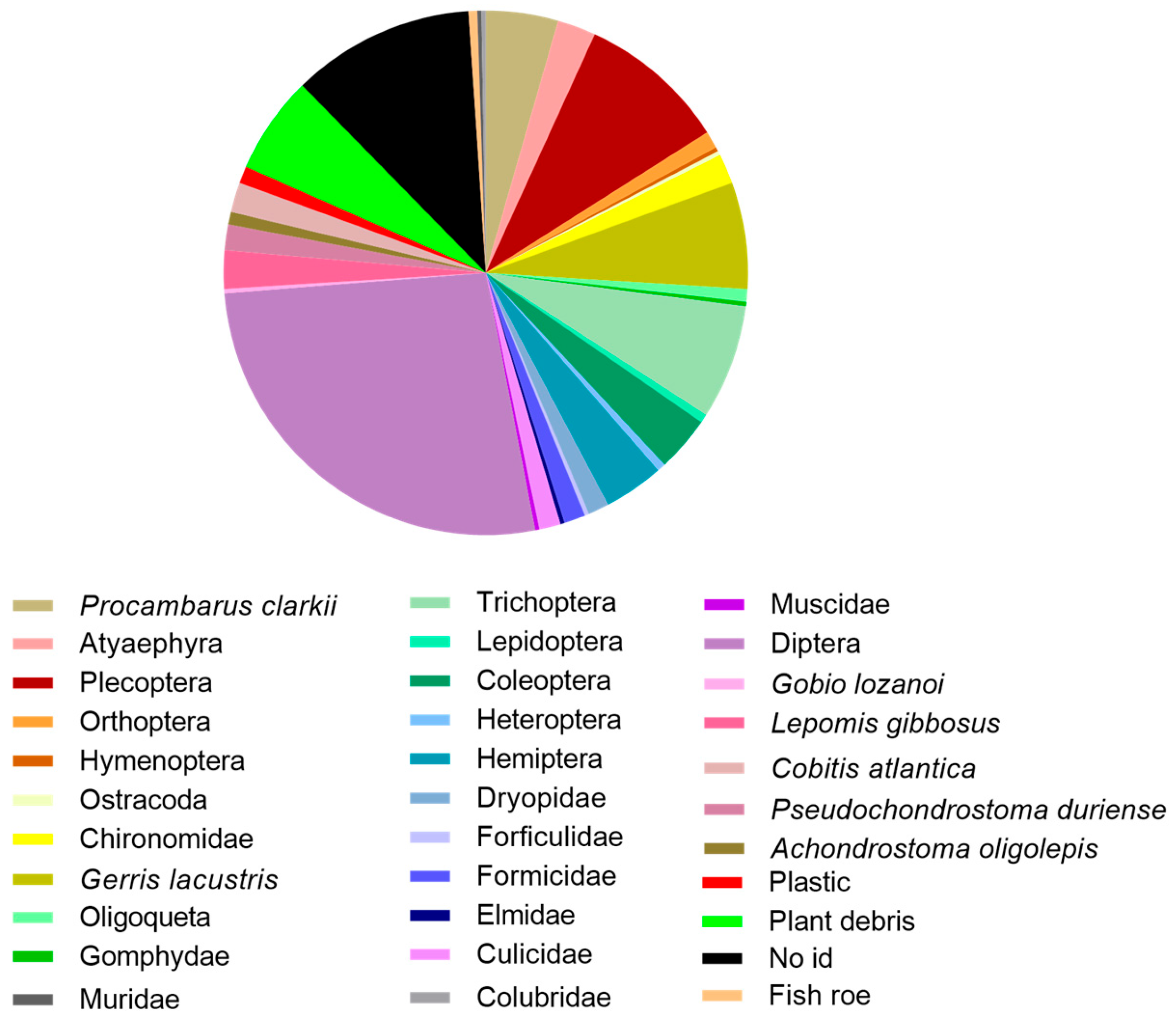
3.4. European Eel (Anguilla anguilla)
4. Discussion
4.1. Sea Lamprey (Petromyzon marinus)
4.2. Shads (Allis Shad Alosa alosa; Twaite Shad Alosa fallax)
4.3. Trout (Salmo trutta)
4.4. European Eel (Anguilla anguilla)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sampling Date | Fyke Nets | Trammel Nets | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. anguilla | S. trutta | P. marinus | Alosa spp. | S. trutta | P. marinus | |||||||
| N | CPUE | N | CPUE | N | CPUE | N | CPUE | N | CPUE | N | CPUE | |
| 12 October 2022 | 0 | 0 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 13 October 2022 | 1 | 0.5 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 15 October 2022 | 0 | 0 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 18 October 2022 | 1 | 0.17 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 21 October 2022 | 1 | 0.11 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 22 October 2022 | 1 | 0.33 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 27 October 2022 | 0 | 0 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 02 November 2022 | 1 | 0.13 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 04November 2022 | 0 | 0 | 1 | 0.5 | 0 | 0 | * | * | * | * | * | * |
| 04 November 2022 | 7 | 1.75 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 05 November 2022 | 0 | 0 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 05 November 2022 | 1 | 1 | 2 | 2 | 0 | 0 | * | * | * | * | * | * |
| 07 November 2022 | 0 | 0 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 07 November 2022 | 2 | 1 | 4 | 2 | 0 | 0 | * | * | * | * | * | * |
| 10 November 2022 | 0 | 0 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 10 November 2022 | 1 | 0.33 | 1 | 0.33 | 0 | 0 | * | * | * | * | * | * |
| 12November 2022 | 2 | 1 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 12 November 2022 | 1 | 0.5 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 19 November 2022 | 3 | 0.5 | 2 | 0.33 | 0 | 0 | * | * | * | * | * | * |
| 25November 2022 | 1 | 0.1 | 1 | 0.1 | 0 | 0 | * | * | * | * | * | * |
| 02 February 2023 | 0 | 0 | 2 | 0.33 | 0 | 0 | * | * | * | * | * | * |
| 03 February 2023 | 0 | 0 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 10 February 2023 | 4 | 0.44 | 3 | 0.33 | 0 | 0 | * | * | * | * | * | * |
| 14 February 2023 | 3 | 0.25 | 3 | 0.25 | 0 | 0 | * | * | * | * | * | * |
| 16 February 2023 | 0 | 0 | 0 | 0 | 1 | 0.25 | * | * | * | * | * | * |
| 16 February 2023 | 1 | 0.5 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 25 February 2023 | 1 | 0.11 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| 28 February 2023 | 1 | 0.11 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 04March 2023 | 1 | 0.11 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 08 March 2023 | 2 | 0.25 | 2 | 0.25 | 0 | 0 | * | * | * | * | * | * |
| 11 March 2023 | 0 | 0 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 17 March 2023 | 0 | 0 | 0 | 0 | 0 | 0 | * | * | * | * | * | * |
| 20 March 2023 | 4 | 0.33 | 1 | 0.08 | 0 | 0 | * | * | * | * | * | * |
| 25 March 2023 | * | * | * | * | * | * | 0 | 0 | 6 | 3 | 0 | 0 |
| 01 April 2023 | * | * | * | * | * | * | 0 | 0 | 4 | 2 | 1 | 0.5 |
| 02 April 2023 | * | * | * | * | * | * | 0 | 0 | 0 | 0 | 1 | 0.5 |
| 03 April 2023 | 1 | 0.13 | 1 | 0.13 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| 04 April 2023 | * | * | * | * | * | * | 0 | 0 | 1 | 0.5 | 0 | 0 |
| 05 April 2023 | * | * | * | * | * | * | 1 | 0.5 | 0 | 0 | 0 | 0 |
| 13 April 2023 | * | * | * | * | * | * | 0 | 0 | 0 | 0 | 5 | 2.5 |
| 21 April 2023 | * | * | * | * | * | * | 0 | 0 | 2 | 1 | 1 | 0.5 |
| 23 April 2023 | * | * | * | * | * | * | 0 | 0 | 1 | 0.5 | 0 | 0 |
| 24 April 2023 | * | * | * | * | * | * | 0 | 0 | 0 | 0 | 1 | 0.5 |
| 25 April 2023 | * | * | * | * | * | * | 0 | 0 | 3 | 1.5 | 0 | 0 |
| 26 April 2023 | * | * | * | * | * | * | 0 | 0 | 0 | 0 | 1 | 0.5 |
| 27 April 2023 | * | * | * | * | * | * | 0 | 0 | 0 | 0 | 3 | 1.5 |
| 28 April 2023 | * | * | * | * | * | * | 0 | 0 | 2 | 1 | 2 | 1 |
| 29 April 2023 | * | * | * | * | * | * | 3 | 1.5 | 3 | 1.5 | 3 | 1.5 |
| 02May 2023 | * | * | * | * | * | * | 3 | 1.5 | 1 | 0.5 | 0 | 0 |
| 03 May 2023 | * | * | * | * | * | * | 0 | 0 | 4 | 2 | 0 | 0 |
| 04 May 2023 | * | * | * | * | * | * | 0 | 0 | 1 | 0.5 | 2 | 1 |
| 06 May 2023 | 1 | 0.17 | 1 | 0.17 | 0 | 0 | 1 | 0.5 | 0 | 0 | 0 | 0 |
| 07 May 2023 | * | * | * | * | * | * | 6 | 3 | 1 | 0.5 | 3 | 1.5 |
| 08 May 2023 | * | * | * | * | * | * | 2 | 1 | 2 | 1 | 3 | 1.5 |
| 09 May 2023 | * | * | * | * | * | * | 1 | 0.5 | 0 | 0 | 0 | 0 |
| 10 May 2023 | * | * | * | * | * | * | 5 | 2.5 | 4 | 2 | 0 | 0 |
| 11 May 2023 | 8 | 0.89 | 7 | 0.78 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1.5 |
| 12 May 2023 | * | * | * | * | * | * | 1 | 0.5 | 0 | 0 | 0 | 0 |
| 13 May 2023 | * | * | * | * | * | * | 2 | 1 | 2 | 1 | 0 | 0 |
| 16 May 2023 | * | * | * | * | * | * | 3 | 1.5 | 0 | 0 | 0 | 0 |
| 17 May 2023 | 2 | 0.13 | 3 | 0.2 | 0 | 0 | 5 | 2.5 | 0 | 0 | 0 | 0 |
| 19 May 2023 | * | * | * | * | * | * | 9 | 4.5 | 2 | 1 | 1 | 0.5 |
| 20 May 2023 | 1 | 0.17 | 1 | 0.17 | 0 | 0 | * | * | * | * | * | * |
| 21 May 2023 | * | * | * | * | * | * | 2 | 1 | 1 | 0.5 | 0 | 0 |
| 24 May 2023 | * | * | * | * | * | * | 3 | 1.5 | 1 | 0.5 | 0 | 0 |
| 25 May 2023 | * | * | * | * | * | * | 6 | 3 | 0 | 0 | 0 | 0 |
| 26 May 2023 | * | * | * | * | * | * | 2 | 1 | 0 | 0 | 0 | 0 |
| 30 May 2023 | * | * | * | * | * | * | 0 | 0 | 0 | 0 | 5 | 2.5 |
| 23 June 2023 | * | * | * | * | * | * | 17 | 8.5 | 0 | 0 | 0 | 0 |
| 13 June 2023 | * | * | * | * | * | * | 8 | 4 | 1 | 0.5 | 0 | 0 |
| 14 June 2023 | * | * | * | * | * | * | 0 | 0 | 0 | 0 | 0 | 0 |
| 27 June 2023 | * | * | * | * | * | * | 0 | 0 | 1 | 0.5 | 0 | 0 |
| Age | Lima River | Tributaries | ||
|---|---|---|---|---|
| Otolith Radius (mm) | N | Otolith Radius (mm) | N | |
| 0 | * | * | 0.34 ± 0.03 | 4 |
| 1 | 0.40 | 1 | 0.46 ± 0.08 | 15 |
| 2 | 0.79 | 1 | 0.78 ± 0.18 | 13 |
| 3 | 1.14 ± 0.20 | 6 | 0.99 ± 0.10 | 7 |
| 4 | 1.32 ± 0.14 | 6 | 1.08 ± 0.11 | 6 |
| 5 | 1.38 ± 0.19 | 15 | * | * |
| 6 | 1.68 ± 0.20 | 8 | * | * |
| 7 | 1.68 ± 0.27 | 8 | * | * |
| 8 | 1.89 ± 0.15 | 3 | 1.52 | 1 |
| 9 | 2.24 ± 0.16 | 2 | * | * |
| 10 | 2.20 ± 0.17 | 2 | * | * |
| Variable | TL (cm) | TW (g) | K | Parasite Number | Parasite Stage | SDI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| L3 | L4 | Male | Female | |||||||
| TL (cm) | 1.000 | 0.968 | 0.357 ** | 0.281 * | −0.149 | 0.122 | 0.333 * | 0.259 | 0.160 | |
| TW (g) | 0.968 | 1.000 | 0.529 ** | 0.241 | −0.197 | 0.101 | 0.281 * | 0.244 | 0.210 | |
| K | 0.357 ** | 0.529 ** | 1.000 | −0.044 | −0.123 | 0.001 | −0.132 | 0.053 | 0.261 | |
| Parasite number | 0.281 * | 0.241 | −0.044 | 1.000 | 0.103 | 0.476 ** | 0.775 ** | 0.858 ** | 0.063 | |
| Parasite stage | L3 | −0.149 | −0.197 | −0.123 | 0.103 | 1.000 | 0.067 | −0.107 | −0.059 | −0.170 |
| L4 | 0.122 | 0.101 | 0.001 | 0.476 | 0.067 | 1.000 | 0.159 | 0.118 | 0.002 | |
| Male | 0.333 * | 0.281 * | −0.132 | 0.775 | −0.107 | 0.159 | 1.000 | 0.655 ** | −0.053 | |
| Female | 0.259 | 0.244 | 0.053 | 0.858 | −0.059 | 0.118 | 0.655 ** | 1.000 | 0.160 | |
| SDI | 0.160 | 0.210 | 0.261 | 0.063 | −0.170 | 0.002 | −0.053 | 0.160 | 1.000 | |
| Variable | TL (cm) | TW (g) | K | Parasite Number | Parasite Stage | SDI | |||
|---|---|---|---|---|---|---|---|---|---|
| L4 | Male | Female | |||||||
| TL (cm) | 1.000 | 0.606 ** | 0.111 | 0.188 | −0.017 | 0.123 | 0.254 | 0.321 * | |
| TW (g) | 0.606 ** | 1.000 | 0.192 | 0.356 * | −0.139 | 0.222 | 0.556** | 0.445 ** | |
| K | 0.111 | 0.192 | 1.000 | 0.098 | −0.005 | −0.005 | 0.226 | −0.041 | |
| Parasite number | 0.188 | 0.356 * | 0.098 | 1.000 | 0.412 ** | 0.884 ** | 0.739 ** | 0.128 | |
| Parasite stage | L4 | −0.017 | −0.139 | −0.005 | 0.412 ** | 1.000 | 0.212 | 0.033 | −0.052 |
| Male | 0.123 | 0.222 | −0.005 | 0.884 ** | 0.212 | 1.000 | 0.439 ** | 0.073 | |
| Female | 0.254 | 0.556 ** | 0.226 | 0.739 ** | 0.033 | 0.439 ** | 1.000 | 0.212 | |
| SDI | 0.321 * | 0.445 ** | −0.041 | 0.128 | −0.052 | 0.073 | 0.212 | 1.000 | |
References
- Guidetti, P.; Fanelli, G.; Fraschetti, S.; Terlizzi, A.; Boero, F. Coastal fish indicate human-induced changes in the Mediterranean littoral. Mar. Environ. Res. 2002, 53, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Moraga, D.; Vivancos, A.; Ruiz, V.H.; Rojas, O.; Díaz, G.; Manosalva, A.; Vega, P.; Habit, E. A century of anthropogenic river alterations in a highly diverse river coastal basin: Effects on fish assemblages. Front. Environ. Sci. 2022, 10, 943586. [Google Scholar] [CrossRef]
- Craig, J.F. Introduction. In Freshwater Fisheries Ecology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–4. [Google Scholar]
- Hughes, R.M. Recreational fisheries in the USA: Economics, management strategies, and ecological threats. Fish. Sci. 2015, 81, 1–9. [Google Scholar] [CrossRef]
- Helfman, G. Fish Conservation: A Guide to Understanding and Restoring Global Aquatic Biodiversity and Fishery Resources; Bibliovault OAI Repository, the University of Chicago Press: Chicago, IL, USA, 2008. [Google Scholar] [CrossRef]
- Darwall, W.R.T.; Freyhof, J. Lost fishes, who is counting? The extent of the threat to freshwater fish biodiversity. In Conservation of Freshwater Fishes; Closs, G.P., Krkosek, M., Olden, J.D., Eds.; Conservation Biology; Cambridge University Press: Cambridge, UK, 2015; pp. 1–36. [Google Scholar]
- Thibault, M. Éléments de la problématique du saumon Atlantique en France. In La Restauration des Rivières à Saumon; Thibault, M., Billard, R., Eds.; INRA: Paris, France, 1987; pp. 413–425. [Google Scholar]
- Dadswell, M.; Spares, A.; Reader, J.; McLean, M.; McDermott, T.; Samways, K.; Lilly, J. The Decline and Impending Collapse of the Atlantic Salmon (Salmo salar) Population in the North Atlantic Ocean: A Review of Possible Causes. Rev. Fish. Sci. Aquac. 2021, 30, 215–258. [Google Scholar] [CrossRef]
- Denis, J.; Lepage, M.; Gruselle, M.-C.; Amara, R. The Influence of Natural and Anthropogenic Environmental Pressures on European Eel Abundances in French Estuaries. Fishes 2024, 9, 44. [Google Scholar] [CrossRef]
- Pelster, B. Swimbladder Function in the European Eel Anguilla anguilla. Fishes 2023, 8, 125. [Google Scholar] [CrossRef]
- Mota, M.; Bio, A.; Bao, M.; Pascual, S.; Rochard, E.; Antunes, C. New insights into biology and ecology of the Minho River Allis shad (Alosa alosa L.): Contribution to the conservation of one of the last European shad populations. Rev. Fish Biol. Fisher 2015, 25, 395–412. [Google Scholar] [CrossRef]
- Azeiteiro, U.M.; Pereira, M.J.; Soares, A.M.V.M.; Braga, H.O.; Morgado, F.; Sousa, M.C.; Dias, J.M.; Antunes, C. Dynamics of Two Anadromous Species in a Dam Intersected River: Analysis of Two 100-Year Datasets. Fishes 2021, 6, 21. [Google Scholar] [CrossRef]
- Antunes, C.; Cobo, F.; Araújo, M.J. Iberian inland fisheries. In Freshwater Fisheries Ecology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 268–282. [Google Scholar]
- Chang, H.Y.; McKown, K.; Chen, Y. A long-term ichthyoplankton monitoring program suggests climate-induced environmental variabilities changed fish communities in the Hudson River estuary. Front. Mar. Sci. 2023, 9, 1077997. [Google Scholar] [CrossRef]
- Palmer, M.A.; Lettenmaier, D.P.; Poff, N.L.; Postel, S.L.; Richter, B.; Warner, R. Climate Change and River Ecosystems: Protection and Adaptation Options. Environ. Manag. 2009, 44, 1053–1068. [Google Scholar] [CrossRef]
- Sousa, J. Fase larvar e metamorfose da população de lampreia (Petromyzon marinus L.) do Rio Lima. Publicações avulsas INIP 1992, 17, 199–227. [Google Scholar]
- Costa, M.J.; Almeida, P.R.; Domingos, I.M.; Costa, J.L.; Correia, M.J.; Chaves, M.L.; Teixeira, C.M. Present status of the main shads’ populations in Portugal. Bull. Fr. Pêche Piscic. 2001, 1109–1116. [Google Scholar] [CrossRef]
- Valente, A. Trout populations in the lima basin, north Portugal. Management of Freshwater Fisheries. In Proceedings of the Symposium EIFAC, Gothenburg, Sweden, 31 May–3 June 1988; Van Densen, W.L., Steinmetz, T.B., Hugues, R.H., Eds.; pp. 437–446. [Google Scholar]
- Sá, F.M.S. Estado de Populações de truta-de-rio em Portugal: Influência de Fatores Ambientais, Bióticos e do Ordenamento da Pesca. Master’s Thesis, Universidade de Lisboa Portugal, Lisboa, Portugal, 2021. [Google Scholar]
- Maia, C.; Valente, A. The brown trout Salmo trutta L. populations in the river Lima catchment. Limnetica 1999, 17, 119–126. [Google Scholar] [CrossRef]
- Santos, J.M.; Ferreira, M.T.; Godinho, F.N.; Bochechas, J. Performance of fish lift recently built at the Touvedo Dam on the Lima River, Portugal. J. Appl. Ichthyol. 2002, 18, 118–123. [Google Scholar] [CrossRef]
- Santos, J.M.; Godinho, F.; Ferreira, M.T.; Cortes, R. The organisation of fish assemblages in the regulated Lima basin, Northern Portugal. Limnologica 2004, 34, 224–235. [Google Scholar] [CrossRef]
- Mameri, D.; Rivaes, R.; Oliveira, J.M.; Pádua, J.; Ferreira, M.T.; Santos, J.M. Passability of Potamodromous Species through a Fish Lift at a Large Hydropower Plant (Touvedo, Portugal). Sustainability 2020, 12, 172. [Google Scholar] [CrossRef]
- Baeta, A.; Vieira, L.R.; Lírio, A.V.; Canhoto, C.; Marques, J.C.; Guilhermino, L. Use of stable isotope ratios of fish larvae as indicators to assess diets and patterns of anthropogenic nitrogen pollution in estuarine ecosystems. Ecol. Indic. 2017, 83, 112–121. [Google Scholar] [CrossRef]
- Pereira, H.; Sousa, M.C.; Vieira, L.R.; Morgado, F.; Dias, J.M. Modelling Salt Intrusion and Estuarine Plumes under Climate Change Scenarios in Two Transitional Ecosystems from the NW Atlantic Coast. J. Mar. Sci. Eng. 2022, 10, 262. [Google Scholar] [CrossRef]
- Caetano, M.; Raimundo, J.; Nogueira, M.; Santos, M.; Mil-Homens, M.; Prego, R.; Vale, C. Defining benchmark values for nutrients under the Water Framework Directive: Application in twelve Portuguese estuaries. Mar. Chem. 2016, 185, 27–37. [Google Scholar] [CrossRef]
- Costa, N.C.; Venâncio, S.; Pinho, J.L.; Vieira, J.M. Morphodynamics of the River Lima Estuary (NW Portugal). In Proceedings of the 2nd International Conference on Integrated Environmental Management for Sustainable Development, Hammamet, Tunisia, 27–30 October 2016. [Google Scholar]
- Oliveira, V.H.; Sousa, M.C.; Picado, A.; Mendes, R.; Ribeiro, A.S.; Morgado, F.; Dias, J.M. Coupled modelling of the interaction between dissolved substances emitted by Minho and Lima estuarine outflows (Portugal). J. Mar. Syst. 2021, 222, 103601. [Google Scholar] [CrossRef]
- Pinho, J.L.S.; Vieira, J.M.P. Mathematical Modelling of Salt Water Intrusion in a Northern Portuguese Estuary; IAHS-Aish Press: Wallingford UK, 2007; Volume 310, p. 277. [Google Scholar]
- Rocha, M.J.; Cruzeiro, C.; Reis, M.; Pardal, M.A.; Rocha, E. Spatial and seasonal distribution of 17 endocrine disruptor compounds in an urban estuary (Mondego River, Portugal): Evaluation of the estrogenic load of the area. Environ. Monit. Assess 2014, 186, 3337–3350. [Google Scholar] [CrossRef]
- Fais, M.; Bellisario, B.; Duarte, S.; Vieira, P.E.; Sousa, R.; Canchaya, C.; Costa, F.O. Meiofauna metabarcoding in Lima estuary (Portugal) suggests high taxon replacement within a background of network stability. Reg. Stud. Mar. Sci. 2020, 38, 101341. [Google Scholar] [CrossRef]
- Sousa, R.; Dias, S.; Antunes, J.C. Spatial Subtidal Macrobenthic Distribution in Relation to Abiotic Conditions in the Lima Estuary, NW of Portugal. Hydrobiologia 2006, 559, 135–148. [Google Scholar] [CrossRef]
- Ilarri, M.; Souza, A.T.; Dias, E.; Antunes, C. Influence of climate change and extreme weather events on an estuarine fish community. Sci. Total Environ. 2022, 827, 154190. [Google Scholar] [CrossRef]
- Tesch, F.W. Age and growth. In Methods for Assessment of Fish Production in Fresh Waters; Ricker, W.E., Ed.; Blackwell Scientific Publications: Oxford, UK, 1971; pp. 99–130. [Google Scholar]
- Pauly, D. A mechanism for the juvenile-to-adult transition in fishes. ICES J. Mar. Sci. 1984, 41, 280–284. [Google Scholar] [CrossRef]
- Fulton, T.W. The Rate of Growth of Fishes. In 20th Annual Report of the Fishery Board of Scotland; London, 1902; Volume 3, pp. 326–446. [Google Scholar]
- Sindhe, V.R.; Kulkarni, R.S. Gonadosomatic and hepatosomatic indices of the freshwater fish Notopterus notopterus (Pallas) in response to some heavy metal exposure. J. Environ. Biol. 2004, 25, 365–368. [Google Scholar]
- Karaytug, S.; Erdem, C.; Cicik, B.; Ay, Ö. Effects of copper on hepatosomatic index, gonadosomatic index and condition factor of Oreochromis niloticus (L. 1758). Fresen Environ. Bull. 2007, 16, 1355–1358. [Google Scholar]
- Alexandrino, P.; Faria, R.; Linhares, D.; Castro, F.; Le Corre, M.; Sabatié, R.; Baglinière, J.L.; Weiss, S. Interspecific differentiation and intraspecific substructure in two closely related clupeids with extensive hybridization, Alosa alosa and Alosa fallax. J. Fish Biol. 2006, 69, 242–259. [Google Scholar] [CrossRef]
- Baglinière, J.L.; Sabatié, M.R.; Aprahamian, M.W.; Alexandrino, P.; Aprahamian, C.D.; Assis, C.A.; Cassou-Leins, J.J.; Le Corre, M.; Mennesson-Boisneau, C.; Martin-Vandembulcke, D.; et al. A guide to scale interpretation and age estimation for the East-Atlantic and West-Mediterranean shads (Alosa spp.). Bull. Fr. Peche Piscic 2001, 357–360, 496–531. [Google Scholar]
- Panfili, J.; De Pontual, H.; Troadec, H.; Wright, P. Manual of Fish Sclerochronology; Ifremer-IRD coedition: Brest, France, 2002; Volume 2004. [Google Scholar]
- ICES. Third Workshop on Age Reading of European and American Eel (WKAREA3). ICES Sci. Rep./Rapp. Sci. Du CIEM 2020, 2, 34. [Google Scholar] [CrossRef]
- Vigliola, L.; Meekan, M. The Back-Calculation of Fish Growth From Otoliths. In Tropical Fish Otoliths: Information for Assessment, Management and Ecology; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 174–211. [Google Scholar]
- Moura, A.; Dias, E.; López, R.; Antunes, C. Regional Population Structure of the European Eel at the Southern Limit of Its Distribution Revealed by Otolith Shape Signature. Fishes 2022, 7, 135. [Google Scholar] [CrossRef]
- Mota, M. Biology and Ecology of the Allis Shad, Alosa alosa (Linnaeus, 1758), in the Minho River. Ph.D Thesis, University of Porto, Porto, Portugal, 2014; p. 200. [Google Scholar]
- Kara, C.; Alp, A. Feeding habits and diet composition of brown trout (Salmo trutta) in the upper streams of River Ceyhan and River Euphrates in Turkey. Turk J. Vet. Anim. Sci. 2005, 29, 417–428. [Google Scholar]
- Durif, C.M.F.; Diserud, O.H.; Sandlund, O.T.; Thorstad, E.B.; Poole, R.; Bergesen, K.; Escobar-Lux, R.H.; Shema, S.; Vollestad, L.A. Age of European silver eels during a period of declining abundance in Norway. Ecol. Evol. 2020, 10, 4801–4815. [Google Scholar] [CrossRef]
- Durif, C.; Guibert, A.; Elie, P. Morphological discrimination of the silvering stages of the European eel. In American Fisheries Society Symposium 2009; American Fisheries Society: New York, NY, USA, 2009; Volume 58, pp. 103–111. [Google Scholar]
- Lefebvre, F.; Contournet, P.; Crivelli, A.J. The health state of the eel swimbladder as a measure of parasite pressure by. Parasitology 2002, 124, 457–463. [Google Scholar] [CrossRef]
- Tesch, F.W. The Eel: Third Edition; Blackwell Science: Oxford, UK, 2007; pp. 1–408. [Google Scholar]
- Rasband, W. ImageJ; v. 1.50i; National Institute of Health: Bethesda, MD, USA, 2009. [Google Scholar]
- Bryan, M.B.; Zalinski, D.; Filcek, K.B.; Libants, S.; Li, W.; Scribner, K.T. Patterns of invasion and colonization of the sea lamprey (Petromyzon marinus) in North America as revealed by microsatellite genotypes. Mol. Ecol. 2005, 14, 3757–3773. [Google Scholar] [CrossRef]
- Stratoudakis, Y.; Mateus, C.S.; Quintella, B.R.; Antunes, C.; de Almeida, P.R. Exploited anadromous fish in Portugal: Suggested direction for conservation and management. Mar. Policy 2016, 73, 92–99. [Google Scholar] [CrossRef]
- Lucas, M.C.; Hume, J.B.; Almeida, P.R.; Aronsuu, K.; Habit, E.; Silva, S.; Wang, C.J.; Zampatti, B. Emerging conservation initiatives for lampreys: Research challenges and opportunities. J. Great Lakes Res. 2021, 47, S690–S703. [Google Scholar] [CrossRef]
- Braga, H.O.; Pereira, M.J.; Morgado, F.; Soares, A.M.; Azeiteiro, U.M. Ethnozoological knowledge of traditional fishing villages about the anadromous sea lamprey (Petromyzon marinus) in the Minho river, Portugal. J. Ethnobiol. Ethnomed. 2019, 15, 71. [Google Scholar] [CrossRef]
- Beaulaton, L.; Taverny, C.; Castelnaud, G. Fishing, abundance and life history traits of the anadromous sea lamprey (Petromyzon marinus) in Europe. Fish. Res. 2008, 92, 90–101. [Google Scholar] [CrossRef]
- Boulêtreau, S.; Carry, L.; Meyer, E.; Filloux, D.; Menchi, O.; Mataix, V.; Santoul, F. High predation of native sea lamprey during spawning migration. Sci. Rep. 2020, 10, 6122. [Google Scholar] [CrossRef]
- Moser, M.L.; Hume, J.B.; Aronsuu, K.K.; Lampman, R.T.; Jackson, A.D. Lamprey Reproduction and Early Life History: Insights from Artificial Propagation. In Lampreys: Biology, Conservation and Control, Fish & Fisheries Series; Docker, M., Ed.; Springer: Dordrecht, The Netherlands, 2019; Volume 38. [Google Scholar] [CrossRef]
- Mateus, C.S.; Rodríguez-Muñoz, R.; Quintella, B.R.; Alves, M.J.; Almeida, P.R. Lampreys of the Iberian Peninsula: Distribution, population status and conservation. Endanger. Species Res. 2012, 16, 183–198. [Google Scholar] [CrossRef]
- Magalhães, M.F.; Amaral, S.D.; Sousa, M.; Alexandre, C.M.; Almeida, P.R.; Alves, M.J.; Cortes, R.; Farrobo, A.; Filipe, A.F.; Franco, A.; et al. Livro Vermelho dos Peixes Dulciaquícolas e Diádromos de Portugal Continental; FCiências.ID & ICNF-Instituto da Conservação da Natureza e das Florestas: Lisboa, Portugal, 2023. [Google Scholar]
- Santos, J.M.; Ferreira, M.T.; Godinho, F.N.; Bochechas, J. Efficacy of a nature-like bypass channel in a Portuguese lowland river. J. Appl. Ichthyol. 2005, 21, 381–388. [Google Scholar] [CrossRef]
- Duarte, A.; Jorge, I.; Sobral, M.; Rebordão, F.; Martins, R.; Carneiro, M. Rendimento do Botirão Usado na Captura da Lampreia Petromyzon marinus L., 1758 no Estuário do rio Mondego; Relatórios Científicos e Técnicos-Série Digital: Lisboa, Portugal, 2003; pp. 1–27. [Google Scholar]
- Quintella, B.R.; Andrade, N.O.; Almeida, P.R. Distribution, larval stage duration and growth of the sea lamprey ammocoetes, Petromyzon marinus L., in a highly modified river basin. Ecol. Freshw. Fish 2003, 12, 286–293. [Google Scholar] [CrossRef]
- Gonçalves, M.S.A. Caracterização da Pesca da Lampreia-Marinha, Petromyzon marinus L., no Estuário do Cávado, em 2002. Master’s Thesis, University of Algarve, Faro, Portugal, 2004. [Google Scholar]
- Beamish, F.W.H.; Potter, I.C.; Thomas, E. Proximate Composition of the Adult Anadromous Sea Lamprey, Petromyzon Marinus, in Relation to Feeding, Migration and Reproduction. J. Anim. Ecol. 1979, 48, 1–19. [Google Scholar] [CrossRef]
- Araújo, M.J.; Ozório, R.O.A.; Bessa, R.J.B.; Kijjoa, A.; Gonçalves, J.F.M.; Antunes, C. Nutritional status of adult sea lamprey (Petromyzon marinus Linnaeus, 1758) during spawning migration in the Minho River, NW Iberian Peninsula. J. Appl. Ichthyol. 2013, 29, 808–814. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Mesa, M.G.; Bayer, J.M.; Seelye, J.G. Swimming performance and physiological responses to exhaustive exercise in radio-tagged and untagged Pacific lampreys. Trans. Am. Fish. Soc. 2003, 132, 483–492. [Google Scholar] [CrossRef]
- Quintella, B.R.; Póva, I.; Almeida, P.R. Swimming behaviour of upriver migrating sea lamprey assessed by electromyogram telemetry. J. Appl. Ichthyol. 2009, 25, 46–54. [Google Scholar] [CrossRef]
- ICES. Report of the Workshop on Lampreys and Shads (WKLS); ICES CM 2014/SSGEF:13; ICES: Lisbon, Portugal, 2015; p. 206. [Google Scholar]
- Volk, J.; Bekkevold, D.; Loeschcke, V. Weak population differentiation in northern European populations of the endangered anadromous clupeid. J. Fish Biol. 2007, 71, 461–469. [Google Scholar] [CrossRef]
- Jager, Z.; Bolle, L.; Dänhardt, A.; Diederichs, B.; Neudecker, T.; Scholle, J.; Vorberg, R. Fish. Thematic Report No. 14: Fishes Checklist; OBIS Secretariat: Wilhelmshaven, Germany, 2009. [Google Scholar] [CrossRef]
- Scholle, J.; Schuchardt, B. A fish-based index of biotic integrity–FAT-TW an assessment tool for transitional waters of the northern German tidal estuaries. Coastline Rep. 2012, 18, 1–73. [Google Scholar]
- Mota, M.; Rochard, E.; Antunes, C. Status of the Diadromous Fish of the Iberian Peninsula: Past, Present and Trends. Limnetica 2016, 35, 1–18. [Google Scholar]
- Alexandrino, P.; Boisneau, P.H. Diversité Génétique; Les Aloses (Alosa alosa et Alosa fallax spp.). In Écobiologie et variabilité des populations; Baglinière, J.L., Elie, P., Eds.; INRA/CEMAGREF: Paris, France, 2000; pp. 179–198. [Google Scholar]
- Alexandrino, P.; Ferrand, N.; Baglinière, J.-L. Preliminary Results on the Genetic Differentiation Between the Two Species of Shad (Alosa alosa and Alosa fallax) Occuring in Portugal; ICES Paper CM-1992/M17; EQHC: Rennes Cedex, France, 1992; pp. 1–10. [Google Scholar]
- Boisneau, P.; Mennesson Boisneau, C.; Guyomard, R. Electrophoretic Identity between Allis Shad, Alosa alosa (L), and Twaite Shad, Alosa fallax (Lacepede). J. Fish Biol. 1992, 40, 731–738. [Google Scholar] [CrossRef]
- Faria, R.; Weiss, S.; Alexandrino, P. Comparative phylogeography and demographic history of European shads (Alosa alosa and A. fallax) inferred from mitochondrial DNA. BMC Evol. Biol. 2012, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Rougier, T.; Lambert, P.; Drouineau, H.; Girardin, M.; Castelnaud, G.; Carry, L.; Aprahamian, M.; Rivot, E.; Rochard, E. Collapse of allis shad, Alosa alosa, in the Gironde system (southwest France): Environmental change, fishing mortality, or Allee effect? Ices J. Mar. Sci. 2012, 69, 1802–1811. [Google Scholar] [CrossRef]
- Maitland, P.S.; Lyle, A.A. Ecology of allis shad Alosa alosa and twaite shad Alosa fallax in the Solway Firth, Scotland. Hydrobiologia 2005, 534, 205–221. [Google Scholar] [CrossRef]
- Faria, R. Estudo de Processos Evolutivos e Filogeografia em Espécies Europeias do Género Alosa. (Study of Evolutionary Processes and Phylogeography in European Species of the Genus Alosa). Ph.D. Thesis, University of Porto, Porto, Portugal, 2007. [Google Scholar]
- Jolly, M.T.; Aprahamian, M.W.; Hawkins, S.J.; Henderson, P.A.; Hillman, R.; O’Maoiléidigh, N.; Maitland, P.S.; Piper, R.; Genner, M.J. Population genetic structure of protected allis shad (Alosa alosa) and twaite shad (Alosa fallax). Mar. Biol. 2012, 159, 675–687. [Google Scholar] [CrossRef]
- Martin, J.; Rougemont, Q.; Drouineau, H.; Launey, S.; Jatteau, P.; Bareille, G.; Berail, S.; Pécheyran, C.; Feunteun, E.; Roques, S.; et al. Dispersal capacities of anadromous Allis shad population inferred from a coupled genetic and otolith approach. Can. J. Fish. Aquat. Sci. 2015, 72, 991–1003. [Google Scholar] [CrossRef]
- Rougemont, Q.; Perrier, C.; Besnard, A.L.; Lebel, I.; Abdallah, Y.; Feunteun, E.; Réveillac, E.; Lasne, E.; Acou, A.; Nachón, D.J.; et al. Population genetics reveals divergent lineages and ongoing hybridization in a declining migratory fish species complex. Heredity 2022, 129, 137–151. [Google Scholar] [CrossRef]
- Boisneau, C.; Aprahamian, M.W.; Sabatié, M.R.; Cassou-Leins, J.J. Remontée migratoire des adultes. In Les aloses (Alosa alosa et Alosa fallax spp.): Ecobiologie et Variabilité des Populations; INRA Editions (coll. Hydrobiologie et Aquaculture): Versailles, France, 2000. [Google Scholar]
- Aprahamian, M.W.; Baglinière, J.L.; Sabatié, M.R.; Alexandrino, P.; Aprahamian, C.D. Synopsis of Biological Data on Alosa alosa and Alosa fallax spp.; Environment Agency R&D Dissemination Center, WRc: Swindon, UK, 2002; p. 314. [Google Scholar]
- Maitland, P.; Hatton-Ellis, T. Ecology of the Allis and Twaite Shad. Conserving Natura 2000 Rivers Ecology Series Nº 3; English Nature: Peterborough, UK, 2003. [Google Scholar]
- Baglinière, J.-L.; Sabatié, M.R.; Rochard, E.; Alexandrino, P.; Aprahamian, M.W. The allis shad Alosa alosa: Biology, ecology, range, and status of populations. Am. Fish. Soc. Symp. 2003, 2003, 85–102. [Google Scholar]
- Eiras, J.C. Contribuição para o Conhecimento da Biologia de Alosa alosa L. Estudo de Algumas Modificações Somáticas, Fisiológicas e Bioquímicas Durante a Migração Anádroma No Rio Douro. Ph.D. Thesis, Faculty of Sciences of University of Porto, Porto, Portugal, 1981. [Google Scholar]
- Sabatié, M.R.; Alexandrino, P.; Baglinière, J.-L. Comparaison des caractéristiques biologiques de la Grande Alose (Alosa alosa) dans l’oued Sebou (façade Nord-Atlantique du Maroc) et dans le fleuve Lima (Portugal). Cybium. Cybium 1996, 20, 59–73. [Google Scholar]
- Aprahamian, M.; Aprahamian, C.; Baglinière, J.; Sabatié, R.; Alexandrino, P. Alosa alosa and Alosa fallax spp.: Literature Review and Bibliography; Environment Agency: Warrington, UK, 2003. [Google Scholar]
- McIntosh, A.; McHugh, P.; Budy, P. Salmo trutta L. (brown trout). In A Handbook of Global Freshwater Invasive Species; Routledge: Oxfordshire, UK, 2011; pp. 285–298. [Google Scholar]
- ICES. Report of the Workshop on Sea Trout (WKTRUTTA); ICES Expert Group Reports (Until 2018); ICES: Toronto, ON, Canada, 2013. [Google Scholar]
- Lundqvist, H.; McKinnell, S.M.; Jonsson, S.; Östergren, J. Is Stocking with Sea Trout Compatible with the Conservation of Wild Trout (Salmo trutta L.)? In Sea Trout Biology, Conservation and Management; Wiley: Hoboken, NJ, USA, 2006; pp. 356–371. [Google Scholar]
- Okumuş, I.; Kurtoglu, I.Z.; Atasaral, Ş. General Overview of Turkish Sea Trout (Salmo trutta L.) Populations. In Sea Trout: Biology, Conservation and Management; Wiley: Hoboken, NJ, USA, 2007; pp. 115–127. [Google Scholar]
- Jonsson, B.; Jonsson, N. Ecology of Atlantic salmon and Brown Trout: Habitat as A Template for Life Histories; Springer: Berlin/Heidelberg, Germany, 2011; Volume 33. [Google Scholar]
- Collares-Pereira, M.J.; Alves, M.J.; Ribeiro, F.; Domingos, I.; Almeida, P.R.; da Costa, L.; Gante, H.; Filipe, A.F.; Aboim, M.A.; Rodrigues, P.M.; et al. Guia dos Peixes de Água Doce e Migradores de Portugal Continental; Edições Afrontamento: Porto, Portugal, 2021. [Google Scholar]
- Rogado, L.C.; Alexandrino, P.; Almeida, P.R.; Alves, J.; Bochechas, J.; Cortes, R.; Domingos, I.; Filipe, F.; Madeira, J.; Magalhães, F. Salmo trutta: Truta-marisca. In Livro Vermelho dos Vertebrados de Portugal; Cabral, M.J., Almeida, J., Almeida, P.R., Dellinger, T., Ferrand de Almeida, N., Oliveira, M.E., Palmeirim, J.M., Queirós, A.I., Rogado, L., Santos-Reis, M., Eds.; Instituto da Conservação da Natureza: Lisbon, Portugal, 2005; pp. 105–106. [Google Scholar]
- Olsen, E.M.; Knutsen, H.; Simonsen, J.H.; Jonsson, B.; Knutsen, J.A. Seasonal variation in marine growth of sea trout, Salmo trutta, in coastal Skagerrak. Ecol. Freshw. Fish 2006, 15, 446–452. [Google Scholar] [CrossRef]
- Arslan, M.; Yildirim, A.; Bektas, S. Length-weight relationship of brown trout, Salmo trutta, inhabiting kan stream coruh basin Nort-Eastren Turkey. Turk. J. Fish. Aquat. Sci. 2004, 4, 45–48. [Google Scholar]
- Costa, M.J.; Cabral, H.N. Changes in the Tagus nursery function for commercial fish species: Some perspectives for management. Aquat. Ecol. 1999, 33, 287–292. [Google Scholar] [CrossRef]
- Wootton, R.J. Ecology of Teleost Fishes; Springer Netherlands: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A Standardized Terminology for Describing Reproductive Development in Fishes. Mar. Coast Fish 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Antunes, C.; Rodrigues, H. Guia Natural do Rio Minho–Os Peixes; Aquamuseu do Rio Minho: Vila Nova de Cerveira, Portugal, 2004; 84p. [Google Scholar]
- Parikh, P.; Sadekarpawar, S. Gonadosomatic and Hepatosomatic Indices of Freshwater Fish Oreochromis mossambicus in Response to a Plant Nutrient. World J. Zool. 2013, 8, 110–118. [Google Scholar] [CrossRef]
- Novais, D. Biologia, ecologia e pesca desportiva da truta, Salmo trutta morpha fario L., no rio Vez. Master Thesis, University of Porto, Porto, Portugal, 2012. [Google Scholar]
- Ergüden, S.A.; Özcan, T.; Ergüden, D. The Occurrence of Atyaephyra desmarestii (Millet, 1831) (Decapoda: Atyidae) in the Seyhan Reservoir (Seyhan River Basin). J. Black Sea/Mediterr. Environ. 2011, 17, 4–10. [Google Scholar]
- Ribeiro, H.A.C. Ecologia e Conservação da Espécie Salmo trutta no Troço Internacional do Rio Minho (Ecology and Conservation of the Especies Salmo trutta in International Section of the river Minho). Master’s Thesis, University of Minho, Braga, Portugal, 2014. [Google Scholar]
- Hernández, J.S. Influencia del estiaje sobre el comportamento alimentário de la trucha común en un río de montaña (Espanha Central): Implicaciones en el estado de repleción y selección del alimento. Ecologia 2012, 24, 61–72. [Google Scholar]
- Ferguson, A.; Reed, T.E.; Cross, T.F.; McGinnity, P.; Prodohl, P.A. Anadromy, potamodromy and residency in brown trout Salmo trutta: The role of genes and the environment. J. Fish Biol. 2019, 95, 692–718. [Google Scholar] [CrossRef]
- Lobon-Cervia, J.; Fernandez-Delgado, C. On the biology of the barbel (Barbus barbus bocagei) in the Jarama river. Folia Zool. 1984, 33, 371–384. [Google Scholar]
- Lobon-Cervia, J.; Montañés, C.; de Sostoa, A. Reproductive ecology and growth of a population of brown trout (Salmo trutta L.) in an aquifer-fed stream of Old Castile (Spain). Hydrobiologia 1986, 135, 81–94. [Google Scholar] [CrossRef]
- Valente, A.C.N. Biologia e dinâmica das populações de truta-de-rio, Salmo trutta L., da bacia hidrográfica do rio Lima. Ph.D. Thesis, University of Porto, Porto, Portugal, 1993. [Google Scholar]
- Formigo, N.M.E. A Bacia Hidrográfica do rio Âncora-Caracterização Ecológica e Potencialidades bio-Económicas para a Prática da Pesca Desportiva. Ph.D. Thesis, University of Porto, Porto, Portugal, 1997. [Google Scholar]
- Martinho, A.M.V.; Cortes, R.M.V. Gestão Sustentável das Populações de Truta (Salmo trutta): O caso do Rio Olo. Master’s Thesis, University of Trás-os-Montes and Alto Douro, Vila Real, Portugal, 2008. [Google Scholar]
- Caballero, P.; Cobo, F.; Gonzàlez, M.A. Life History of a Sea Trout (Salmo trutta L.) Population from the North-West Iberian Peninsula (River Ulla, Galicia, Spain). In Sea Trout: Biology, Conservation and Management; Wiley: Hoboken, NJ, USA, 2007; pp. 234–247. [Google Scholar]
- Caballero Javierre, P. Biología de la Forma Anádroma de la Trucha Común Salmo trutta Linnaeus, 1758, en Galicia. Ph.D. Thesis, University of Santiago de Compostela, Galicia, Spain, 2021. [Google Scholar]
- Domingos, I.M.M. A Enguia-Europeia, Anguilla anguilla (L., 1758), na Bacia Hidrográfica do rio Mondego. Ph.D. Thesis, University of Lisbon, Lisbon, Portugal, 2003. [Google Scholar]
- Bonhommeau, S.; Le Pape, O.; Gascuel, D.; Blanke, B.; Tréguier, A.M.; Grima, N.; Vermard, Y.; Castonguay, M.; Rivot, E. Estimates of the mortality and the duration of the trans-Atlantic migration of European eel Anguilla anguilla leptocephali using a particle tracking model. J. Fish Biol. 2009, 74, 1891–1914. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, C.; Dekker, W. Management of the European Eel; 0332-4338; Marine Institute: St. John’s, NL, Canada, 1997. [Google Scholar]
- Jacoby, D.; Gollock, M. Anguilla anguilla. The IUCN Red List of Threatened Species 2020:e. T60344A45833138. IUCN. 2014. [Google Scholar]
- Kettle, A.J.; Asbjørn Vøllestad, L.; Wibig, J. Where once the eel and the elephant were together: Decline of the European eel because of changing hydrology in southwest Europe and northwest Africa? Fish Fish. 2011, 12, 380–411. [Google Scholar] [CrossRef]
- Doadrio, I. Atlas y Libro rojo de los Peces Continentales de España; Dirección General de Conservación de la Naturaleza; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2002. [Google Scholar]
- Lobón-Cerviá, J. The decline of eel Anguilla anguilla (L.) in a river catchment of northern Spain 1986-1997. Further evidence for a critical status of eel in Iberian waters. Arch. Für Hydrobiologie 1999, 144, 245–253. [Google Scholar] [CrossRef]
- Nicola, G.; Elvira, B.; Almodóvar, A. Dams and fish passage facilities in the large rivers of Spain: Effects on migratory species. Arch. Für Hydrobiologie 1996, 113, 375–379. [Google Scholar] [CrossRef]
- Domingos, I.; Antunes, C.; Oliveira, J.M. Report on the Eel Stock and Fishery in Portugal 2011/2012; FAO: Rome, Italy, 2012; pp. 630–665. [Google Scholar]
- Díaz, E.; Korta, M.; Aranburu, A.; Abaroa, C. Report on the Eel Stock and Fishery in Spain 2010/2011; FAO: Rome, Italy, 2012; pp. 666–699. [Google Scholar]
- Amorim, E.; Ramos, S.; Elliott, M.; Franco, A.; Bordalo, A.A. Habitat loss and gain: Influence on habitat attractiveness for estuarine fish communities. Estuar. Coast. Shelf Sci. 2017, 197, 244–257. [Google Scholar] [CrossRef]
- Costa-Dias, S.; Dias, E.; Lobón-Cerviá, J.; Antunes, C.; Coimbra, J. Infection by Anguillicoloides crassus in a riverine stock of European eel, Anguilla anguilla. Fish. Manag. Ecol. 2010, 17, 485–492. [Google Scholar] [CrossRef]
- Braga, A.C.R. Susceptibilidade da Enguia Europeia, Anguilla anguilla e do parasita Anguillicoloides crassus, às Concentrações de metais Pesados no rio Minho Internacional. Master’s Thesis, School of Medicine and Biomedical Sciences (ICBAS)-University of Porto, Porto, Portugal, 2011. [Google Scholar]
- Bruslé, J. Effects of heavy metals on eels, Anguilla sp. Aquat. Living Resour. 1990, 3, 131–141. [Google Scholar] [CrossRef]
- Belpaire, C.; Goemans, G. Eels: Contaminant cocktails pinpointing environmental contamination. ICES J. Mar. Sci. 2007, 64, 1423–1436. [Google Scholar] [CrossRef][Green Version]
- Imbert, H.; Arrowsmith, R.; Dufour, S.; Elie, P. Relationships between locomotor behavior, morphometric characters and thyroid hormone levels give evidence of stage-dependent mechanisms in European eel upstream migration. Horm. Behav. 2008, 53, 69–81. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melià, P.; Crivelli, A.J.; De Leo, G.A.; Gatto, M. Timing and rate of sexual maturation of European eel in brackish and freshwater environments. J. Fish Biol. 2006, 69, 200–208. [Google Scholar] [CrossRef]
- Edeline, E.; Elie, P. Is salinity choice related to growth in juvenile eel Anguilla anguilla? Cybium 2004, 28, 77–82. [Google Scholar] [CrossRef]
- Daverat, F.; Élie, P.; Lahaye, M. Microchemistry contribution to a first approach to the diversity of life histories of eels from the lower part of the Gironde-Garonne-Dordogne watershed. Cybium 2004, 28, 83–90. [Google Scholar]
- Daverat, F.; Limburg, K.E.; Thibault, I.; Shiao, J.-C.; Dodson, J.J.; Caron, F.; Tzeng, W.-N.; Iizuka, Y.; Wickström, H. Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Mar. Ecol. Prog. Ser. 2006, 308, 231–241. [Google Scholar] [CrossRef]
- Jessop, B.M.; Shiao, J.C.; Iizuka, Y.; Tzeng, W.N. Variation in the annual growth, by sex and migration history, of silver American eels Anguilla rostrata. Mar. Ecol. Prog. Ser. 2004, 272, 231–244. [Google Scholar] [CrossRef]
- Dias, E.; Miranda, M.L.; Sousa, R.; Antunes, C. Riparian vegetation subsidizes sea lamprey ammocoetes in a nursery area. Aquat. Sci. 2019, 81, 44. [Google Scholar] [CrossRef]
- Rooney, N.; McCann, K.S. Integrating food web diversity, structure and stability. Trends Ecol. Evol. 2012, 27, 40–46. [Google Scholar] [CrossRef]
- Dias, E.; Morais, P.; Cotter, A.M.; Antunes, C.; Hoffman, J.C. Estuarine consumers utilize marine, estuarine and terrestrial organic matter and provide connectivity among these food webs. Mar. Ecol. Prog. Ser. 2016, 554, 21–34. [Google Scholar] [CrossRef]
- Rytwinski, T.; Harper, M.; Taylor, J.J.; Bennett, J.R.; Donaldson, L.A.; Smokorowski, K.E.; Clarke, K.; Bradford, M.J.; Ghamry, H.; Olden, J.D.; et al. What are the effects of flow-regime changes on fish productivity in temperate regions? A systematic map. Environ. Evid. 2020, 9, 7. [Google Scholar] [CrossRef]
- Domingos, I.; Costa, J.L.; Costa, M.J. Factors determining length distribution and abundance of the European eel, Anguilla anguilla, in the River Mondego (Portugal). Freshw. Biol. 2006, 51, 2265–2281. [Google Scholar] [CrossRef]
- Gravato, C.; Guimarães, L.; Santos, J.; Faria, M.; Alves, A.; Guilhermino, L. Comparative study about the effects of pollution on glass and yellow eels (Anguilla anguilla) from the estuaries of Minho, Lima and Douro Rivers (NW Portugal). Ecotoxicol. Environ. Saf. 2010, 73, 524–533. [Google Scholar] [CrossRef]
- Marohn, L.; Jakob, E.; Hanel, R. Implications of facultative catadromy in Anguilla anguilla. Does individual migratory behaviour influence eel spawner quality? J. Sea Res. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Simon, J. Age, growth, and condition of European eel (Anguilla anguilla) from six lakes in the River Havel system (Germany). ICES J. Mar. Sci. 2007, 64, 1414–1422. [Google Scholar] [CrossRef]
- Matthews, M.; Evans, D.; Rosell, R.; Moriarty, C.; Marsh, I. The Erne eel enhancement programme. In EU Programme for Peace and Reconciliation; Project No. 15, Ballyshannon; Northern Regional Fisheries Board: Co Donegal, Ireland, 2001; p. 348. [Google Scholar]
- Jörgensen, L. Fischereibiologische Analyse der Altersstruktur der Aalbestände in der Havel, Berlin (West); Projektabschluß: Berlin, Germany, 1988. [Google Scholar]
- Antunes, C.; Cobo, F.; Araújo, M.J.; Braga, C.; Roleira, A.; Mota, M.; Sanchez, J.; Vieira, R.; Servia, M.J.; Couto, M.T.; et al. Contribuição para o plano de gestão da enguia-europeia, Anguilla anguilla no rio Minho internacional. In Valorização dos Recursos Naturais da Bacia Hidrográfica do rio Minho–Projecto Natura Minho-Miño, Relatório Final; CIIMAR: Matosinhos, Portugal, 2011. [Google Scholar]
- Adam, G.; Feunteun, E.; Prouzet, P.; Rigaud, C. L’anguille Européenne. Indicateurs D’abondance et de Colonisation; Editions Quae: Versailles, France, 2008; p. 400. [Google Scholar]
- Simon, J.; Westerberg, H.; Righton, D.; Sjöberg, N.B.; Dorow, M. Diving activity of migrating silver eel with and without Anguillicola crassus infection. J. Appl. Ichthyol. 2018, 34, 659–668. [Google Scholar] [CrossRef]
- Lefebvre, F.; Tobias, S.; Marcel, M.; Mike, H.; and Poulin, R. Anguillicolosis in the short–finned eel Anguilla australis: Epidemiology and pathogenicity. New Zealand J. Mar. Freshw. Res. 2004, 38, 577–583. [Google Scholar] [CrossRef]
- Kennedy, C.R. The pathogenic helminth parasites of eels. J. Fish Dis. 2007, 30, 319–334. [Google Scholar] [CrossRef]
- Taraschewski, H. Hosts and parasites as aliens. J. Helminthol. 2006, 80, 99–128. [Google Scholar] [CrossRef]
- Antunes, C. Anguillicola Infestation of Eel Population from the Rio Minho (North of Portugal); ICES-EIFAC Working Group on Eel: Silkeborg, Denmark, 1999; p. 1. [Google Scholar]
- Passos, D.M. Concentração de Metais Pesados na Enguia Europeia, Anguilla anguilla (Linnaeus, 1758), em Estuários e Lagoas Costeiras de Portugal; University of Aveiro: Aveiro, Portugal, 2008; p. 41. [Google Scholar]
- Jakob, E.; Walter, T.; Hanel, R. A checklist of the protozoan and metazoan parasites of European eel (Anguilla anguilla): Checklist of Anguilla anguilla parasites. J. Appl. Ichthyol. 2016, 32, 757–804. [Google Scholar] [CrossRef]
- Pereira, L.; Braga, A.C.; Moura, A.; Antunes, C. Prevalence of the Anguillicola crassus parasite in the International Minho River. Environ. Smoke 2022, 64–74. [Google Scholar] [CrossRef]
- Heitlinger, E.G.; Laetsch, D.R.; Weclawski, U.; Han, Y.-S.; Taraschewski, H. Massive encapsulation of larval Anguillicoloides crassus in the intestinal wall of Japanese eels. Parasites Vectors 2009, 2, 48. [Google Scholar] [CrossRef]
- Wysujack, K.; Dorow, M.; Ubl, C. The infection of the European eel with the parasitic nematode Anguillicoloides crassus in inland and coastal waters of northern Germany. J. Coast. Conserv. 2014, 18, 121–130. [Google Scholar] [CrossRef]
- Haenen, O.L.M.; Van Banning, P. Detection of larvae of Anguillicola crassus (an eel swimbladder nematode) in freshwater fish species. Aquaculture 1990, 87, 103–109. [Google Scholar] [CrossRef]
- Würtz, J.; Taraschewski, H. Histopathological changes in the swimbladder wall of the European eel Anguilla anguilla due to infections with Anguillicola crassus. Dis. Aquat. Organ. 2000, 39, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Székely, C.; Palstra, A.; Molnár, K.; van den Thillart, G. Impact of the Swim-Bladder Parasite on the Health and Performance of European Eels. In Spawning Migration of the European Eel: Reproduction Index, a Useful Tool for Conservation Management; van den Thillart, G., Dufour, S., Rankin, J.C., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 201–226. [Google Scholar]
- Lefebvre, F.; Fazio, G.; Mounaix, B.; Crivelli, A.J. Is the continental life of the European eel Anguilla anguilla affected by the parasitic invader Anguillicoloides crassus? Proc. R. Soc. B Biol. Sci. 2013, 280, 20122916. [Google Scholar] [CrossRef]
- Neto, A.F.; Costa, J.L.; Costa, M.J.; Domingos, I. Epidemiology and pathology of Anguillicoloides crassus in European eel Anguilla anguilla from the Tagus estuary (Portugal). Dis. Aquat. Organ. 2010, 88, 225–233. [Google Scholar] [CrossRef]
- Denny, S.; Denny, A.; Paul, T. Distribution, prevalence and intensity of Anguillicoloides crassus in the American eel, Anguilla rostrata, in the Bras d’Or Lakes, Nova Scotia. BioInvasions Rec. 2013, 2, 19–26. [Google Scholar] [CrossRef]
- Taverny, C.; Elie, P. Bilan des Connaissances Biologiques et de l’état des Habitats des Lamproies Migratrices dans le Bassin de la Gironde: Propositions D’actions Prioritaires; IRSTEA: Montpellier, France, 2009; p. 110. [Google Scholar]


| Species | Common Name | Classification |
|---|---|---|
| Anguilla anguilla | European eel | Native |
| Salmo trutta | Trout | Native |
| Petromyzon marinus | Sea lamprey | Native |
| Alosa spp. | Allis/Twaite shad | Native |
| Chelon ramada | Thinlip grey mullet | Native |
| Pseudochondrostoma duriense | Northern straight-mouth nase | Native |
| Achondrostoma oligolepis | Portuguese nase | Native |
| Luciobarbus bocagei | Iberain barbel | Native |
| Squalius carolitertii | Northern Iberian chub | Native |
| Cobitis atlantica | Southern Iberian spined loach | Native |
| Gasterosteus aculeatus | Three-spined stickleback | Native |
| Lepomis gibbosus | Pumpkinseed sunfish | Exotic |
| Gobio lozanoi | Iberian gudgeon | Exotic |
| Micropterus salmoides | Largemouth black bass | Exotic |
| Carassius auratus | Goldfish | Exotic |
| Sample | Zone | N | TL (cm) | TW (g) | LWR Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Mean ± SD | Max | Min | Mean ± SD | Max | a | b | 95% CI of b | R2 | |||
| All | Estuary + Upstream | 59 | 63.5 | 81.5 ± 9.2 | 98.6 | 708.0 | 1240.0 ± 301.3 | 2350.0 | 2.380 | 1.421 | 0.9666 to 1.888 | 0.4164 |
| Estuary | 23 | 76.7 | 87.6 ± 6.0 | 98.6 | 795.0 | 1300.0 ± 384.9 | 2350.0 | 0.0007044 | 3.222 | 1.851 to 4.639 | 0.5343 | |
| Upstream | 36 | 63.5 | 77.6 ± 8.8 | 94.0 | 708.0 | 1201.0 ± 231.0 | 1715.0 | 5.847 | 1.224 | 0.8402 to 1.607 | 0.5474 | |
| Female | Estuary | 4 | 83.0 | 87.6 ± 5.4 | 95.0 | 1220.0 | 1276.0 ± 72.3 | 1380.0 | 21.03 | 0.9180 | 0.6052 to 1.228 | 0.9874 |
| Upstream | 18 | 63.5 | 78.9 ± 9.5 | 94.0 | 762.0 | 1261.0 ± 224.7 | 1715.0 | 9.728 | 1.111 | 0.5749 to 1.661 | 0.5505 | |
| Male | Estuary | 4 | 80.4 | 83.5 ± 5.0 | 91.0 | 1020.0 | 1125.0 ± 140.6 | 1330.0 | 0.1716 | 1.985 | 0.8254 to 3.101 | 0.9630 |
| Upstream | 15 | 65.2 | 76.2 ± 8.3 | 92.5 | 708.0 | 1143.0 ± 242.5 | 1491.0 | 3.417 | 1.341 | 0.5743 to 2.084 | 0.5136 | |
| N | K | TL (cm) | TW (g) | LWR Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Mean ± SD | Max | Min | Mean ± SD | Max | a | b | 95% CI of b | R2 | ||||
| A. alosa | All | 51 | 0.88 | 47.9 | 57.3 ± 4.4 | 66.7 | 710.0 | 1712.0 ± 590.3 | 2950.0 | 0.0001362 | 4.029 | 3.386 to 4.689 | 0.7750 |
| Males | 35 | 0.85 | 47.9 | 55.4 ± 3.5 | 62.7 | 710.0 | 1478.0 ± 453.3 | 2433.0 | 0.0002835 | 3.846 | 2.869 to 4.838 | 0.6557 | |
| Females | 16 | 0.94 | 54.6 | 61.5 ± 3.0 | 66.7 | 1203.0 | 2225.0 ± 536.0 | 2950.0 | ~7.327 × 10−5 | ~4.180 | (very wide) | 0.6525 | |
| Hybrids | All | 27 | 0.85 | 44.8 | 49.8 ± 5.1 | 62.6 | 562.0 | 1124.0 ± 581.4 | 2690.0 | 0.0001413 | 4.048 | 3.631 to 4.470 | 0.9276 |
| Males | 23 | 0.83 | 44.8 | 48.4 ± 3.5 | 60.7 | 562.0 | 971.3 ± 374.5 | 2310.0 | 0.0002164 | 3.940 | 3.208 to 4.633 | 0.8033 | |
| Female | 4 | 0.97 | 49.9 | 57.9 ± 6.0 | 62.6 | 902.0 | 2001.0 ± 835.4 | 2690.0 | 2.092 × 10−5 | 4.513 | 3.280 to 5.976 | 0.9942 | |
| N | K | TL (cm) | TW (g) | LWR Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Mean ± SD | Max | Min | Mean ± SD | Max | a | b | 95% CI of b | R2 | ||||
| Lima River | All | 121 | 0.94 | 10.4 | 32.0 ± 7.2 | 54.4 | 14.2 | 354.2 ± 258.8 | 1599.0 | 0.004273 | 3.216 | 3.100 to 3.334 | 0.9602 |
| Male | 16 | 0.94 | 25.8 | 35.1 ± 5.8 | 47.4 | 169.0 | 431.9 ± 208.0 | 940.0 | 0.01813 | 2.814 | 2.605 to 3.027 | 0.9839 | |
| Female | 54 | 0.89 | 17.1 | 31.2 ± 6.1 | 45.2 | 50.2 | 298.7 ± 173.0 | 865.0 | 0.006717 | 3.076 | 2.859 to 3.298 | 0.9476 | |
| Und. | 51 | 0.98 | 10.4 | 31.9 ± 8.5 | 54.4 | 14.2 | 388.6 ± 331.0 | 1599.0 | 0.005345 | 3.169 | 3.005 to 3.336 | 0.9707 | |
| Tributaries | 48 | 1.06 | 3.7 | 12.0 ± 5.5 | 23.5 | 0.5 | 28.4 ± 30.1 | 117.0 | 0.01649 | 2.828 | 2.650 to 3.012 | 0.9757 | |
| Age | Observed (cm) | N | Back-Calculated (cm) | N | Otolith Radius (mm) | N |
|---|---|---|---|---|---|---|
| 1 | 14.3 | 1 | 13.5 ± 2.9 | 115 | 1.46 | 1 |
| 2 | 25.3 ± 4.2 | 30 | 22.7 ± 3.4 | 114 | 2.4 ± 0.44 | 30 |
| 3 | 32.0 ± 3.7 | 50 | 29.8 ± 3.6 | 84 | 3.0 ± 0.26 | 48 |
| 4 | 34.2 ± 2.9 | 21 | 34.8 ± 4.2 | 37 | 3.3 ± 0.26 | 20 |
| 5 | 39.7 ± 4.8 | 10 | 40.5 ± 4.9 | 17 | 3.7 ± 0.26 | 10 |
| 6 | 47.0 ± 3.5 | 8 | 46.1 ± 3.0 | 7 | 4.0 ± 0.24 | 8 |
| Sample | N | K | TL (cm) | TW (g) | LWR Parameters | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | a | b | 95% CI of b | R2 | ||
| Lima | 54 | 0.17 ± 0.03 | 41.3 ± 12.5 (12.2–69.3) | 159.4 ± 167.2 (1.26–845.0) | 0.000046 | 3.914 | 3.617 to 4.235 | 0.9656 |
| Tributaries | 46 | 0.14 ± 0.02 | 19.4 ± 9.2 (7.3–46.4) | 17.9 ± 25.8 (0.4–135.8) | 0.001803 | 2.937 | 2.757 to 3.122 | 0.9667 |
| Age | N | Lima River | N | Tributaries | |
|---|---|---|---|---|---|
| Back calculation Length at age (cm) | 1 | 53 | 9.1–22.2 (14.6 ± 3.1) | 42 | 6.5–21.9 (13.0 ± 4.1) |
| 2 | 52 | 13.3–28.8 (20.2 ± 3.4) | 27 | 13.7–29.0 (20.0 ± 4.0) | |
| 3 | 51 | 17.6–34.8 (26.0 ± 3.6) | 14 | 17.2–29.0 (25.3 ± 3.2) | |
| 4 | 45 | 24.1–41.6 (31.1 ± 3.8) | 7 | 21.4–31.8 (28.7 ± 3.9) | |
| 5 | 39 | 28.2–53.0 (36.7 ± 5.2) | 1 | 24.7 (24.7 ± 0.0) | |
| 6 | 24 | 34.6–58.8 (44.2 ± 6.4) | 1 | 32.3 (32.3 ± 0.0) | |
| 7 | 16 | 36.3–58.3 (48.6 ± 6.3) | 1 | 40.3 (40.3 ± 0.0) | |
| 8 | 8 | 50.0–60.6 (54.1 ± 3.8) | 1 | 45.7 (45.7 ± 0.0) | |
| 9 | 5 | 55.5–64.3 (58.8 ± 3.8) | * | * | |
| 10 | 3 | 59.6–66.1 (63.5 ± 3.6) | * | * |
| Sample | N | Otolith Diameter (mm) | Otolith Radius (mm) | DRR Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Mean ± SD | Max | Min | Mean ± SD | Max | a | b | 95% CI of b | R2 | ||
| Lima River | 53 | 0.7670 | 2.821 ± 0.71 | 4.445 | 0.4010 | 1.516 ± 0.40 | 2.402 | 0.5532 | −0.04495 | −0.1091 to 0.01920 | 0.9803 |
| Tributaries | 46 | 0.3520 | 1.344 ± 0.59 | 2.835 | 0.2950 | 0.737 ± 0.31 | 1.517 | 0.5064 | 0.04298 | −0.00843 to 0.09439 | 0.9503 |
| Sampling Site | Total (N) | Infected (N) | Parasite Stage * | Intensity Range | Mean Intensity | Abundance | Prevalence (%) | 95% CI of Prevalence (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L3 | L4 | Adults | |||||||||
| Males | Females | ||||||||||
| Lima River | 53 | 39 | 3 | 25 | 54 | 79 | 1–15 | 4.1 (±3.5) | 3.0 | 73.6 | 60.4–83.6 |
| Tributaries | 46 | 21 | 0 | 7 | 26 | 21 | 1–9 | 2.6 (±2.5) | 1.2 | 45.7 | 32.2–59.8 |
| All | 99 | 60 | 3 | 32 | 80 | 100 | 1–15 | 3.6 (±3.3) | 2.2 | 60.6 | 50.8–69.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, L.; Azeiteiro, U.; Antunes, C. Assessing Diadromous Fish Populations in the Lima River, Northwest Iberian Peninsula. Fishes 2025, 10, 230. https://doi.org/10.3390/fishes10050230
Pereira L, Azeiteiro U, Antunes C. Assessing Diadromous Fish Populations in the Lima River, Northwest Iberian Peninsula. Fishes. 2025; 10(5):230. https://doi.org/10.3390/fishes10050230
Chicago/Turabian StylePereira, Luís, Ulisses Azeiteiro, and Carlos Antunes. 2025. "Assessing Diadromous Fish Populations in the Lima River, Northwest Iberian Peninsula" Fishes 10, no. 5: 230. https://doi.org/10.3390/fishes10050230
APA StylePereira, L., Azeiteiro, U., & Antunes, C. (2025). Assessing Diadromous Fish Populations in the Lima River, Northwest Iberian Peninsula. Fishes, 10(5), 230. https://doi.org/10.3390/fishes10050230








