Effects of Increased Feeding Rates on Oxidative Stress, Biochemical Indices and Growth of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) Under Mild-Hyperoxia Conditions
Abstract
1. Introduction
2. Materials and Methods
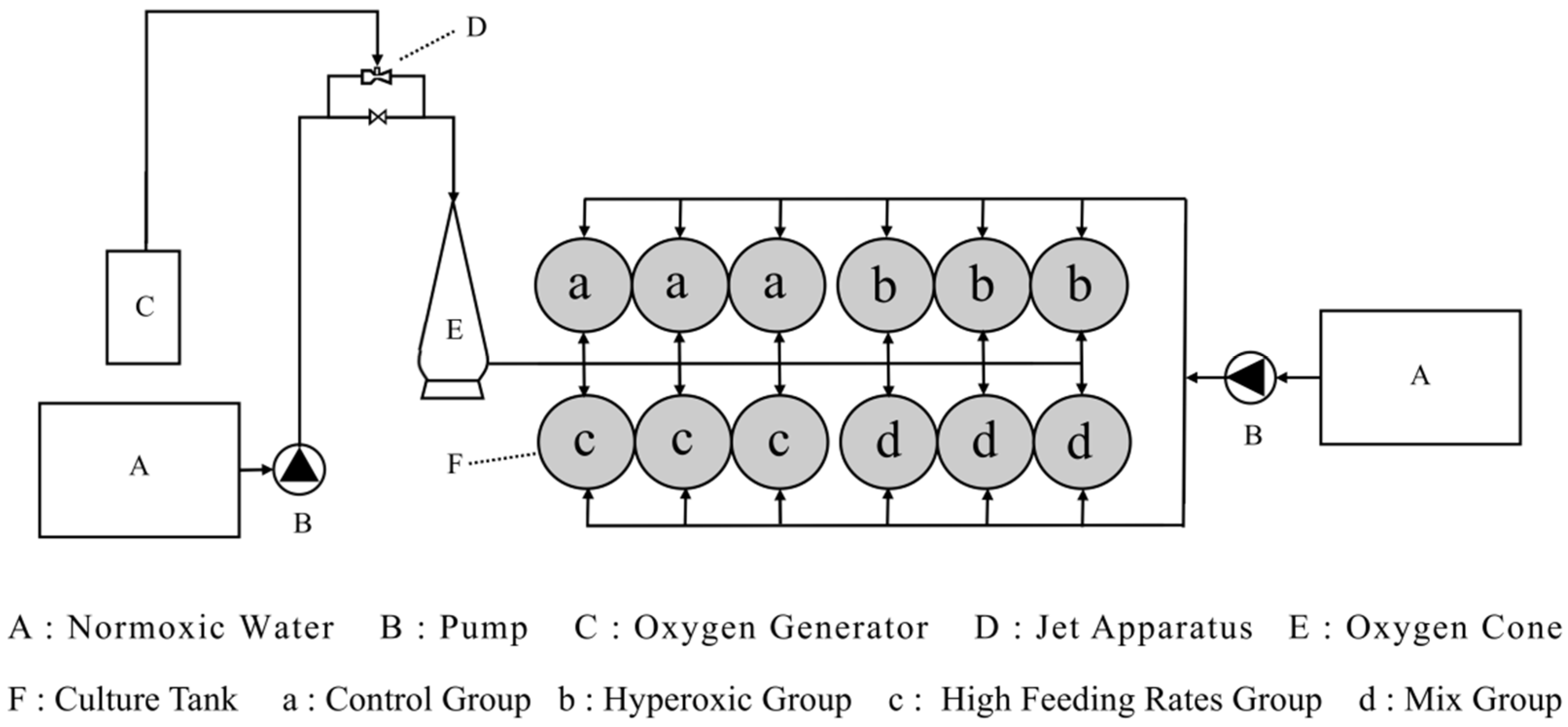
2.1. Fish and Aquaculture Facilities
2.2. Experimental Design
2.3. Sampling
2.4. Untargeted Metabolomic Analysis
2.5. RNA Extraction and Real-Time Fluorescence Quantitative Determination
2.6. Assessment of Serum Biochemical Indices
2.7. Statistical Analysis
3. Results
3.1. Growth Indicator
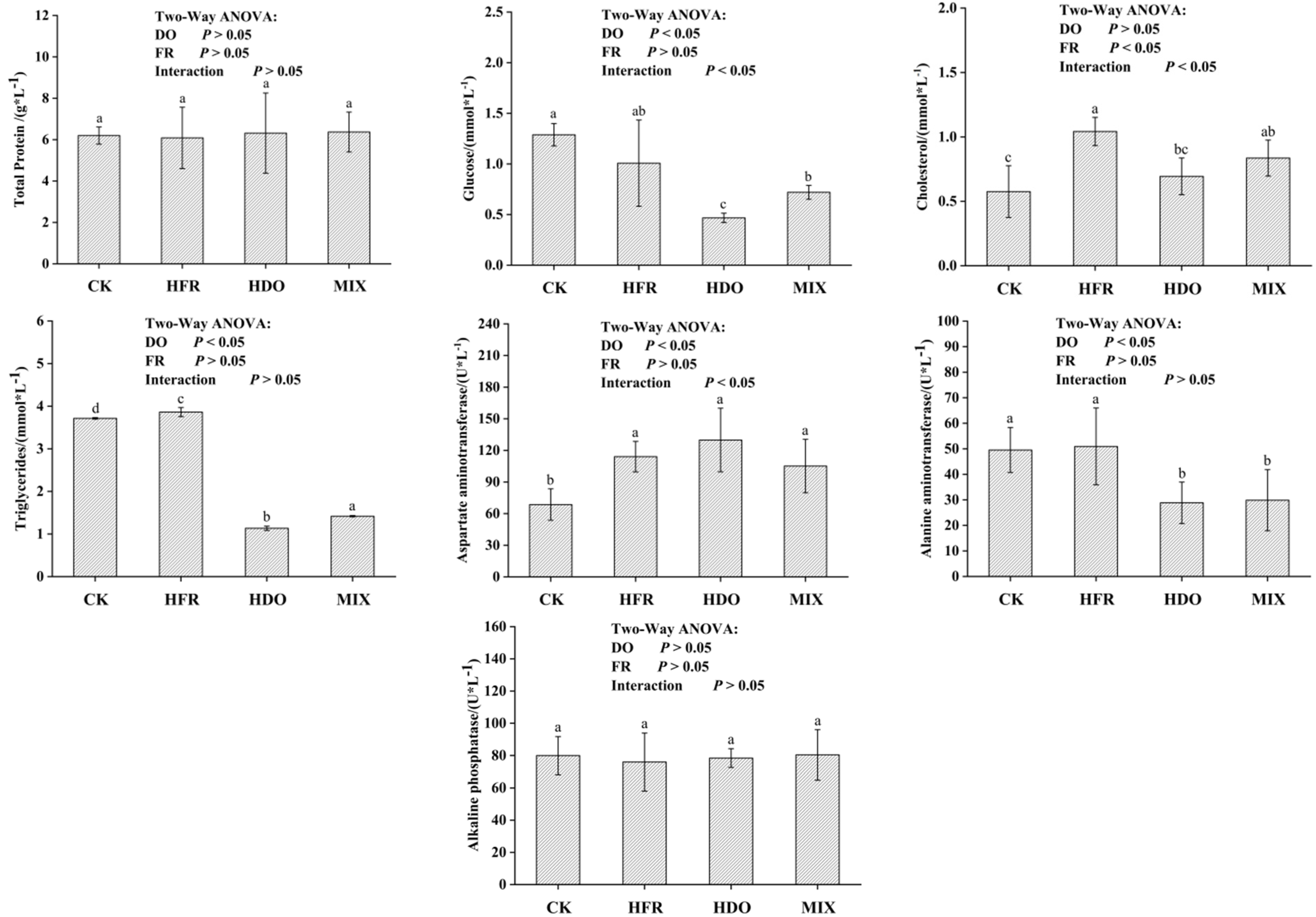
3.2. Physiological and Biochemical Serum Indicators
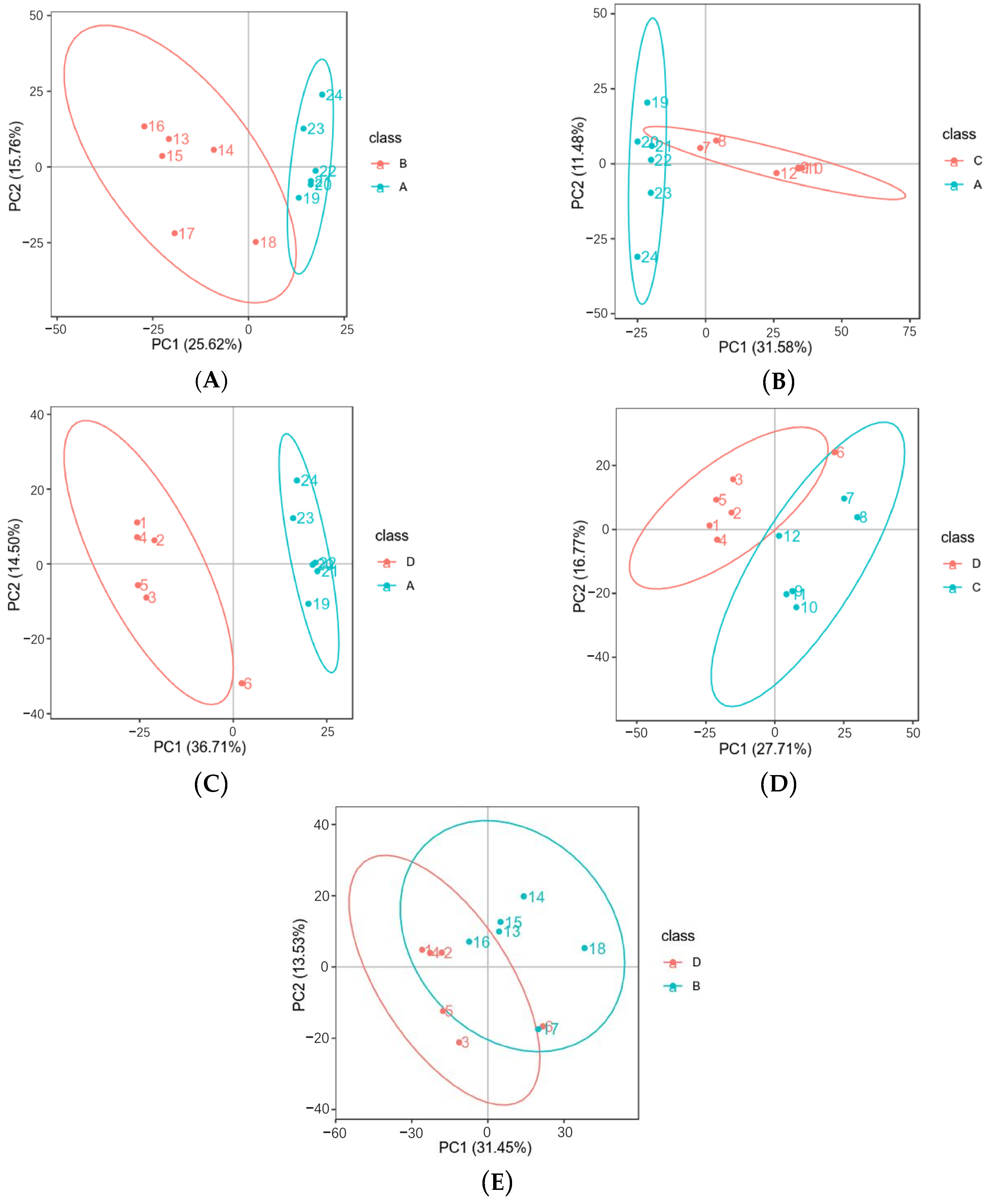
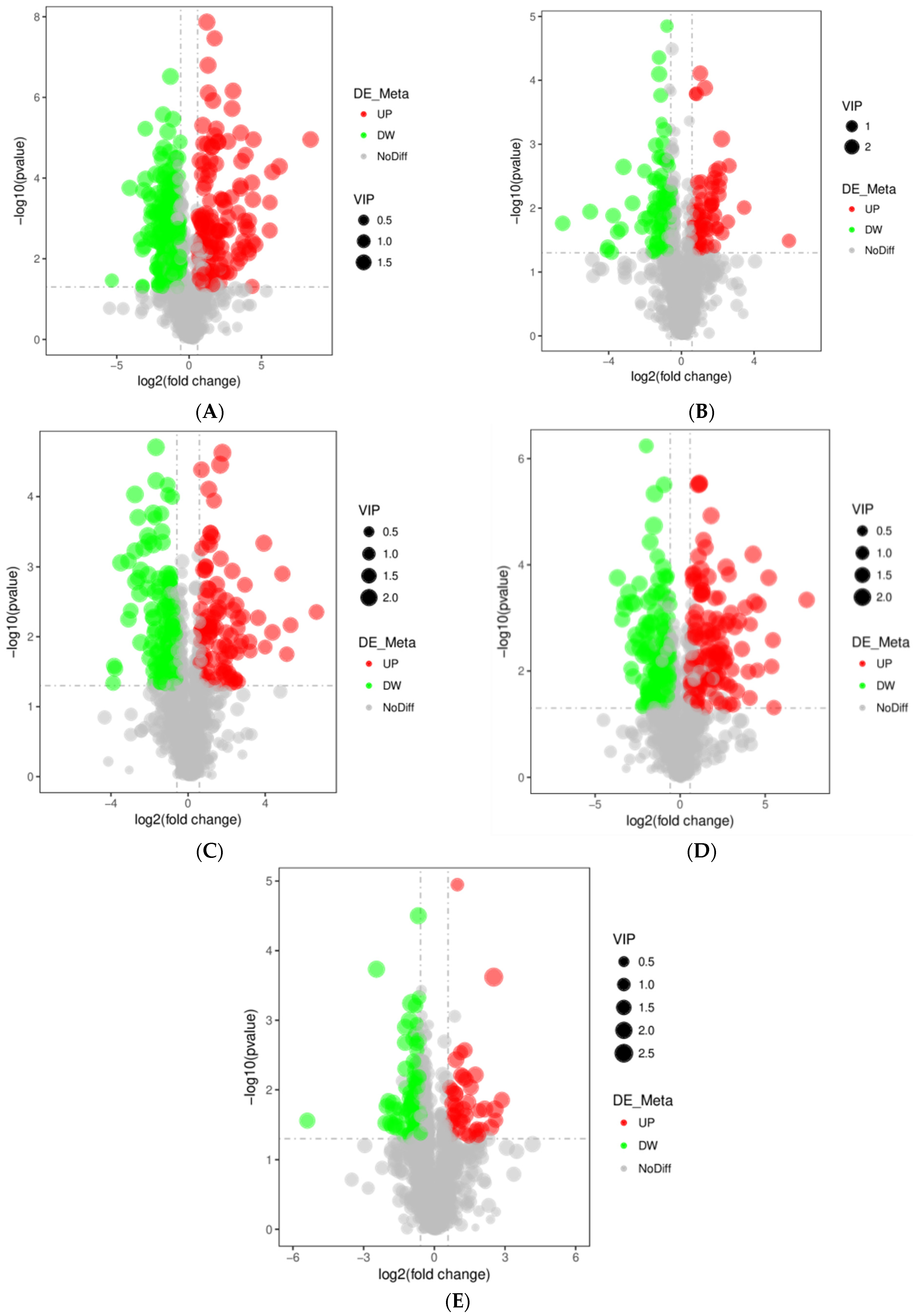
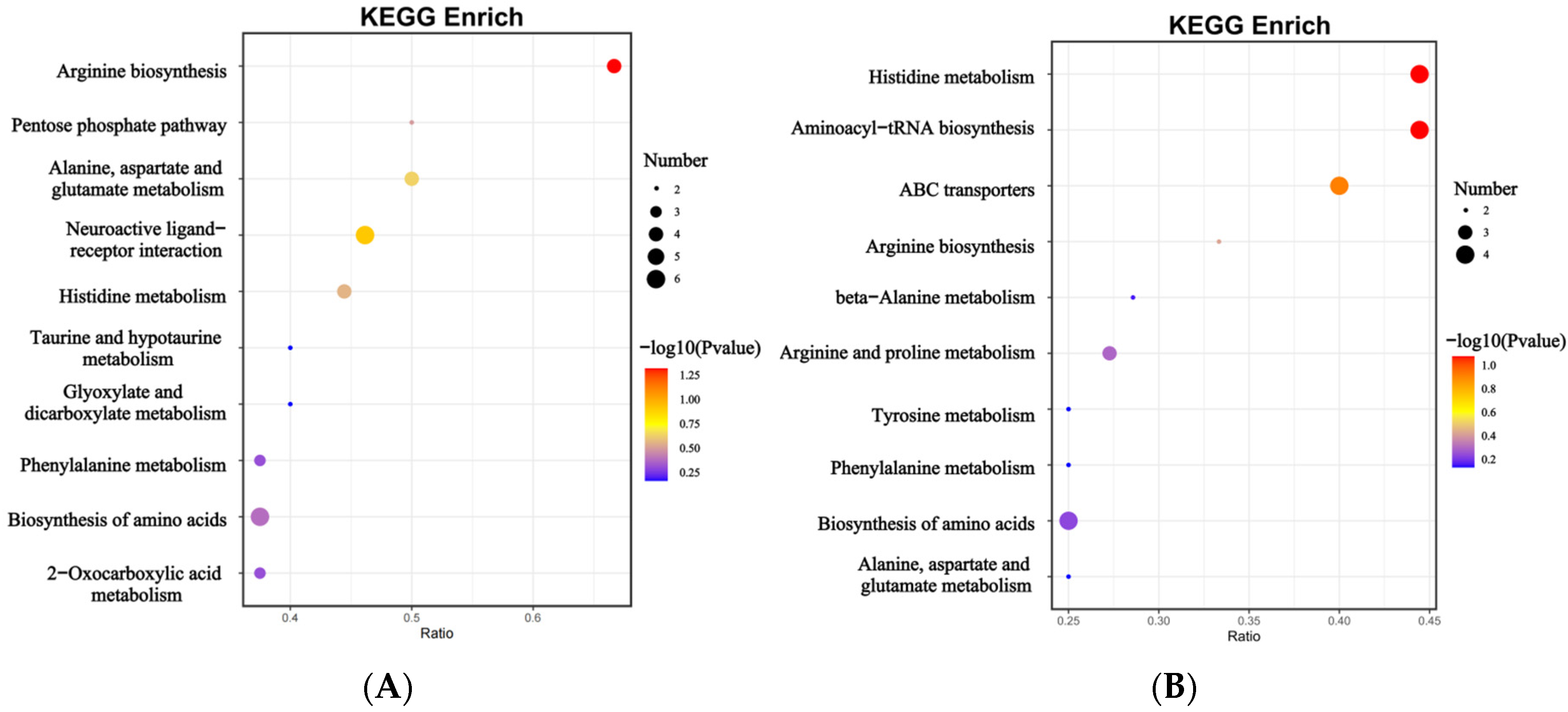
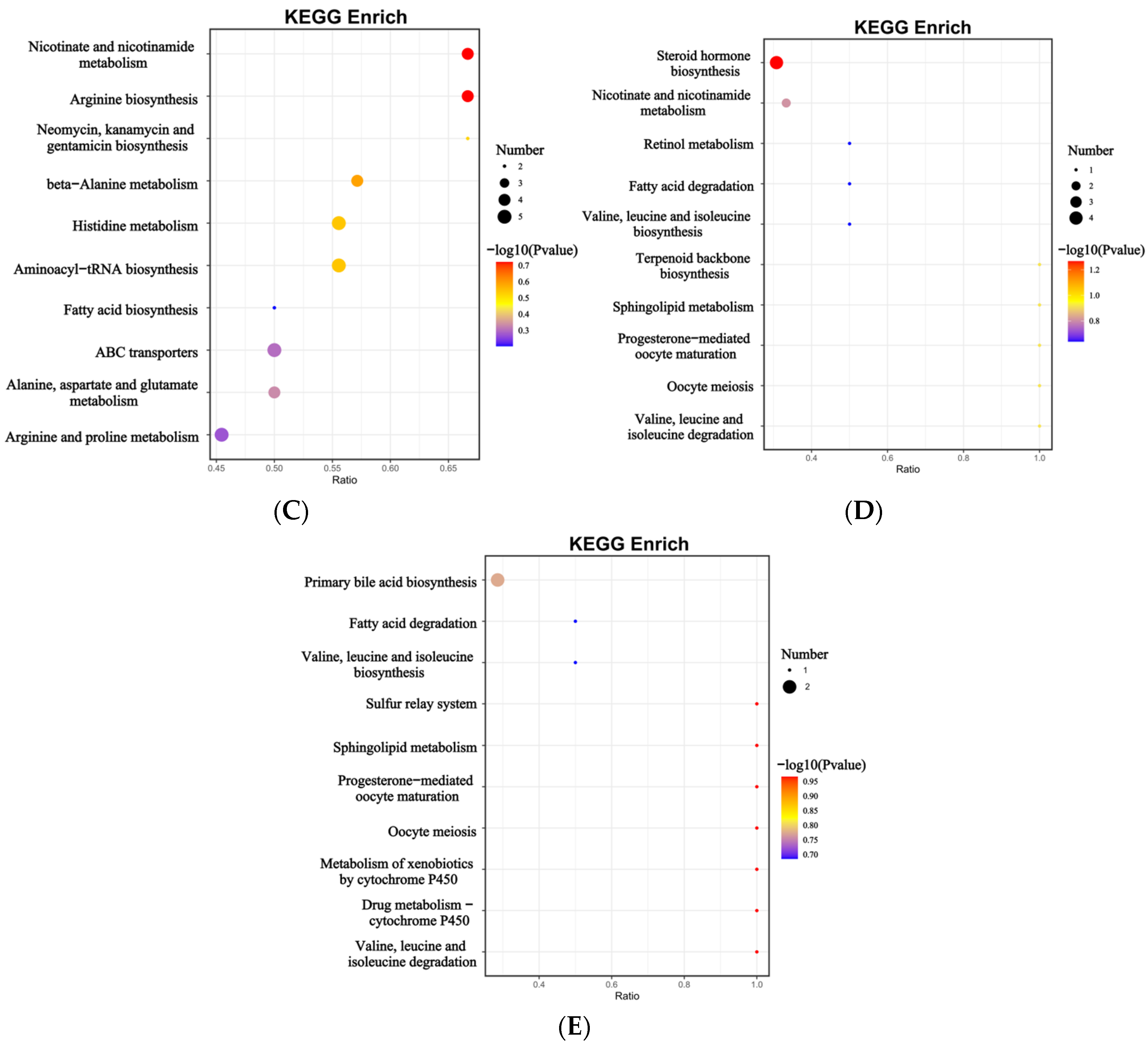
3.3. Differential Metabolites
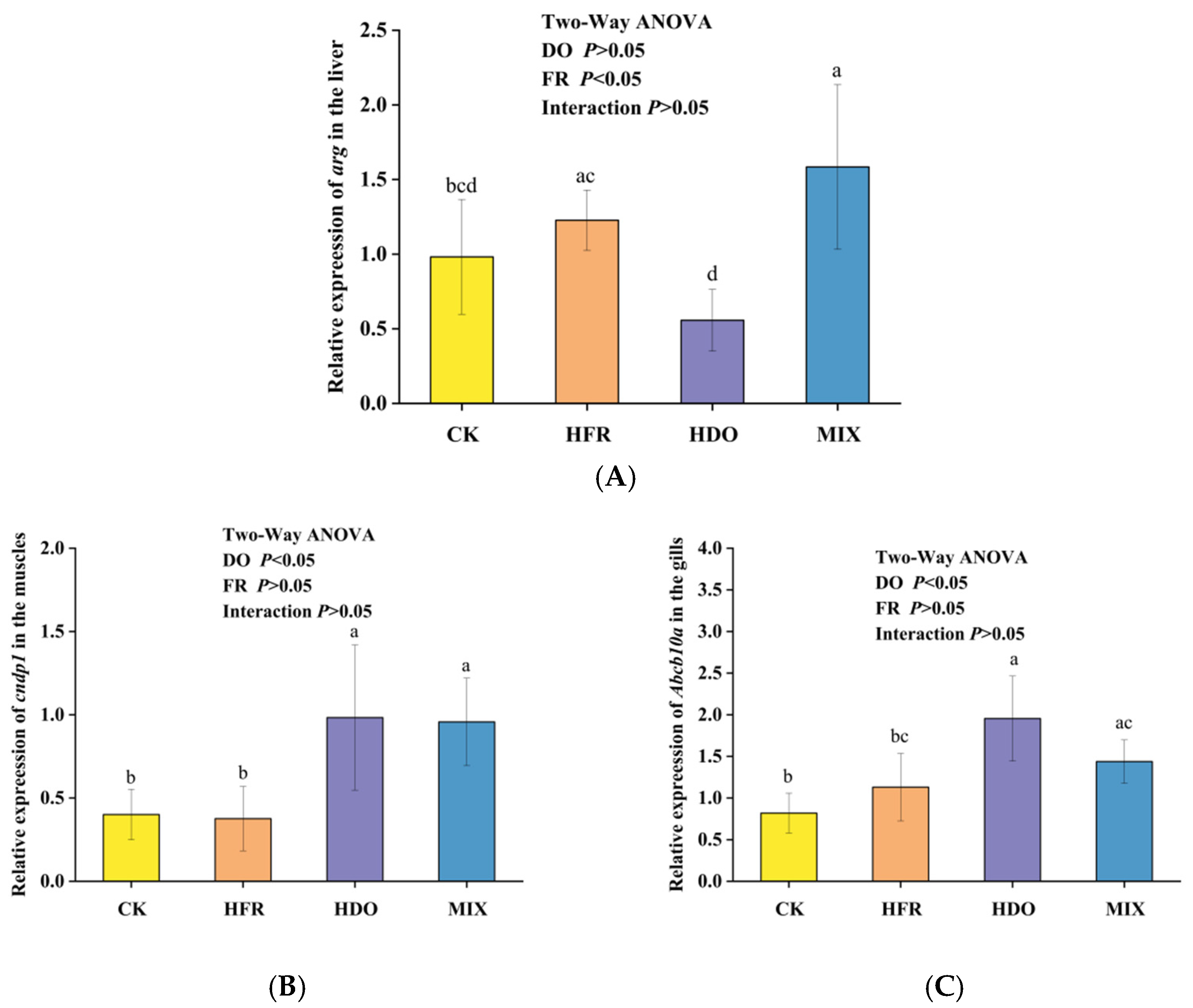
3.4. Metabolomics-Related Gene Expression Results
3.5. Expression of Growth-Related Genes in Juvenile Golden Tiger Epinephelus
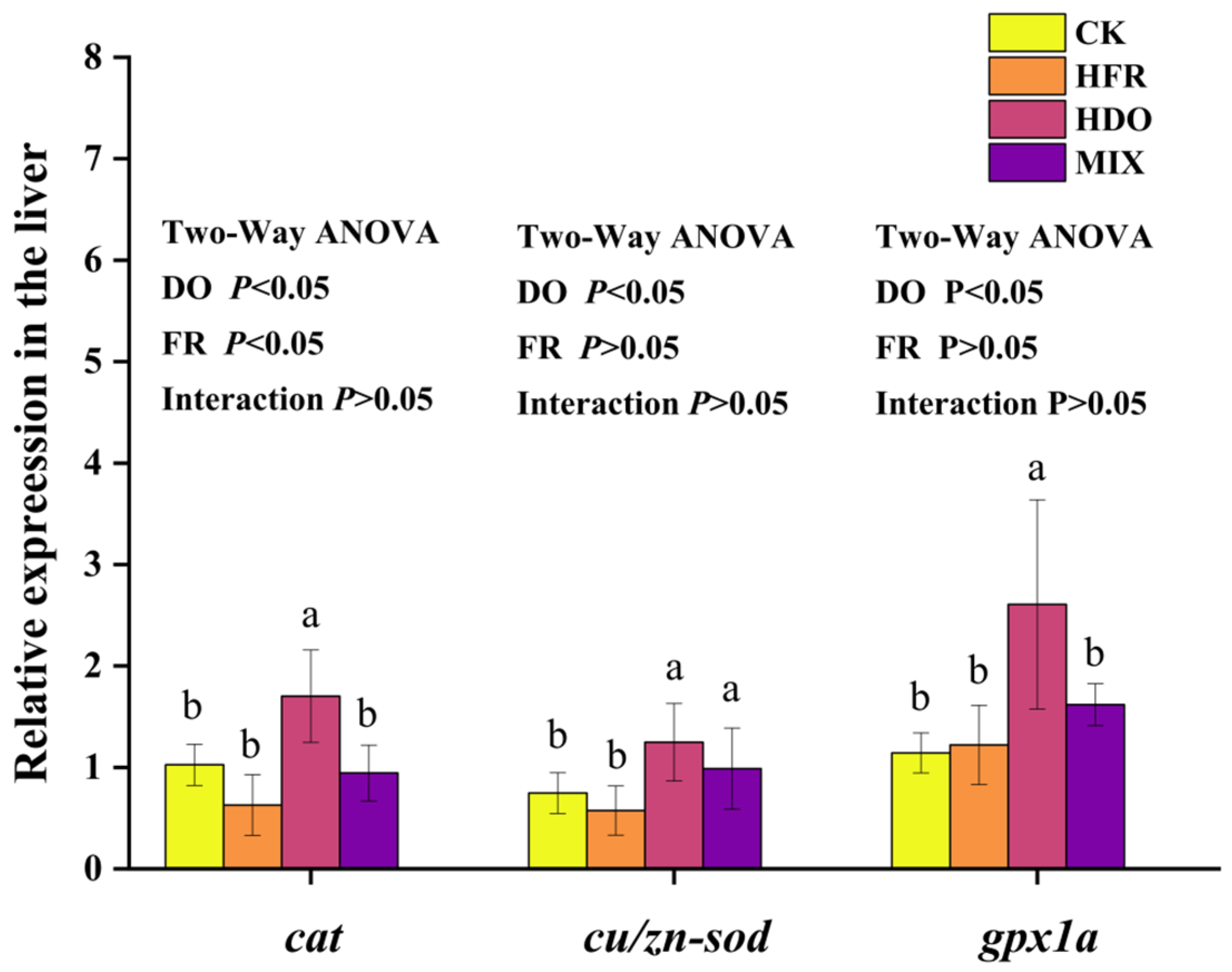
3.6. Assessment of Antioxidative Gene Response in Hybrid Grouper Juveniles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Tawwab, M.; Hagras, A.E.; Elbaghdady, H.A.M.; Monier, M.N. Effects of Dissolved Oxygen and Fish Size on Nile Tilapia, Oreochromis Niloticus (L.): Growth Performance, Whole-Body Composition, and Innate Immunity. Aquacult. Int. 2015, 23, 1261–1274. [Google Scholar] [CrossRef]
- Buentello, J.A.; Gatlin, D.M., III; Neill, W.H. Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquaculture 2000, 182, 339–352. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Zhou, Y.; Zhang, Z.; Chen, G.; Yu, X.; Tong, J. Different Dissolved Oxygen Effects on Growth and O2-Sensing Pathway in Juvenile Bighead Carp (Hypophthalmichthys nobilis). Aquaculture 2024, 584, 740672. [Google Scholar] [CrossRef]
- Wood, A.T.; Clark, T.D.; Elliott, N.G.; Frappell, P.B.; Andrewartha, S.J. Physiological Effects of Dissolved Oxygen Are Stage-Specific in Incubating Atlantic Salmon (Salmo salar). J. Comp. Physiol. B 2019, 189, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Zhong, C.Y.; Bao, J.W.; Liang, M.; Liang, C.; Tao, Y.F.; Li, H.X.; He, J.; Xu, P. Synergistic Effect of Water Temperature and Dissolved Oxygen Concentration on Rates of Fertilization, Hatching and Deformity of Hybrid Yellow Catfish (Tachysurus fulvidraco♀ × Pseudobagrus vachellii♂). J. Therm. Biol. 2019, 83, 47–53. [Google Scholar] [CrossRef]
- Rocha-Santos, C.; Bastos, F.F.; Dantas, R.F.; Hauser-Davis, R.A.; Rodrigues, L.C.; Cunha Bastos, V.L.F.; Cunha Bastos, J. Glutathione Peroxidase and Glutathione S-Transferase in Blood and Liver from a Hypoxia-Tolerant Fish under Oxygen Deprivation. Ecotoxicol. Environ. Saf. 2018, 163, 604–611. [Google Scholar] [CrossRef]
- Yang, K.; Fan, Q.; Zhang, L.; Li, B.; Gao, Y.; Zeng, K.; Wang, Q.; Zhu, S.; Fang, G. Effect of Dissolved Oxygen Levels on Growth Performance, Energy Budget and Antioxidant Responses of Yellow Catfish, Pelteobagrus fulvidraco (Richardson). Aquac. Res. 2015, 46, 2025–2033. [Google Scholar] [CrossRef]
- Espmark, Å.M.; Baeverfjord, G. Effects of Hyperoxia on Behavioural and Physiological Variables in Farmed Atlantic Salmon (Salmo salar) Parr. Aquac. Int. 2009, 17, 341–353. [Google Scholar] [CrossRef]
- Hosfeld, C.D.; Handeland, S.O.; Fivelstad, S.; Stefansson, S.O. Physiological Effects of Normbaric Environmental Hyperoxia on Atlantic Salmon (Salmo salar L.) Presmolts. Aquaculture 2010, 308, 28–33. [Google Scholar] [CrossRef]
- McArley, T.J.; Sandblom, E.; Herbert, N.A. Fish and Hyperoxia—From Cardiorespiratory and Biochemical Adjustments to Aquaculture and Ecophysiology Implications. Fish Fish. 2021, 22, 324–355. [Google Scholar] [CrossRef]
- Hosfeld, C.D.; Engevik, A.; Mollan, T.; Lunde, T.M.; Waagbø, R.; Olsen, A.B.; Breck, O.; Stefansson, S.; Fivelstad, S. Long-Term Separate and Combined Effects of Environmental Hypercapnia and Hyperoxia in Atlantic Salmon (Salmo salar L.) Smolts. Aquaculture 2008, 280, 146–153. [Google Scholar] [CrossRef]
- Guo, H.-Y.; Dong, X.-Y.; Zhang, X.-M.; Zhang, P.-D.; Li, W.-T. Survival, Growth and Physiological Responses of Juvenile Japanese Flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846) Exposed to Different Dissolved Oxygen Concentrations and Stocking Densities. J. Appl. Ichthyol. 2017, 33, 731–739. [Google Scholar]
- Thorarensen, H.; Gústavsson, A.; Gunnarsson, S.; Árnason, J.; Steinarsson, A.; Björnsdóttir, R.; Imsland, A.K.D. The Effect of Oxygen Saturation on the Growth and Feed Conversion of Juvenile Atlantic Cod (Gadus morhua L.). Aquaculture 2017, 475, 24–28. [Google Scholar] [CrossRef]
- Aksakal, E.; Ekinci, D. Effects of Hypoxia and Hyperoxia on Growth Parameters and Transcription Levels of Growth, Immune System and Stress Related Genes in Rainbow Trout. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 262, 111060. [Google Scholar] [CrossRef]
- Phull, A.-R.; Nasir, B.; Haq, I.U.; Kim, S.J. Oxidative Stress, Consequences and ROS Mediated Cellular Signaling in Rheumatoid Arthritis. Chem.-Biol. Interact. 2018, 281, 121–136. [Google Scholar] [CrossRef]
- Wang, M.; Wu, F.; Xie, S.; Zhang, L. Acute Hypoxia and Reoxygenation: Effect on Oxidative Stress and Hypoxia Signal Transduction in the Juvenile Yellow Catfish (Pelteobagrus fulvidraco). Aquaculture 2021, 531, 735903. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.; Zhang, J.; Wang, Z.; Li, H.; Amenyogbe, E.; Chen, G. Effects of Hypoxia Stress on the Intestinal Microflora of Juvenile of Cobia (Rachycentron canadum). Aquaculture 2021, 536, 736419. [Google Scholar] [CrossRef]
- Lygren, B.; Hamre, K.; Waagbø, R. Effect of induced hyperoxia on the antioxidant status of Atlantic salmon Salmo salar L. fed three different levels of dietary vitamin E. Aquac. Res. 2000, 31, 401–407. [Google Scholar]
- Ritola, O.; Tossavainen, K.; Kiuru, T.; Lindstrom-Seppa, P.; Molsa, H. Effects of Continuous and Episodic Hyperoxia on Stress and Hepatic Glutathione Levels in One-Summer-Old Rainbow Trout (Oncorhynchus mykiss). J. Appl. Ichthyol. 2002, 18, 159–164. [Google Scholar] [CrossRef]
- Magnúsdóttir, S.O.; Maltesen, R.G.; Haugaard Banch, L.; Baandrup, U.T.; Valbjørn, H.; Andreassen, T.; Bathen, T.F.; Steen Rasmussen, B.; Kjærgaard, B. Hyperoxia Affects the Lung Tissue: A Porcine Histopathological and Metabolite Study Using Five Hours of Apneic Oxygenation. Metab. Open 2019, 4, 100018. [Google Scholar] [CrossRef]
- Jordan, A.D.; Steffensen, J.F. Effects of Ration Size and Hypoxia on Specific Dynamic Action in the Cod. Physiol. Biochem. Zool. 2007, 80, 178–185. [Google Scholar] [CrossRef]
- Fu, S.J.; Xie, X.J.; Cao, Z.D. Effect of Meal Size on Postprandial Metabolic Response in Southern Catfish (Silurus meridionalis). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 140, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, S.; Dabrowski, K.; Izquierdo, M.; Malinovskyi, O.; Kolářová, J.; Policar, T. Effects of Dietary Protein and Lipid Levels on Growth, Body Composition, Blood Biochemistry, Antioxidant Capacity and Ammonia Excretion of European Grayling (Thymallus thymallus). Front. Mar. Sci. 2021, 8, 715636. [Google Scholar] [CrossRef]
- Barri, T.; Dragsted, L.O. UPLC-ESI-QTOF/MS and Multivariate Data Analysis for Blood Plasma and Serum Metabolomics: Effect of Experimental Artefacts and Anticoagulant. Anal. Chim. Acta 2013, 768, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-Dependent Metabolite Distribution, Clustering, and Protein Extraction for Serum Profiling with Mass Spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global Metabolic Profiling of Animal and Human Tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A Flexible and Comprehensive Software for Processing Metabolomics Data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V. Effects of Different Environmental Oxygen Levels on Free Radical Processes in Fish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 144, 283–289. [Google Scholar] [CrossRef]
- Singh, S.P.; Sharma, J.; Ahmad, T.; Chakrabarti, R. Oxygen Stress: Impact on Innate Immune System, Antioxidant Defence System and Expression of HIF-1α and ATPase 6 Genes in Catla catla. Fish Physiol. Biochem. 2016, 42, 673–688. [Google Scholar] [CrossRef]
- Sollid, J.; Nilsson, G.E. Plasticity of Respiratory Structures—Adaptive Remodeling of Fish Gills Induced by Ambient Oxygen and Temperature. Respir. Physiol. Neurobiol. 2006, 154, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.G.; Zhu, J.-H.; Cheng, W.-H.; Bao, Y.; Ho, Y.-S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S.J. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol. Rev. 2016, 96, 307–364. [Google Scholar] [CrossRef] [PubMed]
- Sabri, D.; Rabie, T.; Ahmed, A.; Zakaria, S.; Bourdineaud, J.-P. Heavy Metals-Induced Expression of ABCB10 Gene in Zebrafish (Danio rerio). Egypt. Acad. J. Biol. Sciences. C Physiol. Mol. Biol. 2012, 4, 97–106. [Google Scholar] [CrossRef]
- McKay, L.I.; Cidlowski, J.A. Molecular Control of Immune/Inflammatory Responses: Interactions Between Nuclear Factor-κB and Steroid Receptor-Signaling Pathways. Endocr. Rev. 1999, 20, 435–459. [Google Scholar] [CrossRef]
- Qi, B.; Wang, J.; Hu, M.; Ma, Y.; Wu, S.; Qi, G.; Qiu, K.; Zhang, H. Influences of Beta-Alanine and l-Histidine Supplementation on Growth Performance, Meat Quality, Carnosine Content, and mRNA Expression of Carnosine-Related Enzymes in Broilers. Animals 2021, 11, 2265. [Google Scholar] [CrossRef]
- Aydın, A.F.; Bingül, İ.; Küçükgergin, C.; Doğan-Ekici, I.; Doğru Abbasoğlu, S.; Uysal, M. Carnosine Decreased Oxidation and Glycation Products in Serum and Liver of High-fat Diet and Low-dose Streptozotocin-induced Diabetic Rats. Int. J. Exp. Path. 2017, 98, 278–288. [Google Scholar] [CrossRef]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From Physiology to Clinical Application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef]
- Ashaf-Ud-Doulah, M.; Mamun, A.A.; Rahman, M.L.; Islam, S.M.M.; Jannat, R.; Hossain, M.A.R.; Shahjahan, M. High Temperature Acclimation Alters Upper Thermal Limits and Growth Performance of Indian Major Carp, Rohu, Labeo Rohita (Hamilton, 1822). J. Therm. Biol. 2020, 93, 102738. [Google Scholar] [CrossRef]
- Yudkoff, M.; Nelson, D.; Daikhin, Y.; Erecińska, M. Tricarboxylic Acid Cycle in Rat Brain Synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J. Biol. Chem. 1994, 269, 27414–27420. [Google Scholar]
- Choi, I.; Son, H.; Baek, J.-H. Tricarboxylic Acid (TCA) Cycle Intermediates: Regulators of Immune Responses. Life 2021, 11, 69. [Google Scholar] [CrossRef]
- MacKenzie, D.S.; VanPutte, C.M.; Leiner, K.A. Nutrient Regulation of Endocrine Function in Fish. Aquaculture 1998, 161, 3–25. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.-L. TSC2 Is Phosphorylated and Inhibited by Akt and Suppresses mTOR Signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Astheimer, R.W. Thermistor Infrared Detectors; Wolfe, W.L., Ed.; SPIE: San Diego, CA, USA, 1983; pp. 95–111. [Google Scholar]
- Murashita, K.; Yoshiura, Y.; Chisada, S.; Furuita, H.; Sugita, T.; Matsunari, H.; Iwashita, Y.; Yamamoto, T. Homologue Gene of Bile Acid Transporters Ntcp, Asbt, and Ost-Alpha in Rainbow Trout Oncorhynchus Mykiss: Tissue Expression, Effect of Fasting, and Response to Bile Acid Administration. Fish Physiol. Biochem. 2014, 40, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.-S.; Chen, J.-H. The Mechanism of Enterohepatic Circulation in the Formation of Gallstone Disease. J. Membr. Biol. 2014, 247, 1067–1082. [Google Scholar] [CrossRef]
- Russell, D.W. Fifty Years of Advances in Bile Acid Synthesis and Metabolism. J. Lipid Res. 2009, 50, S120–S125. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 1191–1212. ISBN 978-0-470-65071-4. [Google Scholar]
- Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1376. [Google Scholar] [CrossRef]

| Gene | Sequences (5′-3′) | Size (bp) | |
|---|---|---|---|
| 18s | F | ATTCCGATAACGAACGAGAC | 20 |
| R | GGACATCTAAGGGCATCACAG | 21 | |
| argH | F | CCAATGAACGCAGACTAAA | 19 |
| R | TGTAACCAGGAAACAGGAC | 19 | |
| cndp1 | F | TGCCCTCCATTCTGCTGC | 18 |
| R | TGCGGTGTAGGTCTGGTCTC | 20 | |
| Abcb10a | F | AGCCAGATTAGCCAACGC | 18 |
| R | TTCCATCAGACGCTCCAA | 18 | |
| IGF-1 | F | TTCAGTAAACCAACAGGCTATG | 22 |
| R | AATGACTATGTCCAGGTAAAGGT | 23 | |
| ghrb | F | ACTACAGCACCGACAGGC | 18 |
| R | CACTGGGAATCTTGACACTTT | 21 | |
| cat | F | GGCATTTGGTTACTTTGAGG | 20 |
| R | CAGTGGAGAAGCGGACAG | 18 | |
| cu/zn-sod | F | GACCAGCGGGACCGTGTAT | 19 |
| R | CCAGCGTTGCCTGTCTTT | 18 | |
| gpx1a | F | CTGAGCGTATTCTGTTTCC | 19 |
| R | CTGAGCGTATTCTGTTTCC | 18 |
| Treatment | Initial Body Weight (g/ind) | Final Body Weight (g/ind) | Specific Growth Rate (SGR) (%) | Feed Conversion Ratio (FCR) | Survival Rate (%) |
|---|---|---|---|---|---|
| CK | 56.53 ± 2.64 a | 158.60 ± 6.42 a | 1.62 ± 0.19 a | 0.98 ± 0.03 a | 88.89 ± 7.70 a |
| HFR | 57.09 ± 0.89 a | 177.05 ± 4.28 b | 1.89 ± 0.02 b | 1.52 ± 0.006 b | 93.33 ± 6.67 a |
| HDO | 57.06 ± 2.13 a | 156.42 ± 6.48 a | 1.68 ± 0.07 a | 0.97 ± 0.05 a | 93.33 ± 6.67 a |
| MIX | 56.93 ± 2.66 a | 191.59 ± 8.09 c | 2.07 ± 0.02 c | 1.43 ± 0.06 b | 95.56 ± 7.70 a |
| Specific growth rate (%) = 100 × (ln(final body weight) − ln(body weight))/breeding time initial body weight | |||||
| Feed conversion rate (%) = dry feed consumed/(final body weight − initial body weight) | |||||
| Survival rate (%) = 100 (final amount of fish)/(initial amount of fish) | |||||
| Final Body Weight | SGR | FCR | |
|---|---|---|---|
| Dissolved oxygen | 0.120 | 0.077 | 0.107 |
| Feeding rates | 0.000 | 0.000 | 0.000 |
| Interaction between dissolved oxygen and feeding rates | 0.048 | 0.010 | 0.178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zheng, Y.; Dong, D.; Song, X.; Li, M. Effects of Increased Feeding Rates on Oxidative Stress, Biochemical Indices and Growth of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) Under Mild-Hyperoxia Conditions. Fishes 2025, 10, 228. https://doi.org/10.3390/fishes10050228
Wang Z, Zheng Y, Dong D, Song X, Li M. Effects of Increased Feeding Rates on Oxidative Stress, Biochemical Indices and Growth of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) Under Mild-Hyperoxia Conditions. Fishes. 2025; 10(5):228. https://doi.org/10.3390/fishes10050228
Chicago/Turabian StyleWang, Zhiyi, Yikai Zheng, Dengpan Dong, Xiefa Song, and Meng Li. 2025. "Effects of Increased Feeding Rates on Oxidative Stress, Biochemical Indices and Growth of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) Under Mild-Hyperoxia Conditions" Fishes 10, no. 5: 228. https://doi.org/10.3390/fishes10050228
APA StyleWang, Z., Zheng, Y., Dong, D., Song, X., & Li, M. (2025). Effects of Increased Feeding Rates on Oxidative Stress, Biochemical Indices and Growth of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) Under Mild-Hyperoxia Conditions. Fishes, 10(5), 228. https://doi.org/10.3390/fishes10050228





