Green Chemistry and Multivariate Optimization in the Extraction of Phenolic Compounds: The Potential of NaDES in Alternative Raw Materials for Expanded Extrudates
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Experimental Design
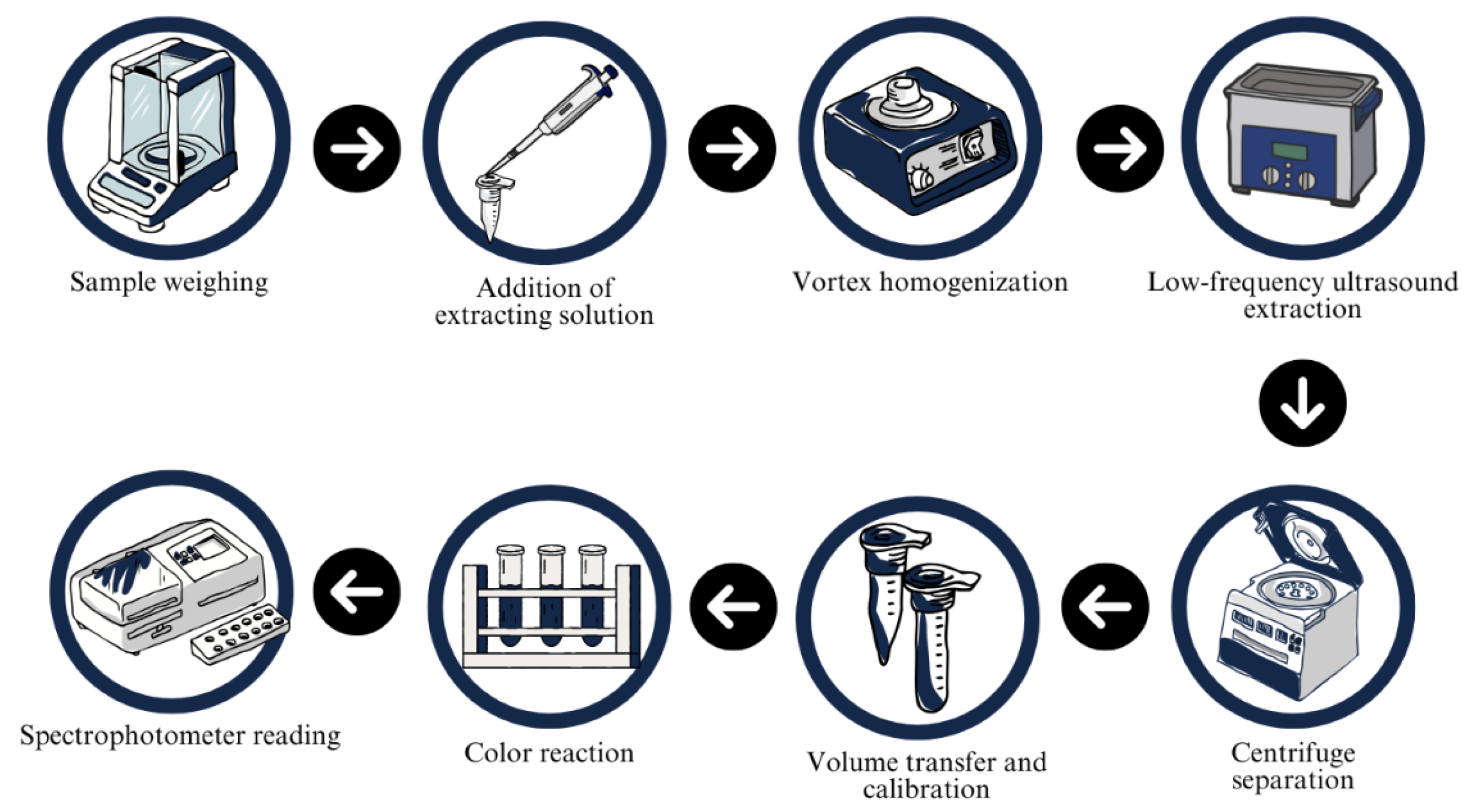
2.3. Extraction of TSPC and Absorbance Quantification
2.4. Optimization of Extraction Cycles
2.5. Production of Expanded Extrudates
2.6. Quantification of TSPC
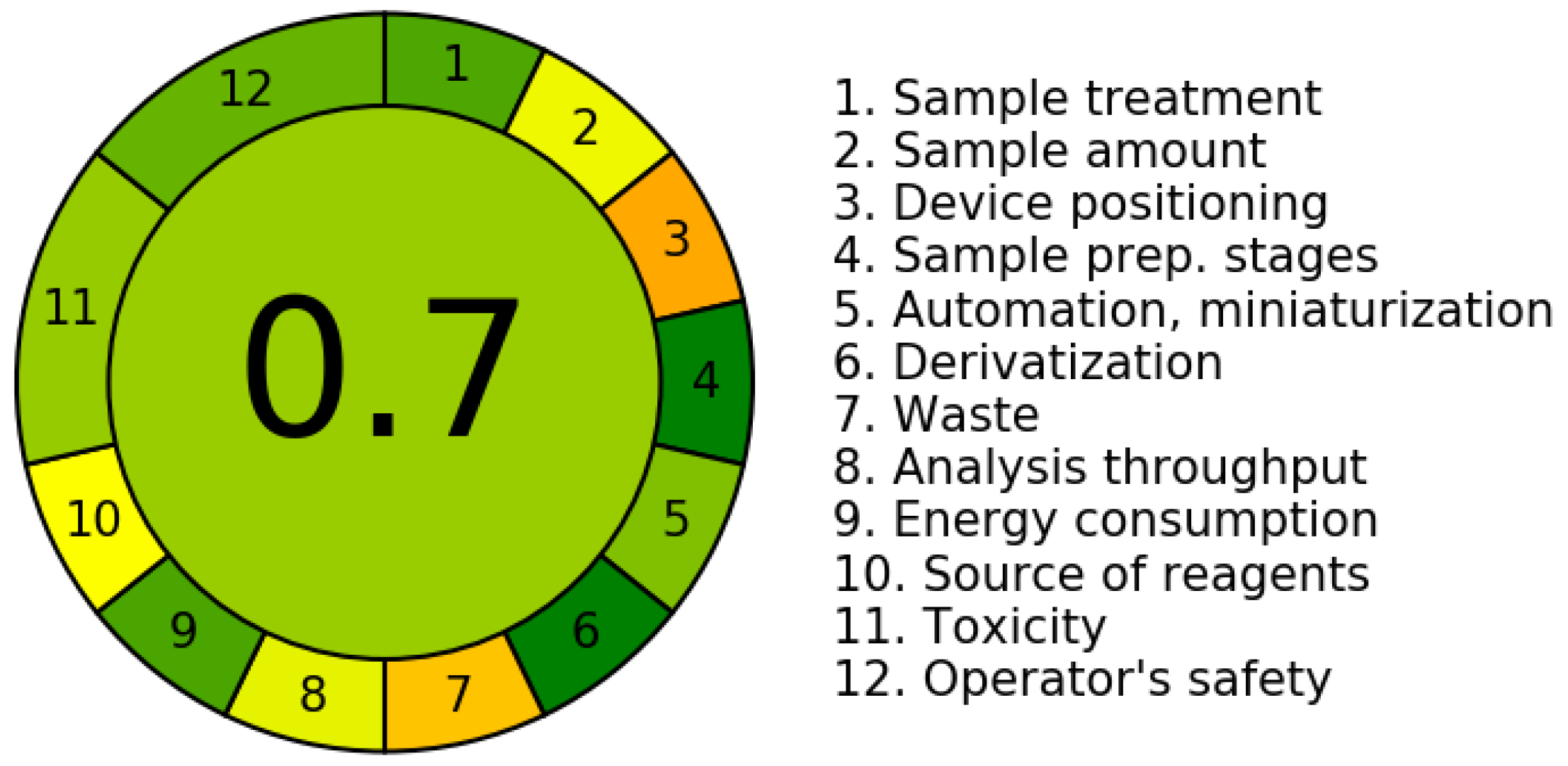
2.7. Determination of the AGREE Index (Analytical Greenness Metric Approach)
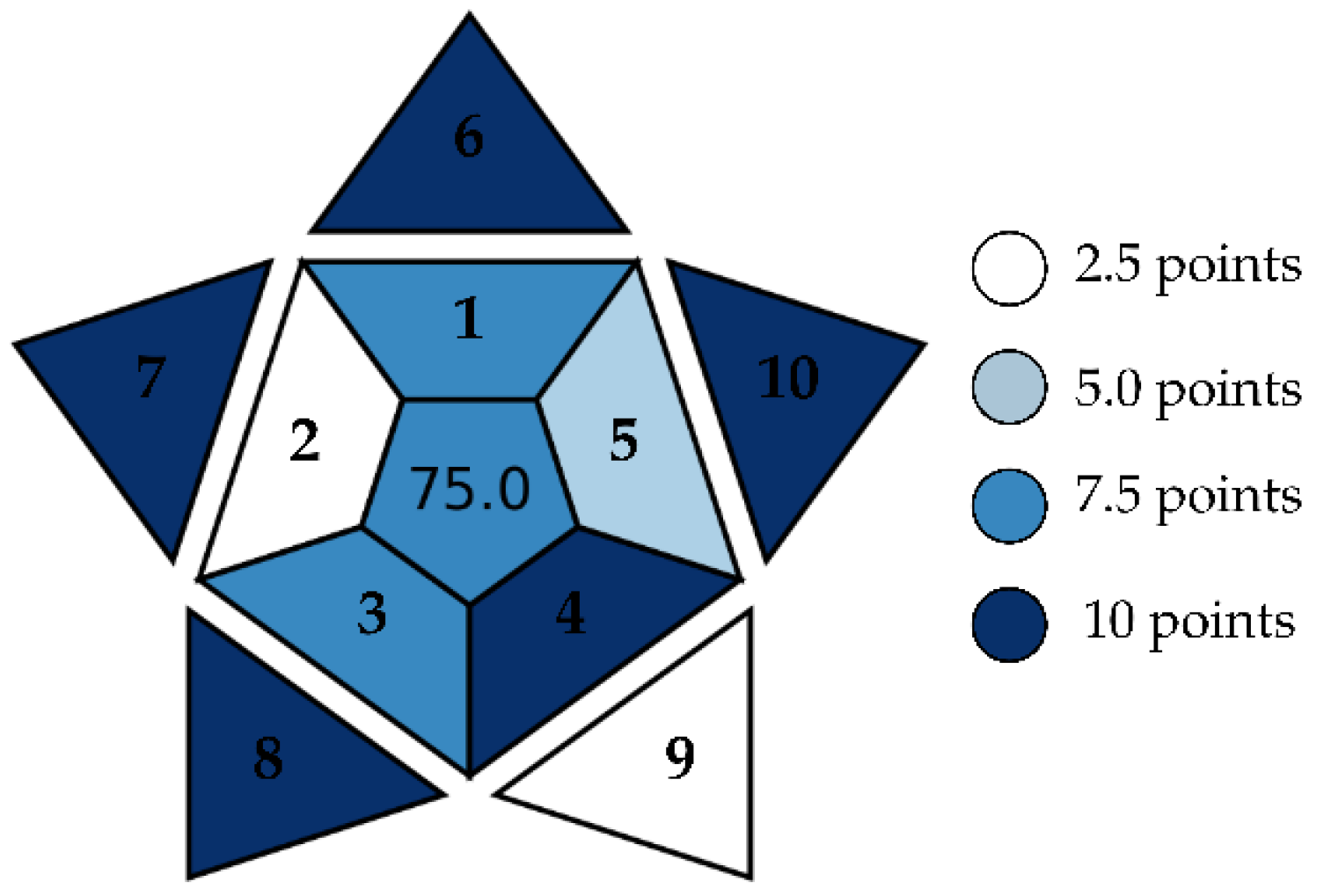
2.8. Determination of the BAGI (Blue Applicability Grade Index)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the Extracting Solution
3.2. Numerical Optimization and Validation of the Mathematical Models
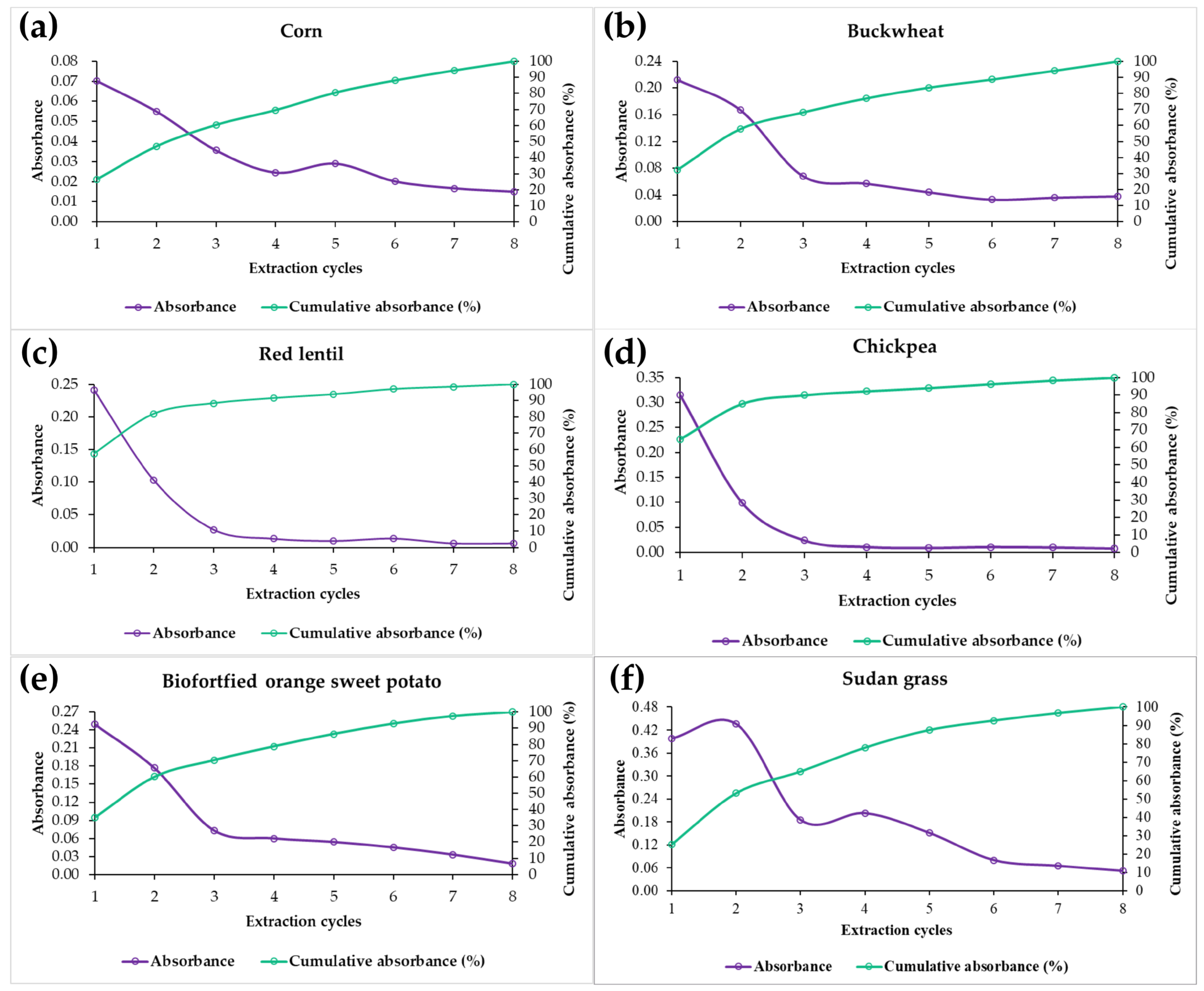
3.3. Optimization of Extraction Cycles
3.4. Quantification of TSPCs
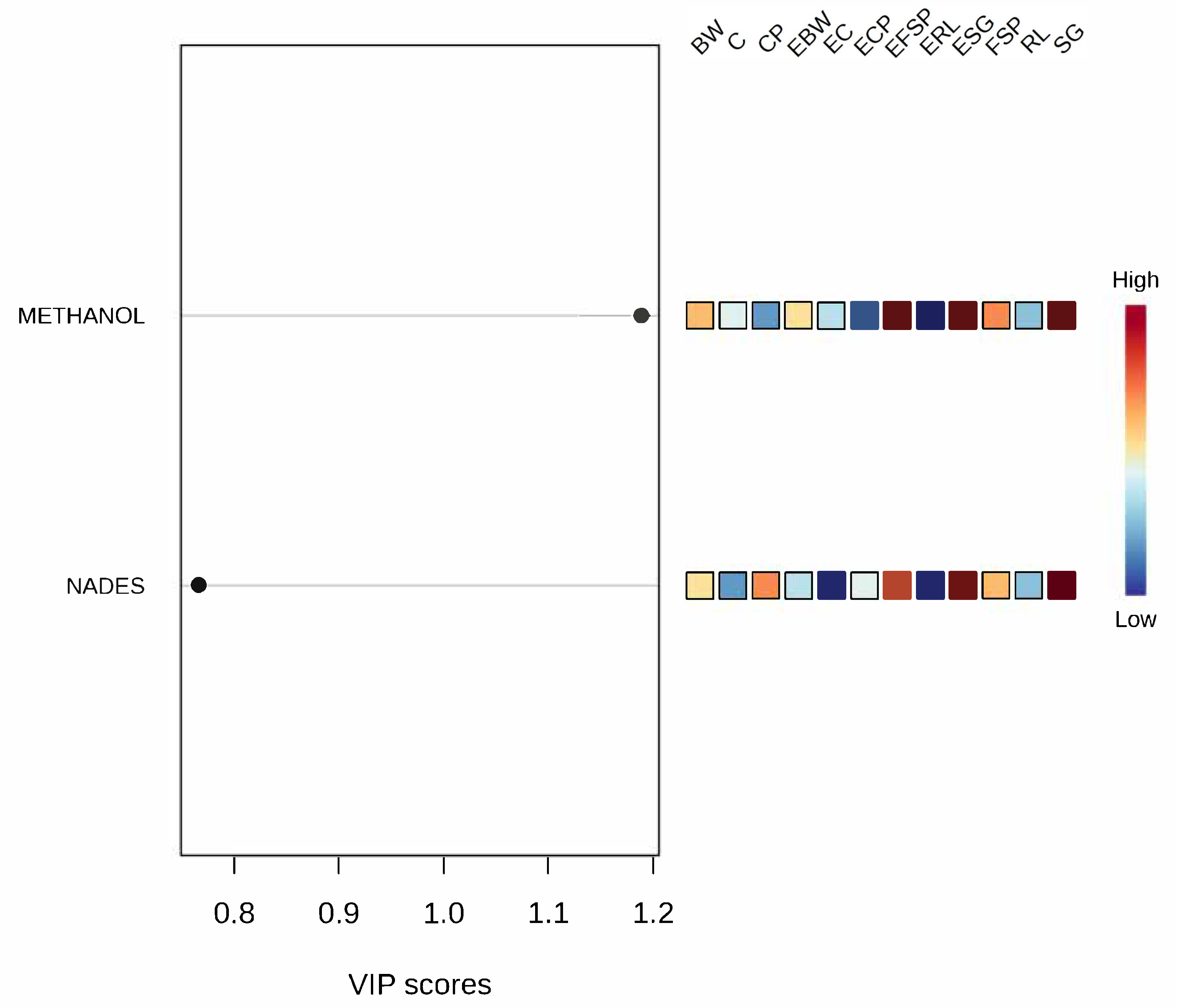
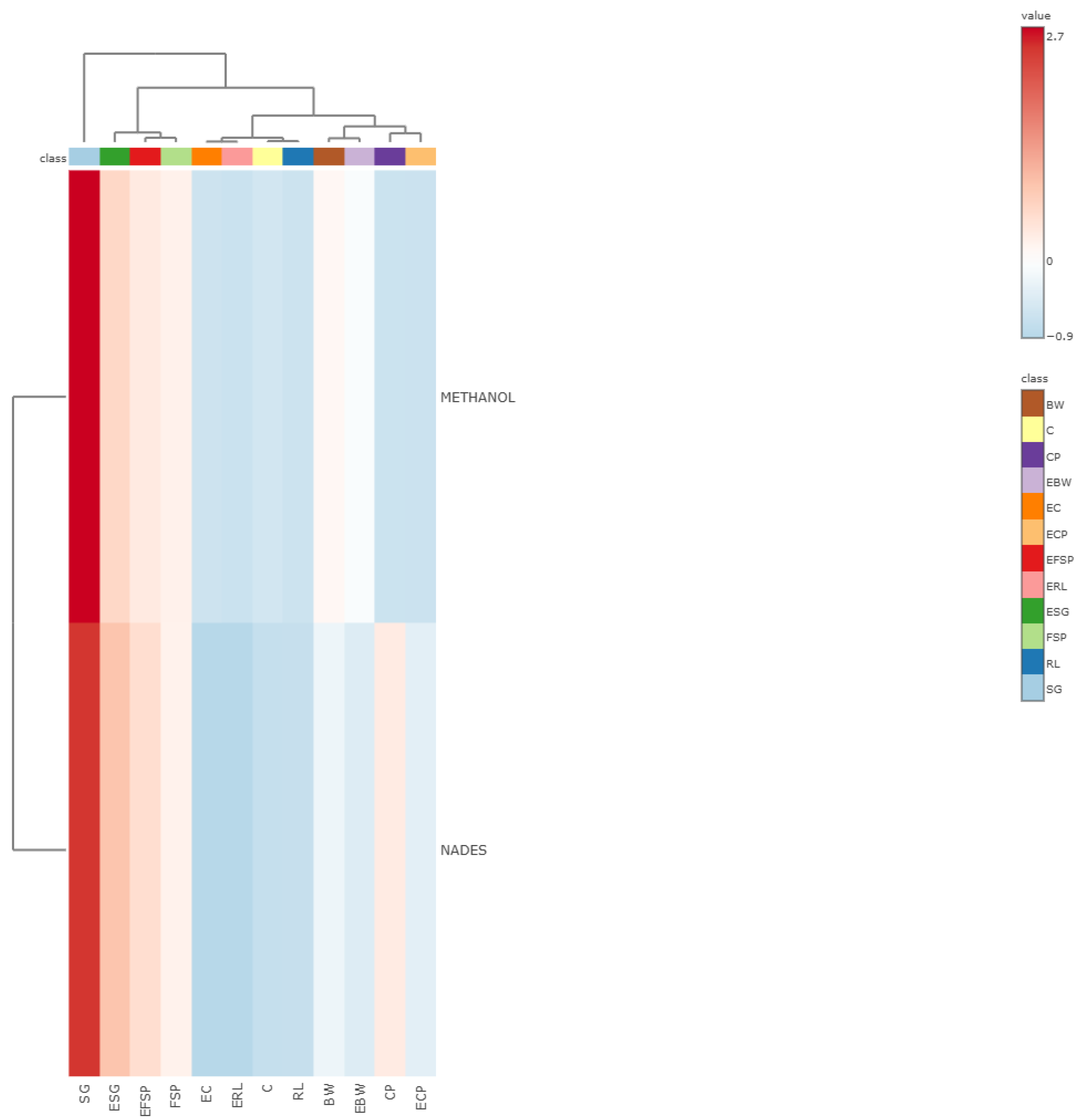
3.5. Multivariate Analysis
3.6. Determination of the AGREE Index (Analytical Greenness Metric Approach)
3.7. Determination of the BAGI (Blue Applicability Grade Index)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, A.; Dutra, F.S.; Brito, S.N.S.; Pereira-Vasques, M.S.; Azevedo, G.Z.; Schneider, A.R.; Oliveira, E.R.; Santos, A.A.; Maraschin, M.; Vianello, F.; et al. Effect of Biomass Drying Protocols on Bioactive Compounds and Antioxidant and Enzymatic Activities of Red Macroalga Kappaphycus Alvarezii. Methods Protoc. 2024, 7, 88. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic Compounds: From Plants to Foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Mihaylova, D.; Dimitrova-Dimova, M.; Popova, A. Dietary Phenolic Compounds—Wellbeing and Perspective Applications. Int. J. Mol. Sci. 2024, 25, 4769–4784. [Google Scholar] [CrossRef] [PubMed]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Ivanović, M.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and Green Extraction of Bioactive Compounds from Lippia Citriodora by Tailor-Made Natural Deep Eutectic Solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Airouyuwa, J.O.; Mostafa, H.; Ranasinghe, M.; Maqsood, S. Influence of Physicochemical Properties of Carboxylic Acid-Based Natural Deep Eutectic Solvents (CA-NADES) on Extraction and Stability of Bioactive Compounds from Date (Phoenix dactylifera L.) Seeds: An Innovative and Sustainable Extraction Technique. J. Mol. Liq. 2023, 388, 122767. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Zhao, J.; Wang, Y.; Yao, Q.; Li, S. Development and Optimization of Green Extraction of Polyphenols in Michelia Alba Using Natural Deep Eutectic Solvents (NADES) and Evaluation of Bioactivity. Sustain. Chem. Pharm. 2024, 37, 101425. [Google Scholar] [CrossRef]
- Spaggiari, C.; Carbonell-Rozas, L.; Zuilhof, H.; Costantino, G.; Righetti, L. Structural Elucidation and Long-Term Stability of Synthesized NADES: A Detailed Physicochemical Analysis. J. Mol. Liq. 2025, 424, 127105. [Google Scholar] [CrossRef]
- González-Laredo, R.F.; Sayago-Monreal, V.I.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Landeros-Macías, L.F.; Rosales-Castro, M. Natural Deep Eutectic Solvents (NaDES) as an Emerging Technology for the Valorisation of Natural Products and Agro-Food Residues: A Review. Int. J. Food Sci. Technol. 2023, 58, 6660–6673. [Google Scholar] [CrossRef]
- Maia, N.M.A.; Andressa, I.; Cunha, J.S.; Costa, N.A.; Borges, L.L.R.; Fontes, E.A.F.; Oliveira, E.B.; Leite Júnior, B.R.C.; Bhering, L.L.; Saldaña, M.D.A.; et al. Optimization of Ultrasound-Assisted Obtention of Bluish Anthocyanin Extracts from Butterfly Pea (Clitoria ternatea) Petal Powders Using Natural Deep Eutectic Solvents. Plants 2025, 14, 1042. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Rodrigues, M.I.; Iemma, A.F. Experiment Planning and Process Optimization: A Sequential Planning Strategy, 3rd ed.; Casa do Pão: Campinas, SP, Brazil, 2014; p. 100. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid Microplate High-Throughput Methodology for Assessment of Folin-Ciocalteu Reducing Capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remón, A.; Barrionuevo-González, A.; Zamora-Ros, R.; Andres-Lacueva, C.; Estruch, R.; Martínez-González, M.Á.; Diez-Espino, J.; Lamuela-Raventos, R.M. Rapid Folin–Ciocalteu Method Using Microtiter 96-Well Plate Cartridges for Solid Phase Extraction to Assess Urinary Total Phenolic Compounds, as a Biomarker of Total Polyphenols Intake. Anal. Chim. Acta 2009, 634, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A Reproducible, Rapid and Inexpensive Folin–Ciocalteu Micro-Method in Determining Phenolics of Plant Methanol Extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Araújo, M.; Jesus, L.K.; Rodrigues, S.M.; Jesus, A.A.S.N.; Nascimento, G.C.; Soares, M.A.; Clerici, M.T.P.S.; Schmiele, M. Development of second-generation extruded snacks with innovative raw materials: Evaluation of instrumental texture and bowl-life properties. In Proceedings of the Brazilian Congress on Cereal and Baking Technology, Sete Lagoas, Brazil, 4–7 December 2024. [Google Scholar]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue Applicability Grade Index (BAGI) and Software: A New Tool for the Evaluation of Method Practicality. Green. Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Massaretto, I.L.; Rivero Meza, S.L.; Schmiele, M.; Lanfer Marquez, U.M.; Sinnecker, P. Nutritional Characterization and Effect of Cooking on Phenolic Compounds, Antioxidant Capacity and Sensory Acceptability of Commercial Wild Rice (Zizania aquatica L.). Biocatal. Agric. Biotechnol. 2023, 50, 102705. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Zhao, J.; Ou, P.; Yao, Q.; Wang, W. Ultrasound-Assisted Extraction of Phenolic Compounds from Celtuce (Lactuca sativa Var. Augustana) Leaves Using Natural Deep Eutectic Solvents (NADES): Process Optimization and Extraction Mechanism Research. Molecules 2024, 29, 2385. [Google Scholar] [CrossRef] [PubMed]
- Pires, I.V.; Sakurai, Y.C.N.; Ferreira, N.R.; Moreira, S.G.C.; Cruz Rodrigues, A.M.; Silva, L.H.M. Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from Pitaya. Molecules 2022, 27, 8310. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gómez-Alonso, S.; Pastene-Navarrete, E.; Pérez-Navarro, J. Green Extraction of Alkaloids and Polyphenols from Peumus Boldus Leaves with Natural Deep Eutectic Solvents and Profiling by HPLC-PDA-IT-MS/MS and HPLC-QTOF-MS/MS. Plants 2020, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Rasera, G.B.; Castro, R.J.S. Grain germination: A systematic review of how the biochemical process involved affect the content and profile of phenolic compouds and their antioxidant properties. Braz. J. Nat. Sci. 2020, 3, 287. [Google Scholar] [CrossRef]
- Boeing, A.H.; Longo, C.; Kuhnen, S. Perfil de compostos fenólicos solúveis e insolúveis e atividade antioxidante em farinhas de variedades crioulas de milho após processamento térmico para produção de polenta. In Ciência e Tecnologia de Alimentos: Pesquisa e Práticas Contemporâneas, 1st ed.; Científica Digital: São Paulo, Brazil, 2008; pp. 154–196. [Google Scholar]
- Podolska, G.; Gujska, E.; Klepacka, J.; Aleksandrowicz, E. Bioactive Compounds in Different Buckwheat Species. Plants 2021, 10, 961. [Google Scholar] [CrossRef]
- Aleksenko, S.S.; Kazimirova, K.O.; Shtykov, S.N. Comparative Evaluation of the Concentration of Free Phenolic Compounds and the Antioxidant Activity of Various Buckwheat Samples. J. Anal. Chem. 2022, 77, 948–956. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kinabo, J.; Uebersax, M.A. Nutrient Profile and Effect of Processing Methods on the Composition and Functional Properties of Lentils (Lens culinaris Medik): A Review. Legume Sci. 2023, 5, e156. [Google Scholar] [CrossRef]
- Xia, M.; Li, M.; Souza, T.S.P.; Barrow, C.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS2 Characterization of Phenolic Compounds in Different Lentil (Lens culinaris M.) Samples and Their Antioxidant Capacity. Front. Biosci.-Landmark 2023, 28, 44. [Google Scholar] [CrossRef]
- Begum, N.; Khan, Q.U.; Liu, L.G.; Li, W.; Liu, D.; Haq, I.U. Nutritional Composition, Health Benefits and Bio-Active Compounds of Chickpea (Cicer arietinum L.). Front. Nutr. 2023, 10, 1218468. [Google Scholar] [CrossRef]
- Klongklaew, A.; Banwo, K.; Soodsawaeng, P.; Christopher, A.; Khanongnuch, C.; Sarkar, D.; Shetty, K. Lactic Acid Bacteria Based Fermentation Strategy to Improve Phenolic Bioactive-Linked Functional Qualities of Select Chickpea (Cicer arietinum L.) Varieties. NFS J. 2022, 27, 36–46. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Pazos, J.; Zema, P.; Corbino, G.B.; Gabilondo, J.; Borioni, R.; Malec, L.S. Growing Location and Root Maturity Impact on the Phenolic Compounds, Antioxidant Activity and Nutritional Profile of Different Sweet Potato Genotypes. Food Chem. Mol. Sci. 2022, 5, 100125. [Google Scholar] [CrossRef]
- Sinha, N.; Thomas, A. Nutritional, Therapeutic, and Functional Applications of Sorghum: A Comprehensive Review. Medicon Med. Sci. 2025, 8, 41–48. [Google Scholar] [CrossRef]
- Carvalho, H.J.M.; Oliveira, L.H.R.; Souza, G.J.S.; Pinto, C.C.; Souza, S.M.; Carvalho, C.W.P.; Nabeshima, E.H.; Clerici, M.T.P.S.; Barcia, M.T.; Schmiele, M. Unraveling Sudan Grass Starch: A First Report of Its Physicochemical, Structural, Technological, and Nutritional Properties. Food Humanit. 2025, 4, 100627. [Google Scholar] [CrossRef]
- Chumsri, P.; Panpipat, W.; Cheong, L.Z.; Nisoa, M.; Chaijan, M. Comparative Evaluation of Hydrothermally Produced Rice Starch–Phenolic Complexes: Contributions of Phenolic Type, Plasma-Activated Water, and Ultrasonication. Foods 2022, 11, 3826. [Google Scholar] [CrossRef]
- D’costa, A.S.; Bordenave, N. Whole Grain Chemistry and Nutrition from a Health Perspective: Understanding the Fibre-Phenolic-Starch Ménage à Trois. Trends Food Sci. Technol. 2023, 141, 104196. [Google Scholar] [CrossRef]
- Šárka, E.; Sluková, M.; Henke, S. Changes in Phenolics during Cooking Extrusion: A Review. Foods 2021, 10, 2100. [Google Scholar] [CrossRef]
- Andressa, I.; Paiva, M.J.A.; Pacheco, F.C.; Santos, F.R.; Cunha, J.S.; Pacheco, A.F.C.; Neves, N.A.; Vendruscolo, R.G.; Schmiele, M.; Tribst, A.A.L.; et al. Germination as a Strategy to Improve the Characteristics of Flour and Water-Soluble Extracts Obtained from Sunflower Seed. Food Biosci. 2024, 61, 104763. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Castillo-Martínez, W.E.; Simpalo-Lopez, W.D.; Verona-Ruiz, A.; Lavado-Cruz, A.; Martínez-Villaluenga, C.; Peñas, E.; Frias, J.; Schmiele, M. Performance of Thermoplastic Extrusion, Germination, Fermentation, and Hydrolysis Techniques on Phenolic Compounds in Cereals and Pseudocereals. Foods 2022, 11, 1957. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, X.; Guo, R.; Zhu, L.; Qiu, X.; Yu, X.; Chai, J.; Gu, C.; Feng, Z. Insights into the Effects of Extractable Phenolic Compounds and Maillard Reaction Products on the Antioxidant Activity of Roasted Wheat Flours with Different Maturities. Food Chem. X 2023, 17, 100548. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A Modified Folin-Ciocalteu Assay for the Determination of Total Phenolics Content in Honey. Appl. Sci. 2023, 13, 2135. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L. Fennema’s Food Chemistry, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4822-0812-0. [Google Scholar]
- Feng, J.; Berton-Carabin, C.C.; Fogliano, V.; Schroën, K. Maillard Reaction Products as Functional Components in Oil-in-Water Emulsions: A Review Highlighting Interfacial and Antioxidant Properties. Trends Food Sci. Technol. 2022, 121, 129–141. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Ribéreau, S.; Mondor, M.; Cuevas-Rodríguez, E.O.; Arcand, Y.; Hernández-Alvarez, Á.J. Impact of Processing on the in Vitro Protein Quality, Bioactive Compounds, and Antioxidant Potential of 10 Selected Pulses. Legume Sci. 2021, 3, e88. [Google Scholar] [CrossRef]
- Vashishth, R.; Semwal, A.D.; Naika, M.; Sharma, G.K.; Kumar, R. Influence of Cooking Methods on Antinutritional Factors, Oligosaccharides and Protein Quality of Underutilized Legume Macrotyloma Uniflorum. Food Res. Int. 2021, 143, 110299. [Google Scholar] [CrossRef]
- Mironeasa, S.; Coţovanu, I.; Mironeasa, C.; Ungureanu-Iuga, M. A Review of the Changes Produced by Extrusion Cooking on the Bioactive Compounds from Vegetal Sources. Antioxidants 2023, 12, 1453. [Google Scholar] [CrossRef]
- Rizvi, N.B.; Aleem, S.; Khan, M.R.; Ashraf, S.; Busquets, R. Quantitative Estimation of Protein in Sprouts of Vigna Radiate (Mung beans), Lens Culinaris (Lentils), and Cicer Arietinum (Chickpeas) by Kjeldahl and Lowry Methods. Molecules 2022, 27, 814. [Google Scholar] [CrossRef]
- Guo, F.; Danielski, R.; Santhiravel, S.; Shahidi, F. Unlocking the Nutraceutical Potential of Legumes and Their By-Products: Paving the Way for the Circular Economy in the Agri-Food Industry. Antioxidants 2024, 13, 636. [Google Scholar] [CrossRef]
- Alam, M.K.; Sams, S.; Rana, Z.H.; Akhtaruzzaman, M.; Islam, S.N. Minerals, Vitamin C, and Effect of Thermal Processing on Carotenoids Composition in Nine Varieties Orange-Fleshed Sweet Potato (Ipomoea batatas L.). J. Food Compos. Anal. 2020, 92, 103582. [Google Scholar] [CrossRef]
- Setoguchi, Y.; Narasako, Y.; Hirano, T.; Otani, M.; Kunitake, H. Changes in Carotenoids and Polyphenols during the Growth Stages of Orange-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam.). Horticulturae 2024, 10, 629. [Google Scholar] [CrossRef]
- Yu, Q.; Su, Y.; Xi, Y.; Rong, Y.; Long, Y.; Xie, Y.; Sun, H.; Dong, R.; Hao, J.; Yang, F.; et al. Comparison of the Impacts of Cellulase and Laccase on Fermentation Quality, Bacterial Composition and in Vitro Degradability of Anaerobic Cofermentation Derived from Sudan Grass with Mulberry under Lactobacillus Plantarum and Different Lignocellulolytic Enzyme Inoculation. Chem. Biol. Technol. Agric. 2025, 12, 41. [Google Scholar] [CrossRef]
- Zeb, L.; Shafiq, M.; Jordheim, M. Advancements in Sustainable Extraction of Seaweed Phenolics: Integrating Extraction Technologies with Natural Deep Eutectic Solvents (NADES). Food Chem. 2025, 489, 144941. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Urios, C.; Puchades-Colera, P.; Frigola, A.; Esteve, M.J.; Blesa, J.; Lopez-Malo, D. Natural Deep Eutectic Solvents: A Paradigm of Stability and Permeability in the Design of New Ingredients. J. Mol. Liq. 2024, 412, 125864. [Google Scholar] [CrossRef]
- Bi, Y.; Chi, X.; Zhang, R.; Lu, Y.; Wang, Z.; Dong, Q.; Ding, C.; Yang, R.; Jiang, L. Highly Efficient Extraction of Mulberry Anthocyanins in Deep Eutectic Solvents: Insights of Degradation Kinetics and Stability Evaluation. Innov. Food Sci. Emerg. Technol. 2020, 66, 102512. [Google Scholar] [CrossRef]
- Xu, L.; Liaqat, F.; Khazi, M.I.; Sun, J.; Zhu, D. Natural Deep Eutectic Solvents-Based Green Extraction of Vanillin: Optimization, Purification, and Bioactivity Assessment. Front. Nutr. 2023, 10, 1279552. [Google Scholar] [CrossRef]
- Julshahril, N.H.; Phuah, E.T.; Rambli, M.M. Deep Eutectic Solvents in the Extraction of Bioactive Compounds in Agri-Food Industry. Food Humanit. 2025, 4, 100468. [Google Scholar] [CrossRef]
- Stanisz, M.; Stanisz, B.J.; Cielecka-Piontek, J. A Comprehensive Review on Deep Eutectic Solvents: Their Current Status and Potential for Extracting Active Compounds from Adaptogenic Plants. Molecules 2024, 29, 4767. [Google Scholar] [CrossRef]
- Li, D. Natural Deep Eutectic Solvents in Phytonutrient Extraction and Other Applications. Front. Plant Sci. 2022, 13, 1004332. [Google Scholar] [CrossRef]
- Welton, T. Solvents and Sustainable Chemistry. Proc. R. Soc. A 2015, 471, 20150502. [Google Scholar] [CrossRef]
- Huang, M.M.; Yiin, C.L.; Mun Lock, S.S.; Fui Chin, B.L.; Othman, I.; Ahmad Zauzi, N.S.; Chan, Y.H. Natural Deep Eutectic Solvents (NADES) for Sustainable Extraction of Bioactive Compounds from Medicinal Plants: Recent Advances, Challenges, and Future Directions. J. Mol. Liq. 2025, 425, 127202. [Google Scholar] [CrossRef]
- Verep, D.; Ateş, S.; Karaoğul, E.; Bazlı, B.; Biyoaktif, H.; Elde, B.; İçin, E.; İncelenmesi, E.Y.; Üniversitesi, B.; Yüksekokulu, G.M.; et al. A Review of Extraction Methods for Obtaining Bioactive Compounds in Plant-Based Raw Materials. J. Bartın For. Fac. 2023, 25, 492–513. [Google Scholar] [CrossRef]
- Agrawal, A.; Keçili, R.; Ghorbani-Bidkorbeh, F.; Hussain, C.M. Green Miniaturized Technologies in Analytical and Bioanalytical Chemistry. TrAC Trends Anal. Chem. 2021, 143, 116383. [Google Scholar] [CrossRef]
- Sajid, M. Greening the Derivatization Step in Analytical Extractions: Recent Strategies and Future Directions. Chem. Rev. 2015, 115, 4693–4718. [Google Scholar]
- Raposo, F.; Borja, R.; Gutiérrez-González, J.A. A Comprehensive and Critical Review of the Unstandardized Folin-Ciocalteu Assay to Determine the Total Content of Polyphenols: The Conundrum of the Experimental Factors and Method Validation. Talanta 2024, 272, 125771. [Google Scholar] [CrossRef] [PubMed]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2023, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Stoenescu, A.M.; Trandafir, I.; Cosmulescu, S. Determination of Phenolic Compounds Using HPLC-UV Method in Wild Fruit Species. Horticulturae 2022, 8, 84. [Google Scholar] [CrossRef]



| Trials | Coded Levels | Real Proportions (%) | ||||
|---|---|---|---|---|---|---|
| x1 | x2 | x3 | X1 | X2 | X3 | |
| 1 | 1.0 | 0.0 | 0.0 | 98.0 | 1.0 | 1.0 |
| 2 | 0.0 | 1.0 | 0.0 | 1.0 | 98.0 | 1.0 |
| 3 | 0.0 | 0.0 | 1.0 | 1.0 | 1.0 | 98.0 |
| 4 | 0.5 | 0.5 | 0.0 | 49.5 | 49.5 | 1.0 |
| 5 | 0.5 | 0.0 | 0.5 | 49.5 | 1.0 | 49.5 |
| 6 | 0.0 | 0.5 | 0.5 | 1.0 | 49.5 | 49.5 |
| 7 | 0.6667 | 0.1667 | 0.1667 | 65.7 | 17.2 | 17.2 |
| 8 | 0.1667 | 0.6667 | 0.1667 | 17.2 | 65.7 | 17.2 |
| 9 | 0.1667 | 0.1667 | 0.6667 | 17.2 | 17.2 | 65.7 |
| 10 | 0.3333 | 0.3333 | 0.3333 | 33.3 | 33.3 | 33.3 |
| 11 | 0.3333 | 0.3333 | 0.3333 | 33.3 | 33.3 | 33.3 |
| 12 | 0.3333 | 0.3333 | 0.3333 | 33.3 | 33.3 | 33.3 |
| 13 | 0.3333 | 0.3333 | 0.3333 | 33.3 | 33.3 | 33.3 |
| Raw Material | Independent Variables # | Numerical Optimization and Validation | |||||
|---|---|---|---|---|---|---|---|
| Sorbitol 3 M | Citric Acid 60 mM | Glycine 300 mM | Predicted Value | Experimental Value δ | Relative Deviation | Desirability | |
| Corn | 0.9782 | 0.1180 | 0.1000 | 0.087 | 0.097 ± 0.009 | 10.62 | 0.9303 |
| Buckwheat | 0.9800 | 0.0100 | 0.0100 | 0.284 | 0.236 ± 0.019 | −16.98 | 0.9272 |
| Red lentil | 0.0102 | 0.0100 | 0.9798 | 0.220 | 0.262 ± 0.012 | 18.81 | 0.9611 |
| Chickpea | 0.0100 | 0.0100 | 0.9800 | 0.290 | 0.317 ± 0.030 | 9.22 | 0.8598 |
| Sweet potato | 0.0100 | 0.9800 | 0.0100 | 0.235 | 0.213 ± 0.030 | −9.07 | 0.4567 |
| Sudan grass | 0.9241 | 0.0658 | 0.0100 | 0.405 | 0.436 ± 0.065 | 7.49 | 0.9827 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, M.A.; Rodrigues Morais, B.; da Silva Santos, J.P.; de Jesus, L.K.; Aurélio Lomba, K.; do Nascimento, G.C.; Soares, M.A.; Neves, N.d.A.; Andressa, I.; Pedrosa Silva Clerici, M.T.; et al. Green Chemistry and Multivariate Optimization in the Extraction of Phenolic Compounds: The Potential of NaDES in Alternative Raw Materials for Expanded Extrudates. Methods Protoc. 2025, 8, 82. https://doi.org/10.3390/mps8040082
Araújo MA, Rodrigues Morais B, da Silva Santos JP, de Jesus LK, Aurélio Lomba K, do Nascimento GC, Soares MA, Neves NdA, Andressa I, Pedrosa Silva Clerici MT, et al. Green Chemistry and Multivariate Optimization in the Extraction of Phenolic Compounds: The Potential of NaDES in Alternative Raw Materials for Expanded Extrudates. Methods and Protocols. 2025; 8(4):82. https://doi.org/10.3390/mps8040082
Chicago/Turabian StyleAraújo, Mateus Alves, Bianca Rodrigues Morais, João Pedro da Silva Santos, Larissa Karla de Jesus, Kaliston Aurélio Lomba, Gustavo Costa do Nascimento, Marcus Alvarenga Soares, Nathalia de Andrade Neves, Irene Andressa, Maria Teresa Pedrosa Silva Clerici, and et al. 2025. "Green Chemistry and Multivariate Optimization in the Extraction of Phenolic Compounds: The Potential of NaDES in Alternative Raw Materials for Expanded Extrudates" Methods and Protocols 8, no. 4: 82. https://doi.org/10.3390/mps8040082
APA StyleAraújo, M. A., Rodrigues Morais, B., da Silva Santos, J. P., de Jesus, L. K., Aurélio Lomba, K., do Nascimento, G. C., Soares, M. A., Neves, N. d. A., Andressa, I., Pedrosa Silva Clerici, M. T., & Schmiele, M. (2025). Green Chemistry and Multivariate Optimization in the Extraction of Phenolic Compounds: The Potential of NaDES in Alternative Raw Materials for Expanded Extrudates. Methods and Protocols, 8(4), 82. https://doi.org/10.3390/mps8040082










