Development and Evaluation of a Nanoparticle-Based Immunoassay for Rotavirus Detection: A Suitable Alternative to ELISA and PCR in Low-Income Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design, Location and Sample Characteristics
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Sample Collection
2.4. Rotavirus Assay Development
2.4.1. Materials for Assay Development
2.4.2. Optimisation of Periodate Oxidisation of Cotton Swabs
2.4.3. Immobilisation of Lactoferrin on Oxidised Cotton Swab
2.4.4. Activation of Nanobeads and Conjugation of RVA-VP6 Monoclonal Antibody on Carboxyl-Functionalized Coloured Nanobeads
2.4.5. Detection of Rotavirus as Proof of Concept
2.5. Sample Evaluation by ELISA, Newly Developed RV Nanoparticle Immunoassay and Molecular Methods
2.5.1. Stool Preparation
2.5.2. Rotavirus Detection by ELISA
2.5.3. Rotavirus Detection Using Our Developed RV Nanoparticle-Based Immunoassay
Principle of the Test
Test Procedure
2.5.4. Rotavirus Detection by Real-Time Quantitative Reverse Transcription PCR (qRT-PCR)
Viral RNA Extraction
2.6. Data Analysis
3. Results
3.1. Optimal Oxidation Conditions for Cotton Swabs
Effect of Periodate Concentration
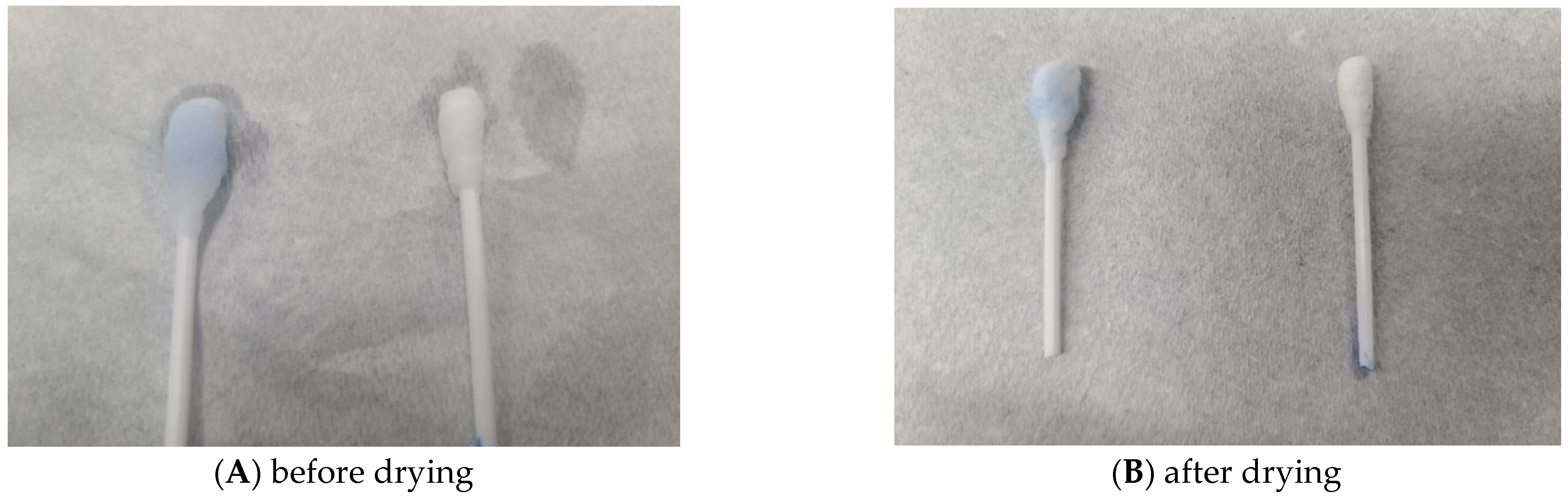
3.2. Lactoferrin Immobilisation on Oxidised Cotton Swab
3.3. Experimental Setup and Detection of Rotavirus as Proof of Concept
3.4. Rotavirus Detection Using PCR, ELISA and Nanoparticle Developed Kit
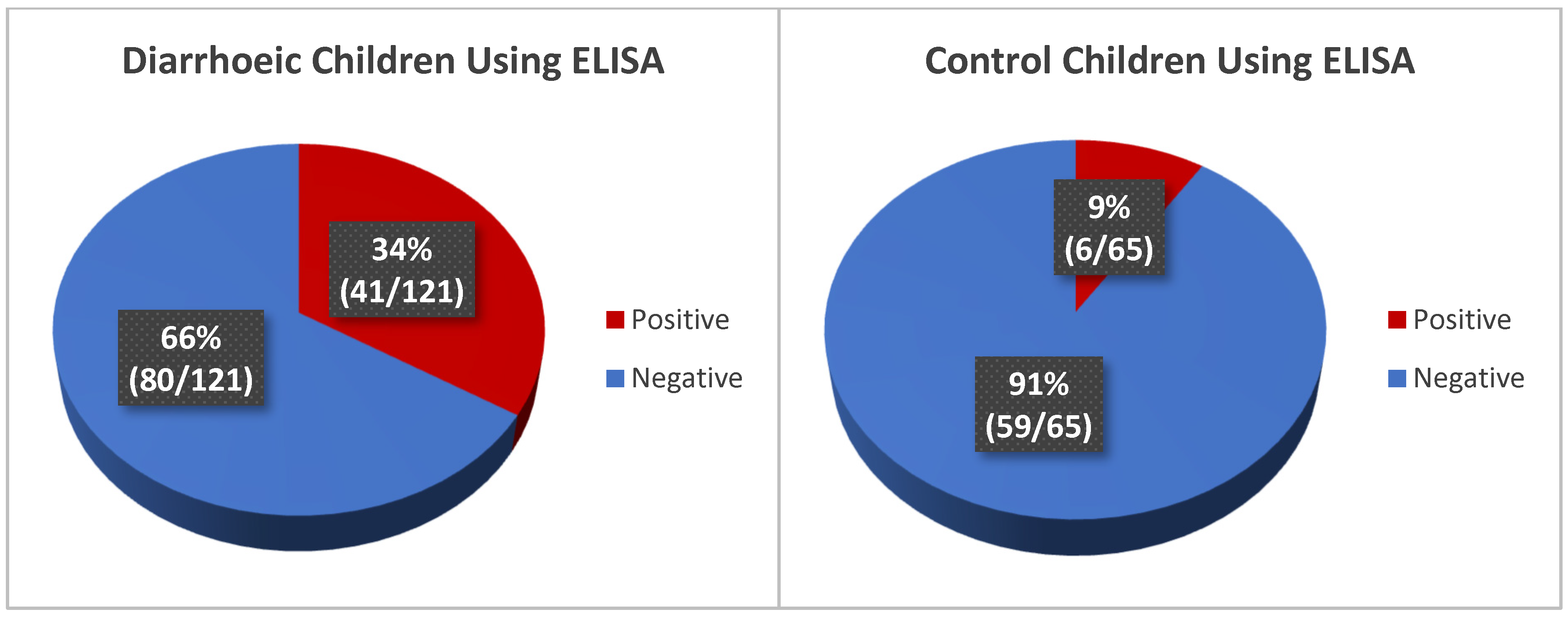
3.4.1. Detection of Rotavirus from Stool Samples Using ELISA
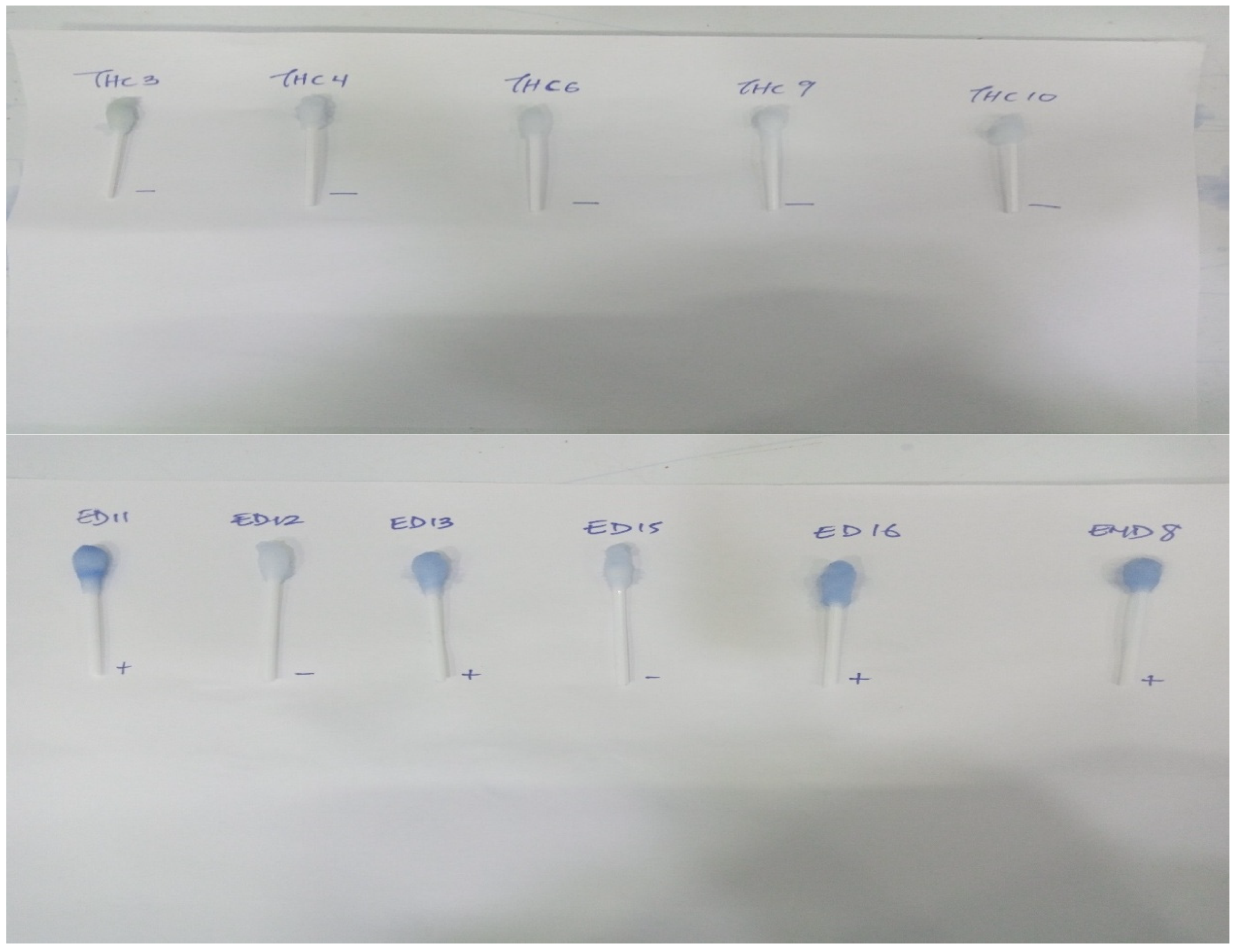
3.4.2. Detection of Rotavirus Using the Developed RV Nanoparticle-Based Immunoassay
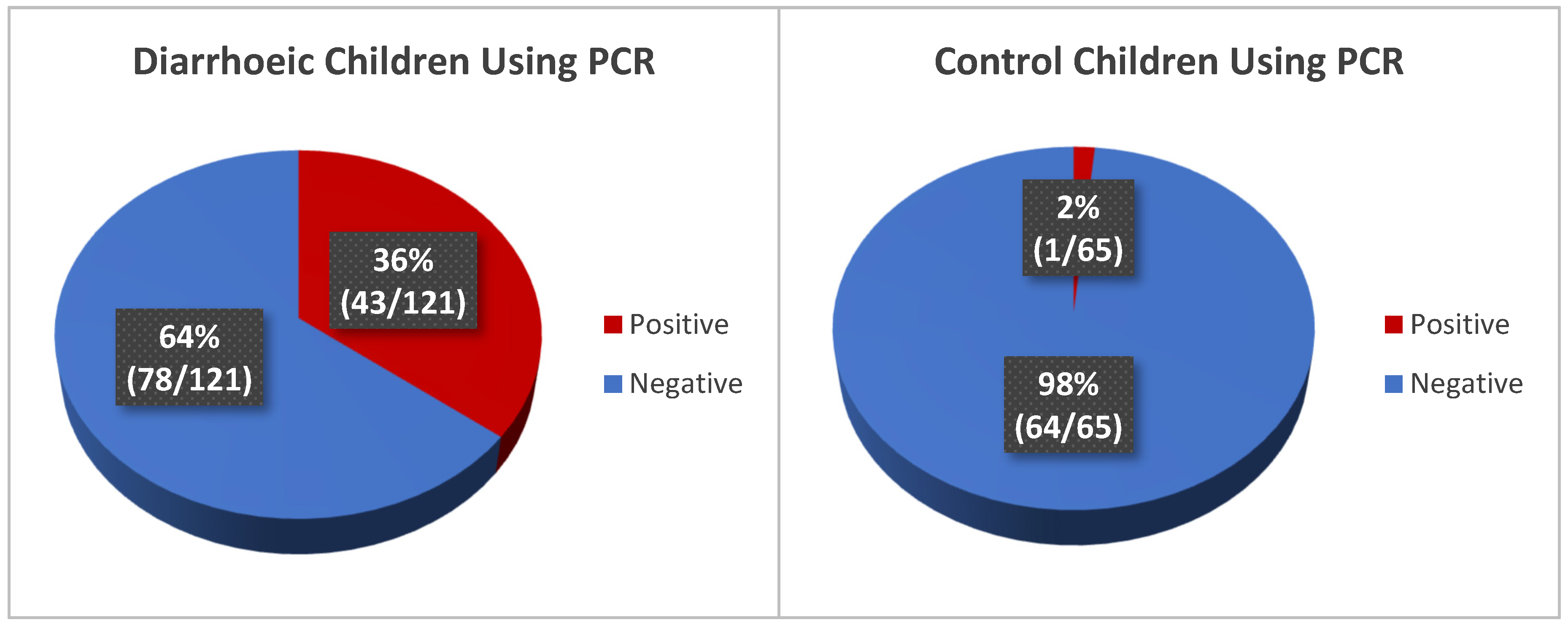
3.4.3. Detection of Rotavirus in Diarrhoeic Children Using Molecular Method (qRT-PCR)
3.4.4. Comparison of RV Detection by ELISA, the Developed Nanoparticle-Based Immunoassay and Quantitative PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RVA | Group A rotavirus (RVA) |
| LF | Lactoferrin |
| RV | Rotavirus |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| LA | Latex agglutination |
| ELISA | Enzyme Linked Immunosorbent Assay |
| NaIO4 | Sodium periodate |
| PBS | Phosphate-buffered saline |
| EDC | 1-ethyl-3-(3-dimethylaminopropy)carbodiimide hydrochloride |
| BSA | Bovine serum albumin |
| qRT-PCR | Quantitative Reverse Transcription PCR |
| MS2 | 2-(N-morpholino) ethanesulfonic acid |
| NHS | N-hydroxy succinimide |
| Ag | Antigen |
| PCR | Polymerase chain reaction |
References
- GBD 2021 Diarrhoeal Diseases Collaborators. Global, regional, and national age-sex-specific burden of diarrhoeal diseases, their risk factors, and aetiologies, 1990–2021, for 204 countries and territories: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2025, 25, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Bakir, H.; Hadi, M.; Jurdi, M. Towards a renewed public health regulatory and surveillance role in water, sanitation and hygiene. East. Mediterr. Health J. 2017, 23, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Demissie, G.D.; Yeshaw, Y.; Aleminew, W.; Akalu, Y. Diarrhea and associated factors among under five children in sub-Saharan Africa: Evidence from demographic and health surveys of 34 sub-Saharan countries. PLoS ONE 2021, 16, e0257522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Black, R.E.; Perin, J.; Yeung, D.; Rajeev, T.; Miller, J.; Elwood, S.E.; Platts-Mills, J.A. Estimated global and regional causes of deaths from diarrhoea in children younger than 5 years during 2000-21: A systematic review and Bayesian multinomial analysis. Lancet Glob. Health 2024, 12, e919–e928. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.K.; Umar, U. Combating diarrhoea in Nigeria: The way forward. J. Microbiol. Exp. 2018, 6, 191–197. [Google Scholar] [CrossRef]
- Becker-Dreps, S.; Bucardo, F.; Vilchez, S.; Zambrana, L.E.; Liu, L.; Weber, D.J.; Peña, R.; Barclay, L.; Vinjé, J.; Hudgens, M.G.; et al. Etiology of childhood diarrhea after rotavirus vaccine introduction: A prospective, population-based study in Nicaragua. Pediatr. Infect. Dis. J. 2014, 33, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.C.; Armah, G.; Fuentes, S.; Korpela, K.E.; Parashar, U.; Victor, J.C.; Tate, J.; de Weerth, C.; Giaquinto, C.; Wiersinga, W.J.; et al. Significant Correlation Between the Infant Gut Microbiome and Rotavirus Vaccine Response in Rural Ghana. J. Infect. Dis. 2017, 215, 34–41. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wang, L.; Liu, S.; Li, S.; Zhou, X.; Xiao, Y.; Zhong, P.; Chen, Y.; Wang, C.; Xu, S.; et al. Global Incidence of Diarrheal Diseases-An Update Using an Interpretable Predictive Model Based on XGBoost and SHAP: A Systematic Analysis. Nutrients 2024, 16, 3217. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diarrhoeal Disease. 2024. Available online: http://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 7 March 2024).
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D.; World Health Organization—Coordinated Global Rotavirus Surveillance Network; Agocs, M.; Serhan, F.; de Oliveira, L.; Mwenda, J.M.; Mihigo, R.; et al. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin. Infect. Dis. 2016, 62 (Suppl. S2), S96–S105. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, A.G.; Verani, J.R.; Keita, A.M.; Jahangir Hossain, M.; Roose, A.; Sow, S.O.; Omore, R.; Doh, S.; Jones, J.C.M.; Nasrin, D.; et al. Etiology, Presentation, and Risk Factors for Diarrheal Syndromes in 3 Sub-Saharan African Countries After the Introduction of Rotavirus Vaccines from the Vaccine Impact on Diarrhea in Africa (VIDA) Study. Clin. Infect. Dis. 2023, 76 (Suppl. S1), S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Thystrup, C.; Majowicz, S.E.; Kitila, D.B.; Desta, B.N.; Fayemi, O.E.; Ayolabi, C.I.; Hugho, E.; Buys, E.M.; Akanni, G.B.; Machava, N.E.; et al. Etiology-specific incidence and mortality of diarrheal diseases in the African region: A systematic review and meta-analysis. BMC Public Health 2024, 24, 1864. [Google Scholar] [CrossRef] [PubMed]
- Efunshile, A.M.; Ezeanosike, O.; Onyekachi, O.N.I.; Ugwu, M.I.; König, B.; Robertson, L.J. Apparent absence of Giardia infections among children under 5-years of age with acute watery diarrhoea in Abakaliki, Nigeria. Epidemiol. Infect. 2019, 147, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Salência-Ferrão, J.; Chissaque, A.; Manhique-Coutinho, L.; Kenga, A.N.; Cassocera, M.; de Deus, N. Inappropriate use of antibiotics in the management of diarrhoea in children under five years admitted with acute diarrhoea in four provinces of Mozambique 2014–2019. BMC Infect. Dis. 2025, 25, 209. [Google Scholar] [CrossRef] [PubMed]
- AL-Khafaji, Y.A.; AL-Jiboury, H.J. Detection of Rotavirus in Diarrhea Stool Samples of Children with Acute Gastroenteritis in Babylon Governorate, Iraq. Int. Res. J. Microbiol. (IRJM) 2013, 4, 84–88. [Google Scholar]
- Eibach, D.; Krumkamp, R.; Hahn, A.; Sarpong, N.; Adu-Sarkodie, Y.; Leva, A.; Käsmaier, J.; Panning, M.; May, J.; Tannich, E. Application of a multiplex PCR assay for the detection of gastrointestinal pathogens in a rural African setting. BMC Infect. Dis. 2016, 16, 150. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.E.; Mijatovic-Rustempasic, S.; Tam, K.I.; Lyde, F.C.; Payne, D.C.; Szilagyi, P.; Edwards, K.; Staat, M.A.; Weinberg, G.A.; Hall, C.B.; et al. Comparison of 2 assays for diagnosing rotavirus and evaluating vaccine effectiveness in children with gastroenteritis. Emerg. Infect. Dis. 2013, 19, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, M.S.; van Well, G.T.J.; van Loo, I.H.M. Diagnosis of viral gastroenteritis in children: Interpretation of real-time PCR results and relation to clinical symptoms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Sidoti, F.; Rittà, M.; Costa, C.; Cavallo, R. Diagnosis of viral gastroenteritis: Limits and potential of currently available procedures. J. Infect. Dev. Ctries. 2015, 9, 551–561. [Google Scholar] [CrossRef] [PubMed]
- González-Serrano, L.; Muñoz-Algarra, M.; González-Sanz, R.; Portero-Azorín, M.F.; Amaro, M.J.; Higueras, P.; Cabrerizo, M. Viral gastroenteritis in hospitalized patients: Evaluation of immunochromatographic methods for rapid detection in stool samples. J. Clin. Virol. 2020, 128, 104420. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; Gregoricus, N.; Weinberg, G.A.; Halasa, N.; Chappell, J.; Hassan, F.; Selvarangan, R.; Mijatovic-Rustempasic, S.; Ward, M.L.; Bowen, M.; et al. Comparison of three multiplex gastrointestinal platforms for the detection of gastroenteritis viruses. J. Clin. Virol. 2017, 95, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.Y.; Wu, Y.H.; Xiong, Q.R.; Xu, H.Y.; Xiong, Y.H.; Liu, K.; Jin, Y.; Lai, W.H. Advantages of fluorescent microspheres compared with colloidal gold as a label in immunochromatographic lateral flow assays. Biosens. Bioelectron. 2014, 54, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Shi, L.; Lin, J.; Zhang, L.; Peng, Y.; Sheng, H.; Wu, P.; Pan, Q. Comparison of two different combined test strips with fluorescent microspheres or colored microspheres as tracers for rotavirus and adenovirus detection. Virol. J. 2018, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Watzinger, F.; Ebner, K.; Lion, T. Detection and monitoring of virus infections by real-time PCR. Mol. Asp. Med. 2006, 27, 254–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, J.; Gao, E.B.; Lu, Y.; Qiu, H. A rapid visualization method for detecting rotavirus A by combining nuclear acid sequence-based amplification with the CRISPR-Cas12a assay. J. Med. Microbiol. 2024, 73, 001892. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lim, E.; Park, G.; Park, C.; Lim, J.; Lee, H.; Na, W.; Yeom, M.; Kim, J.; Song, D.; et al. Advanced Nanomaterials for Preparedness Against (Re-)Emerging Viral Diseases. Adv. Mater. 2021, 33, e2005927. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.C.; Patani, B.O.; Ekpa, D.E. Nanotechnology in Diagnosis: A Review. Adv. Nanopart. 2017, 6, 93–102. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Sai Krishna, M.; Nalluru, S.; Sampath Kumar, N.S. Nanotechnology: An emerging approach to combat COVID-19. Emergent Mater. 2021, 4, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Alamer, S.; Eissa, S.; Chinnappan, R.; Herron, P.; Zourob, M. Rapid colorimetric lactoferrin-based sandwich immunoassay on cotton swabs for the detection of foodborne pathogenic bacteria. Talanta 2018, 185, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Alhadrami, H.A.; Suaifan, G.A.R.Y.; Zourob, M.M. A Portable Nanoprobe for Rapid and Sensitive Detection of SARS-CoV-2 S1 Protein. Biosensors 2022, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Raji, M.A.; Aloraij, Y.; Alhamlan, F.; Suaifan, G.; Weber, K.; Cialla-May, D.; Popp, J.; Zourob, M. Development of rapid colorimetric assay for the detection of Influenza A and B viruses. Talanta 2021, 221, 121468. [Google Scholar] [CrossRef] [PubMed]
- Aloraij, Y.M.; Suaifan, G.A.R.Y.; Shibl, A.; Al-Kattan, K.; Zourob, M.M. Development of Rapid Aptamer-Based Screening Assay for the Detection of COVID-19 Variants. ACS Omega 2023, 8, 32877–32883. [Google Scholar] [CrossRef] [PubMed]
- Naidu, A.S.; Arnold, R.R. Lactoferrin interaction with salmonellae potentiates antibiotic susceptibility in vitro. Diagn. Microbiol. Infect. Dis. 1994, 20, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.G.; Lewis, D.M.; Tapley, K.N. Characterization of cellulose aldehyde using Fourier transform infrared spectroscopy. J. Appl. Polym. Sci. 2001, 82, 1195–1202. [Google Scholar] [CrossRef]
- Naidu, A.S.; Andersson, M.; Forsgren, A. Identification of a human lactoferrin-binding protein in Staphylococcus aureus. J. Med. Microbiol. 1992, 36, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Cleary, T.G. Effect of lactoferrin on enteric pathogens. Biochimie 2009, 91, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Japhet, M.O.; Famurewa, O.; Iturriza-Gomara, M.; Adesina, O.A.; Opaleye, O.O.; Niendorf, S.; Bock, C.T.; Marques, A.M. Group A rotaviruses circulating prior to a national immunization programme in Nigeria: Clinical manifestations, high G12P[8] frequency, intra-genotypic divergence of VP4 and VP7. J. Med. Virol. 2018, 90, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, Y.; Hsu, S.-H. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.S.; Finn, M.G. Click chemistry in complex mixtures: Bioorthogonal bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, J.A.; Liu, J.; Rogawski, E.T.; Kabir, F.; Lertsethtakarn, P.; Siguas, M.; Khan, S.S.; Praharaj, I.; Murei, A.; Nshama, R.; et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: A reanalysis of the MAL-ED cohort study. Lancet Glob. Health 2018, 6, e1309–e1318. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, T.; Kostic, M.; Praskalo, J.; Pejic, B.; Petronijevic, Z.; Skundric, P. Sodium periodate oxidized cotton yarn as carrier for immobilization of trypsin. Carbohydr. Polym. 2010, 82, 976–981. [Google Scholar] [CrossRef]
- Varma, A.; Kulkarni, M. Oxidation of cellulose under controlled conditions. Polym. Degrad. Stab. 2002, 77, 25–27. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, M.; Wang, Q.; Li, B. A novel durable flame retardant cotton fabric produced by surface chemical grafting of phosphorus- and nitrogen-containing compounds. Cellulose 2017, 24, 4069–4081. [Google Scholar] [CrossRef]
- Duceac, I.A.; Tanasa, F.; Coseri, S. Selective Oxidation of Cellulose-A Multitask Platform with Significant Environmental Impact. Materials 2022, 15, 5076. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, C.; Fan, X.; Wang, P.; Cui, L. Immobilization of catalase on cotton fabric oxidized by sodium periodate. Biocatal. Biotransformation 2009, 26, 437–443. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Shao, S.; Chang, X.; Li, M. Periodate Oxidation of Carboxymethyl Cellulose under Controlled Conditions. ChemistrySelect 2020, 5, 6765–6773. [Google Scholar] [CrossRef]
- Wei, S.; Kumar, V.; Banker, G.S. Phosphoric acid mediated depolymerization and decrystallization of cellulose: Preparation of low crystallinity cellulose—A new pharmaceutical excipient. Int. J. Pharm. 1996, 142, 175–181. [Google Scholar] [CrossRef]
- Madivoli, E.S.; Kareru, P.G.; Gachanja, A.N.; Mugo, S.M.; Makhanu, D.S. Synthesis and characterization of dialdehyde cellulose nanofibers from O.sativa husks. SN Appl. Sci. 2019, 1, 723. [Google Scholar] [CrossRef]
- Höglund, E. Production of Dialdehyde Cellulose and Periodate Regeneration: Towards Feasible Oxidation Processes. Master’s Thesis, Karlstad University, Karlstad, Sweden, 2015. [Google Scholar]
- Wang, H. Study on the process and properties of selective oxidized cotton fiber by sodium periodate. J. Anhui Agric. Univ. 2011, 38, 812–816. [Google Scholar]
- Wilham, C.A.; McGuire, T.A.; Mehltretter, C.L. Hydrolysis of Dialdehyde Starch with Sulfurous Acid. Starch—Stärke 1971, 23, 201–203. [Google Scholar] [CrossRef]
- Meng, S.; Feng, Y.; Liang, Z.; Fu, Q.; Zhang, E. Oxidizing cellulose to 2,3-dialdehyde cellulose by sodium periodate. Trans. Tianjin Univ. 2005, 11, 250–254. [Google Scholar]
- Li, H.; Wu, B.; Mu, C.; Lin, W. Concomitant degradation in periodate oxidation of carboxymethyl cellulose. Carbohydr. Polym. 2011, 84, 881–886. [Google Scholar] [CrossRef]
- Driskell, J.D.; Zhu, Y.; Kirkwood, C.D.; Zhao, Y.; Dluhy, R.A.; Tripp, R.A. Rapid and sensitive detection of rotavirus molecular signatures using surface enhanced raman spectroscopy. PLoS ONE 2010, 5, e10222. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fan, B.; Zhang, X.; Guo, R.; Zhao, Y.; Zhou, J.; Zhou, J.; Peng, Q.; Zhu, M.; Li, J.; et al. Development of a colloidal gold immunochromatographic assay strip using monoclonal antibody for rapid detection of porcine deltacoronavirus. Front. Microbiol. 2022, 13, 1074513. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, F.; Tang, T.; Wang, M.; Yu, X.; Wang, R.; Li, Y.; Xu, Y.; Tang, L.; Wang, L.; et al. Development of a Colloidal Gold Immunochromatographic Strip Assay for Rapid Detection of Bovine Rotavirus. Viral Immunol. 2019, 32, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.; Yoo, I.Y.; Yun, S.A.; Chung, Y.N.; Huh, H.J.; Lee, N.Y. Performance evaluation of Automated Fluorescent Immunoassay System ROTA and NORO for detection of rotavirus and norovirus: A comparative study of assay performance with RIDASCREEN® Rotavirus and Norovirus. J. Clin. Lab. Anal. 2021, 35, e23585. [Google Scholar] [CrossRef] [PubMed]
- Malla, B.A.; Dubal, Z.B.; Saini, S.; VinodhKumar, O.R.; Mohmad, A.; Mirsab, P.W.; Beegum, M.; Singh, V.; Bhilegaonkar, K.N. Evaluation of a commercial immunochromatographic strip assay (ICT) for rapid detection of bovine, porcine and human Rotavirus A. Microbe 2024, 5, 100182. [Google Scholar] [CrossRef]
- Biswas, S.K.; Sultana, N.; Badruddoza; Azim, A.; Biswas, R.S.R. Immunochromatographic Test and ELISA for Detection of Rotavirus in Fecal Sample: A Comparative Study. Chattagram Maa-O-Shishu Hosp. Med. Coll. J. 2020, 18, 7–11. [Google Scholar] [CrossRef]
- Shaker, R.; Abdalrahman, E.; Ali, Z.; Reslan, L.; Harastani, H.; Haidar, A.; Ghanem, S.; Hajar, F.; Inati, A.; Rajab, M.; et al. Wide Variability in the Sensitivity and Specificity of Rotavirus Immunoassay Diagnostic Kits in Practice. J. Infect. Dev. Ctries. 2021, 15, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Manual of Rotavirus Detection and Characterization Methods (No. WHO/IVB/08.17); World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Hamzavi, H.; Azaran, A.; Makvandi, M.; Karami, S.; Roayaei Ardakani, M.; Mozaffari Nejad, A.S. Performance of Latex agglutination, ELISA and RT-PCR for diagnosis of Rotavirus nfection. J. Biol. Res. 2017, 90, 92–95. [Google Scholar] [CrossRef]
- Hoque, S.A.; Iizuka, I.; Kobayashi, M.; Takanashi, S.; Anwar, K.S.; Islam, M.T.; Hoque, S.A.; Khamrin, P.; Okitsu, S.; Hayakawa, S.; et al. Determining effectiveness of rotavirus vaccine by immunochromatography and reverse transcriptase polymerase chain reaction: A comparison. Vaccine 2019, 37, 5886–5890. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, Z. Rotavirus and adenovirus detecting method: Sensitivity and specificity of rapid antigen testing: Prospective study in one region of Ireland. Virusdisease 2020, 31, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Yandle, Z.; Coughlan, S.; Drew, R.J.; Cleary, J.; De Gascun, C. Diagnosis of rotavirus infection in a vaccinated population: Is a less sensitive immunochromatographic method more suitable for detecting wild-type rotavirus than real-time RT-PCR? J. Clin. Virol. 2018, 109, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Moutelíková, R.; Dvořáková Heroldová, M.; Holá, V.; Sauer, P.; Prodělalová, J. Human rotavirus A detection: Comparison of enzymatic immunoassay and rapid chromatographic test with two quantitative RT-PCR assays. Epidemiol. Mikrobiol. Imunol. 2018, 67, 110–113. [Google Scholar] [PubMed]
- Weitzel, T.; Reither, K.; Mockenhaupt, F.P.; Stark, K.; Ignatius, R.; Saad, E.; Seidu-Korkor, A.; Bienzle, U.; Schreier, E. Field Evaluation of a Rota- and Adenovirus Immunochromatographic Assay Using Stool Samples from Children with Acute Diarrhea in Ghana. J. Clin. Microbiol. 2007, 45, 2695–2697. [Google Scholar] [CrossRef] [PubMed]
- De Grazia, S.; Bonura, F.; Pepe, A.; Muli, S.L.; Cappa, V.; Collura, A.; Terranova, D.; Urone, N.; Di Bernardo, F.; Matranga, D.; et al. Performance analysis of two immunochromatographic assays for the diagnosis of rotavirus infection. J. Virol. Methods 2017, 243, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Lagare, A.; Moumouni, A.; Kaplon, J.; Langendorf, C.; Pothier, P.; Grais, R.F.; Issaka, B.; Page, A.-L. Diagnostic accuracy of VIKIA® Rota-Adeno and PremierTM Rotaclone® tests for the detection of rotavirus in Niger. BMC Res. Notes 2017, 10, 505. [Google Scholar] [CrossRef] [PubMed]



| Result | ELISA | Nanoparticle Based Kit | qRT-PCR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Diarrhoeic Group | Control | Total | Diarrhoeic Group | Control | Total | Diarrhoeic Group | Control | Total | |
| Positive | 41 (34%) | 6 (9%) | 47 (25%) | 45 (37%) | 0 (0%) | 45 | 43 (36%) | 1 (2%) | 44 (24%) |
| Negative | 80 (66%) | 59 (91%) | 139 (75%) | 76 (63%) | 65 (100%) | 141 (76%) | 78 (65%) | 64 (99%) | 142 (76%) |
| Total | 121 | 65 | 186 | 121 | 65 | 186 | 121 | 65 | 186 |
| (a) | ||||
| Nanoparticle-Based Kit | Cases | Control | PCR | |
| True +ve | 37 | 37 | 0 | 44 |
| True −ve | 136 | 72 | 64 | 142 |
| False +ve | 8 | 8 | 0 | |
| False −ve | 5 | 4 | 1 | |
| 186 | 121 | 65 | ||
| (b) | ||||
| ELISA | Cases | Controls | PCR | |
| True +ve | 25 | 25 | 0 | 44 |
| True −ve | 122 | 64 | 58 | 142 |
| False +ve | 22 | 16 | 6 | |
| False −ve | 17 | 16 | 1 | |
| 186 | 121 | 65 | ||
| Assay | Sensitivity (95% Cl) | Specificity (95% Cl) | PPV (95% Cl) | NPV (95%Cl) |
|---|---|---|---|---|
| ELISA | 0.60 (0.43–0.74) | 0.84 (0.78–0.90) | 0.53 (0.38–0.68) | 0.88 (0.81–0.93) |
| Nanoparticle Kit | 0.88 (0.74–0.96) | 0.94 (0.89–0.98) | 0.82 (0.68–0.92) | 0.96 (0.92–0.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Japhet, M.O.; Bankole, A.T.; Omotade, T.I.; Adeoye, O.E.; Famurewa, O.; Adesina, S.K. Development and Evaluation of a Nanoparticle-Based Immunoassay for Rotavirus Detection: A Suitable Alternative to ELISA and PCR in Low-Income Setting. Methods Protoc. 2025, 8, 81. https://doi.org/10.3390/mps8040081
Japhet MO, Bankole AT, Omotade TI, Adeoye OE, Famurewa O, Adesina SK. Development and Evaluation of a Nanoparticle-Based Immunoassay for Rotavirus Detection: A Suitable Alternative to ELISA and PCR in Low-Income Setting. Methods and Protocols. 2025; 8(4):81. https://doi.org/10.3390/mps8040081
Chicago/Turabian StyleJaphet, Margaret Oluwatoyin, Adeogo Timilehin Bankole, Temiloluwa Ifeoluwa Omotade, Oyelola Eyinade Adeoye, Oladiran Famurewa, and Simeon K. Adesina. 2025. "Development and Evaluation of a Nanoparticle-Based Immunoassay for Rotavirus Detection: A Suitable Alternative to ELISA and PCR in Low-Income Setting" Methods and Protocols 8, no. 4: 81. https://doi.org/10.3390/mps8040081
APA StyleJaphet, M. O., Bankole, A. T., Omotade, T. I., Adeoye, O. E., Famurewa, O., & Adesina, S. K. (2025). Development and Evaluation of a Nanoparticle-Based Immunoassay for Rotavirus Detection: A Suitable Alternative to ELISA and PCR in Low-Income Setting. Methods and Protocols, 8(4), 81. https://doi.org/10.3390/mps8040081





