Point-of-Care Testing for G6PD Deficiency: Opportunities for Screening
Abstract
:1. Introduction
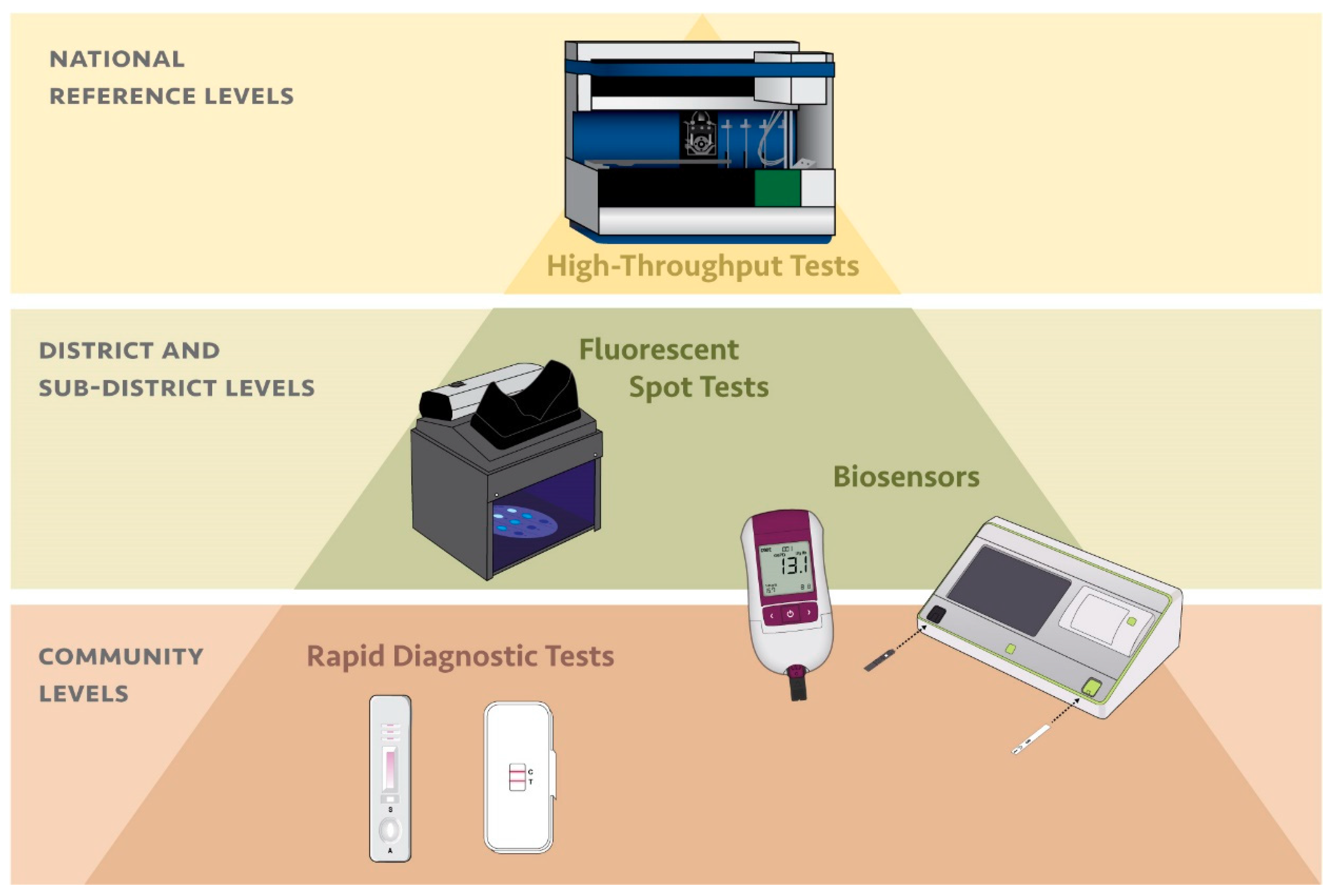
2. Testing for G6PD Deficiency
3. Newborn Screening Practices for G6PD Deficiency
3.1. Newborn Screening for G6PD Deficiency in the United States and Europe
3.2. Newborn Screening for G6PD Deficiency in the Asia Pacific
4. G6PD Testing for Malaria Case Management
5. New Opportunities for G6PD Screening: Synergies and Considerations
5.1. Overlap in Desired Product Characteristics
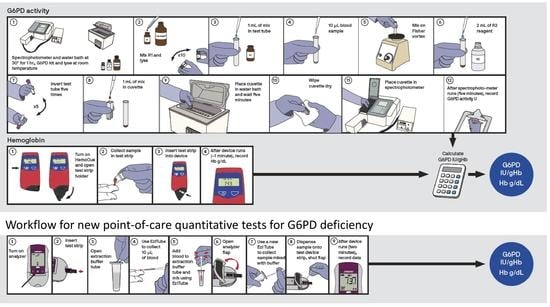
5.2. Work Flow and Sample Type
5.3. External Quality Assurance
5.4. Record Keeping
5.5. Awareness and Sensitization
5.6. Cost-Effectiveness
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cappellini, M.D.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 371, 64–74. [Google Scholar] [CrossRef]
- Luzzatto, L.; Nannelli, C.; Notaro, R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol./Oncol. Clin. N. Am. 2016, 30, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Bancone, G.; Kalnoky, M.; Chu, C.S.; Chowwiwat, N.; Kahn, M.; Malleret, B.; Wilaisrisak, P.; Renia, L.; Domingo, G.J.; Nosten, F. The G6PD flow-cytometric assay is a reliable tool for diagnosis of G6PD deficiency in women and anaemic subjects. Sci. Rep. 2017, 7, 9822. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Yeh, M.; Fairbanks, V.F. The normal human female as a mosaic of X-chromosome activity: Studies using the gene for C-6-PD-deficiency as a marker. Proc. Natl. Acad. Sci. USA 1962, 48, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kalnoky, M.; Bancone, G.; Kahn, M.; Chu, C.S.; Chowwiwat, N.; Wilaisrisak, P.; Pal, S.; LaRue, N.; Leader, B.; Nosten, F.; et al. Cytochemical flow analysis of intracellular G6PD and aggregate analysis of mosaic G6PD expression. Eur. J. Haematol. 2018, 100, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Nantakomol, D.; Paul, R.; Palasuwan, A.; Day, N.P.; White, N.J.; Imwong, M. Evaluation of the phenotypic test and genetic analysis in the detection of glucose-6-phosphate dehydrogenase deficiency. Malar. J. 2013, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.L.; Veldthuis, M.; van Leeuwen, K.; Bossuyt, P.M.M.; Vlaar, A.P.J.; van Bruggen, R.; de Korte, D.; Van Noorden, C.J.F.; van Zwieten, R. Comparison of Spectrophotometry, Chromate Inhibition, and Cytofluorometry Versus Gene Sequencing for Detection of Heterozygously Glucose-6-Phosphate Dehydrogenase-Deficient Females. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2017, 65, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.D.; Hwang, S.; Mochly-Rosen, D. Glucose-6-Phosphate Dehydrogenase Deficiency and the Need for a Novel Treatment to Prevent Kernicterus. Clin. Perinatal. 2016, 43, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Hammerman, C. Glucose-6-phosphate dehydrogenase deficiency and severe neonatal hyperbilirubinemia: A complexity of interactions between genes and environment. Semin. Fetal Neonatal Med. 2010, 15, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, B.O.; Emokpae, A.A.; Zamora, T.G.; Slusher, T.M. Addressing the burden of neonatal hyperbilirubinaemia in countries with significant glucose-6-phosphate dehydrogenase deficiency. Acta Paediatr. 2014, 103, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- WHO Working Group. Glucose-6-phosphate dehydrogenase deficiency. Bull. World Health Organ. 1989, 67, 601–611. [Google Scholar]
- Howes, R.E.; Piel, F.B.; Patil, A.P.; Nyangiri, O.A.; Gething, P.W.; Dewi, M.; Hogg, M.M.; Battle, K.E.; Padilla, C.D.; Baird, J.K.; et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: A geostatistical model-based map. PLoS Med. 2012, 9, e1001339. [Google Scholar] [CrossRef] [PubMed]
- Nkhoma, E.T.; Poole, C.; Vannappagari, V.; Hall, S.A.; Beutler, E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: A systematic review and meta-analysis. Blood Cells Mol. Dis. 2009, 42, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.M.; Rockett, K.; Kivinen, K.; Hubbart, C.; Jeffreys, A.E.; Rowlands, K.; Jallow, M.; Conway, D.J.; Bojang, K.A.; Pinder, M.; et al. Characterisation of the opposing effects of G6PD deficiency on cerebral malaria and severe malarial anaemia. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Ruwende, C.; Khoo, S.C.; Snow, R.W.; Yates, S.N.; Kwiatkowski, D.; Gupta, S.; Warn, P.; Allsopp, C.E.; Gilbert, S.C.; Peschu, N.; et al. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature 1995, 376, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Cappadoro, M.; Giribaldi, G.; O’Brien, E.; Turrini, F.; Mannu, F.; Ulliers, D.; Simula, G.; Luzzatto, L.; Arese, P. Early phagocytosis of glucose-6-phosphate dehydrogenase (G6PD)-deficient erythrocytes parasitized by Plasmodium falciparum may explain malaria protection in G6PD deficiency. Blood 1998, 92, 2527–2534. [Google Scholar] [PubMed]
- Bancone, G.; Malleret, B.; Suwanarusk, R.; Chowwiwat, N.; Chu, C.S.; McGready, R.; Renia, L.; Nosten, F.; Russell, B. Asian G6PD-Mahidol Reticulocytes Sustain Normal Plasmodium Vivax Development. J. Infect. Dis. 2017, 216, 263–266. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Malaria Report 2017; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization (WHO). Control and Elimination of Plasmodium Vivax Malaria—A Technical Brief; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Baird, K.J.; Maguire, J.D.; Price, R.N. Diagnosis and treatment of Plasmodium vivax malaria. Adv. Parasitol. 2012, 80, 203–270. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; White, N.J. Management of relapsing Plasmodium vivax malaria. Expert Rev. Anti-Infect. Ther. 2016, 14, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Luxemburger, C.; van Vugt, M.; Jonathan, S.; McGready, R.; Looareesuwan, S.; White, N.J.; Nosten, F. Treatment of vivax malaria on the western border of Thailand. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 433–438. [Google Scholar] [CrossRef]
- Robinson, L.J.; Wampfler, R.; Betuela, I.; Karl, S.; White, M.T.; Li Wai Suen, C.S.; Hofmann, N.E.; Kinboro, B.; Waltmann, A.; Brewster, J.; et al. Strategies for Understanding and Reducing the Plasmodium vivax and Plasmodium ovale Hypnozoite Reservoir in Papua New Guinean Children: A Randomised Placebo-Controlled Trial and Mathematical Model. PLoS Med. 2015, 12, e1001891. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for the Treatment of Malaria, 3rd ed.; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Zhang, L.; Yang, Y.; Liu, R.; Li, Q.; Yang, F.; Ma, L.; Liu, H.; Chen, X.; Yang, Z.; Cui, L.; et al. A multiplex method for detection of glucose-6-phosphate dehydrogenase (G6PD) gene mutations. Int. J. Lab. Hematol. 2015, 37, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.S.; Agrawal, P.B.; Bailey, D.B., Jr.; Beggs, A.H.; Brenner, S.E.; Brower, A.M.; Cakici, J.A.; Ceyhan-Birsoy, O.; Chan, K.; Chen, F.; et al. Newborn Sequencing in Genomic Medicine and Public Health. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [PubMed]
- Bogari, N.M. Next generation sequencing (NGS) in glucose-6-phosphate dehydrogenase (G6PD) deficiency studies. Bioinformation 2016, 12, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Holm, I.A.; Agrawal, P.B.; Ceyhan-Birsoy, O.; Christensen, K.D.; Fayer, S.; Frankel, L.A.; Genetti, C.A.; Krier, J.B.; LaMay, R.C.; Levy, H.L.; et al. The BabySeq project: Implementing genomic sequencing in newborns. BMC Pediatr. 2018, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Technical Specifications Series for Submission to WHO Prequalification—Diagnostic Assessment: In Vitro Diagnostics Medical Devices to Identify Glucose-6-Phosphate Dehydrogenase (G6PD) Activity; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Domingo, G.J.; Satyagraha, A.W.; Anvikar, A.; Baird, K.; Bancone, G.; Bansil, P.; Carter, N.; Cheng, Q.; Culpepper, J.; Eziefula, C.; et al. G6PD testing in support of treatment and elimination of malaria: Recommendations for evaluation of G6PD tests. Malar. J. 2013, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- LaRue, N.; Kahn, M.; Murray, M.; Leader, B.T.; Bansil, P.; McGray, S.; Kalnoky, M.; Zhang, H.; Huang, H.; Jiang, H.; et al. Comparison of quantitative and qualitative tests for glucose-6-phosphate dehydrogenase deficiency. Am. J. Trop. Med. Hyg. 2014, 91, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Mitchell, M. Special modifications of the fluorescent screening method for glucose-6-phosphate dehydrogenase deficiency. Blood 1968, 32, 816–818. [Google Scholar] [PubMed]
- Fu, C.; Luo, S.; Li, Q.; Xie, B.; Yang, Q.; Geng, G.; Lin, C.; Su, J.; Zhang, Y.; Wang, J.; et al. Newborn screening of glucose-6-phosphate dehydrogenase deficiency in Guangxi, China: Determination of optimal cutoff value to identify heterozygous female neonates. Sci. Rep. 2018, 8, 833. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Hammerman, C.; Vreman, H.J.; Stevenson, D.K.; Beutler, E. Acute hemolysis and severe neonatal hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient heterozygotes. J. Pediatr. 2001, 139, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Riskin, A.; Gery, N.; Kugelman, A.; Hemo, M.; Spevak, I.; Bader, D. Glucose-6-phosphate dehydrogenase deficiency and borderline deficiency: Association with neonatal hyperbilirubinemia. J. Pediatr. 2012, 161, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.L.; Boo, N.Y.; Ainoon, O.; Wong, M.K. Comparison of detection of glucose-6-phosphate dehydrogenase deficiency using fluorescent spot test, enzyme assay and molecular method for prediction of severe neonatal hyperbilirubinaemia. Singap. Med. J. 2009, 50, 62–67. [Google Scholar]
- Chiang, S.H.; Wu, K.F.; Liu, T.T.; Wu, S.J.; Hsiao, K.J. Quality assurance program for neonatal screening of glucose-6-phosphate dehydrogenase deficiency. Southeast Asian J. Trop. Med. Public Health 2003, 34 (Suppl. 3), 130–134. [Google Scholar] [PubMed]
- Chiang, S.H.; Wu, S.J.; Wu, K.F.; Hsiao, K.J. Neonatal screening for glucose-6-phosphate dehydrogenase deficiency in Taiwan. Southeast Asian J. Trop. Med. Public Health 1999, 30 (Suppl. 2), 72–74. [Google Scholar] [PubMed]
- Domingo, G.J.; Advani, N.; Satyagraha, A.W.; Sibley, C.H.; Rowley, E.; Kalnoky, M.; Cohen, J.; Parker, M.; Kelley, M. Addressing the gender-knowledge gap in glucose-6-phosphate dehydrogenase deficiency: Challenges and opportunities. Int. Health 2018. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Bancone, G.; Nosten, F.; White, N.J.; Luzzatto, L. Primaquine-induced haemolysis in females heterozygous for G6PD deficiency. Malar. J. 2018, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatal. 2015, 39, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Leong, A. Is There a Need for Neonatal Screening of Glucose-6-Phosphate Dehydrogenase Deficiency in Canada? McGill J. Med. 2007, 10, 31–34. [Google Scholar] [PubMed]
- Watchko, J.F.; Kaplan, M.; Stark, A.R.; Stevenson, D.K.; Bhutani, V.K. Should we screen newborns for glucose-6-phosphate dehydrogenase deficiency in the United States? J. Perinatal. Off. J. Calif. Perinat. Assoc. 2013, 33, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, R.; McInnes, R.; Willard, H. Thompson & Thompson Genetics in Medicine, 8th ed.; Elsevier/Saunders: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Watchko, J.F. Screening for glucose-6-phosphate dehydrogenase deficiency in newborns-practical considerations. J. Pediatr. 2012, 161, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.E. Diagnosis and management of G6PD deficiency. Am. Fam. Phys. 2005, 72, 1277–1282. [Google Scholar]
- Nock, M.L.; Johnson, E.M.; Krugman, R.R.; Di Fiore, J.M.; Fitzgerald, S.; Sandhaus, L.M.; Walsh, M.C. Implementation and analysis of a pilot in-hospital newborn screening program for glucose-6-phosphate dehydrogenase deficiency in the United States. J. Perinatal. Off. J. Calif. Perinat. Assoc. 2011, 31, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quiroga, G.; Ramirez del Rio, J.L.; Ortiz-Jalomo, R.; Garcia-Contreras, R.F.; Cerda-Flores, R.M.; Mata-Cardenas, B.D.; Garza-Chapa, R. Relative frequency of glucose-6-phosphate dehydrogenase deficiency in jaundiced newborn infants in the metropolitan area of Monterrey, Nuevo Leon. Arch. Investig. Med. 1990, 21, 223–227. [Google Scholar]
- Manu Pereira Mdel, M.; Cabot, A.; Martinez Gonzalez, A.; Sitja Navarro, E.; Cararach, V.; Sabria, J.; Boixaderas, J.; Teixidor, R.; Bosch, A.; Lopez Vilchez, M.A.; et al. Neonatal screening of hemoglobinopathies and G6PD deficiency in Catalonia (Spain). Molecular study of sickle cell disease associated with alpha thalassemia and G6PD deficiency. Med. Clin. 2007, 129, 161–164. [Google Scholar]
- Kaplan, M.; Hammerman, C. Severe neonatal hyperbilirubinemia. A potential complication of glucose-6-phosphate dehydrogenase deficiency. Clin. Perinatal. 1998, 25, 575–590. [Google Scholar] [CrossRef]
- Valaes, T. Severe neonatal jaundice associated with glucose-6-phosphate dehydrogenase deficiency: Pathogenesis and global epidemiology. Acta Paediatr. 1994, 394, 58–76. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef]
- Lin, Z.; Fontaine, J.M.; Freer, D.E.; Naylor, E.W. Alternative DNA-based newborn screening for glucose-6-phosphate dehydrogenase deficiency. Mol. Genet. Metab. 2005, 86, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Reclos, G.J.; Hatzidakis, C.J.; Schulpis, K.H. Glucose-6-phosphate dehydrogenase deficiency neonatal screening: Preliminary evidence that a high percentage of partially deficient female neonates are missed during routine screening. J. Med. Screen. 2000, 7, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.; Li, H.; Nock, M.L. Assessment of G6PD screening program in premature infants in a NICU. J. Perinatol. 2015, 35, 1027. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, V.K.; Kaplan, M.; Glader, B.; Cotten, M.; Kleinert, J.; Pamula, V. Point-of-Care Quantitative Measure of Glucose-6-Phosphate Dehydrogenase Enzyme Deficiency. Pediatrics 2015, 136, e1268–e1275. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Rugolotto, S.; Zamboni, G.; Gaudino, R.; Tato, L. Neonatal screening for glucose-6-phosphate dehydrogenase deficiency fails to detect heterozygote females. Eur. J. Epidemiol. 2004, 19, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Stuhrman, G.; Perez Juanazo, S.J.; Crivelly, K.; Smith, J.; Andersson, H.; Morava, E. False-Positive Newborn Screen Using the Beutler Spot Assay for Galactosemia in Glucose-6-Phosphate Dehydrogenase Deficiency. JIMD Rep. 2017, 36, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Hammerman, C. Glucose-6-phosphate dehydrogenase deficiency: A hidden risk for kernicterus. Semin. Perinatal. 2004, 28, 356–364. [Google Scholar] [CrossRef]
- Koopmans, J.; Ross, L.F. Does familiarity breed acceptance? The influence of policy on physicians’ attitudes toward newborn screening programs. Pediatrics 2006, 117, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, J.; Nock, M. Pediatric Provider Insight into Newborn Screening for G6PD Deficiency. Clin. Pediatr. 2015, 54, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.D.; Nussenzveig, R.H.; Yaish, H.M.; Henry, E.; Eggert, L.D.; Agarwal, A.M. Causes of hemolysis in neonates with extreme hyperbilirubinemia. J. Perinatal. Off. J. Calif. Perinat. Assoc. 2014, 34, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Watchko, J.F. Hyperbilirubinemia and Bilirubin Toxicity in the Late Preterm Infant. Clin. Perinatal. 2006, 33, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Missiou-Tsagaraki, S. Screening for glucose-6-phosphate dehydrogenase deficiency as a preventive measure: Prevalence among 1,286,000 Greek newborn infants. J. Pediatr. 1991, 119, 293–299. [Google Scholar] [CrossRef]
- Piomelli, S.; Wolff, J.A. Neonatal screening for glucose-6-phosphate dehydrogenase deficiency. J. Pediatr. 1992, 121, 497. [Google Scholar] [CrossRef]
- Meloni, T.; Forteleoni, G.; Meloni, G.F. Marked decline of favism after neonatal glucose-6-phosphate dehydrogenase screening and health education: The northern Sardinian experience. Acta Haematol. 1992, 87, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Padilla, C.D.; Therrell, B.L. Newborn screening in the Asia Pacific region. J. Inherit. Metab. Dis. 2007, 30, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Charoenkwan, P.; Tantiprabha, W.; Sirichotiyakul, S.; Phusua, A.; Sanguansermsri, T. Prevalence and molecular characterization of glucose-6-phosphate dehydrogenase deficiency in northern Thailand. Southeast Asian J. Trop. Med. Public Health 2014, 45, 187–193. [Google Scholar] [PubMed]
- Ratrisawadi, V.; Horpaopan, S.; Chotigeat, U.; Sangtawesin, V.; Kanjanapattanakul, W.; Ningsanond, V.; Sunthornthepvarakul, T.; Khooarmompatana, S.; Charoensiriwatana, W. Neonatal screening program in Rajavithi Hospital, Thailand. Southeast Asian J. Trop. Med. Public Health 1999, 30 (Suppl. 2), 28–32. [Google Scholar]
- Sanpavat, S.; Nuchprayoon, I.; Kittikalayawong, A.; Ungbumnet, W. The value of methemoglobin reduction test as a screening test for neonatal glucose 6-phosphate dehydrogenase deficiency. J. Med. Assoc. Thail. 2001, 84 (Suppl. 1), S91–S98. [Google Scholar]
- Tanphaichitr, V.S. Glucose-6-phosphate dehydrogenase deficiency in Thailand; its significance in the newborn. Southeast Asian J. Trop. Med. Public Health 1999, 30 (Suppl. 2), 75–78. [Google Scholar]
- Tanphaichitr, V.S.; Pung-amritt, P.; Yodthong, S.; Soongswang, J.; Mahasandana, C.; Suvatte, V. Glucose-6-phosphate dehydrogenase deficiency in the newborn: Its prevalence and relation to neonatal jaundice. Southeast Asian J. Trop. Med. Public Health 1995, 26 (Suppl. 1), 137–141. [Google Scholar]
- Rustama, D.S.; Fadil, M.R.; Harahap, E.R.; Primadi, A. Newborn screening in Indonesia. Southeast Asian J. Trop. Med. Public Health 2003, 34 (Suppl. 3), 76–79. [Google Scholar]
- Padilla, C.D. Newborn screening in the Philippines. Southeast Asian J. Trop. Med. Public Health 2003, 34 (Suppl. 3), 87–88. [Google Scholar]
- Joseph, R. Mass newborn screening in Singapore--position and projections. Ann. Acad. Med. Singap. 2003, 32, 318–323. [Google Scholar] [PubMed]
- Care of the Newborn. Available online: https://www.healthhub.sg/live-healthy/1047/pregnancy-care-of-the-newborn (accessed on 13 September 2018).
- Espino, F.E.; Bibit, J.A.; Sornillo, J.B.; Tan, A.; von Seidlein, L.; Ley, B. Comparison of Three Screening Test Kits for G6PD Enzyme Deficiency: Implications for Its Use in the Radical Cure of Vivax Malaria in Remote and Resource-Poor Areas in the Philippines. PLoS ONE 2016, 11, e0148172. [Google Scholar] [CrossRef] [PubMed]
- Henriques, G.; Phommasone, K.; Tripura, R.; Peto, T.J.; Raut, S.; Snethlage, C.; Sambo, I.; Sanann, N.; Nguon, C.; Adhikari, B.; et al. Comparison of glucose-6 phosphate dehydrogenase status by fluorescent spot test and rapid diagnostic test in Lao PDR and Cambodia. Malaria J. 2018, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Roca-Feltrer, A.; Khim, N.; Kim, S.; Chy, S.; Canier, L.; Kerleguer, A.; Tor, P.; Chuor, C.M.; Kheng, S.; Siv, S.; et al. Field trial evaluation of the performances of point-of-care tests for screening G6PD deficiency in Cambodia. PLoS ONE 2014, 9, e116143. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Bansil, P.; Bancone, G.; Hrutkay, S.; Kahn, M.; Gornsawun, G.; Penpitchaporn, P.; Chu, C.S.; Nosten, F.; Domingo, G.J. Evaluation of a novel quantitative test for G6PD deficiency: Bringing quantitative testing for G6PD deficiency closer to the patient. Am. J. Trop. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bancone, G.; Gornsawun, G.; Chu, C.S.; Porn, P.; Pal, S.; Bansil, P.; Domingo, G.J.; Nosten, F. Validation of the quantitative point-of-care CareStart biosensor for assessment of G6PD activity in venous blood. PLoS ONE 2018, 13, e0196716. [Google Scholar] [CrossRef] [PubMed]
| Genotype | Phenotype | ||
|---|---|---|---|
| % RBC with High G6PD Activity (Cytometry) | % Normal G6PD Activity (Spectrophotometry) | ||
| Males | |||
| hemizygous normal | (+) | >85% | >30% |
| hemizygous deficient | (−) | <10% | ≤30% |
| Females | |||
| homozygous normal | (+1/+1) | >85% | >70% |
| heterozygous normal | (+1/+2) | ||
| heterozygous normal/deficient | (+/−) | 10–85% | ~20–80% |
| heterozygous deficient | (−1/−2) | <10% | ≤30% |
| homozygous deficient | (−1/−1) | ||
| Qualitative | Quantitative |
|---|---|
| Accurately classifies males | Accurately classifies males |
| Females with intermediate G6PD activity classified as normal | Accurately classifies females |
| Does not require an instrument | Requires an instrument |
| Cannot correct for operating temperature, typically resulting in a more limited operating temperature range | Corrects for temperature allowing for a broader operating temperature range |
| Time-to-result < 10 min | Time-to-result < 10 min |
| Low to moderate complexity | Moderate complexity |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderle, A.; Bancone, G.; Domingo, G.J.; Gerth-Guyette, E.; Pal, S.; Satyagraha, A.W. Point-of-Care Testing for G6PD Deficiency: Opportunities for Screening. Int. J. Neonatal Screen. 2018, 4, 34. https://doi.org/10.3390/ijns4040034
Anderle A, Bancone G, Domingo GJ, Gerth-Guyette E, Pal S, Satyagraha AW. Point-of-Care Testing for G6PD Deficiency: Opportunities for Screening. International Journal of Neonatal Screening. 2018; 4(4):34. https://doi.org/10.3390/ijns4040034
Chicago/Turabian StyleAnderle, Athena, Germana Bancone, Gonzalo J. Domingo, Emily Gerth-Guyette, Sampa Pal, and Ari W. Satyagraha. 2018. "Point-of-Care Testing for G6PD Deficiency: Opportunities for Screening" International Journal of Neonatal Screening 4, no. 4: 34. https://doi.org/10.3390/ijns4040034
APA StyleAnderle, A., Bancone, G., Domingo, G. J., Gerth-Guyette, E., Pal, S., & Satyagraha, A. W. (2018). Point-of-Care Testing for G6PD Deficiency: Opportunities for Screening. International Journal of Neonatal Screening, 4(4), 34. https://doi.org/10.3390/ijns4040034