Managing Newborn Screening Repeat Collections for Sick and Preterm Neonates
Abstract
1. Introduction
2. Materials and Methods
2.1. Victorian NBS Process at Time of Audit Initiation
- All babies—between 48 and 72 h of life. From 2020, i.e., the start of the COVID-19 pandemic, samples were accepted down to 36 h of age without the need for a repeat collection.
- Low birthweight babies—babies with a birth weight < 1500 g required a regular collection and then a follow-up collection at 2 weeks (1000–<1500 g at birth) or 3 weeks (<1000 g at birth) of age. Organization of the repeat samples was managed independently by the maternity service provider without prompting from the NBS laboratory. The NBS laboratory was only involved as needed to prompt follow-up of borderline or screen positive results. Therefore, if the repeat collection did not happen because it was missed by the maternity service provider, the NBS lab did not have a role in recognizing this.
- Transfused neonates—babies who received any form of transfusion prior to the routine NBS collection, required an early sample (pre-transfusion), a 48 h post-transfusion, plus a 3-weeks post-transfusion. These repeat collections were managed by the NBS laboratory; i.e., where electronic letters were sent out to remind providers to perform the repeat collection and follow-up of outstanding samples.
2.2. Audit of Repeat Collection Guideline for Preterm Neonates
2.3. Review of Evidence and Harmonization
- The USA’s Clinical Laboratory Standards Institute (CLSI) document NBS03 was extensively reviewed as part of the literature and review looking for their recommendations and the evidence to support this [1].
- The Human Genetics Society of Australasia NBS Committee was the professional body consulted, and each of the five other jurisdictions was asked (1) what their current recommendations were for preterm and transfused neonates, and (2) what is the evidence for their recommendation [14]?
2.4. New Protocol Design
2.5. One Year On—Review of Impact of New Protocol
2.6. Statistical Analysis
3. Results
3.1. Audit of Current Repeat Collection Guideline for Preterm Neonates
3.2. Review of Evidence and Harmonization
3.3. New Sick–Prem (SP) Protocol
3.4. Adherence to New SP Protocol
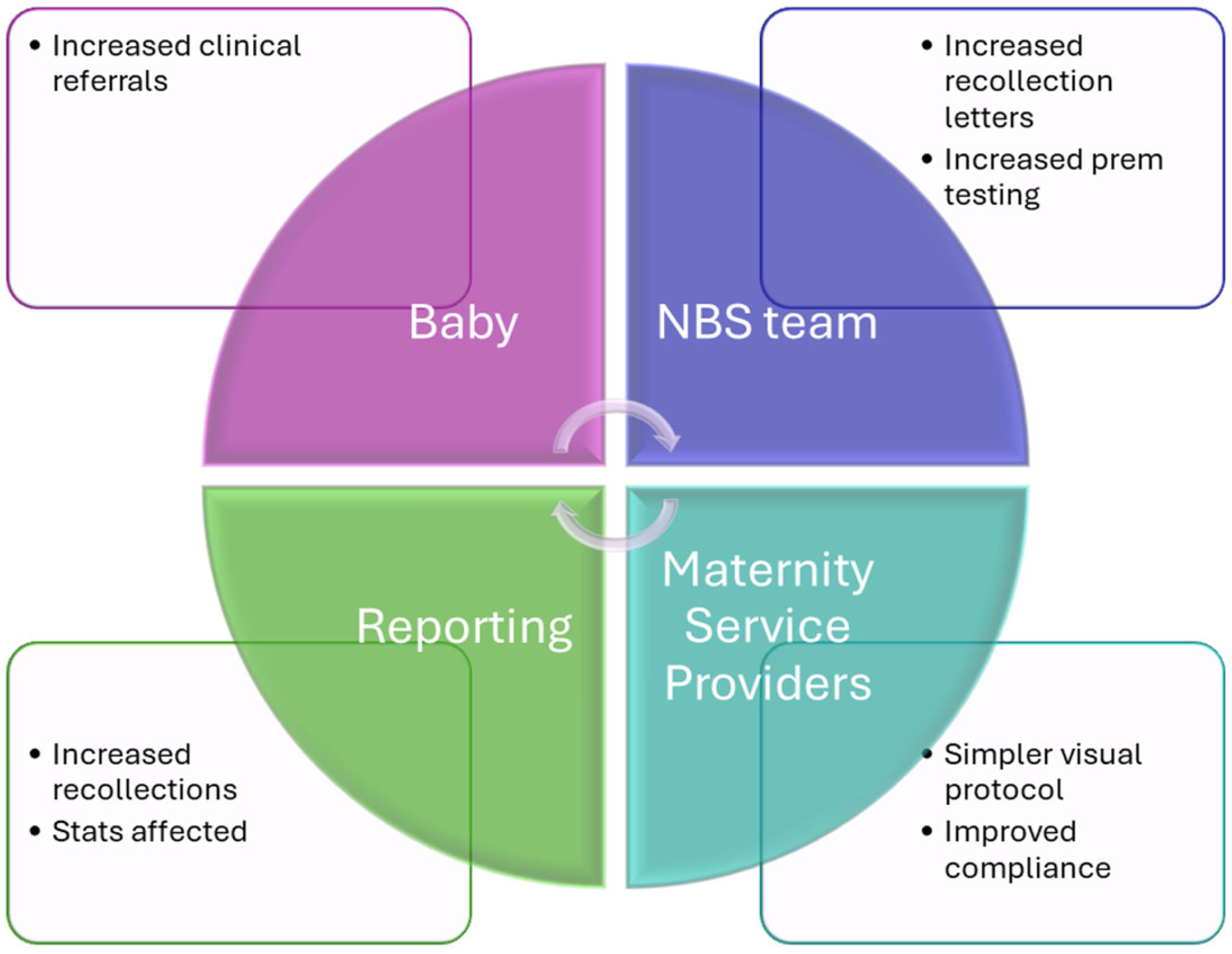
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clinical and Laboratory Standards Institute. Newborn Screening for Preterm, Low Birth Weight and Sick Newborns, 2nd ed.; CLSI Guideline NBS03; CLSI: Wayne, PA, USA, 2019; p. 82. [Google Scholar]
- Rose, S.R.; Wassner, A.J.; Wintergerst, K.A.; Yayah-Jones, N.H.; Hopkin, R.J.; Chuang, J.; Smith, J.R.; Abell, K.; LaFranchi, S.H. Congenital Hypothyroidism: Screening and Management. Pediatrics 2023, 151, e2022060419. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Preterm Birth; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Australian Institute of Health and Welfare (AIHW) Australia’s Mothers and Babies; AIHW: Canberra, Australia, 2023.
- Australian Institute of Health and Welfare (AIHW). Preliminary Data Tables: National Perinatal Data Collection Annual Update 2022; Australian Institute of Health and Welfare, Ed.; Australian Government: Canberra, Australia, 2022. [Google Scholar]
- UNICEF; WHO. Country Consultation on Low Birthweight and Preterm Birth Estimates—Technical Note; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Huynh, T.; Greaves, R.; Mawad, N.; Greed, L.; Wotton, T.; Wiley, V.; Ranieri, E.; Rankin, W.; Ungerer, J.; Price, R.; et al. Fifty years of newborn screening for congenital hypothyroidism: Current status in Australasia and the case for harmonisation. Clin. Chem. Lab. Med. 2022, 60, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Van Wassenaer, A.G.; Kok, J.H.; Dekker, F.W.; de Vijlder, J.J. Thyroid function in very preterm infants: Influences of gestational age and disease. Pediatr. Res. 1997, 42, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.L.; Walraven, C.; Rojas, D.A.; McIntosh, K.F.; Hermos, R.J. Screening very-low-birthweight infants for congenital hypothyroidism. Lancet 1994, 343, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Nolan, B.; Uy, C.; Stablein, L.; Bany-Mohammed, F. Screening for Delayed Thyroid Stimulation Hormone Rise and Atypical Congenital Hypothyroidism in Infants Born Very Preterm and Infants with Very Low Birth Weight. J. Pediatr. 2024, 269, 113974. [Google Scholar] [CrossRef] [PubMed]
- Midgley, P.C.; Russell, K.; Oates, N.; Shaw, J.C.; Honour, J.W. Activity of the adrenal fetal zone in preterm infants continues to term. Endocr. Res. 1996, 22, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Greaves, R.F.; Jevalikar, G.; Hewitt, J.K.; Zacharin, M.R. A guide to understanding the steroid pathway: New insights and diagnostic implications. Clin. Biochem. 2014, 47, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Greaves, R.F.; Kumar, M.; Mawad, N.; Francescon, A.; Le, C.; O’Connell, M.; Chi, J.; Pitt, J. Best Practice for Identification of Classical 21-Hydroxylase Deficiency Should Include 21 Deoxycortisol Analysis with Appropriate Isomeric Steroid Separation. Int. J. Neonatal Screen. 2023, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Human Genetics Society of Australasia (HGSA). Newborn Screening Committee; HGSA: Mascot, NSW, Australia, 2024. [Google Scholar]
- Cutland, C.L.; Lackritz, E.M.; Mallett-Moore, T.; Bardají, A.; Chandrasekaran, R.; Lahariya, C.; Nisar, M.I.; Tapia, M.D.; Pathirana, J.; Kochhar, S.; et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017, 35 Pt A, 6492–6500. [Google Scholar] [CrossRef]
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Victorian Newborn Bloodspot Screening Program. Newborn Bloodspot Screening Comprehensive Collection Guideline, 4th ed.; VCGS, Ed.; Victorian Clinical Genetics Services (VCGS): Parkville, Australia, 2021. [Google Scholar]
- Greaves, R.; Kricka, L.; Gruson, D.; Ferrari, M.; Martin, H.; Loh, T.P.; Bernardini, S. Toolkit for emerging technologies in laboratory medicine. Clin. Chem. Lab. Med. 2023, 61, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Connelly, J.F.; Coakley, J.C.; Gold, H.; Francis, I.; Mathur, K.S.; Rickards, A.L.; Price, G.J.; Halliday, J.L.; Wolfe, R. Newborn screening for congenital hypothyroidism, Victoria, Australia, 1977–1997. Part 1: The screening programme, demography, baseline perinatal data and diagnostic classification. J. Pediatr. Endocrinol. Metab. JPEM 2001, 14, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Connelly, J.F.; Rickards, A.L.; Coakley, J.C.; Price, G.J.; Francis, I.; Mathur, K.S.; Wolfe, R. Newborn screening for congenital hypothyroidism, Victoria, Australia, 1977–1997. Part 2: Treatment, progress and outcome. J. Pediatr. Endocrinol. Metab. JPEM 2001, 14, 1611–1634. [Google Scholar] [CrossRef] [PubMed]
- Coakley, J.C.; Francis, I.; Gold, H.; Mathur, K.; Connelly, J.F. Transient primary hypothyroidism in the newborn: Experience of the Victorian Neonatal Thyroid Screening Programme. Aust. Paediatr. J. 1989, 25, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Mercado, M.; Yu, V.Y.; Francis, I.; Szymonowicz, W.; Gold, H. Thyroid function in very preterm infants. Early Hum. Dev. 1988, 16, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Greaves, R.F.; Zacharin, M.R.; Donath, S.M.; Inder, T.E.; Doyle, L.W.; Hunt, R.W. Establishment of hormone reference intervals for infants born <30 weeks’ gestation. Clin. Biochem. 2014, 47, 101–108. [Google Scholar] [PubMed]
- Ares Segura, S.; Casano-Sancho, P.; Chueca Guindulain, M. Assessment of thyroid function in the preterm and/or very low birth weight newborn. An. De Pediatría (Engl. Ed.) 2021, 95, 277.e1–277.e8. [Google Scholar] [CrossRef] [PubMed]
- Newborn Bloodspot Screening: What is Screened in the Program; Department of Health and Aged Care, Australian Government: Canberra, Australia, 2024.
- Greaves, R.F.; Pitt, J.; McGregor, C.; Wall, M.; Christodoulou, J. Newborn bloodspot screening in the time of COVID-19. Genet. Med. 2021, 23, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
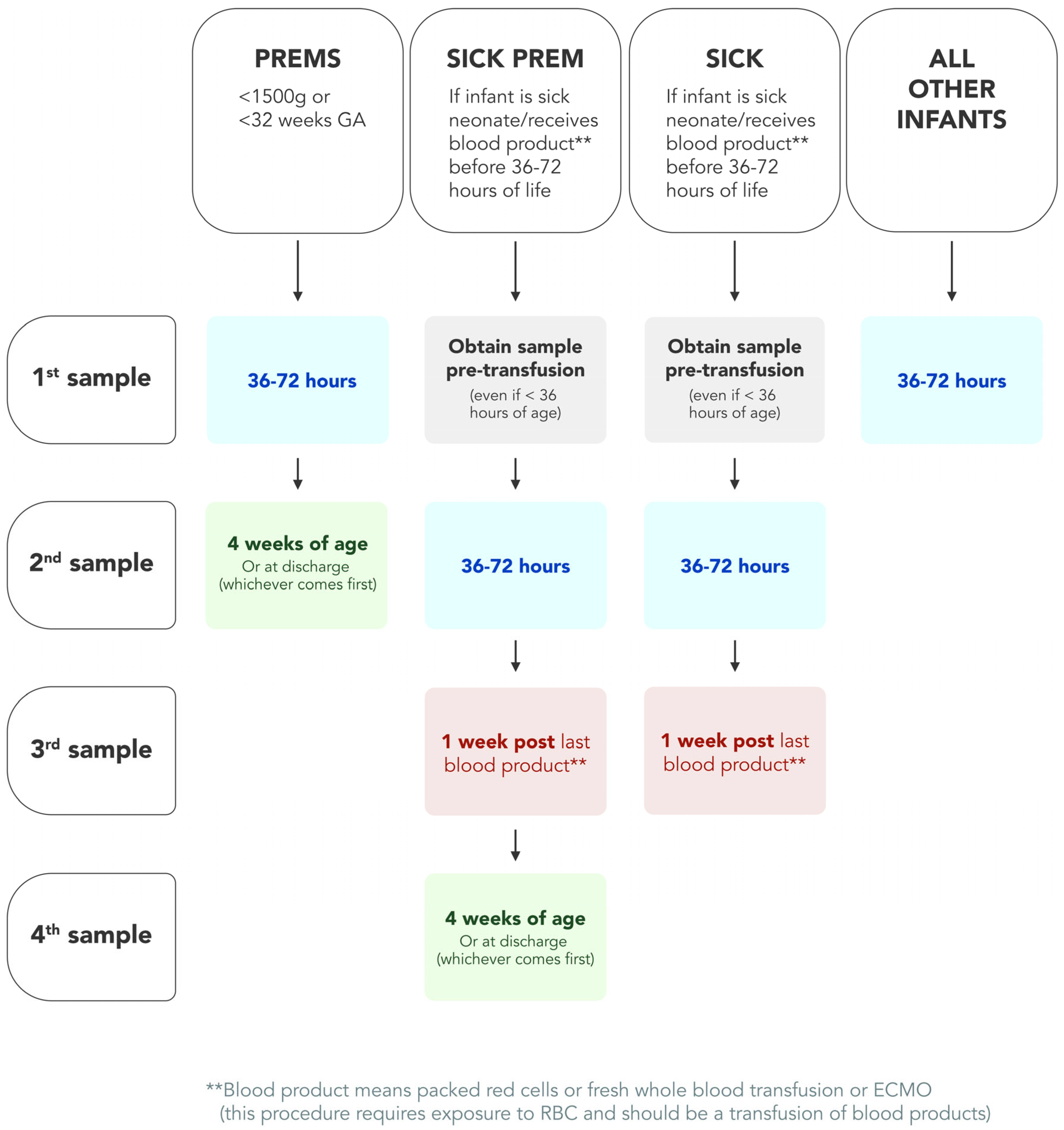
| Birthweight (BW) | Preterm (PT) by Gestational Age (GA) |
|---|---|
|
|
| Jurisdiction | Preterm—Second Sample, Adapted from [7] | Post-Transfusion Sample |
|---|---|---|
| NSW |
| 48 h + 2 weeks |
| QLD |
| 48 h + 2 weeks |
| SA |
| 1 week |
| VIC |
| 48 h + 3 weeks |
| WA |
| 48 h |
| NZ |
| 1 week |
| CLSI 2009 [1] * |
| 120 days * |
| AAP 2023 [2] ** |
| 2–4 weeks of age |
| SP Criteria | First Sample | Second Sample | Percentage of Second Samples |
|---|---|---|---|
| GA < 32 weeks | 566 | 546 | 96% |
| BW < 1500 g | 635 | 606 | 95% |
| Both BW and GA | 523 | 507 | 97% |
| GA < 32 weeks and no BW | 4 | N/A | N/A |
| BW < 1500 g and no GA | 333 | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greaves, R.F.; Northfield, J.-A.; Cross, L.; Mawad, N.; Nguyen, T.; Tan, M.; O’Connell, M.A.; Pitt, J. Managing Newborn Screening Repeat Collections for Sick and Preterm Neonates. Int. J. Neonatal Screen. 2024, 10, 63. https://doi.org/10.3390/ijns10030063
Greaves RF, Northfield J-A, Cross L, Mawad N, Nguyen T, Tan M, O’Connell MA, Pitt J. Managing Newborn Screening Repeat Collections for Sick and Preterm Neonates. International Journal of Neonatal Screening. 2024; 10(3):63. https://doi.org/10.3390/ijns10030063
Chicago/Turabian StyleGreaves, Ronda F., Jo-Ann Northfield, Lauren Cross, Nazha Mawad, Thanh Nguyen, Maggie Tan, Michele A. O’Connell, and James Pitt. 2024. "Managing Newborn Screening Repeat Collections for Sick and Preterm Neonates" International Journal of Neonatal Screening 10, no. 3: 63. https://doi.org/10.3390/ijns10030063
APA StyleGreaves, R. F., Northfield, J.-A., Cross, L., Mawad, N., Nguyen, T., Tan, M., O’Connell, M. A., & Pitt, J. (2024). Managing Newborn Screening Repeat Collections for Sick and Preterm Neonates. International Journal of Neonatal Screening, 10(3), 63. https://doi.org/10.3390/ijns10030063








