Wilson and Jungner Revisited: Are Screening Criteria Fit for the 21st Century?
Abstract
1. Introduction
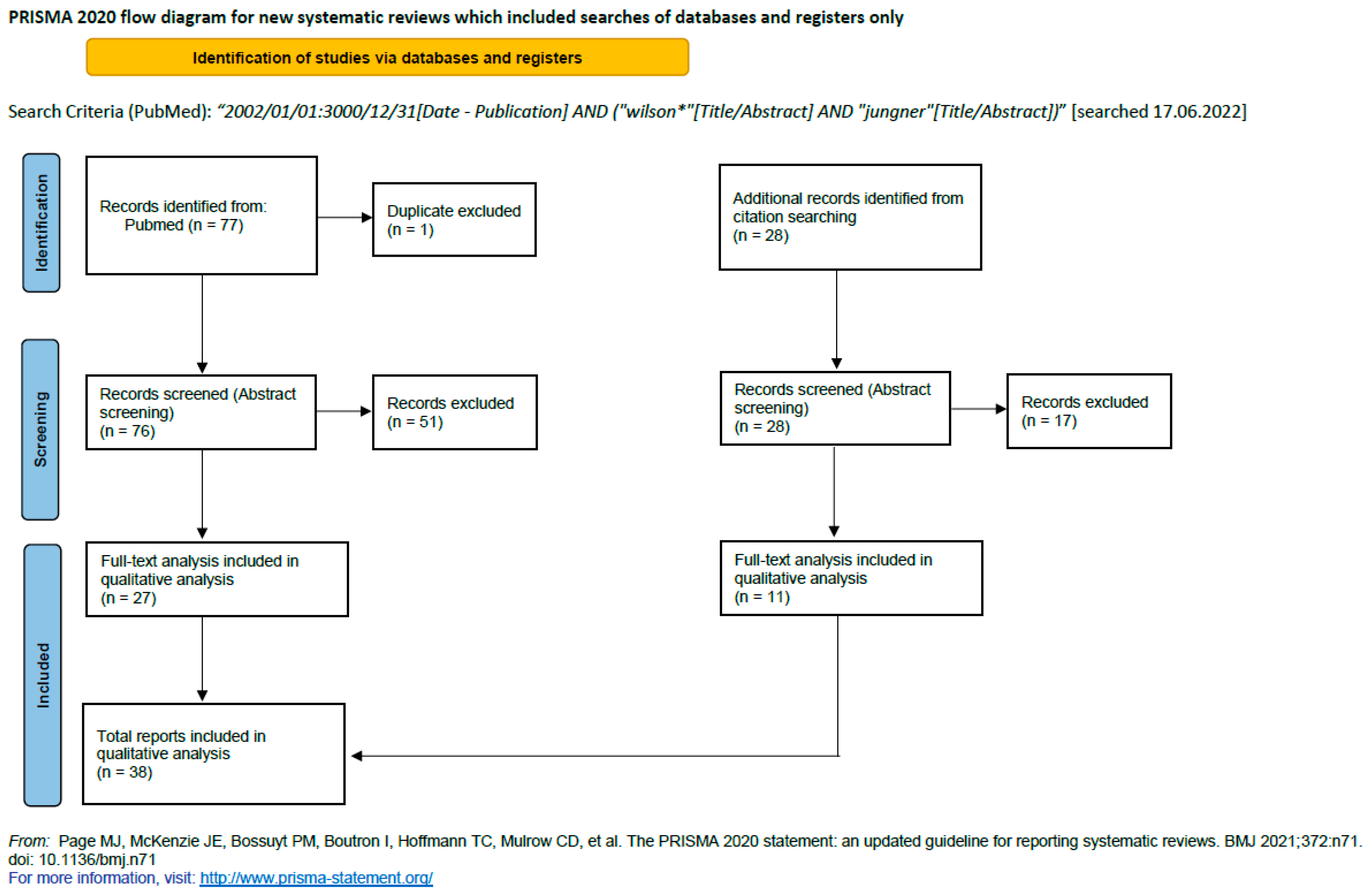
2. Materials and Methods
3. Results
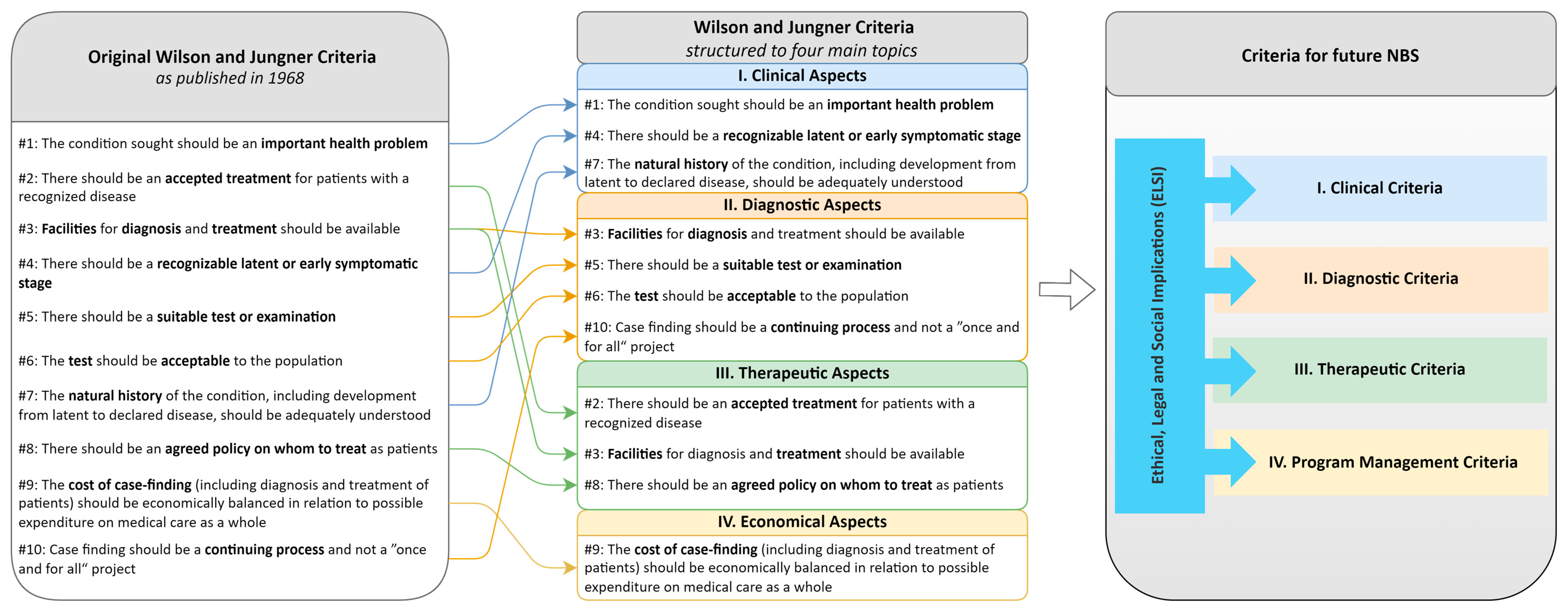
3.1. General Limitations of the WJ Criteria
3.2. Specific Aspects of Criticism of WJ Criteria
3.2.1. Clinical Aspects
3.2.2. Diagnostic Aspects
3.2.3. Therapeutic Aspects
3.2.4. Economical Aspects
3.3. Missing Criteria and Sub-Categories
4. Discussion
4.1. Are We Moving towards Consensus on the Selection of Target Diseases for NBS Programs?
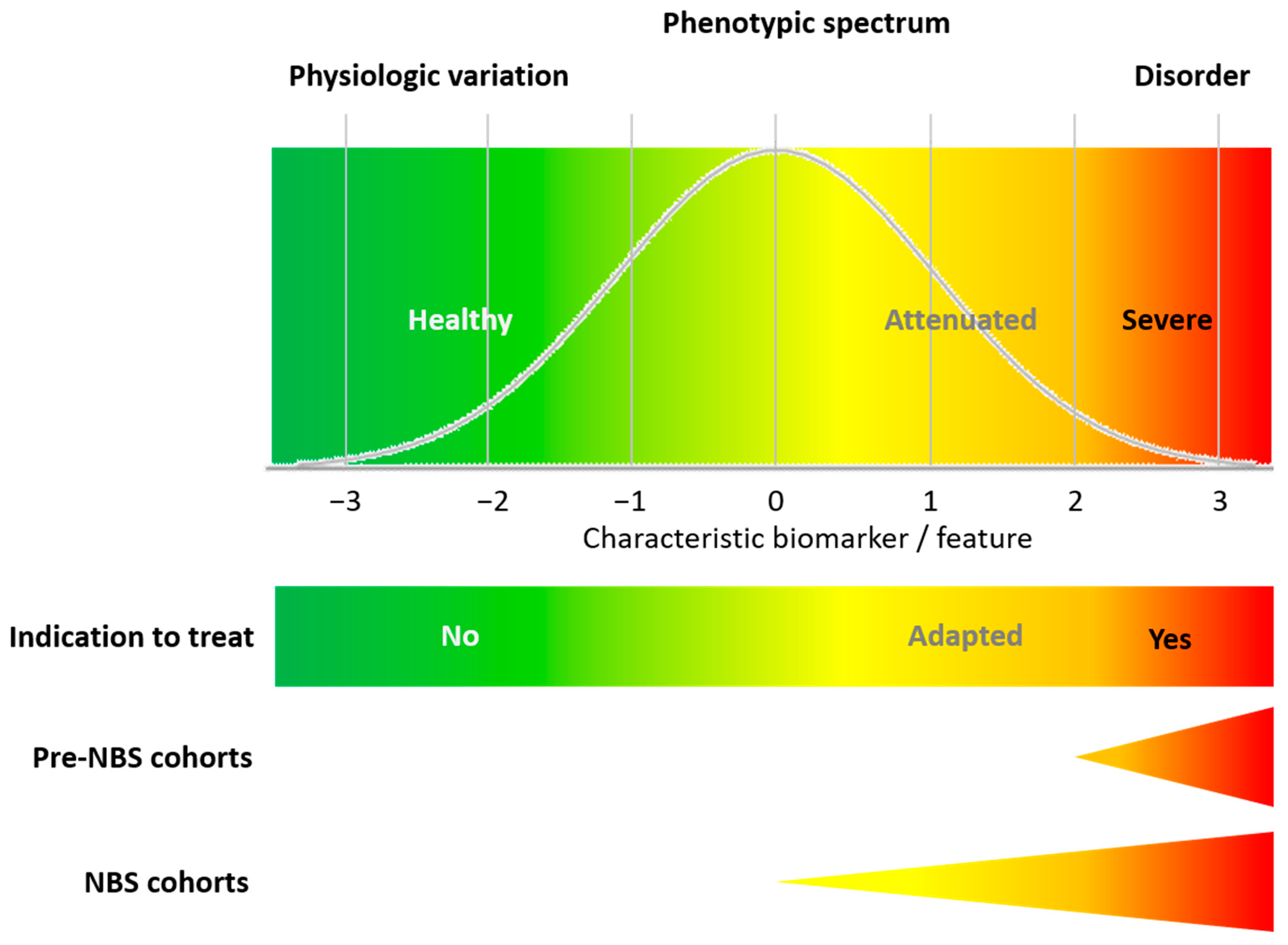
4.2. In Practice, There Are Snags: The Importance of Closing the Knowledge Gap
4.3. Managing Change as a Project: NBS as an Integrated Public Health Program
4.4. Limitations
5. Conclusions: Developing a Multi-Dimensional Framework for Future NBS Programs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Study Registration and Protocol
Abbreviations
| gNBS | Genomic newborn screening |
| NBS | Newborn screening |
| WJ criteria | Wilson and Jungner criteria |
References
- Mütze, U.; Mengler, K.; Boy, N.; Gleich, F.; Opladen, T.; Garbade, S.F.; Kölker, S. How Longitudinal Observational Studies Can Guide Screening Strategy for Rare Diseases. J. Inherit. Metab. Dis. 2022, 45, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, R.; Susi, A. A Simple Phenylalanine Method for Detecting Phenylketonuria in Large Populations of Newborn Infants. Pediatrics 1963, 32, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B.; Wiley, V.; Hammond, J.; Carpenter, K. Screening Newborns for Inborn Errors of Metabolism by Tandem Mass Spectrometry. N. Engl. J. Med. 2003, 348, 2304–2312. [Google Scholar] [CrossRef]
- Schulze, A.; Lindner, M.; Kohlmüller, D.; Olgemöller, K.; Mayatepek, E.; Hoffmann, G.F. Expanded Newborn Screening for Inborn Errors of Metabolism by Electrospray Ionization-Tandem Mass Spectrometry: Results, Outcome, and Implications. Pediatrics 2003, 111, 1399–1406. [Google Scholar] [CrossRef]
- Watson, M.S.; Lloyd-Puryear, M.A.; Mann, M.Y.; Rinaldo, P.; Howell, R. (Eds.) Newborn Screening: Toward a Uniform Screening Panel and System. Genet. Med. 2006, 8 (Suppl. S1), 1s–252s. [Google Scholar] [CrossRef]
- Sommerburg, O.; Lindner, M.; Muckenthaler, M.; Kohlmueller, D.; Leible, S.; Feneberg, R.; Kulozik, A.E.; Mall, M.A.; Hoffmann, G.F. Initial Evaluation of a Biochemical Cystic Fibrosis Newborn Screening by Sequential Analysis of Immunoreactive Trypsinogen and Pancreatitis-Associated Protein (IRT/PAP) as a Strategy That Does Not Involve DNA Testing in a Northern European Population. J. Inherit. Metab. Dis. 2010, 33, S263–S271. [Google Scholar] [CrossRef]
- Vill, K.; Kölbel, H.; Schwartz, O.; Blaschek, A.; Olgemöller, B.; Harms, E.; Burggraf, S.; Röschinger, W.; Durner, J.; Gläser, D.; et al. One Year of Newborn Screening for SMA Results of a German Pilot Project. J. Neuromuscul. Dis. 2019, 6, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Czibere, L.; Burggraf, S.; Fleige, T.; Glück, B.; Keitel, L.M.; Landt, O.; Durner, J.; Röschinger, W.; Hohenfellner, K.; Wirth, B.; et al. High-Throughput Genetic Newborn Screening for Spinal Muscular Atrophy by Rapid Nucleic Acid Extraction from Dried Blood Spots and 384-Well qPCR. Eur. J. Hum. Genet. 2020, 28, 23–30. [Google Scholar] [CrossRef]
- Tesorero, R.; Janda, J.; Hörster, F.; Feyh, P.; Mütze, U.; Hauke, J.; Schwarz, K.; Kunz, J.B.; Hoffmann, G.F.; Okun, J.G. A High-Throughput Newborn Screening Approach for SCID, SMA, and SCD Combining Multiplex qPCR and Tandem Mass Spectrometry. PLoS ONE 2023, 18, e0283024. [Google Scholar] [CrossRef]
- Mütze, U.; Garbade, S.F.; Gramer, G.; Lindner, M.; Freisinger, P.; Grünert, S.C.; Hennermann, J.; Ensenauer, R.; Thimm, E.; Zirnbauer, J.; et al. Long-Term Outcomes of Individuals with Metabolic Diseases Identified through Newborn Screening. Pediatrics 2020, 146, e20200444. [Google Scholar] [CrossRef]
- Mütze, U.; Garbade, S.F.; Gleich, F.; Lindner, M.; Freisinger, P.; Hennermann, J.B.; Thimm, E.; Gramer, G.; Posset, R.; Krämer, J.; et al. Long-Term Anthropometric Development of Individuals with Inherited Metabolic Diseases Identified by Newborn Screening. J. Inherit. Metab. Dis. 2023, 46, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Boy, N.; Mengler, K.; Heringer-Seifert, J.; Hoffmann, G.F.; Garbade, S.F.; Kölker, S. Impact of Newborn Screening and Quality of Therapy on the Neurological Outcome in Glutaric Aciduria Type 1: A Meta-Analysis. Genet. Med. 2021, 23, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Boy, N.; Mengler, K.; Thimm, E.; Schiergens, K.A.; Marquardt, T.; Weinhold, N.; Marquardt, I.; Das, A.M.; Freisinger, P.; Grünert, S.C.; et al. Newborn Screening: A Disease-Changing Intervention for Glutaric Aciduria Type 1. Ann. Neurol. 2018, 83, 970–979. [Google Scholar] [CrossRef]
- Landau, Y.E.; Waisbren, S.E.; Chan, L.M.; Levy, H.L. Long-Term Outcome of Expanded Newborn Screening at Boston Children’s Hospital: Benefits and Challenges in Defining True Disease. J. Inherit. Metab. Dis. 2017, 40, 209–218. [Google Scholar] [CrossRef]
- Wilcken, B.; Haas, M.; Joy, P.; Wiley, V.; Bowling, F.; Carpenter, K.; Christodoulou, J.; Cowley, D.; Ellaway, C.; Fletcher, J.; et al. Expanded Newborn Screening: Outcome in Screened and Unscreened Patients at Age 6 Year. Pediatrics 2009, 124, e241–e248. [Google Scholar] [CrossRef]
- Couce, M.L.; Baña, A.; Bóveda, M.D.; Pérez-Muñuzuri, A.; Fernández-Lorenzo, J.R.; Fraga, J.M. Inborn Errors of Metabolism in a Neonatology Unit: Impact and Long-Term Result. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2011, 53, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Gramer, G.; Haege, G.; Fang-Hoffmann, J.; Schwab, K.O.; Tacke, U.; Trefz, F.K.; Mengel, E.; Wendel, U.; Leichsenring, M.; et al. Efficacy and Outcome of Expanded Newborn Screening for Metabolic Diseases—Report of 10 Years from South-West Germany. Orphanet J. Rare Dis. 2011, 6, 44. [Google Scholar] [CrossRef]
- Mengler, K.; Garbade, S.F.; Gleich, F.; Thimm, E.; May, P.; Lindner, M.; Lüsebrink, N.; Marquardt, T.; Hübner, V.; Krämer, J.; et al. Treatment Outcomes for Maple Syrup Urine Disease Detected by Newborn Screening. Pediatrics 2024, 154, e2023064370. [Google Scholar] [CrossRef]
- Gray, J.A.; Patnick, J.; Blanks, R.G. Maximising Benefit and Minimising Harm of Screening. BMJ 2008, 336, 480–483. [Google Scholar] [CrossRef]
- Kingsmore, S.F.; Smith, L.D.; Kunard, C.M.; Bainbridge, M.; Batalov, S.; Benson, W.; Blincow, E.; Caylor, S.; Chambers, C.; Del Angel, G.; et al. A Genome Sequencing System for Universal Newborn Screening, Diagnosis, and Precision Medicine for Severe Genetic Diseases. Am. J. Hum. Genet. 2022, 109, 1605–1619. [Google Scholar] [CrossRef]
- Schaaf, C.P.; Kölker, S.; Hoffmann, G.F. Genomic Newborn Screening: Proposal of a Two-Stage Approach. J. Inherit. Metab. Dis. 2021, 44, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Betzler, I.R.; Hempel, M.; Mütze, U.; Kölker, S.; Winkler, E.; Dikow, N.; Garbade, S.F.; Schaaf, C.P.; Brennenstuhl, H. Comparative Analysis of Gene and Disease Selection in Genomic Newborn Screening Studies. J. Inherit. Metab. Dis. 2024. [Google Scholar] [CrossRef]
- Stark, Z.; Scott, R.H. Genomic Newborn Screening for Rare Diseases. Nat. Rev. Genet. 2023, 24, 755–766. [Google Scholar] [CrossRef]
- Roman, T.S.; Crowley, S.B.; Roche, M.I.; Foreman, A.K.M.; O’Daniel, J.M.; Seifert, B.A.; Lee, K.; Brandt, A.; Gustafson, C.; DeCristo, D.M.; et al. Genomic Sequencing for Newborn Screening: Results of the Nc Nexus Project. Am. J. Hum. Genet. 2020, 107, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Kingsmore, S.F. Dispatches from Biotech Beginning Beginngs: Rapid Newborn Genome Sequencing to End the Diagnostic and Therapeutic Odyssey. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 243–256. [Google Scholar] [CrossRef]
- BabyDetect. Baby Detect. Available online: https://babydetect.com/ (accessed on 15 April 2024).
- Holm, I.A.; Agrawal, P.B.; Ceyhan-Birsoy, O.; Christensen, K.D.; Fayer, S.; Frankel, L.A.; Genetti, C.A.; Krier, J.B.; LaMay, R.C.; Levy, H.L.; et al. The Babyseq Project: Implementing Genomic Sequencing in Newborns. BMC Pediatr. 2018, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Dangouloff, T.; Hovhannesyan, K.; Piazzon, F.; Mashhadizadeh, D.; Helou, L.; Palmeira, L.; Boemer, F.; Servais, L. O10 Universal Genomic Newborn Screening for Early, Treatable, and Severe Conditions- Including 33 Genes of Nmd: Baby Detect. Neuromuscul. Disord. 2023, 33, S130–S131. [Google Scholar] [CrossRef]
- Guardian-Study. Available online: https://guardian-study.org/ (accessed on 15 April 2024).
- GenomicsEngland. Available online: https://www.genomicsengland.co.uk/ (accessed on 15 April 2024).
- BabyScreen+. Available online: https://babyscreen.mcri.edu.au/ (accessed on 15 April 2024).
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease; World Health Organization: Geneva, Switzerland, 1968. [Google Scholar]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Costea, I. Guiding Policy Decisions for Genetic Screening: Developing a Systematic and Transparent Approach. Public Health Genom. 2011, 14, 9–16. [Google Scholar] [CrossRef]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisiting Wilson and Jungner in the Genomic Age: A Review of Screening Criteria over the Past 40 Years. Bull. World Health Organ. 2008, 86, 317–319. [Google Scholar] [CrossRef]
- Petros, M. Revisiting the Wilson-Jungner Criteria: How Can Supplemental Criteria Guide Public Health in the Era of Genetic Screening? Genet. Med. 2012, 14, 129–134. [Google Scholar] [CrossRef]
- Dobrow, M.J.; Hagens, V.; Chafe, R.; Sullivan, T.; Rabeneck, L. Consolidated Principles for Screening Based on a Systematic Review and Consensus Process. CMAJ 2018, 190, E422–E429. [Google Scholar] [CrossRef] [PubMed]
- Burlina, A.; Jones, S.A.; Chakrapani, A.; Church, H.J.; Heales, S.; Wu, T.H.Y.; Morton, G.; Roberts, P.; Sluys, E.F.; Cheillan, D. A New Approach to Objectively Evaluate Inherited Metabolic Diseases for Inclusion on Newborn Screening Programmes. Int. J. Neonatal Screen. 2022, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. Prisma 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Goel, V. Appraising Organised Screening Programmes for Testing for Genetic Susceptibility to Cancer. BMJ 2001, 322, 1174–1178. [Google Scholar] [CrossRef]
- Acharya, K.; Ackerman, P.D.; Ross, L.F. Pediatricians’ Attitudes toward Expanding Newborn Screening. Pediatrics 2005, 116, e476–e484. [Google Scholar] [CrossRef]
- Balayla, J. On the Formalism of the Screening Paradox. PLoS ONE 2021, 16, e0256645. [Google Scholar] [CrossRef]
- Cornel, M.C.; Rigter, T.; Weinreich, S.S.; Burgard, P.; Hoffmann, G.F.; Lindner, M.; Gerard Loeber, J.; Rupp, K.; Taruscio, D.; Vittozzi, L. A Framework to Start the Debate on Neonatal Screening Policies in the Eu: An Expert Opinion Document. Eur. J. Hum. Genet. 2014, 22, 12–17. [Google Scholar] [CrossRef]
- Cragun, D.; DeBate, R.D.; Pal, T. Applying Public Health Screening Criteria: How Does Universal Newborn Screening Compare to Universal Tumor Screening for Lynch Syndrome in Adults with Colorectal Cancer? J. Genet. Couns. 2015, 24, 409–420. [Google Scholar] [CrossRef]
- Dombrádi, V.; Pitini, E.; van El, C.G.; Jani, A.; Cornel, M.; Villari, P.; Gray, M.; Bíró, K. Value-Based Genomic Screening: Exploring Genomic Screening for Chronic Diseases Using Triple Value Principles. BMC Health Serv. Res. 2019, 19, 823. [Google Scholar] [CrossRef]
- Fidan, Ç.; Örün, H.; Alper, A.B.; Ünver, Ç.N.; Şahin, Ö.C.; Uğurlu, Z.; Akdur, R.; Taruscio, D. Expanded Newborn Bloodspot Screening: Developed Country Examples and What Can Be Done in Turkey. Intractable Rare Dis. Res. 2022, 11, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.E.; Chowdhury, S.; Pashayan, N.; Hallowell, N.; Pharoah, P.; Burton, H. What Ethical and Legal Principles Should Guide the Genotyping of Children as Part of a Personalised Screening Programme for Common Cancer? J. Med. Ethics 2014, 40, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Sawaya, G.F.; Moyer, V.A.; Calonge, N. Reconsidering the Criteria for Evaluating Proposed Screening Programs: Reflections from 4 Current and Former Members of the U.S. Preventive Services Task Force. Epidemiol. Rev. 2011, 33, 20–35. [Google Scholar] [CrossRef]
- Hiraki, S.; Ormond, K.E.; Kim, K.; Ross, L.F. Attitudes of Genetic Counselors towards Expanding Newborn Screening and Offering Predictive Genetic Testing to Children. Am. J. Med. Genet. Part. A 2006, 140, 2312–2319. [Google Scholar] [CrossRef]
- Jones, S.A.; Cheillan, D.; Chakrapani, A.; Church, H.J.; Heales, S.; Wu, T.H.Y.; Morton, G.; Roberts, P.; Sluys, E.F.; Burlina, A. Application of a Novel Algorithm for Expanding Newborn Screening for Inherited Metabolic Disorders across Europe. Int. J. Neonatal Screen. 2022, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- King, J.R.; Notarangelo, L.D.; Hammarström, L. An Appraisal of the Wilson & Jungner Criteria in the Context of Genomic-Based Newborn Screening for Inborn Errors of Immunity. J. Allergy Clin. Immunol. 2021, 147, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Pitini, E.; De Vito, C.; Marzuillo, C.; D’Andrea, E.; Rosso, A.; Federici, A.; Di Maria, E.; Villari, P. How Is Genetic Testing Evaluated? A Systematic Review of the Literature. Eur. J. Hum. Genet. 2018, 26, 605–615. [Google Scholar] [CrossRef]
- Plass, A.M.; van El, C.G.; Pieters, T.; Cornel, M.C. Neonatal Screening for Treatable and Untreatable Disorders: Prospective Parents’ Opinions. Pediatrics 2010, 125, e99–e106. [Google Scholar] [CrossRef]
- Pollitt, R.J. Different Viewpoints: International Perspectives on Newborn Screening. J. Med. Biochem. 2015, 34, 18–22. [Google Scholar] [CrossRef][Green Version]
- Pollitt, R.J. Newborn Blood Spot Screening: New Opportunities, Old Problems. J. Inherit. Metab. Dis. 2009, 32, 395–399. [Google Scholar] [CrossRef]
- Pollitt, R.J. Introducing New Screens: Why Are We All Doing Different Things? J. Inherit. Metab. Dis. 2007, 30, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, R.J. International Perspectives on Newborn Screening. J. Inherit. Metab. Dis. 2006, 29, 390–396. [Google Scholar] [CrossRef]
- Ross, L.F. Screening for Conditions That Do Not Meet the Wilson and Jungner Criteria: The Case of Duchenne Muscular Dystrophy. Am. J. Med. Genet. Part A 2006, 140, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Sagan, A.; McDaid, D.; Rajan, S.; Farrington, J.; McKee, M. European Observatory Policy Briefs. In Screening: When Is It Appropriate and How Can We Get It Right? European Observatory on Health Systems and Policies: Copenhagen, Denmark, 2020. [Google Scholar]
- Smith, R.A. Can We Improve on Wilson and Jungner’s Principles of Screening for Disease? CMAJ 2018, 190, E414–E415. [Google Scholar] [CrossRef]
- Sturdy, S.; Miller, F.; Hogarth, S.; Armstrong, N.; Chakraborty, P.; Cressman, C.; Dobrow, M.; Flitcroft, K.; Grossman, D.; Harris, R.; et al. Half a Century of Wilson & Jungner: Reflections on the Governance of Population Screening. Wellcome Open Res. 2020, 5, 158. [Google Scholar] [CrossRef]
- Timmermans, S.; Buchbinder, M. Expanded Newborn Screening: Articulating the Ontology of Diseases with Bridging Work in the Clinic. Sociol. Health Illn. 2012, 34, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Eurordis Rare Diseases Europe. Key Principles for Newborn Screening. Available online: https://www.eurordis.org/publications/key-principles-for-newborn-screening/ (accessed on 9 September 2024).
- Hendricks-Sturrup, R.M.; Lu, C.Y. When Should Genomic and Exome Sequencing Be Implemented in Newborns? A Call for an Update to Newborn Screening Guidelines. Genet. Med. 2020, 22, 809–810. [Google Scholar] [CrossRef]
- Botkin, J.R. Assessing the New Criteria for Newborn Screening. Health Matrix 2009, 19, 163–186. [Google Scholar]
- Forman, J.; Coyle, F.; Levy-Fisch, J.; Roberts, P.; Terry, S.; Legge, M. Screening Criteria: The Need to Deal with New Developments and Ethical Issues in Newborn Metabolic Screening. J. Community Genet. 2013, 4, 59–67. [Google Scholar] [CrossRef]
- Palmboom, G.; Willems, D. Risk Detection in Individual Health Care: Any Limits? Bioethics 2010, 24, 431–438. [Google Scholar] [CrossRef]
- Hasegawa, L.E.; Fergus, K.A.; Ojeda, N.; Au, S.M. Parental Attitudes toward Ethical and Social Issues Surrounding the Expansion of Newborn Screening Using New Technologies. Public Health Genom. 2011, 14, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Calonge, N.; Green, N.S.; Rinaldo, P.; Lloyd-Puryear, M.; Dougherty, D.; Boyle, C.; Watson, M.; Trotter, T.; Terry, S.F.; Howell, R.R. Committee Report: Method for Evaluating Conditions Nominated for Population-Based Screening of Newborns and Children. Genet. Med. 2010, 12, 153–159. [Google Scholar] [CrossRef] [PubMed]
- New Zealand National Health Commitee. Screening to Improve Health in New Zealand Criteria to Assess Screening Programmes. Available online: https://www.tewhatuora.govt.nz/publications/screening-to-improve-health-in-new-zealand-criteria-to-assess-screening-programmes (accessed on 9 September 2024).
- UK National Screning Committee. Review of the UK National Screening Committee 2015. Available online: https://www.gov.uk/government/publications/review-of-the-uk-national-screening-committee-2015 (accessed on 9 September 2024).
- Doran, G.T. There’s a S.M.A.R.T. Way to Write Managements’s Goals and Objectives. Manag. Rev. 1981, 70, 35–36. [Google Scholar]
- Berg, J.S.; Foreman, A.K.; O’Daniel, J.M.; Booker, J.K.; Boshe, L.; Carey, T.; Crooks, K.R.; Jensen, B.C.; Juengst, E.T.; Lee, K.; et al. A Semiquantitative Metric for Evaluating Clinical Actionability of Incidental or Secondary Findings from Genome-Scale Sequencing. Genet. Med. 2016, 18, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Posset, R.; Zielonka, M.; Gleich, F.; Garbade, S.F.; Hoffmann, G.F.; Kölker, S. The Challenge of Understanding and Predicting Phenotypic Diversity in Urea Cycle Disorders. J. Inherit. Metab. Dis. 2023, 46, 1007–1016. [Google Scholar] [CrossRef]
- Scharre, S.; Posset, R.; Garbade, S.F.; Gleich, F.; Seidl, M.J.; Druck, A.C.; Okun, J.G.; Gropman, A.L.; Nagamani, S.C.S.; Hoffmann, G.F.; et al. Predicting the Disease Severity in Male Individuals with Ornithine Transcarbamylase Deficiency. Ann. Clin. Transl. Neurol. 2022, 9, 1715–1726. [Google Scholar] [CrossRef]
- Zielonka, M.; Garbade, S.F.; Gleich, F.; Okun, J.G.; Nagamani, S.C.S.; Gropman, A.L.; Hoffmann, G.F.; Kölker, S.; Posset, R. From Genotype to Phenotype: Early Prediction of Disease Severity in Argininosuccinic Aciduria. Hum. Mutat. 2020, 41, 946–960. [Google Scholar] [CrossRef]
- Zielonka, M.; Kölker, S.; Gleich, F.; Stützenberger, N.; Nagamani, S.C.S.; Gropman, A.L.; Hoffmann, G.F.; Garbade, S.F.; Posset, R. Early Prediction of Phenotypic Severity in Citrullinemia Type 1. Ann. Clin. Transl. Neurol. 2019, 6, 1858–1871. [Google Scholar] [CrossRef]
- Mütze, U.; Henze, L.; Schröter, J.; Gleich, F.; Lindner, M.; Grünert, S.C.; Spiekerkoetter, U.; Santer, R.; Thimm, E.; Ensenauer, R.; et al. Isovaleric Aciduria Identified by Newborn Screening: Strategies to Predict Disease Severity and Stratify Treatment. J. Inherit. Metab. Dis. 2023, 46, 1063–1077. [Google Scholar] [CrossRef]
- Märtner, E.M.C.; Thimm, E.; Guder, P.; Schiergens, K.A.; Rutsch, F.; Roloff, S.; Marquardt, I.; Das, A.M.; Freisinger, P.; Grünert, S.C.; et al. The Biochemical Subtype Is a Predictor for Cognitive Function in Glutaric Aciduria Type 1: A National Prospective Follow-up Study. Sci. Rep. 2021, 11, 19300. [Google Scholar] [CrossRef]
- Heringer, J.; Valayannopoulos, V.; Lund, A.M.; Wijburg, F.A.; Freisinger, P.; Barić, I.; Baumgartner, M.R.; Burgard, P.; Burlina, A.B.; Chapman, K.A.; et al. Impact of Age at Onset and Newborn Screening on Outcome in Organic Acidurias. J. Inherit. Metab. Dis. 2016, 39, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Mütze, U.; Ottenberger, A.; Gleich, F.; Maier, E.M.; Lindner, M.; Husain, R.A.; Palm, K.; Beblo, S.; Freisinger, P.; Santer, R.; et al. Neurological Outcome in Long-Chain Hydroxy Fatty Acid Oxidation Disorders. Ann. Clin. Transl. Neurol. 2024, 11, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, J.L. Expanded Newborn Screening: Social and Ethical Issues. J. Inherit. Metab. Dis. 2010, 33, S211–S217. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.P.; Clarke, A.J.; Hood, K.; Lycett, E.; Bradley, D.M. Newborn Screening for Duchenne Muscular Dystrophy: A Psychosocial Study. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 86, F91–F95. [Google Scholar] [CrossRef]
- Hopkins, H.; Kinsella, S.; Evans, G. Implications of Whole Genome Sequencing for Newborn Screening. Findings from a Public Dialogue. Available online: https://files.genomicsengland.co.uk/documents/public-dialogue-wgs-for-nbs-final-report.pdf (accessed on 9 September 2024).
- Loeber, J.G.; Burgard, P.; Cornel, M.C.; Rigter, T.; Weinreich, S.S.; Rupp, K.; Hoffmann, G.F.; Vittozzi, L. Newborn Screening Programmes in Europe; Arguments and Efforts Regarding Harmonization. Part 1. From Blood Spot to Screening Result. J. Inherit. Metab. Dis. 2012, 35, 603–611. [Google Scholar] [CrossRef]
- Loeber, J.G.; Platis, D.; Zetterström, R.H.; Almashanu, S.; Boemer, F.; Bonham, J.R.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Neonatal Screening in Europe Revisited: An Isns Perspective on the Current State and Developments since 2010. Int. J. Neonatal Screen. 2021, 7, 15. [Google Scholar] [CrossRef]
- Sikonja, J.; Groselj, U.; Scarpa, M.; la Marca, G.; Cheillan, D.; Kölker, S.; Zetterström, R.H.; Kožich, V.; Le Cam, Y.; Gumus, G.; et al. Towards Achieving Equity and Innovation in Newborn Screening across Europe. Int. J. Neonatal Screen. 2022, 8, 31. [Google Scholar] [CrossRef]
- Therrell, B.L.; Padilla, C.D.; Borrajo, G.J.C.; Khneisser, I.; Schielen, P.; Knight-Madden, J.; Malherbe, H.L.; Kase, M. Current Status of Newborn Bloodspot Screening Worldwide 2024: A Comprehensive Review of Recent Activities (2020–2023). Int. J. Neonatal Screen. 2024, 10, 38. [Google Scholar] [CrossRef]
- Watson, M.S.; Lloyd-Puryear, M.A.; Howell, R.R. The Progress and Future of US Newborn Screening. Int. J. Neonatal Screen. 2022, 8, 41. [Google Scholar] [CrossRef]
- Downie, L.; Bouffler, S.E.; Amor, D.J.; Christodoulou, J.; Yeung, A.; Horton, A.E.; Macciocca, I.; Archibald, A.D.; Wall, M.; Caruana, J.; et al. Gene Selection for Genomic Newborn Screening: Moving toward Consensus? Genet. Med. 2024, 26, 101077. [Google Scholar] [CrossRef]
- van Spronsen, F.J. Mild Hyperphenylalaninemia: To Treat or Not to Treat. J. Inherit. Metab. Dis. 2011, 34, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Liebig, M.; Schymik, I.; Mueller, M.; Wendel, U.; Mayatepek, E.; Ruiter, J.; Strauss, A.W.; Wanders, R.J.; Spiekerkoetter, U. Neonatal Screening for Very Long-Chain Acyl-Coa Dehydrogenase Deficiency: Enzymatic and Molecular Evaluation of Neonates with Elevated C14:1-Carnitine Levels. Pediatrics 2006, 118, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Mütze, U.; Henze, L.; Gleich, F.; Lindner, M.; Grünert, S.C.; Spiekerkoetter, U.; Santer, R.; Blessing, H.; Thimm, E.; Ensenauer, R.; et al. Newborn Screening and Disease Variants Predict Neurological Outcome in Isovaleric Aciduria. J. Inherit. Metab. Dis. 2021, 44, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Maier, E.M.; Liebl, B.; Röschinger, W.; Nennstiel-Ratzel, U.; Fingerhut, R.; Olgemöller, B.; Busch, U.; Krone, N.; v Kries, R.; Roscher, A.A. Population Spectrum of Acadm Genotypes Correlated to Biochemical Phenotypes in Newborn Screening for Medium-Chain Acyl-Coa Dehydrogenase Deficiency. Hum. Mutat. 2005, 25, 443–452. [Google Scholar] [CrossRef]
- Carlock, G.; Fischer, S.T.; Lynch, M.E.; Potter, N.L.; Coles, C.D.; Epstein, M.P.; Mulle, J.G.; Kable, J.A.; Barrett, C.E.; Edwards, S.M.; et al. Developmental Outcomes in Duarte Galactosemia. Pediatrics 2019, 143, e20182516. [Google Scholar] [CrossRef]
- AWMF. S1-Leitlinie Konfirmationsdiagnostik Bei Verdacht auf Angeborene Stoffwechselkrankheiten aus dem Neugeborenenscreening. Available online: https://register.awmf.org/assets/guidelines/027-021l_S1_Konfirmationsdiagnostik-Stoffwechselkrankheiten-Neugeborenenscreening_2020-05.pdf (accessed on 9 September 2024).
- Blom, M.; Zetterström, R.H.; Stray-Pedersen, A.; Gilmour, K.; Gennery, A.R.; Puck, J.M.; van der Burg, M. Recommendations for Uniform Definitions Used in Newborn Screening for Severe Combined Immunodeficiency. J. Allergy Clin. Immunol. 2022, 149, 1428–1436. [Google Scholar] [CrossRef]
- Farrell, P.M.; Sommerburg, O. Toward Quality Improvement in Cystic Fibrosis Newborn Screening: Progress and Continuing Challenges. J. Cyst. Fibros. 2016, 15, 267–269. [Google Scholar] [CrossRef]
- Janda, J.; Hegert, S.; Bzdok, J.; Tesorero, R.; Holtkamp, U.; Burggraf, S.; Schuhmann, E.; Hörster, F.; Hoffmann, G.F.; Janzen, N.; et al. High Throughput Newborn Screening for Sickle Cell Disease—Application of Two-Tiered Testing with a Qpcr-Based Primary Screen. Klin. Padiatr. 2023, 235, 366–372. [Google Scholar] [CrossRef]
- Schnabel, E.; Kölker, S.; Gleich, F.; Feyh, P.; Hörster, F.; Haas, D.; Fang-Hoffmann, J.; Morath, M.; Gramer, G.; Röschinger, W.; et al. Combined Newborn Screening Allows Comprehensive Identification Also of Attenuated Phenotypes for Methylmalonic Acidurias and Homocystinuria. Nutrients 2023, 15, 3355. [Google Scholar] [CrossRef]
- Zaunseder, E.; Haupt, S.; Mütze, U.; Garbade, S.F.; Kölker, S.; Heuveline, V. Opportunities and Challenges in Machine Learning-Based Newborn Screening—A Systematic Literature Review. JIMD Rep. 2022, 63, 250–261. [Google Scholar] [CrossRef]
- Zaunseder, E.; Mütze, U.; Garbade, S.F.; Haupt, S.; Feyh, P.; Hoffmann, G.F.; Heuveline, V.; Kölker, S. Machine Learning Methods Improve Specificity in Newborn Screening for Isovaleric Aciduria. Metabolites 2023, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Zaunseder, E.; Mütze, U.; Okun, J.G.; Hoffmann, G.F.; Kölker, S.; Heuveline, V.; Thiele, I. Personalized Metabolic Whole-Body Models for Newborns and Infants Predict Growth and Biomarkers of Inherited Metabolic Diseases. Cell Metab. 2024, 8, 1882–1897.e7. [Google Scholar] [CrossRef] [PubMed]
- Nennstiel, U.; Odenwald, B.; Throner, V.; Blankenstein, O.; Vieth, A.; Ratzel, R.; Coenen, M.; Brockow, I. Newborn Blood Spot Screening (Nbs) in Germany: Status Quo and Presentation of A concept for Further Development. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2023, 66, 1195–1204. [Google Scholar] [CrossRef]
- Boy, N.; Mühlhausen, C.; Maier, E.M.; Ballhausen, D.; Baumgartner, M.R.; Beblo, S.; Burgard, P.; Chapman, K.A.; Dobbelaere, D.; Heringer-Seifert, J.; et al. Recommendations for Diagnosing and Managing Individuals with Glutaric Aciduria Type 1: Third Revision. J. Inherit. Metab. Dis. 2023, 46, 482–519. [Google Scholar] [CrossRef] [PubMed]
- Pfeil, J.; Listl, S.; Hoffmann, G.F.; Kölker, S.; Lindner, M.; Burgard, P. Newborn Screening by Tandem Mass Spectrometry for Glutaric Aciduria Type 1: A Cost-Effectiveness Analysis. Orphanet J. Rare Dis. 2013, 8, 167. [Google Scholar] [CrossRef]
- Forny, P.; Hörster, F.; Baumgartner, M.R.; Kölker, S.; Boy, N. How Guideline Development Has Informed Clinical Research for Organic Acidurias (Et Vice Versa). J. Inherit. Metab. Dis. 2023, 46, 520–535. [Google Scholar] [CrossRef]
- Gramer, G.; Haege, G.; Glahn, E.M.; Hoffmann, G.F.; Lindner, M.; Burgard, P. Living with an Inborn Error of Metabolism Detected by Newborn Screening-Parents’ Perspectives on Child Development and Impact on Family Life. J. Inherit. Metab. Dis. 2014, 37, 189–195. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Rones, M.; Read, C.Y.; Marsden, D.; Levy, H.L. Brief Report: Predictors of Parenting Stress among Parents of Children with Biochemical Genetic Disorders. J. Pediatr. Psychol. 2004, 29, 565–570. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Albers, S.; Amato, S.; Ampola, M.; Brewster, T.G.; Demmer, L.; Eaton, R.B.; Greenstein, R.; Korson, M.; Larson, C.; et al. Effect of Expanded Newborn Screening for Biochemical Genetic Disorders on Child Outcomes and Parental Stress. JAMA 2003, 290, 2564–2572. [Google Scholar] [CrossRef]
- Schnabel-Besson, E.; Garbade, S.F.; Gleich, F.; Grünert, S.C.; Krämer, J.; Thimm, E.; Hennermann, J.B.; Freisinger, P.; Burgard, P.; Gramer, G.; et al. Parental and Child’s Psychosocial and Financial Burden Living with an Inherited Metabolic Disease Identified by Newborn Screening. J. Inherit. Metab. Dis. 2024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnabel-Besson, E.; Mütze, U.; Dikow, N.; Hörster, F.; Morath, M.A.; Alex, K.; Brennenstuhl, H.; Settegast, S.; Okun, J.G.; Schaaf, C.P.; et al. Wilson and Jungner Revisited: Are Screening Criteria Fit for the 21st Century? Int. J. Neonatal Screen. 2024, 10, 62. https://doi.org/10.3390/ijns10030062
Schnabel-Besson E, Mütze U, Dikow N, Hörster F, Morath MA, Alex K, Brennenstuhl H, Settegast S, Okun JG, Schaaf CP, et al. Wilson and Jungner Revisited: Are Screening Criteria Fit for the 21st Century? International Journal of Neonatal Screening. 2024; 10(3):62. https://doi.org/10.3390/ijns10030062
Chicago/Turabian StyleSchnabel-Besson, Elena, Ulrike Mütze, Nicola Dikow, Friederike Hörster, Marina A. Morath, Karla Alex, Heiko Brennenstuhl, Sascha Settegast, Jürgen G. Okun, Christian P. Schaaf, and et al. 2024. "Wilson and Jungner Revisited: Are Screening Criteria Fit for the 21st Century?" International Journal of Neonatal Screening 10, no. 3: 62. https://doi.org/10.3390/ijns10030062
APA StyleSchnabel-Besson, E., Mütze, U., Dikow, N., Hörster, F., Morath, M. A., Alex, K., Brennenstuhl, H., Settegast, S., Okun, J. G., Schaaf, C. P., Winkler, E. C., & Kölker, S. (2024). Wilson and Jungner Revisited: Are Screening Criteria Fit for the 21st Century? International Journal of Neonatal Screening, 10(3), 62. https://doi.org/10.3390/ijns10030062





