Epicatechin-Enriched Cacao Subproducts Improve Cognition in Older Subjects: Proof of Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion Criteria
2.2.1. Handgrip Strength
2.2.2. Up and Go Test [8]
2.2.3. Skeletal Muscle Index (SMI)
2.3. Non-Inclusion Criteria
2.4. Study Design
2.5. Treatment
2.6. Statistical Analysis
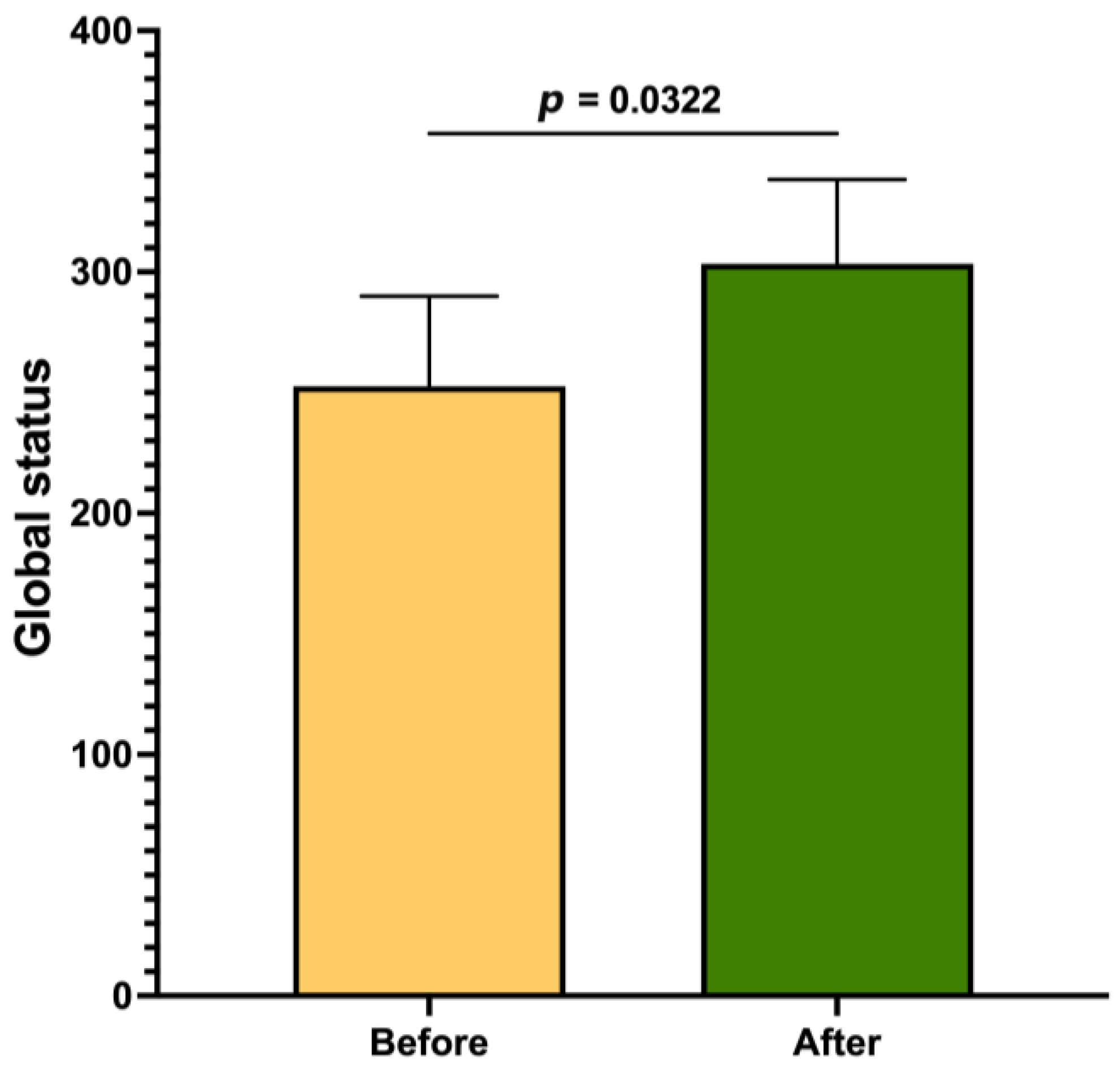
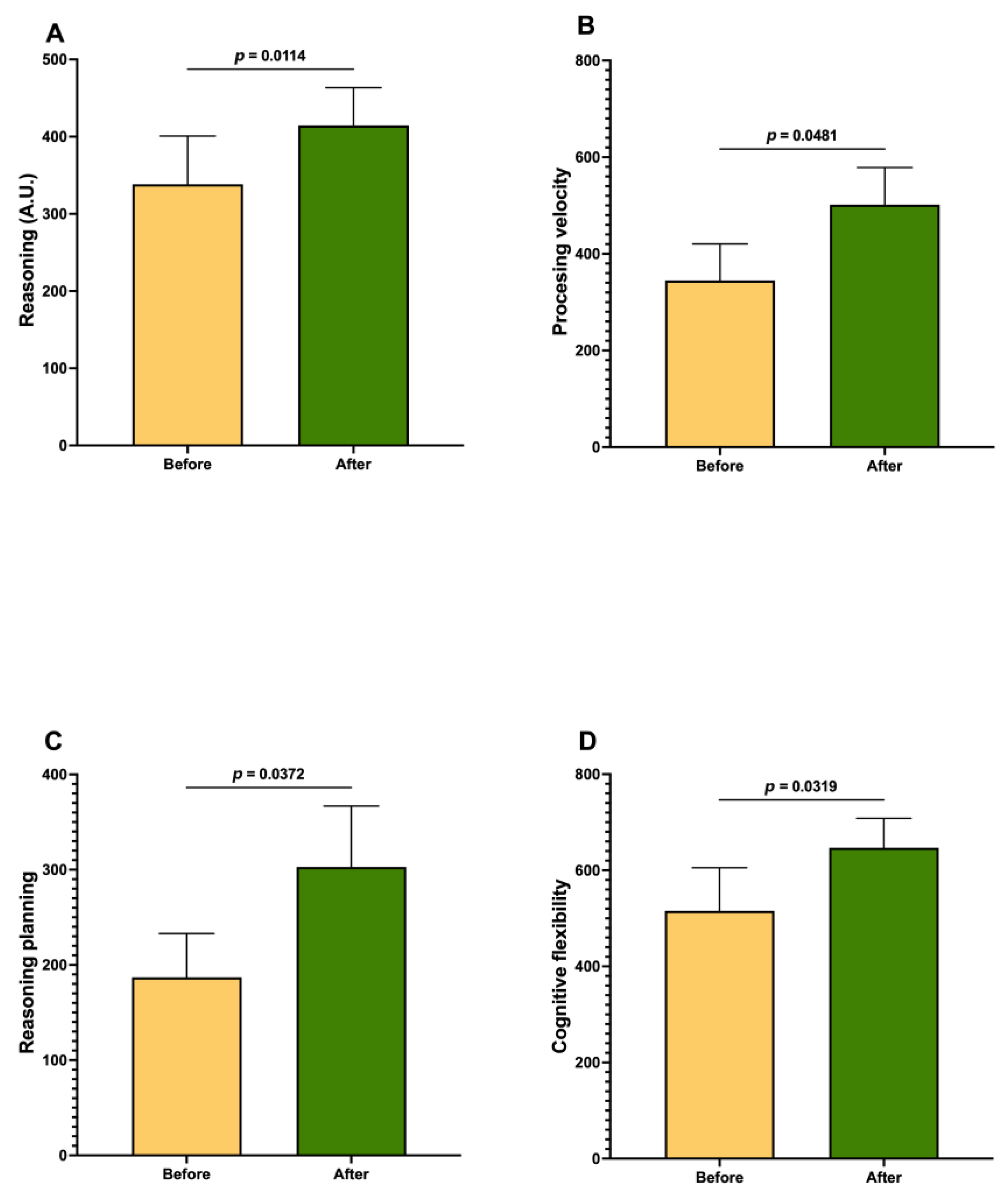
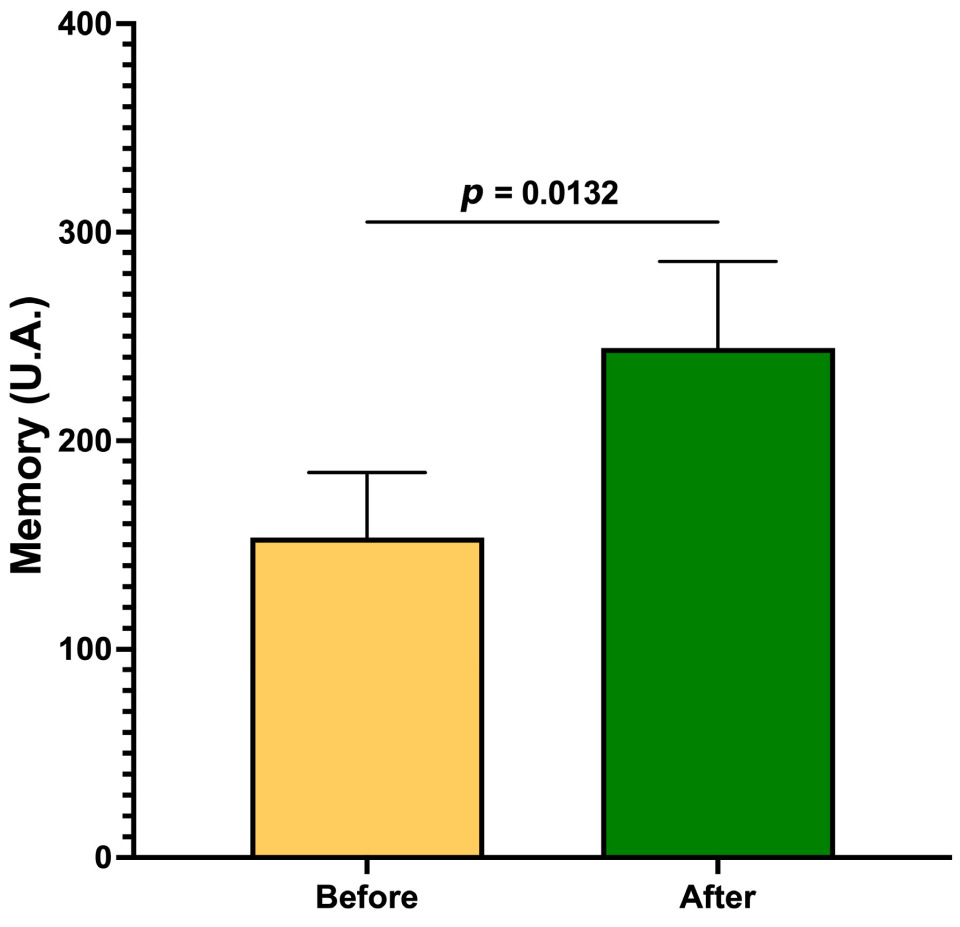
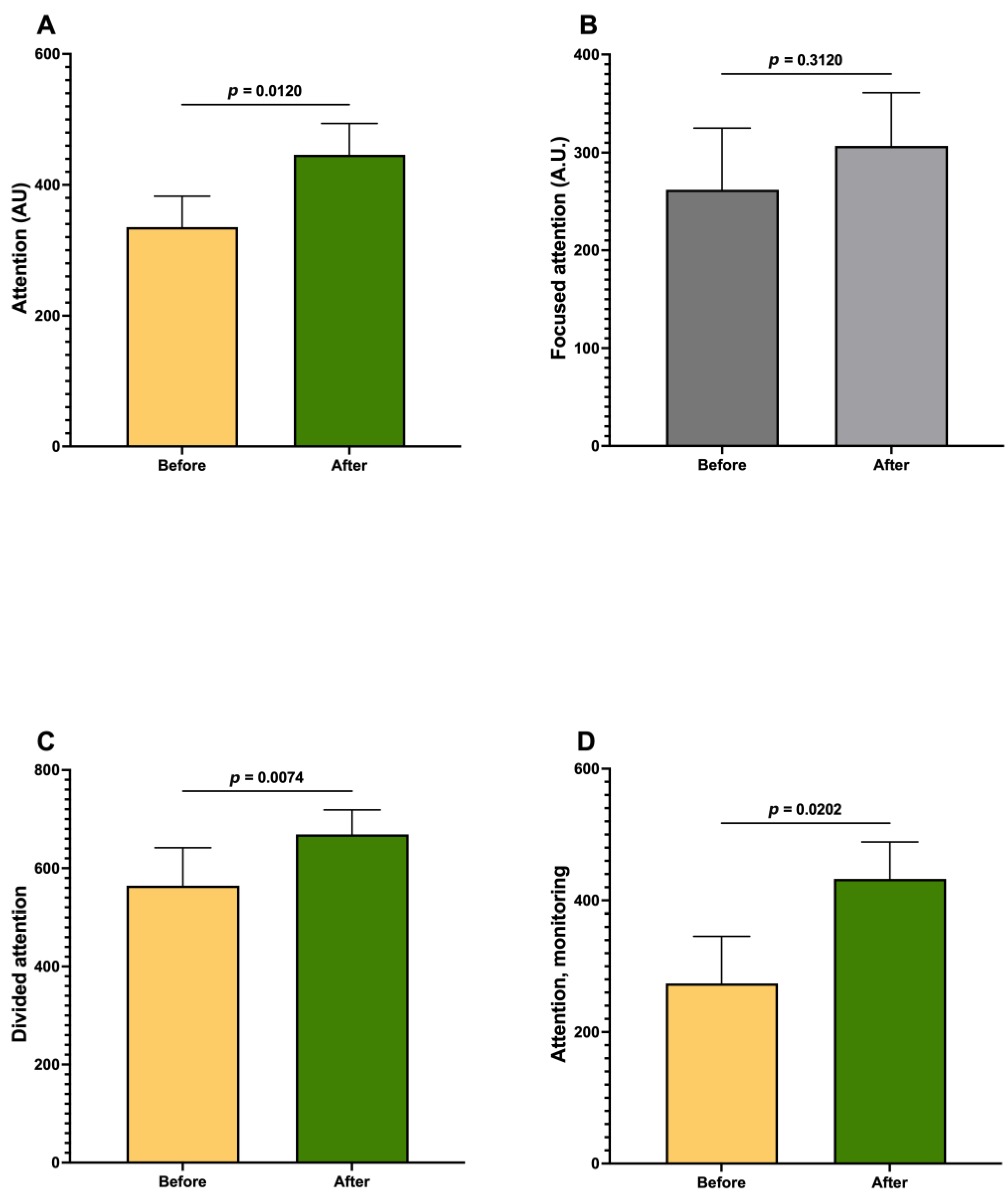
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef]
- Peters, R. Ageing and the brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef]
- Puri, S.; Shaheen, M.; Grover, B. Nutrition and cognitive health: A life course approach. Front. Public Health 2023, 11, 1023907. [Google Scholar] [CrossRef]
- Desideri, G.; Bocale, R. Correlation between cardiovascular risk factors and cognitive decline. Eur. Heart J. Suppl. 2021, 23, E73–E76. [Google Scholar] [CrossRef]
- Cipriani, G.; Danti, S.; Picchi, L.; Nuti, A.; Fiorino, M.D. Daily functioning and dementia. Dement. Neuropsychol. 2020, 14, 93–102. [Google Scholar] [CrossRef]
- Langa, K.; Levine, D.A. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA 2014, 312, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Munguia, L.; Rubio-Gayosso, I.; Ramirez-Sanchez, I.; Ortiz, A.; Hidalgo, I.; Gonzalez, C.; Meaney, E.; Villarreal, F.; Najera, N.; Ceballos, G. High Flavonoid Cocoa Supplement Ameliorates Plasma Oxidative Stress and Inflammation Levels While Improving Mobility and Quality of Life in Older Subjects: A Double-Blind Randomized Clinical Trial. J. Gerontol. Ser. A 2019, 74, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid Intake and Cognitive Decline over a 10-Year Period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef]
- Dodd, G.; Williams, C.; Butler, L.; Spencer, J. Acute effects of flavonoid-rich blueberry on cognitive and vascular function in healthy older adults. Nutr. Healthy Aging 2019, 5, 119–132. [Google Scholar] [CrossRef]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019, 10, S422–S436. [Google Scholar] [CrossRef]
- Morais, C.A.; De Rosso, V.V.; Estadella, D.; Pisani, L. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Warner, E.F.; Smith, M.J.; Zhang, Q.; Raheem, K.S.; O’Hagan, D.; O’Connell, M.A.; Kay, C.D. Signatures of anthocyanin metabolites identified in humans inhibit biomarkers of vascular inflammation in human endothelial cells. Mol. Nutr. Food Res. 2017, 61, 1700053. [Google Scholar] [CrossRef]
- Grosso, G. Nutrition and aging: Is there a link to cognitive health? Int. J. Food Sci. Nutr. 2020, 71, 265–266. [Google Scholar] [CrossRef]
- Berbegal, M.; Tomé, M.; Sánchez-SanSegundo, M.; Zaragoza-Martí, A.; Hurtado-Sánchez, J.A. Memory function performance in individuals classified as overweight, obese, and normal weight. Front. Nutr. 2022, 9, 932323. [Google Scholar] [CrossRef]
- León-Flores, P.; Nájera, N.; Pérez, E.; Pardo, B.; Jimenez, F.; Diaz-Chiguer, D.; Villarreal, F.; Hidalgo, I.; Ceballos, G.; Meaney, M. Effects of Cacao By-Products and a Modest Weight Loss Intervention on the Concentration of Serum Triglycerides in Overweight Subjects: Proof of Concept. J. Med. Food 2020, 23, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Rosso, A.L.; Studenski, S.A.; Chen, W.G.; Aizenstein, H.J.; Alexander, N.B.; Bennett, D.A.; Black, S.E.; Camicioli, R.; Carlson, M.C.; Ferrucci, L.; et al. Aging, the Central Nervous System, and Mobility. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, M.; Navarro, E.; Calero, D. Effectiveness of Cognitive Interventions in Older Adults: A Review. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 876–898. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Soria, I.; Iguacel, I.; Aguilar-Latorre, A.; Peralta-Marrupe, P.; Latorre, E.; Cuenca Zaldívar, J.N.; Calatayud, E. Cognitive stimulation and cognitive results in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 104, 104807. [Google Scholar] [CrossRef]
- Munguia, L.; Ramirez-Sanchez, I.; Meaney, E.; Villarreal, F.; Ceballos, G.; Najera, N. Flavonoids from dark chocolate and (−)-epicatechin ameliorate high-fat diet-induced decreases in mobility and muscle damage in aging mice. Food Biosci. 2020, 37, 100710. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Effect of flavonoids on learning, memory and neurocognitive performance: Relevance and potential implications for Alzheimer’s disease pathophysiology. J. Sci. Food Agric. 2014, 94, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Bell, L.; Lamport, D.J.; Williams, C.M. Dietary Flavonoids and Human Cognition: A Meta-Analysis. Mol. Nutr. Food Res. 2022, 66, 2100976. [Google Scholar] [CrossRef] [PubMed]

| Mean ± SEM | Men | Women | |
|---|---|---|---|
| Age (years) | 67.75 ± 1.927 | 67 ± 2.07 | 68.5 ± 3.44 |
| Body weight (kg) | 69.85 ± 11.5 | 68.3 ± 11.2 | 71.4 ± 11.4 |
| Initial values; n (%) | |||
| Low hand strength | 12 (100) | 6 (100) <30 kg | 6 (100) <20 kg |
| Low up and go test >10 s | 12 (100) | 6 (100) | 6 (100) |
| Skeletal muscle index | 12 (100) | 6 (100) <8.87 kg/m2 | 6 (100) <6.42 kg/m2 |
| Z-Score (A) | Z-Score (B) | |
|---|---|---|
| Global status | 0.4195 | |
| Reasoning | 0.4497 | |
| Processing velocity | 2.0435 | |
| Reasoning planning | 1.8124 | |
| Cognitive flexibility | 2.1345 | |
| Memory | 0.6350 | |
| Short-term memory | 2.0000 | |
| Non-verbal memory | 1.4944 | |
| Working memory | 1.8650 | |
| Contextual memory | 1.2429 | |
| Attention | 2.3225 | |
| Focused attention | 0.8283 | |
| Divided attention | 2.1055 | |
| Attention monitoring | 2.8322 | |
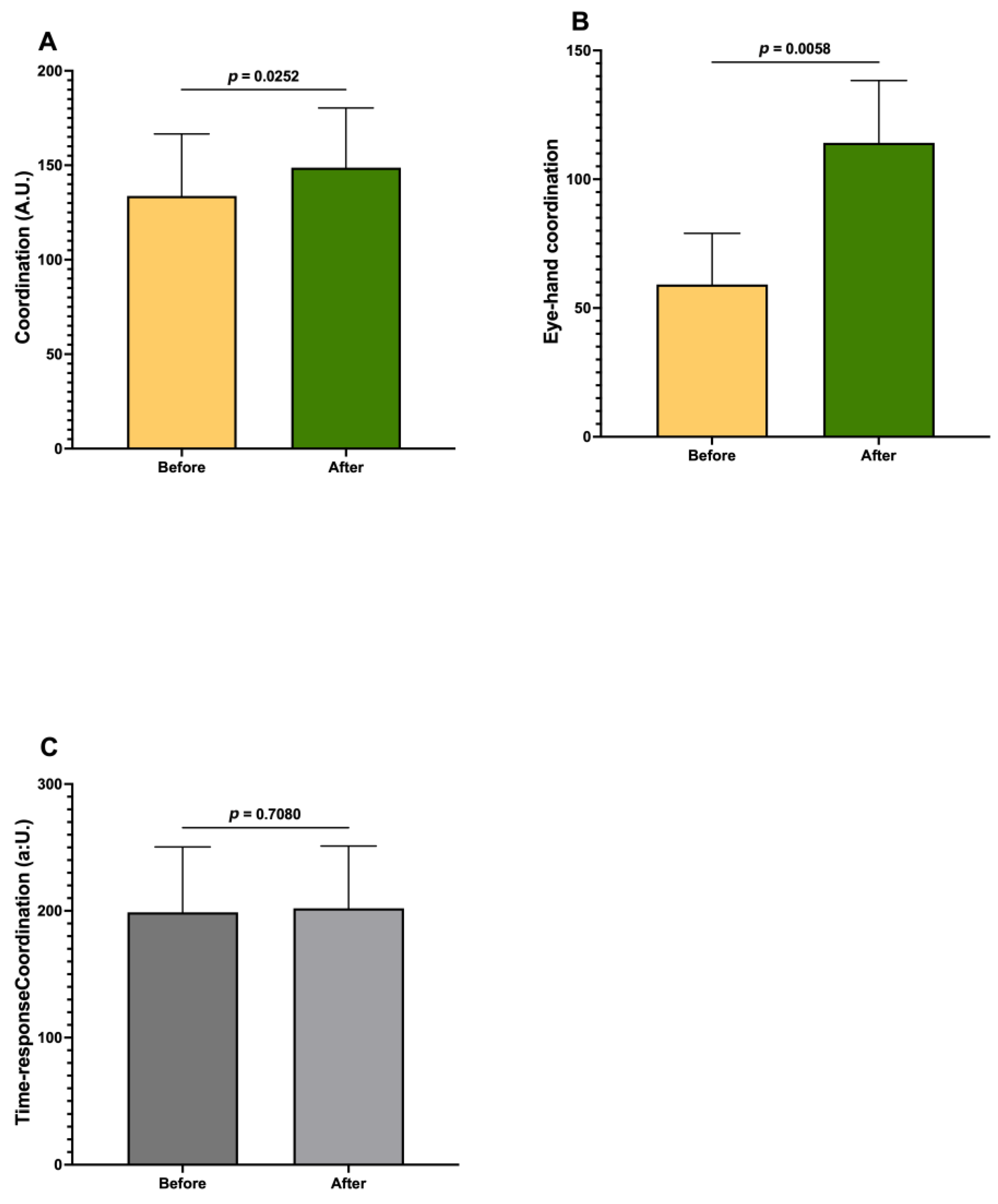
| Coordination | 0.4745 | |
| Eye–hand coordination | 2.2768 | |
| Time–response coordination | 0.0651 | |
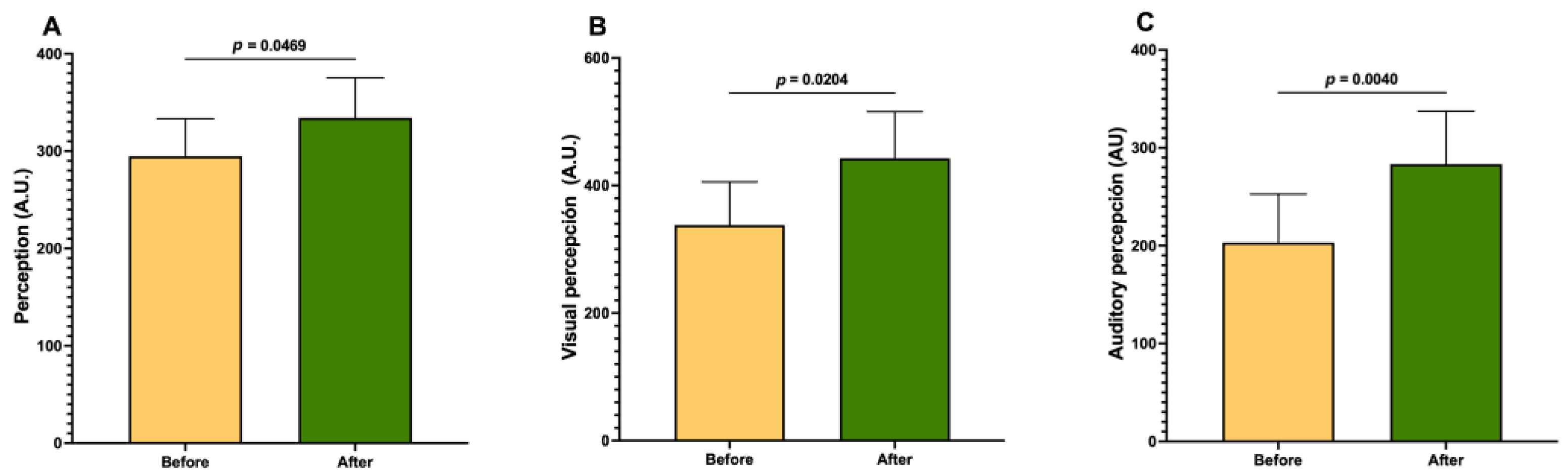
| Perception | 0.9589 | |
| Visual perception | 1.4303 | |
| Auditory perception | 1.4839 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the JMMS. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nájera, N.; Munguía, L.; Ortiz, M.; Villarreal, F.; Martínez-Meza, Y.; Gómez-Cotero, A.; Ceballos, G. Epicatechin-Enriched Cacao Subproducts Improve Cognition in Older Subjects: Proof of Concept. J. Mind Med. Sci. 2025, 12, 41. https://doi.org/10.3390/jmms12020041
Nájera N, Munguía L, Ortiz M, Villarreal F, Martínez-Meza Y, Gómez-Cotero A, Ceballos G. Epicatechin-Enriched Cacao Subproducts Improve Cognition in Older Subjects: Proof of Concept. Journal of Mind and Medical Sciences. 2025; 12(2):41. https://doi.org/10.3390/jmms12020041
Chicago/Turabian StyleNájera, Nayelli, Levy Munguía, Miguel Ortiz, Francisco Villarreal, Yuridia Martínez-Meza, Amalia Gómez-Cotero, and Guillermo Ceballos. 2025. "Epicatechin-Enriched Cacao Subproducts Improve Cognition in Older Subjects: Proof of Concept" Journal of Mind and Medical Sciences 12, no. 2: 41. https://doi.org/10.3390/jmms12020041
APA StyleNájera, N., Munguía, L., Ortiz, M., Villarreal, F., Martínez-Meza, Y., Gómez-Cotero, A., & Ceballos, G. (2025). Epicatechin-Enriched Cacao Subproducts Improve Cognition in Older Subjects: Proof of Concept. Journal of Mind and Medical Sciences, 12(2), 41. https://doi.org/10.3390/jmms12020041






