Overview on Adjunct Ingredients Used in Hydroxyapatite-Based Oral Care Products
Abstract
1. Introduction
2. Literature Search
3. Overview on Adjunct Ingredients Used in Hydroxyapatite-Based Oral Care Products
3.1. Antibacterial Ingredients
3.1.1. Lactoferrin
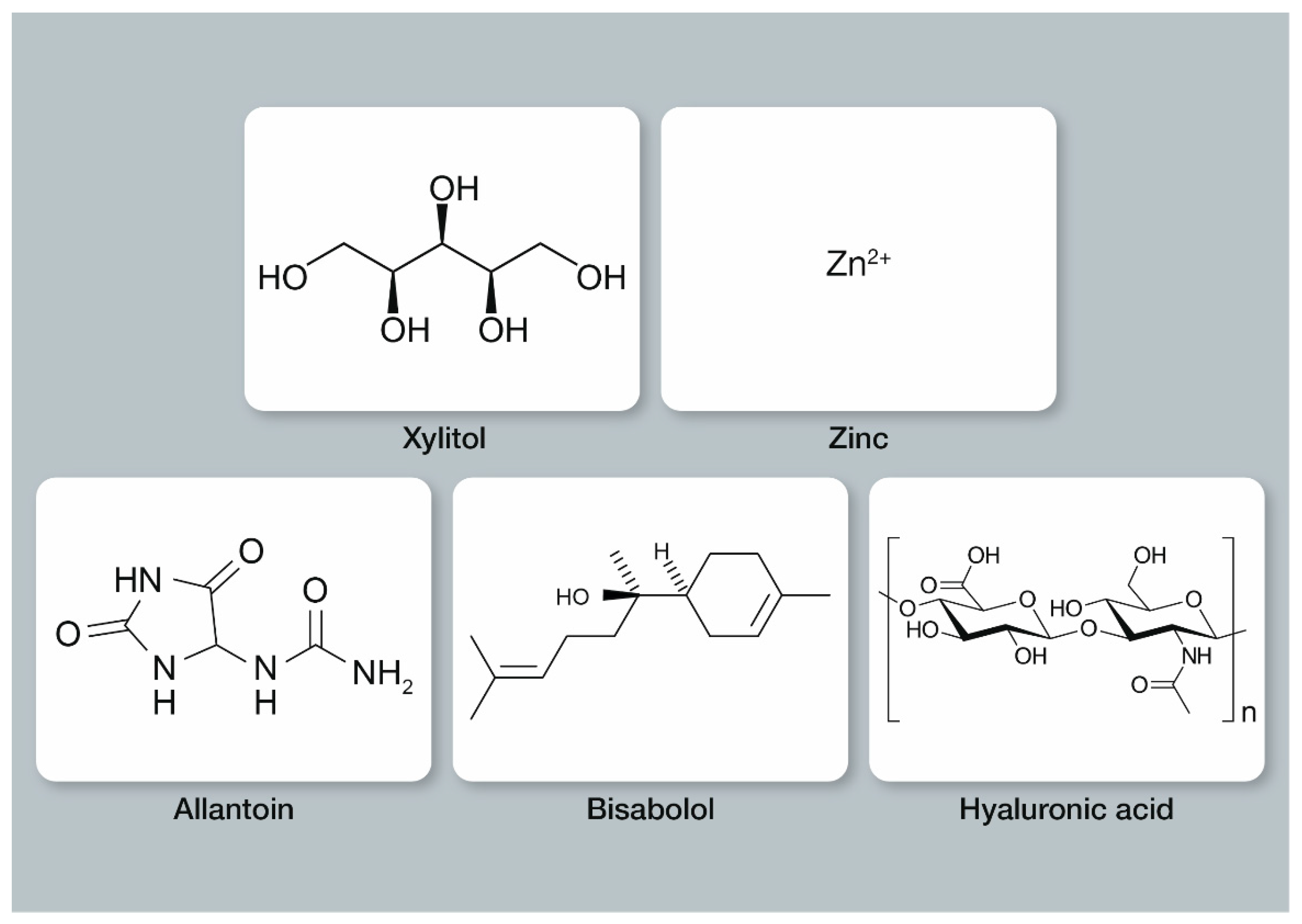
3.1.2. Xylitol
3.1.3. Zinc
3.2. Ingredients for Gum Care
3.2.1. Allantoin
3.2.2. Bisabolol
3.2.3. Hyaluronic Acid
3.3. Additional Ingredients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Loveren, C.V. Toothpastes; Karger: Basel, Switzerland, 2013; Volume 23. [Google Scholar]
- Epple, M.; Enax, J. Moderne Zahnpflege aus chemischer Sicht. Chem. Unserer Zeit 2018, 52, 218–228. [Google Scholar] [CrossRef]
- Hu, M.L.; Zheng, G.; Lin, H.; Yang, M.; Zhang, Y.D.; Han, J.M. Network meta-analysis on the effect of desensitizing toothpastes on dentine hypersensitivity. J. Dent. 2019, 88, 103170. [Google Scholar] [CrossRef] [PubMed]
- Gillam, D.G. Dentine Hypersensitivity: Advances in Diagnosis, Management, and Treatment; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Epple, M.; Meyer, F.; Enax, J. A critical review of modern concepts for teeth whitening. Dent. J. 2019, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Limeback, H.; Enax, J.; Meyer, F. Biomimetic hydroxyapatite and caries prevention: A systematic review and meta-analysis. Can. J. Dent. Hyg. 2021, 55, 148–159. [Google Scholar] [PubMed]
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily application of a toothpaste with biomimetic hydroxyapatite and its subjective impact on dentin hypersensitivity, tooth smoothness, tooth whitening, gum bleeding, and feeling of freshness. Biomimetics 2020, 5, 17. [Google Scholar] [CrossRef]
- Steinert, S.; Kuchenbecker, J.; Meyer, F.; Simader, B.; Zwanzig, K.; Enax, J. Whitening effects of a novel oral care gel with biomimetic hydroxyapatite: A 4-week observational pilot study. Biomimetics 2020, 5, 65. [Google Scholar] [CrossRef]
- Fabritius, H.-O.; Enax, J.; Meyer, F. Eine Reise ins Innere unserer Zähne. Titus-Verlag: Bielefeld, Germany, 2021. [Google Scholar]
- Fabritius-Vilpoux, K.; Enax, J.; Herbig, M.; Raabe, D.; Fabritius, H.-O. Quantitative affinity parameters of synthetic hydroxyapatite and enamel surfaces in vitro. Bioinspired Biomim. Nanobiomaterials 2019, 8, 141–153. [Google Scholar] [CrossRef]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef]
- Enax, J.; Meyer, F.; Schulze zur Wiesche, E.; Epple, M. On the application of calcium phosphate micro- and nanoparticles as food additive. Nanomaterials 2022, 12, 4075. [Google Scholar] [CrossRef]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Enax, J.; Amaechi, B.T.; Limeback, H.; Fabritius, H.-O.; Ganss, B.; Pawinska, M.; Paszynska, E. Hydroxyapatite as remineralization agent for children’s dental care. Front. Dent. Med. 2022, 3, 859560. [Google Scholar] [CrossRef]
- Enax, J.; Fabritius, H.-O.; Fabritius-Vilpoux, K.; Amaechi, B.T.; Meyer, F. Modes of action and clinical efficacy of particulate hydroxyapatite in preventive oral health care—state of the art. Open Dent. J. 2019, 13, 274–287. [Google Scholar] [CrossRef]
- Meyer, F.; Enax, J. Hydroxyapatite in oral biofilm management. Eur. J. Dent. 2019, 13, 287–290. [Google Scholar] [CrossRef]
- Izzetti, R.; Gennai, S.; Nisi, M.; Gulia, F.; Miceli, M.; Giuca, M.R. Clinical applications of nano-hydroxyapatite in dentistry. Appl. Sci. 2022, 12, 10762. [Google Scholar] [CrossRef]
- Kensche, A.; Holder, C.; Basche, S.; Tahan, N.; Hannig, C.; Hannig, M. Efficacy of a mouthrinse based on hydroxyapatite to reduce initial bacterial colonisation in situ. Arch. Oral Biol. 2017, 80, 18–26. [Google Scholar] [CrossRef]
- Wikipedia. Available online: en.wikipedia.org (accessed on 24 October 2022).
- Baker, E.N.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2531. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Tonguc-Altin, K.; Sandalli, N.; Duman, G.; Selvi-Kuvvetli, S.; Topcuoglu, N.; Kulekci, G. Development of novel formulations containing lysozyme and lactoferrin and evaluation of antibacterial effects on mutans streptococci and lactobacilli. Arch. Oral Biol. 2015, 60, 706–714. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Furmanski, P.; Li, Z.P.; Fortuna, M.B.; Swamy, C.V.; Das, M.R. Multiple molecular forms of human lactoferrin. Identification of a class of lactoferrins that possess ribonuclease activity and lack iron-binding capacity. J. Exp. Med. 1989, 170, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Jameson, G.B.; Anderson, B.F.; Norris, G.E.; Thomas, D.H.; Baker, E.N. Structure of human apolactoferrin at 2.0 A resolution. Refinement and analysis of ligand-induced conformational change. Acta Crystallogr. D Biol. Crystallogr. 1998, 54, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Krupińska, A.M.; Bogucki, Z. Clinical aspects of the use of lactoferrin in dentistry. J. Oral Biosci. 2021, 63, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and oral diseases: Current status and perspective in periodontitis. Ann. Stomatol. 2011, 2, 10–18. [Google Scholar]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef] [PubMed]
- Hatti, S.; Ravindra, S.; Satpathy, A.; Kulkarni, R.D.; Parande, M.V. Biofilm inhibition and antimicrobial activity of a dentifrice containing salivary substitutes. Int. J. Dent. Hyg. 2007, 5, 218–224. [Google Scholar] [CrossRef]
- Daly, S.; Seong, J.; Newcombe, R.; Davies, M.; Nicholson, J.; Edwards, M.; West, N. A randomised clinical trial to determine the effect of a toothpaste containing enzymes and proteins on gum health over 3 months. J. Dent. 2019, 80 (Suppl. S1), S26–S32. [Google Scholar] [CrossRef]
- Cunha, E.J.; Auersvald, C.M.; Deliberador, T.M.; Gonzaga, C.C.; Esteban Florez, F.L.; Correr, G.M.; Storrer, C.L.M. Effects of active oxygen toothpaste in supragingival biofilm reduction: A randomized controlled clinical trial. Int. J. Dent. 2019, 2019, 3938214. [Google Scholar] [CrossRef]
- Cawley, A.; Golding, S.; Goulsbra, A.; Hoptroff, M.; Kumaran, S.; Marriott, R. Microbiology insights into boosting salivary defences through the use of enzymes and proteins. J. Dent. 2019, 80 (Suppl. S1), S19–S25. [Google Scholar] [CrossRef]
- Marconcini, S.; Giammarinaro, E.; Cosola, S.; Oldoini, G.; Genovesi, A.; Covani, U. Effects of non-surgical periodontal treatment on reactive oxygen metabolites and glycemic control in diabetic patients with chronic periodontitis. Antioxidants 2021, 10, 1056. [Google Scholar] [CrossRef]
- Gudipaneni, R.K.; Kumar, R.V.; Jesudass, G.; Peddengatagari, S.; Duddu, Y. Short term comparative evaluation of antimicrobial efficacy of tooth paste containing lactoferrin, lysozyme, lactoperoxidase in children with severe early childhood caries: A clinical study. J. Clin. Diagn. Res. 2014, 8, Zc18-20. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.Y.; Leung, K.P.; Wu, C.D. The effect of lactoferrin on oral bacterial attachment. Oral Microbiol. Immunol. 2009, 24, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Nagano-Takebe, F.; Miyakawa, H.; Nakazawa, F.; Endo, K. Inhibition of initial bacterial adhesion on titanium surfaces by lactoferrin coating. Biointerphases 2014, 9, 029006. [Google Scholar] [CrossRef]
- Modesto, A.; Lima, K.C.; de Uzeda, M. Effects of three different infant dentifrices on biofilms and oral microorganisms. J. Clin. Pediatr. Dent. 2000, 24, 237–243. [Google Scholar] [PubMed]
- Pizzo, G.; Guiglia, R.; La Cara, M.; Giuliana, G.; D’Angelo, M. The effects of an amine fluoride/stannous fluoride and an antimicrobial host protein mouthrinse on supragingival plaque regrowth. J. Periodontol. 2004, 75, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Guardia-López, I.; González-Moles, M.A. Evaluation of the clinical efficacy of a mouthwash and oral gel containing the antimicrobial proteins lactoperoxidase, lysozyme and lactoferrin in elderly patients with dry mouth—A pilot study. Gerodontology 2008, 25, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kirstilä, V.; Lenander-Lumikari, M.; Söderling, E.; Tenovuo, J. Effects of oral hygiene products containing lactoperoxidase, lysozyme, and lactoferrin on the composition of whole saliva and on subjective oral symptoms in patients with xerostomia. Acta Odontol. Scand. 1996, 54, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Dirix, P.; Nuyts, S.; Poorten, V.V.; Delaere, P.; Bogaert, W.V.d. Efficacy of the BioXtra dry mouth care system in the treatment of radiotherapy-induced xerostomia. Support. Care Cancer 2007, 15, 1429–1436. [Google Scholar] [CrossRef]
- Silva, M.P.; Chibebe Junior, J.; Jorjão, A.L.; Machado, A.K.; Oliveira, L.D.; Junqueira, J.C.; Jorge, A.O. Influence of artificial saliva in biofilm formation of Candida albicans in vitro. Braz. Oral Res. 2012, 26, 24–28. [Google Scholar] [CrossRef]
- Jager, D.H.; Vissink, A.; Timmer, C.J.; Bronkhorst, E.; Vieira, A.M.; Huysmans, M.C. Reduction of erosion by protein-containing toothpastes. Caries Res. 2013, 47, 135–140. [Google Scholar] [CrossRef]
- Fejerskov, O.; Nyvad, B.; Kidd, E. Dental Caries: The Disease and Its Clinical Management, 3rd ed.; Wiley Blackwell: Oxford, UK, 2015. [Google Scholar]
- Limeback, H. Comprehensive Preventive Dentistry; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Nayak, P.A.; Nayak, U.A.; Khandelwal, V. The effect of xylitol on dental caries and oral flora. Clin. Cosmet. Investig. Dent. 2014, 10, 89–94. [Google Scholar] [CrossRef] [PubMed]
- ALHumaid, J.; Bamashmous, M. Meta-analysis on the Effectiveness of Xylitol in Caries Prevention. J. Int. Soc. Prev. Community Dent. 2022, 12, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Stefanidou, M.; Maravelias, C.; Dona, A.; Spiliopoulou, C. Zinc: A multipurpose trace element. Arch. Toxicol. 2006, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Takino, Y.; Okura, F.; Kitazawa, M.; Iwasaki, K.; Tagami, H. Zinc l-pyrrolidone carboxylate inhibits the UVA-induced production of matrix metalloproteinase-1 by in vitro cultured skin fibroblasts, whereas it enhances their collagen synthesis. Int. J. Cosmet. Sci. 2012, 34, 23–28. [Google Scholar] [CrossRef]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent development of active ingredients in mouthwashes and toothpastes for periodontal diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef]
- Fatima, T.; Haji Abdul Rahim, Z.B.; Lin, C.W.; Qamar, Z. Zinc: A precious trace element for oral health care? J. Pak. Med. Assoc. 2016, 66, 1019–1023. [Google Scholar]
- Brading, M.G.; Marsh, P.D. The oral environment: The challenge for antimicrobials in oral care products. Int. Dent. J. 2003, 53, 353–362. [Google Scholar] [CrossRef]
- Enax, J.; Epple, M. Synthetic hydroxyapatite as a biomimetic oral care agent. Oral Health Prev. Dent. 2018, 16, 7–19. [Google Scholar]
- Lelli, M.; Marchetti, M.; Foltran, I.; Roveri, N.; Putignano, A.; Procaccini, M.; Orsini, G.; Mangani, F. Remineralization and repair of enamel surface by biomimetic Zn-carbonate hydroxyapatite containing toothpaste: A comparative in vivo study. Front. Physiol. 2014, 5, 333. [Google Scholar] [CrossRef]
- Lynch, R.J. Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int. Dent. J. 2011, 61 (Suppl. S3), 46–54. [Google Scholar] [CrossRef]
- Marsh, P.D. Controlling the oral biofilm with antimicrobials. J. Dent. 2010, 38 (Suppl. S1), S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Alan Andersen, F. Final report of the safety assessment of allantoin and its related complexes. Int. J. Toxicol. 2010, 29, 84s–97s. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Abe-yutori, M.; Chikazawa, T.; Shibasaki, K. Effects of allantoin on periodontal tissue cells. In Proceedings of the 2016 IADR/APR General Session (Seoul, Korea), Seoul, Republic of Korea, 21–25 June 2016. [Google Scholar]
- Magaz, V.R.; Llovera, B.F.; Martí, M.; Garre, A. Clinical Impact and Cosmetic Acceptability of Chlorhexidine-enriched Toothpaste and Mouthwash Application on Periodontal Disease: A Randomized Clinical Study. J. Contemp. Dent. Pract. 2018, 19, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Madrazo-Jiménez, M.; Rodríguez-Caballero, Á.; Serrera-Figallo, M.; Garrido-Serrano, R.; Gutiérrez-Corrales, A.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. The effects of a topical gel containing chitosan, 0,2% chlorhexidine, allantoin and despanthenol on the wound healing process subsequent to impacted lower third molar extraction. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e696–e702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sáez-Alcaide, L.M.; Molinero-Mourelle, P.; González-Serrano, J.; Rubio-Alonso, L.; Bornstein, M.M.; López-Quiles, J. Efficacy of a topical gel containing chitosan, chlorhexidine, allantoin and dexpanthenol for pain and inflammation control after third molar surgery: A randomized and placebo-controlled clinical trial. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e644–e651. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, J.; Jan-Pallí, E.; lez-Navarro, B.G.; Jané-Salas, E.; Estrugo-Devesa, A.; Milani, M. Efficacy of chlorhexidine, dexpanthenol, allantoin and chitosan gel in comparison with bicarbonate oral rinse in controlling post-interventional inflammation, pain and cicatrization in subjects undergoing dental surgery. Curr. Med. Res. Opin. 2015, 31, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Kőhidai, Z.; Takács, A.; Lajkó, E.; Géczi, Z.; Pállinger, É.; Láng, O.; Kőhidai, L. The effects of mouthwashes in human gingiva epithelial progenitor (HGEPp) cells. Clin. Oral Investig. 2022, 26, 4559–4574. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. A review of the application and pharmacological properties of a-bisabolol and a-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Amora-Silva, B.F.; Ribeiro, S.C.; Vieira, C.L.; Mendes, F.R.; Vieira-Neto, A.E.; Abdon, A.P.V.; Costa, F.N.; Campos, A.R. Clinical efficacy of new α-bisabolol mouthwashes in postoperative complications of maxillofacial surgeries: A randomized, controlled, triple-blind clinical trial. Clin. Oral Investig. 2019, 23, 577–584. [Google Scholar] [CrossRef]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Pharmacological and biological effects of alpha-bisabolol: An updated review of the molecular mechanisms. Life Sci. 2022, 304, 120728. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health benefits, pharmacological effects, molecular mechanisms, and therapeutic potential of α-bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef] [PubMed]
- Carl, W.; Emrich, L.S. Management of oral mucositis during local radiation and systemic chemotherapy: A study of 98 patients. J. Prosthet. Dent. 1991, 66, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Forrer, M.; Kulik, E.M.; Filippi, A.; Waltimo, T. The antimicrobial activity of alpha-bisabolol and tea tree oil against Solobacterium moorei, a Gram-positive bacterium associated with halitosis. Arch. Oral Biol. 2013, 58, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Neo, B.H.; Betts, R.J. Glycosaminoglycans: Sweet as sugar targets for topical skin anti-aging. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1227–1246. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Low, M.A.; Stern, R. Hyaluronan (hyaluronic acid) and its regulation in human saliva by hyaluronidase and its inhibitors. J. Oral Sci. 2003, 45, 85–91. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.V.; Acharya, A.B.; Bhadbhade, S.; Thakur, S.L. Hyaluronan-containing mouthwash as an adjunctive plaque-control agent. Oral Health Prev. Dent. 2010, 8, 389–394. [Google Scholar] [PubMed]
- Gizligoz, B.; Ince Kuka, G.; Tunar, O.L.; Ozkan Karaca, E.; Gursoy, H.; Kuru, B. Plaque inhibitory effect of hyaluronan-containing mouthwash in a 4-day non-brushing model. Oral Health Prev. Dent. 2020, 18, 61–70. [Google Scholar] [PubMed]
- Trombelli, L.; Simonelli, A.; Pramstraller, M.; Guarnelli, M.E.; Fabbri, C.; Maietti, E.; Farina, R. Clinical efficacy of a chlorhexidine-based mouthrinse containing hyaluronic acid and an antidiscoloration system in patients undergoing flap surgery: A triple-blind, parallel-arm, randomized controlled trial. Int. J. Dent. Hyg. 2018, 16, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, A.; Barone, A.; Toti, P.; Covani, U. The efficacy of 0.12% chlorhexidine versus 0.12% chlorhexidine plus hyaluronic acid mouthwash on healing of submerged single implant insertion areas: A short-term randomized controlled clinical trial. Int. J. Dent. Hyg. 2017, 15, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tüzüner, T.; Ulusoy, A.T.; Baygin, O.; Yahyaoglu, G.; Yalcin, I.; Buruk, K.; Nicholson, J. Direct and transdentinal (indirect) antibacterial activity of commercially available dental gel formulations against Streptococcus mutans. Med. Princ. Pract. 2013, 22, 397–401. [Google Scholar] [CrossRef]
- Talib, H.J.; Mousa, H.A.; Mahmood, A.A. Assessment of the plaque-induced gingivitis patient with and without hyaluronic acid and xylitol toothpaste. J. Int. Soc. Prev. Community Dent. 2021, 11, 138–143. [Google Scholar] [PubMed]
- Aydinyurt, H.S.; Akbal, D.; Altindal, D.; Bozoglan, A.; Ertugrul, A.S.; Demir, H. Evaluation of biochemical and clinical effects of hyaluronic acid on non-surgical periodontal treatment: A randomized controlled trial. Ir. J. Med. Sci. 2020, 189, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Boccalari, E.; Tadakamadla, S.K.; Occhipinti, C.; Lanteri, V.; Maspero, C. Evaluation of the effectiveness of a novel mouth rinse containing hyaluronic acid and hydrogen peroxide on gingivitis: A randomized pilot controlled trial. Clin. Exp. Dent. Res. 2022, 8, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, A.A.; Al Marah, Z.A.; Abdulbaqi, H.R.; Alshaeli, A.J.; Milward, M.R. A randomized double-blind clinical trial to evaluate the efficacy of chlorhexidine, antioxidant, and hyaluronic acid mouthwashes in the management of biofilm-induced gingivitis. Int. J. Dent. Hyg. 2020, 18, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Tadakamadla, S.K.; Bharathwaj, V.V.; Duraiswamy, P.; Sforza, C.; Tartaglia, G.M. Clinical efficacy of a new cetylpyridinium chloride-hyaluronic acid-based mouthrinse compared to chlorhexidine and placebo mouthrinses-A 21-day randomized clinical trial. Int. J. Dent. Hyg. 2020, 18, 116–123. [Google Scholar] [CrossRef]
- Muñoz-Cámara, D.; Pardo-Zamora, G.; Camacho-Alonso, F. Postoperative effects of intra-alveolar application of 0.2% chlorhexidine or 1% hyaluronic acid bioadhesive gels after mandibular third molar extraction: A double-blind randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 617–625. [Google Scholar] [CrossRef]
- Yang, H.; Kim, J.; Kim, J.; Kim, D.; Kim, H.J. Non-inferiority study of the efficacy of two hyaluronic acid products in post-extraction sockets of impacted third molars. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 40. [Google Scholar] [CrossRef]
- Rodríguez Zorrilla, S.; Blanco Carrión, A.; García García, A.; Galindo Moreno, P.; Marichalar Mendía, X.; Seoane Prado, R.; Pérez Estévez, A.J.; Pérez-Sayáns, M. Effect of antiseptic gels in the microbiologic colonization of the suture threads after oral surgery. Sci. Rep. 2020, 10, 8360. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Pourpasha, M.; Amanlou, M.; Moosavi, M.S. Mouthwash containing vitamin E, triamcinolon, and hyaluronic acid compared to triamcinolone mouthwash alone in patients with radiotherapy-induced oral mucositis: Randomized clinical trial. Front. Oncol. 2021, 11, 614877. [Google Scholar] [CrossRef]
- Bardellini, E.; Amadori, F.; Schumacher, R.F.; D’Ippolito, C.; Porta, F.; Majorana, A. Efficacy of a solution composed by verbascoside, polyvinylpyrrolidone (PVP) and sodium hyaluronate in the treatment of chemotherapy-induced oral mucositis in children with acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2016, 38, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Vokurka, S.; Skardova, J.; Hruskova, R.; Kabatova-Maxova, K.; Svoboda, T.; Bystricka, E.; Steinerova, K.; Koza, V. The effect of polyvinylpyrrolidone-sodium hyaluronate gel (Gelclair) on oral microbial colonization and pain control compared with other rinsing solutions in patients with oral mucositis after allogeneic stem cells transplantation. Med. Sci. Monit. 2011, 17, Cr572–Cr576. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, T.; Pol, R.; Camisassa, D.; Arata, V.; Martino, I.; Giaccone, L.; Carossa, S. Use of sodium hyaluronate and synthetic amino acid precursors of collagen for the symptomatic treatment of mucositis in patients undergoing haematopoietic stem cell transplants. J. Biol. Regul. Homeost. Agents 2016, 30, 889–894. [Google Scholar] [PubMed]
- Polizzi, A.; Santonocito, S.; Lo Giudice, A.; Alibrandi, A.; De Pasquale, R.; Isola, G. Analysis of the response to two pharmacological protocols in patients with oral lichen planus: A randomized clinical trial. Oral Dis. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Polizzi, A.; De Pasquale, R.; Ronsivalle, V.; Lo Giudice, A.; Isola, G. Analysis of the efficacy of two treatment protocols for patients with symptomatic oral lichen planus: A randomized clinical trial. Int. J. Environ. Res. Public Health 2020, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Nolan, A.; Badminton, J.; Maguire, J.; Seymour, R.A. The efficacy of topical hyaluronic acid in the management of oral lichen planus. J. Oral Pathol. Med. 2009, 38, 299–303. [Google Scholar] [CrossRef]
- Dalessandri, D.; Zotti, F.; Laffranchi, L.; Migliorati, M.; Isola, G.; Bonetti, S.; Visconti, L. Treatment of recurrent aphthous stomatitis (RAS; aphthae; canker sores) with a barrier forming mouth rinse or topical gel formulation containing hyaluronic acid: A retrospective clinical study. BMC Oral Health 2019, 19, 153. [Google Scholar] [CrossRef]
- De Araújo Nobre, M.; Cintra, N.; Maló, P. Peri-implant maintenance of immediate function implants: A pilot study comparing hyaluronic acid and chlorhexidine. Int. J. Dent. Hyg. 2007, 5, 87–94. [Google Scholar] [CrossRef]
- Park, M.S.; Chang, J.Y.; Kang, J.H.; Park, K.P.; Kho, H.S. Rheological properties of hyaluronic acid and its effects on salivary enzymes and candida. Oral Dis. 2010, 16, 382–387. [Google Scholar] [CrossRef]
- López-Jornet, P.; Camacho-Alonso, F.; Martinez-Canovas, A. Clinical evaluation of polyvinylpyrrolidone sodium hyaluronate gel and 0.2% chlorhexidine gel for pain after oral mucosa biopsy: A preliminary study. J. Oral Maxillofac. Surg. 2010, 68, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
- Palaia, G.; Tenore, G.; Tribolati, L.; Russo, C.; Gaimari, G.; Del Vecchio, A.; Romeo, U. Evaluation of wound healing and postoperative pain after oral mucosa laser biopsy with the aid of compound with chlorhexidine and sodium hyaluronate: A randomized double blind clinical trial. Clin. Oral Investig. 2019, 23, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Bural, C.; Güven, M.; Kayacıoğlu, B.; Ak, G.; Bayraktar, G.; Bilhan, H. Effect of over-the-counter topical agents on denture-induced traumatic lesions: A clinical study. Int. J. Prosthodont. 2018, 31, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhong, X.; Zhang, Y.; Bao, B.; Liu, L.; Bao, H.; Bao, C.; Cheng, X.; Zhu, L.; Lin, Q. Hyaluronic acid-based antibacterial hydrogels constructed by a hybrid crosslinking strategy for pacemaker pocket infection prevention. Carbohydr. Polym. 2020, 245, 116525. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Ndokaj, A.; Bietolini, S.; Nisii, V.; Duś-Ilnicka, I.; Ottolenghi, L. Green dentistry: Organic toothpaste formulations. A literature review. Dent. Med. Probl. 2022, 59, 461–474. [Google Scholar] [CrossRef]
- Cieplik, F.; Kara, E.; Muehler, D.; Enax, J.; Hiller, K.-A.; Maisch, T.; Buchalla, W. Antimicrobial efficacy of alternative compounds for use in oral care towards biofilms from caries-associated bacteria in vitro. MicrobiologyOpen 2018, 8, e00695. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Farah, R.; Liu, J.A.; Phillips, T.S.; Perozo, B.I.; Kataoka, Y.; Meyer, F.; Enax, J. Remineralization of molar incisor hypomineralization (MIH) with a hydroxyapatite toothpaste: An in-situ study. BDJ Open 2022, 8, 33. [Google Scholar] [CrossRef]
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Lima, P.P.d.C.S.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 18. [Google Scholar] [CrossRef]
- Amaechi, B.T.; AbdulAzees, P.A.; Okoye, L.O.; Meyer, F.; Enax, J. Comparison of hydroxyapatite and fluoride oral care gels for remineralization of initial caries: A pH-cycling study. BDJ Open 2020, 6, 9. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Phillips, T.S.; Evans, V.; Ugwokaegbe, C.P.; Luong, M.N.; Okoye, L.O.; Meyer, F.; Enax, J. The potential of hydroxyapatite toothpaste to prevent root caries: A pH-cycling study. Clin. Cosmet. Investig. Dent. 2021, 13, 315–324. [Google Scholar] [CrossRef]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef]
- Cieplik, F.; Rupp, C.M.; Hirsch, S.; Muehler, D.; Enax, J.; Meyer, F.; Hiller, K.-A.; Buchalla, W. Ca2+ release and buffering effects of synthetic hydroxyapatite following bacterial acid challenge. BMC Oral Health 2020, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G. Chlorhexidine: Is it still the gold standard? Periodontology 2000 1997, 15, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Frese, C.; Wohlrab, T.; Sheng, L.; Kieser, M.; Krisam, J.; Wolff, D. Clinical effect of stannous fluoride and amine fluoride containing oral hygiene products: A 4-year randomized controlled pilot study. Sci. Rep. 2019, 9, 7681. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, M.; Itthagarun, A.; King, N.M. Ingestion of fluoride from dentifrices by young children and fluorosis of the teeth—A literature review. J. Clin. Pediatr. Dent. 2011, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance toward chlorhexidine in oral bacteria—Is there cause for concern? Front Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.H.; Mahounak, F.S.; Asgari, N.; Moradi, Z. Cytotoxicity of the ingredients of commonly used toothpastes and nouthwashes on human gingival fibroblasts. Front. Dent. 2019, 16, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, N.; Fulgione, A.; Iannaccone, M.; Tomasetta, L.; Ianniello, F.; Martora, F.; Lelli, M.; Roveri, N.; Capuano, F.; Capparelli, R. Biological activity of lactoferrin-functionalized biomimetic hydroxyapatite nanocrystals. Int. J. Nanomed. 2014, 9, 1175–1184. [Google Scholar]
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowska, K.; Olszewska, A.; Schlagenhauf, U.; et al. Impact of a toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: A 1-year randomized clinical trial. Sci. Rep. 2021, 11, 2650. [Google Scholar] [CrossRef]
- Hannig, C.; Basche, S.; Burghardt, T.; Al-Ahmad, A.; Hannig, M. Influence of a mouthwash containing hydroxyapatite microclusters on bacterial adherence in situ. Clin. Oral Investig. 2013, 17, 805–814. [Google Scholar] [CrossRef]
- Brauner, E.; Di Cosola, M.; Ambrosino, M.; Cazzolla, A.P.; Dioguardi, M.; Nocini, R.; Topi, S.; Mancini, A.; Maggiore, M.E.; Scacco, S.; et al. Efficacy of bio-activated anti-calculus toothpaste on oral health: A single-blind, parallel-group clinical study. Minerva Dent. Oral Sci. 2021, 71, 31–38. [Google Scholar]
- Fabritius-Vilpoux, K.; Enax, J.; Mayweg, D.; Meyer, F.; Herbig, M.; Raabe, D.; Fabritius, H.-O. Ultrastructural changes of bovine tooth surfaces under erosion in presence of biomimetic hydroxyapatite. Bioinspired Biomim. Nanobiomater. 2021, 10, 132–145. [Google Scholar] [CrossRef]
| Ingredient | Total Number of Results | Number of Included Studies | Excluded Studies |
|---|---|---|---|
| lactoferrin | 34 | 16 | Not in the scope of this review: 13 Review articles: 3 Articles in other languages than English: 1 Case reports: 1 Animal studies: 0 |
| xylitol | 244 | Due to the high number of studies, evaluation will be referred to other reviews | |
| zinc | 2026 | Due to the high number of studies, evaluation will be referred to other reviews | |
| allantoin | 13 | 5 | Not in the scope of this review: 3 Review articles: 0 Articles in other languages than English: 4 Case reports: 0 Animal studies: 1 |
| bisabolol | 10 | 3 | Not in the scope of this review: 6 Review articles: 0 Articles in other languages than English: 1 Case reports: 0 Animal studies: 0 |
| hyaluronic acid/hyaluronate | 52 | 27 | Not in the scope of this review: 15 Review articles: 6 Articles in other languages than English: 3 Case reports: 0 Animal studies: 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enax, J.; Amaechi, B.T.; Schulze zur Wiesche, E.; Meyer, F. Overview on Adjunct Ingredients Used in Hydroxyapatite-Based Oral Care Products. Biomimetics 2022, 7, 250. https://doi.org/10.3390/biomimetics7040250
Enax J, Amaechi BT, Schulze zur Wiesche E, Meyer F. Overview on Adjunct Ingredients Used in Hydroxyapatite-Based Oral Care Products. Biomimetics. 2022; 7(4):250. https://doi.org/10.3390/biomimetics7040250
Chicago/Turabian StyleEnax, Joachim, Bennett T. Amaechi, Erik Schulze zur Wiesche, and Frederic Meyer. 2022. "Overview on Adjunct Ingredients Used in Hydroxyapatite-Based Oral Care Products" Biomimetics 7, no. 4: 250. https://doi.org/10.3390/biomimetics7040250
APA StyleEnax, J., Amaechi, B. T., Schulze zur Wiesche, E., & Meyer, F. (2022). Overview on Adjunct Ingredients Used in Hydroxyapatite-Based Oral Care Products. Biomimetics, 7(4), 250. https://doi.org/10.3390/biomimetics7040250





