Strategies for Growing Large-Scale Mycelium Structures
Abstract
:1. Introduction
1.1. Mycelium Composite Materials
1.2. Brick and Block Myco-Structures
1.3. Monolithic and Bio-Welded Myco-Structures
1.3.1. Monolithic Myco-Structures
1.3.2. Bio-Welded Myco-Structures
1.4. Aims and Scope of This Research
2. Context, Design, and Methods
2.1. Parametric Design for Serpentine Walls
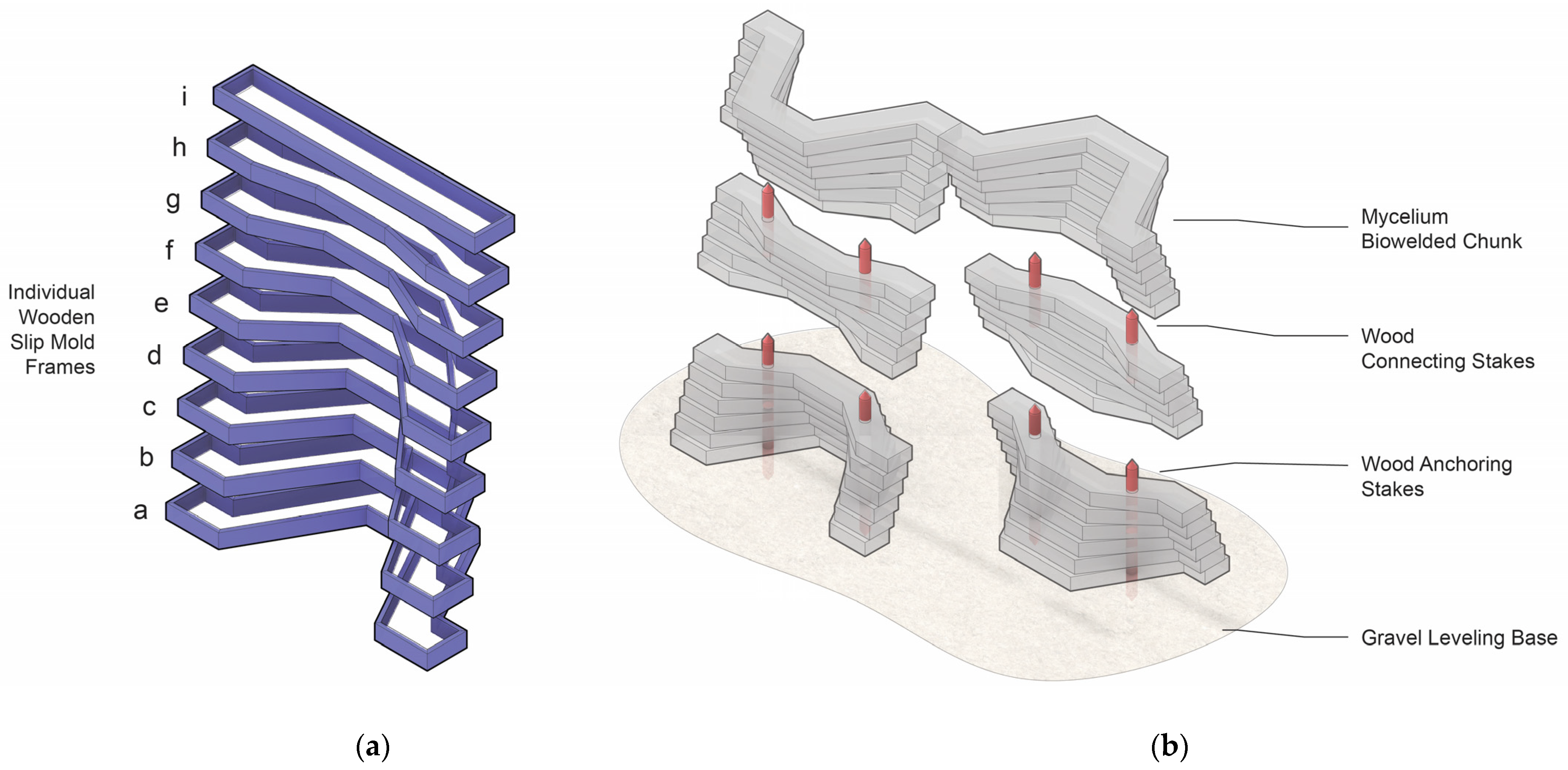
2.2. Summary of Myco-Fabrication Methods
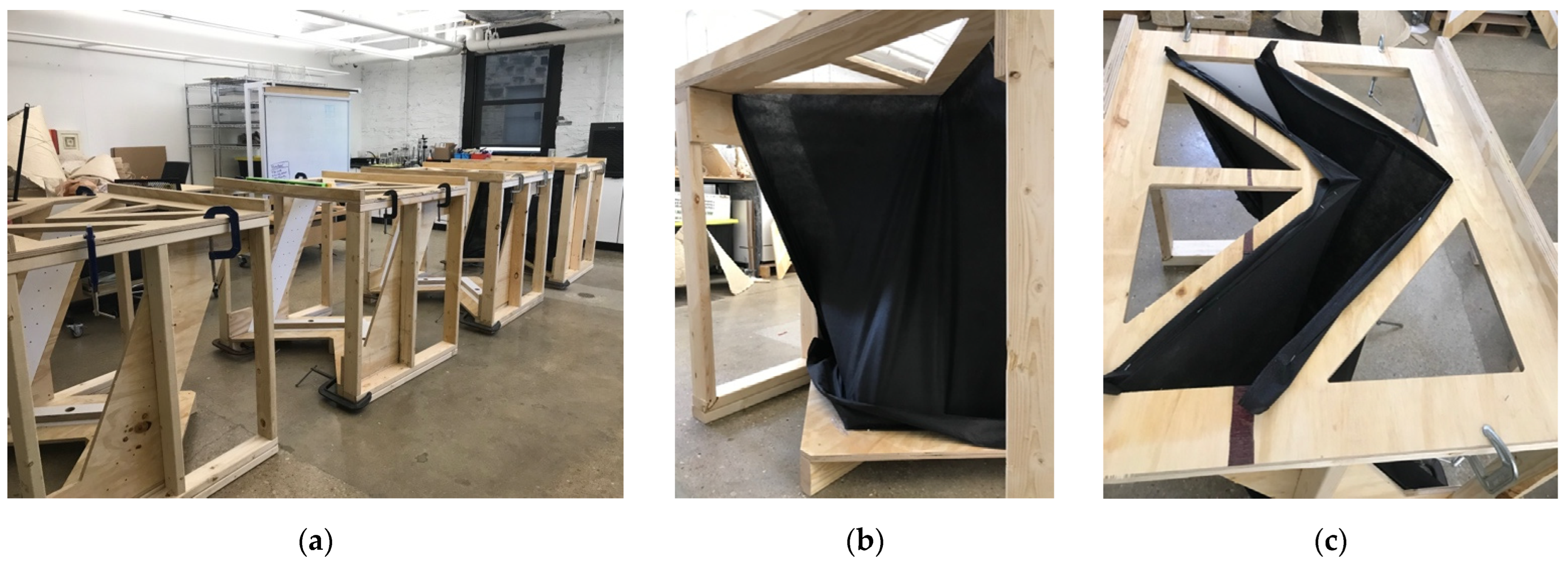
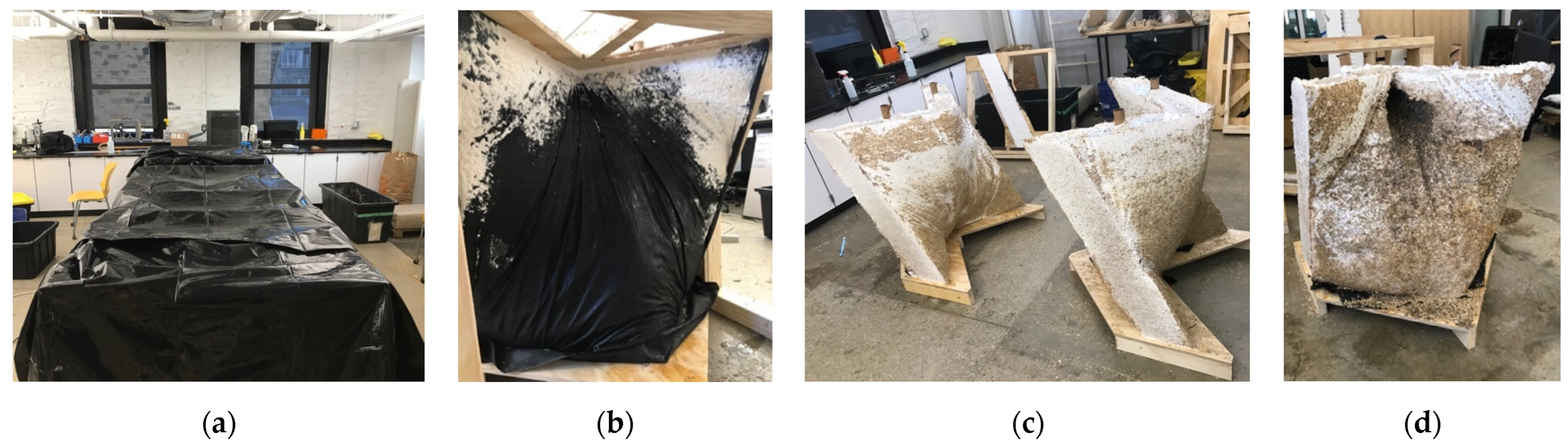
2.2.1. Myco-Welding Slabs into Monolithic Building Units
2.2.2. Fabric-Forming Monolithic Units
2.3. Materials
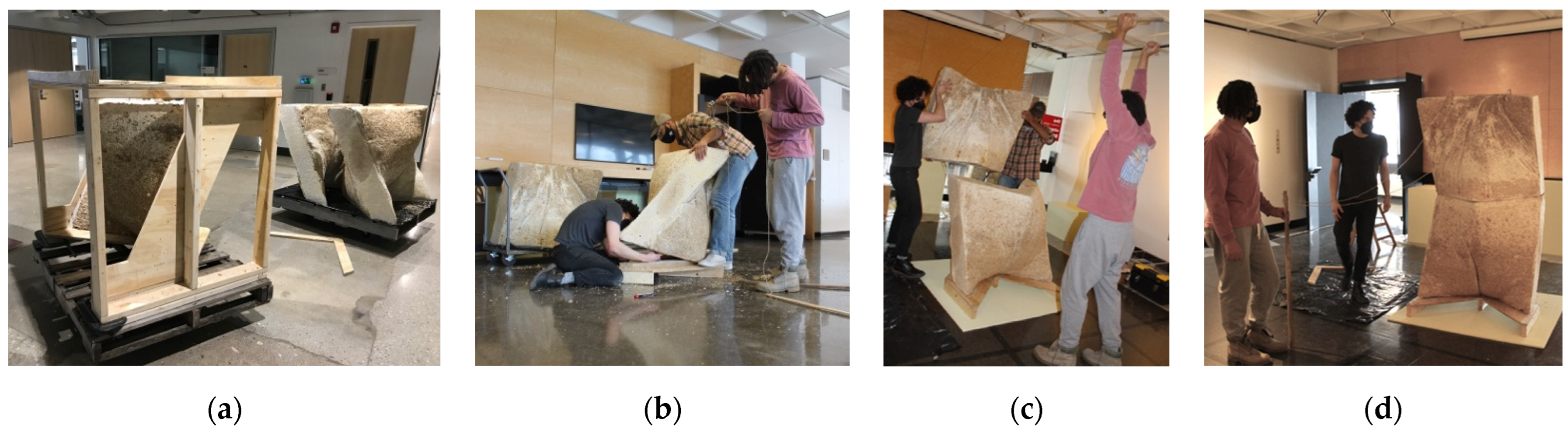
3. Results and Discussion: Two Serpentine Wall Prototypes
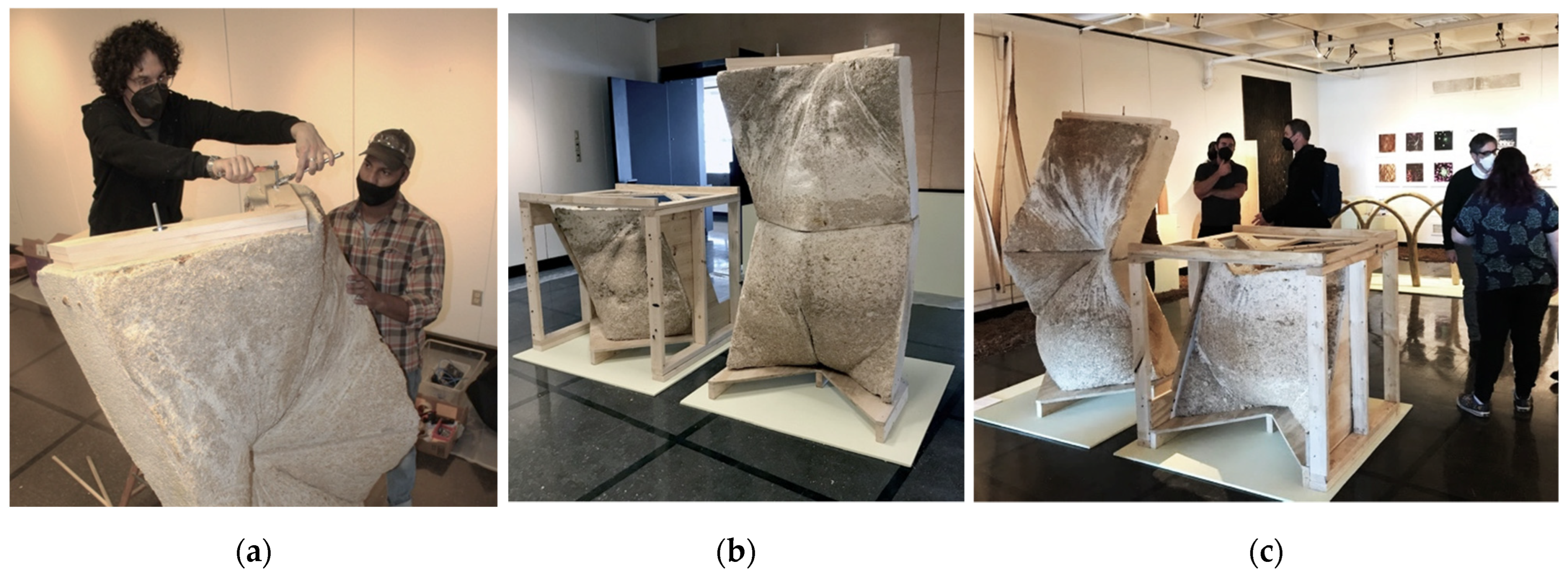
3.1. La Parete Fungina
3.2. L’Orso Fungino
3.3. Discussion and Future Work
4. Summary and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, G.; Xu, K.; Chang, X.; Chang, G. Factors influencing the service lifespan of buildings: An improved hedonic model. Habitat Int. 2014, 43, 274–282. [Google Scholar] [CrossRef]
- Wuyts, W.; Miatto, A.; Sedlitzky, R.; Tanikawa, H. Extending or ending the life of residential buildings in Japan: A social circular economy approach to the problem of short-lived constructions. J. Clean. Prod. 2019, 231, 660–670. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Sustainable Management of Construction and Demolition Waste. 2018. Available online: https://www.epa.gov/smm/sustainable-management-construction-and-demolition-materials (accessed on 15 June 2022).
- Trubiano, F.; Onbargi, C.; Finaldi, A.; Whitlock, Z. Fossil Fuels, The Building Industry, and Human Health; Kleinman Center for Energy Policy: Philadelphia, PA, USA, 2019; Available online: https://kleinmanenergy.upenn.edu/research/publications/fossil-fuels-the-building-industry-and-human-health-evaluating-toxicity-in-architectural-plastics/ (accessed on 21 August 2022).
- National Institute of Environmental Health Science. Endocrine Disruptors; National Institutes of Health: Washington, DC, USA, 2010. Available online: https://www.niehs.nih.gov/health/materials/endocrine_disruptors_508.pdf (accessed on 21 August 2022).
- Stichnothe, H.; Azapagic, A. Life cycle assessment of recycling PVC window frames. Resour. Conserv. Recycl. 2013, 71, 40–47. [Google Scholar] [CrossRef]
- Marten, B.; Hicks, A. Expanded Polystyrene Life Cycle Analysis Literature Review: An Analysis for Different Disposal Scenarios. Sustainability 2018, 11, 29–35. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Municipal Solid Waste Generation, Recycling, and Disposal in the United States. 2014. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/2012_msw_dat_tbls.pdf (accessed on 21 August 2022).
- Gray, A. Germany Recycles More Than Any Other Country. World Economic Forum. 2017. Available online: https://www.weforum.org/agenda/2017/12/germany-recycles-more-than-any-other-country/ (accessed on 21 August 2022).
- Geyer, R.; Jambeck, J.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, V. How the Pandemic Drove up The Cost of Wood Products. U.S. Department of Agriculture. 13 May 2022. Available online: https://www.fs.usda.gov/features/how-pandemic-drove-cost-wood-products (accessed on 21 August 2022).
- Stamets, P. Mycelium Running: How Mushrooms Can Help Save the World; 10 Speed Press: Berkeley, CA, USA, 2005. [Google Scholar]
- Ecovative Design. Available online: https://ecovativedesign.com/ (accessed on 15 June 2022).
- Mycoworks. Available online: https://www.mycoworks.com/ (accessed on 15 June 2022).
- Mogu.bio. Available online: https://mogu.bio/ (accessed on 15 June 2022).
- Holt, G.A.; McIntyre, G.; Flagg, D.; Bayer, E.; Wanjura, J.D.; Pelletier, M.G. Fungal Mycelium and Cotton Plant Materials in the Manufacture of Biodegradable Molded Packaging Material: Evaluation Study of Select Blends of Cotton Byproducts. J. Biobased Mater. Bioenergy 2012, 6, 431–439. [Google Scholar] [CrossRef]
- Mushroom Packaging. Available online: https://mushroompackaging.com/ (accessed on 15 June 2022).
- Magical Mushroom Company. Available online: https://magicalmushroom.com/ (accessed on 15 June 2022).
- Grown.Bio. Available online: https://www.grown.bio/shop/ (accessed on 15 June 2022).
- Forager. Available online: https://forager.bio/ (accessed on 15 June 2022).
- Bolt Threads. Available online: https://boltthreads.com/ (accessed on 15 June 2022).
- Gandia, A.; van den Brandhoff, J.G.; Appels, F.V.W.; Jones, M.P. Flexible Fungal Materials: Shaping the Future. Trends Biotechnol. 2021, 39, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.; McIntyre, G. Method for Producing Grown Materials and Products Made Thereby. U.S. Patent 9485917B2, 8 November 2016. Available online: https://patents.google.com/patent/US9485917B2/en?oq=US9485917 (accessed on 15 June 2022).
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, physical, and chemical characterization of mycelium-based composites with different types of lignocellulosic substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Mautner, A.; Luenco, S.; Bismark, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Girometta, C.; Picco, A.M.; Baiguera, R.M.; Dondi, D.; Babbini, S.; Cartabia, M.; Pellegrini, M.; Savino, E. Physico-Mechanical and Thermodynamic Properties of Mycelium-Based Bio-composites: A Review. Sustainability 2019, 11, 281. [Google Scholar] [CrossRef] [Green Version]
- Appels, F.V.W.; Camere, S.; Montaliti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A.B. Fabrication factors influencing mechanical, moisture- and water-related properties of mycelium-based composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Hsu, T.; Dessi-Olive, J. A design framework for absorption and diffusion panels with sustainable materials. In Proceedings of the 2021 Inter-Noise Conference, Washington, DC, USA, 1 August 2021. [Google Scholar]
- Jones, M.; Bhat, T.; Kandare, E.; Thomas, A.; Joseph, P.; Dekiwadia, C.; Yuen, R.; John, S.; Ma, J.; Wang, C. Thermal Degradation and Fire Properties of Fungal Mycelium and Mycelium—Biomass Composite Materials. Sci. Rep. 2018, 8, 17583. [Google Scholar] [CrossRef] [PubMed]
- Van Wylick, A.; Elsacker, E.; Yap, L.L.; Peeters, E.; de Laet, L. Mycelium Composites and their Biodegradability: An Exploration on the Disintegration of Mycelium-Based Materials in Soil. In Construction Technologies and Architecture, 4th ed.; International Conference on Bio-Based Building Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2022. [Google Scholar]
- Sydor, M.; Bonenberg, A.; Doczekalska, B.; Cofta, G. Mycelium-Based Composites in Art, Architecture, and Interior Design: A Review. Polymers 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Zeitoun, L. Shell Mycelium: Exploring Fungus Growth as a Possible Building Block. Designboom. 25 July 2017. Available online: https://www.designboom.com/architecture/shell-mycelium-degradation-movement-manifesto-07-25-2017/ (accessed on 15 June 2022).
- The Growing Pavilion. Available online: https://thegrowingpavilion.com/ (accessed on 15 June 2022).
- Rensselaer Polytechnic Institute Summer Architecture Studio, Mycelium Pavilion. 2019. Available online: https://www.arch.rpi.edu/2019/09/2019su-summerstudio/ (accessed on 15 June 2022).
- Mushroom Tiny House. Available online: https://mushroomtinyhouse.com/ (accessed on 15 June 2022).
- Mok, K. Mycotecture: Building with Mushrooms? Inventor Says Yes. Treehugger. 26 September 2012. Available online: https://www.treehugger.com/mycotecture-mushroom-bricks-philip-ross-4857225 (accessed on 15 June 2022).
- Saporta, S.; Yang, F.; Clark, M. Design and delivery of structural material innovations. Struct. Congr. 2015, 2015, 1253–1265. [Google Scholar]
- Heisel, F.; Lee, J.; Schlesier, K.; Rippmann, M.; Saeidi, N.; Javadian, A.; Nugroho, A.R.; Van Mele, T.; Block, P.; Hebel, D.E. Design, Cultivation and Application of Load-Bearing Mycelium Components: The MycoTree at the 2017 Seoul Biennale of Architecture and Urbanism. Int. J. Sustain. Energy Dev. (IJSED) 2018, 6, 296–303. [Google Scholar] [CrossRef]
- Soh, E.; Chew, Z.Y.; Saeidi, N.; Javadian, A.; Hebel, D.; Le Ferrand, H. Development of an extrudable paste to build mycelium-bound composites. Mater. Des. 2020, 195, 109058. [Google Scholar] [CrossRef]
- Goidea, A.; Floudas, D.; Andreen, D. Pulp Faction: 3d printed material assemblies through microbial biotransformation. In Fabricate 2020; UCL Press: London, UK, 2020. [Google Scholar]
- Blast Studio. Available online: https://www.blast-studio.com/ (accessed on 15 June 2022).
- Baricci, R.A. Structural Analysis and Form-Finding of Mycelium-Based Monolithic Domes. Master’s Thesis, University of Miami, Coral Gables, FL, USA, 2016. [Google Scholar]
- Pallister, J. 3D-Printed Mushroom Roots Could Be Used to Build Houses. Dezeen. 6 March 2014. Available online: https://www.dezeen.com/2014/03/06/movie-eric-klarenbeek-mushroom-roots-fungus-3d-printed-chair/ (accessed on 15 June 2022).
- Mycelium Chair by Ecovative at Biodesign: From Inspiration to Integration, an Exhibition Curated in Collaboration with William Myers at the Road Island School of Design (RISD). Available online: https://naturelab.risd.edu/events/biodesign-from-inspiration-to-integration/ (accessed on 15 June 2022).
- Piórecka, N. MYCOsella: Growing the Mycelium Chair. Bachelor’s Thesis in Architecture Dissertation, Newcastle University, Newcastle upon Tyne, UK, 2019. Available online: https://issuu.com/nataliapiorecka/docs/dissertation_project_ba_architectur (accessed on 15 June 2022).
- Kuta, S. Is Fungus the Answer to Climate Change? Student Who Grew a Mushroom Canoe Says Yes. Available online: https://www.nbcnews.com/news/us-news/fungus-answer-climate-change-student-who-grew-mushroom-canoe-says-n1185401 (accessed on 15 June 2022).
- Dessi-Olive, J. Monolithic Mycelium: Growing vault structures. In Proceedings of the International Conference on Non-Conventional Materials and Technologies (NOCMAT), Nairobi, Kenya, 24–26 July 2019; pp. 2–15. [Google Scholar]
- Adamatzky, A.; Ayres, P.; Belotti, G.; Wösten, H. Fungal Architecture. Int. J. Unconv. Comput. 2019, 14, 397–441. [Google Scholar]
- Dessi-Olive, J. Craft and structural innovation of mycelium-structures in architectural education. In Structures and Architecture A Viable Urban Perspective? 1st ed.; Hvejsel, M.F., Cruz, P.J.S., Eds.; CRC Press: London, UK, 2022. [Google Scholar] [CrossRef]
- Modanloo, B.; Ghazvinian, A.; Matini, M.; Andaroodi, E. Tilted Arch; Implementation of Additive Manufacturing and Bio-welding of Mycelium-Based Composites. Biomimetics 2021, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Dahmen, J. Soft Futures: Mushrooms and Regenerative Design. J. Archit. Educ. 2017, 71, 57–64. [Google Scholar] [CrossRef]
- Elsacker, E.; Søndergaard, A.; Van Wylick, A.; Peeters, E.; De Laet, L. Growing living and multifunctional mycelium composites for large-scale formwork applications using robotic abrasive wire cutting. Constr. Build. Mater. 2021, 283, 122732. [Google Scholar] [CrossRef]
- Saporta, S.; Clark, M. “Bio-Welding” of Mycelium-based Materials. In Proceedings of the 2019 IASS Symposium, Barcelona, Spain, 7–10 October 2019. [Google Scholar]
- Biomaterials Building Exposition, Curated by Katie MacDonald and Kyle Schumann. Available online: https://www.biomaterialbuilding.com/ (accessed on 15 June 2022).
- President’s Commision on Slavery and the University. University of Virginia. 2018. Available online: https://dei.virginia.edu/sites/g/files/jsddwu511/files/inline-files/PCSU%20Report%20FINAL_July%202018.pdf (accessed on 15 June 2022).
- Trimble, B.E. Design of unique landscape walls and their use in building facades. In Proceedings of the 12th Canadian Masonry Symposium, Vancouver, BC, Canada, 2–5 June 2013. [Google Scholar]
- Rhinoceros by McNeal. Available online: https://www.rhino3d.com/ (accessed on 15 June 2022).
- Mushroom Production in Pennsylvania. Available online: https://extension.psu.edu/forage-and-food-crops/mushrooms (accessed on 15 June 2022).











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dessi-Olive, J. Strategies for Growing Large-Scale Mycelium Structures. Biomimetics 2022, 7, 129. https://doi.org/10.3390/biomimetics7030129
Dessi-Olive J. Strategies for Growing Large-Scale Mycelium Structures. Biomimetics. 2022; 7(3):129. https://doi.org/10.3390/biomimetics7030129
Chicago/Turabian StyleDessi-Olive, Jonathan. 2022. "Strategies for Growing Large-Scale Mycelium Structures" Biomimetics 7, no. 3: 129. https://doi.org/10.3390/biomimetics7030129
APA StyleDessi-Olive, J. (2022). Strategies for Growing Large-Scale Mycelium Structures. Biomimetics, 7(3), 129. https://doi.org/10.3390/biomimetics7030129








