Growth and Mechanical Characterization of Mycelium-Based Composites towards Future Bioremediation and Food Production in the Material Manufacturing Cycle
Abstract
:1. Introduction
1.1. Problem Statement of Current Building Materials
1.2. Towards Living Materials
1.3. Mycelium-Based Materials
1.4. State of the Art of the Production and Mechanical Properties of Mycelium-Based Materials
1.5. Underutilized Benefits of Fungal Mycelium
1.6. Overall Goal of the Project
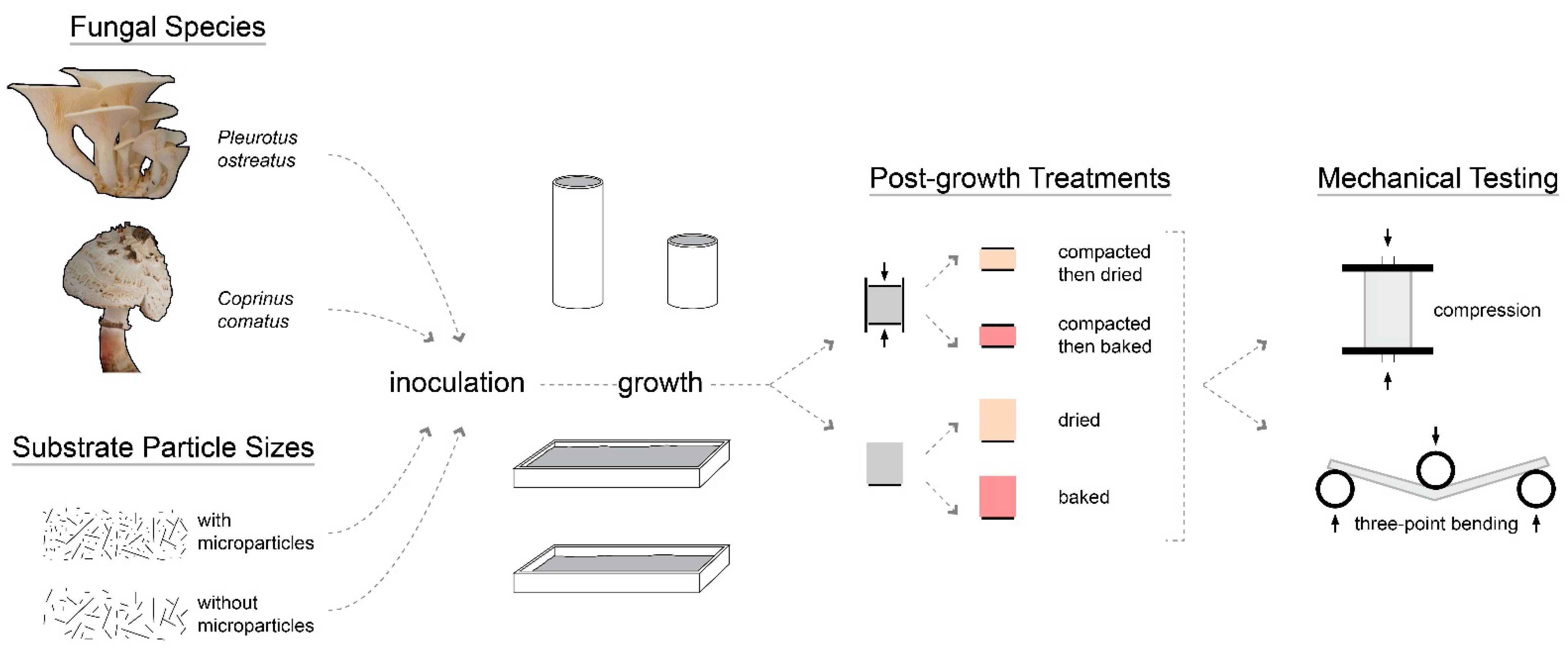
2. Materials and Methods
2.1. Substrate and Fungal Species
2.2. Substrate and Mold Preparation
2.2.1. Chipping
2.2.2. Sieving
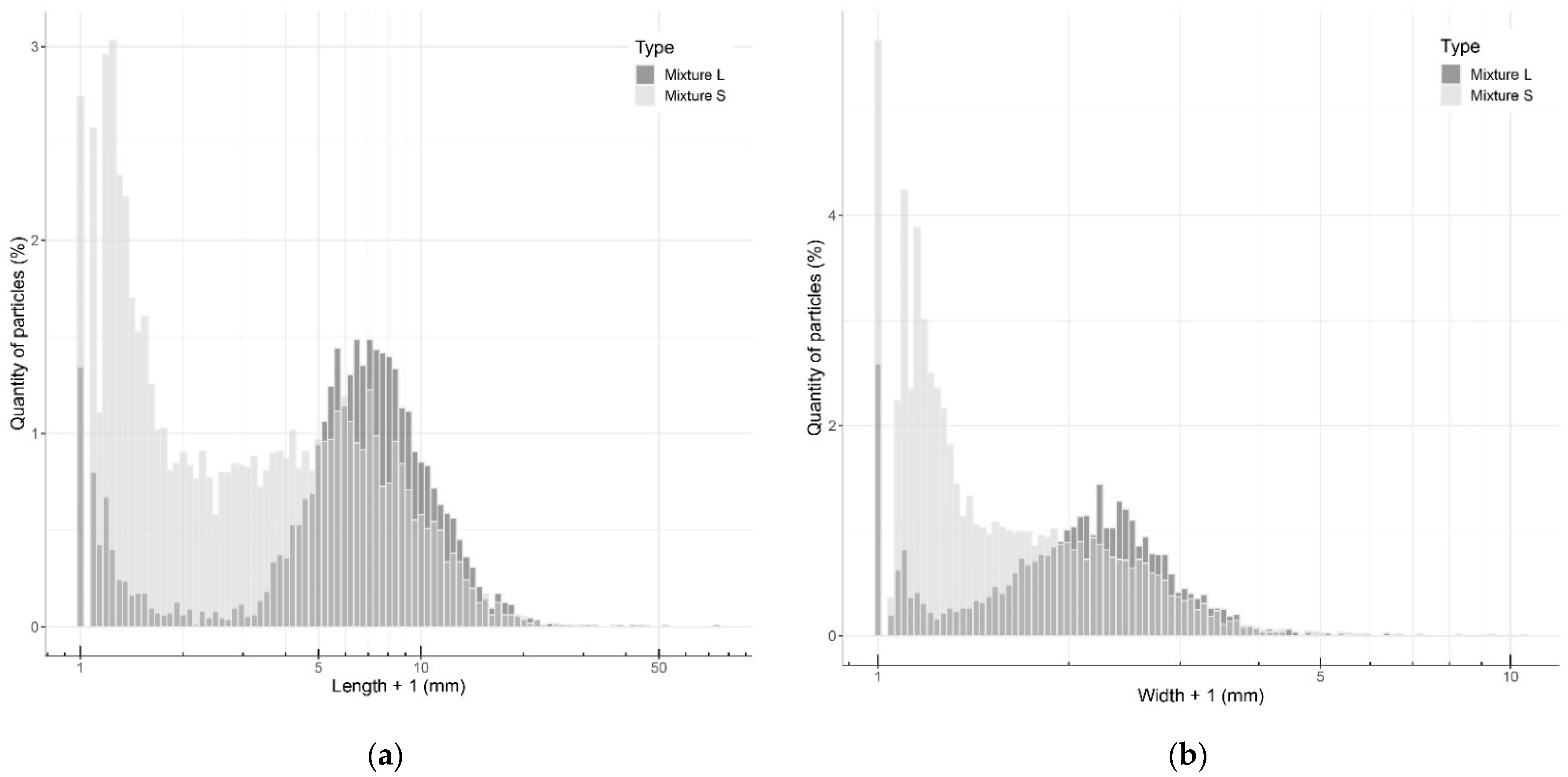
2.2.3. Quantifying Particle Sizes
2.2.4. Sterilization and Pasteurization
2.2.5. Mold Preparation
2.3. Inoculation
2.4. Mycelium Growth
2.5. Application of Post-Growth Treatments
2.6. Mechanical Testing Procedure
2.6.1. Compression Testing
2.6.2. Three-Point Bending Testing
3. Results
3.1. Quantifying Particle Sizes
3.2. Growth of Mycelium-Based Materials with Pleurotus ostreatus
3.2.1. Growth Assessment
3.2.2. Shrinkage and Weight Reduction of Cylindrical Specimens Grown for Compression Testing
3.2.3. Shrinkage and Weight Reduction of Panel Specimens Grown for Bending Testing
3.3. Growth of Mycelium-Based Materials with Coprinus comatus
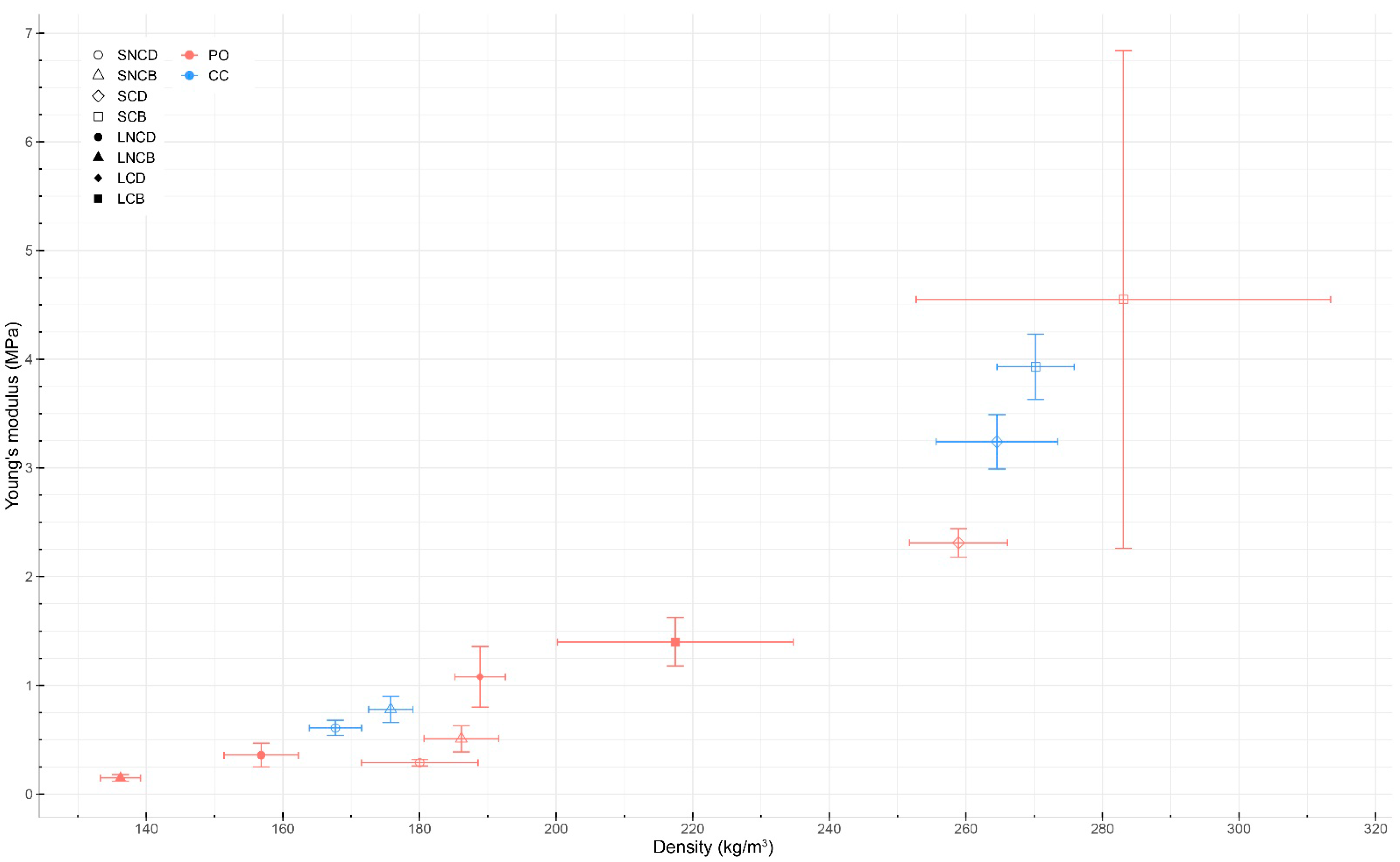
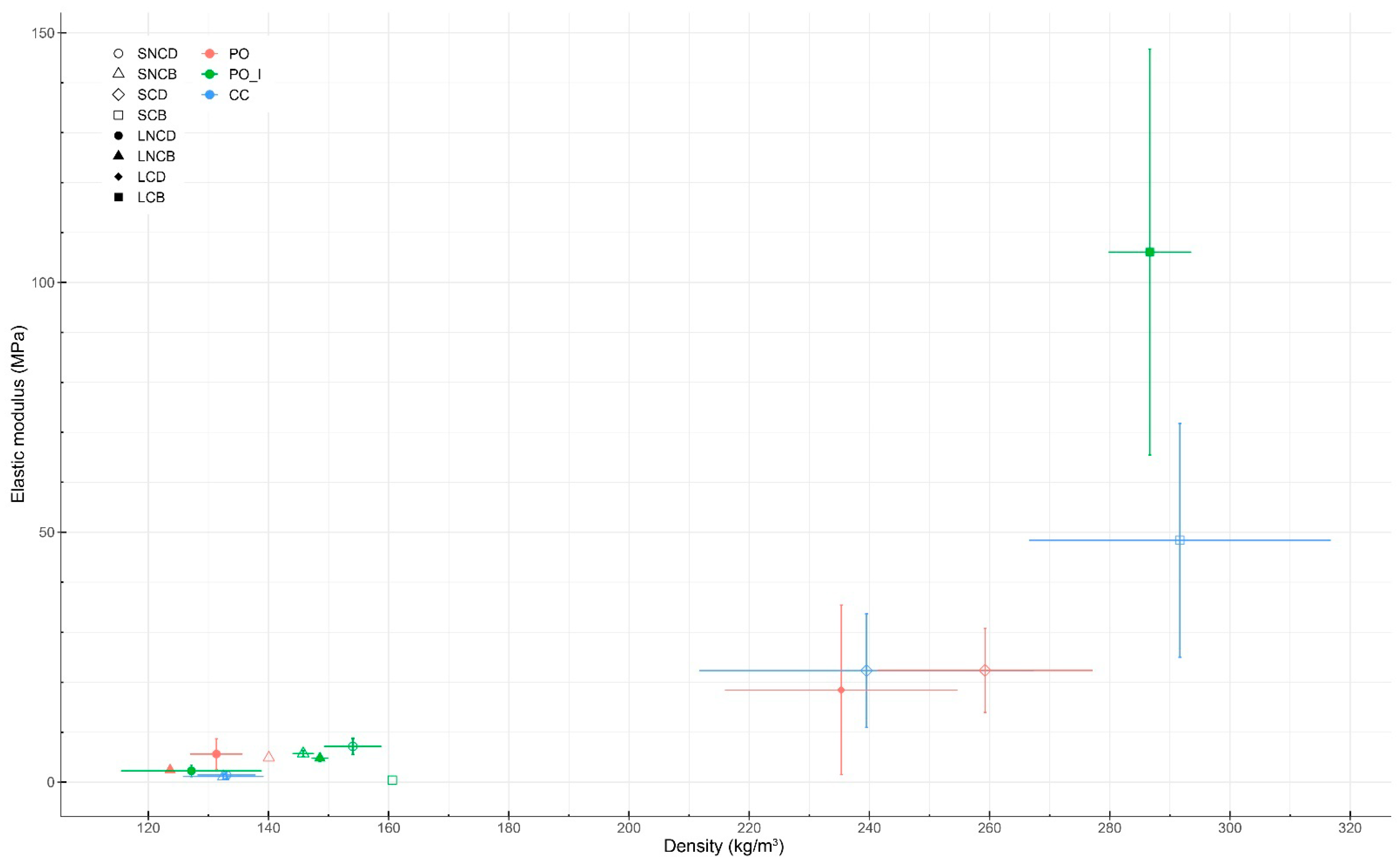
3.4. Mechanical Testing
3.4.1. Compression Testing
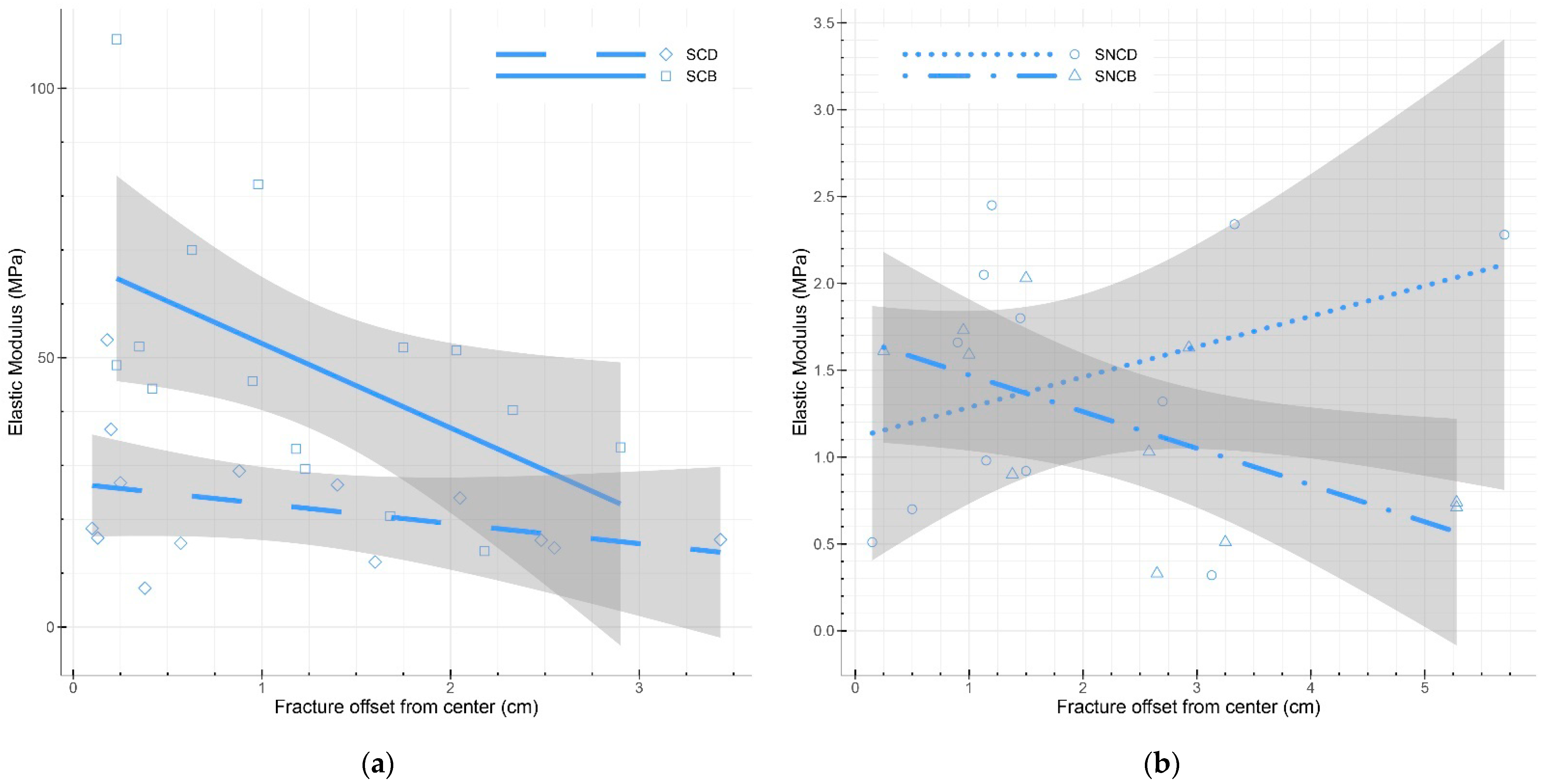
3.4.2. Bending Testing
4. Discussion
4.1. Mycelial Growth
4.1.1. Reducing Contamination and Promote Mycelial Growth
4.1.2. Food Production
4.1.3. Material Shrinkage
4.2. Compression Testing
4.3. Bending Testing
4.4. Emerging Synergies between Bioremediation, and the Production of Food, Materials, and Medicine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix C
Appendix D
Appendix E
Appendix F
Appendix G
Appendix H
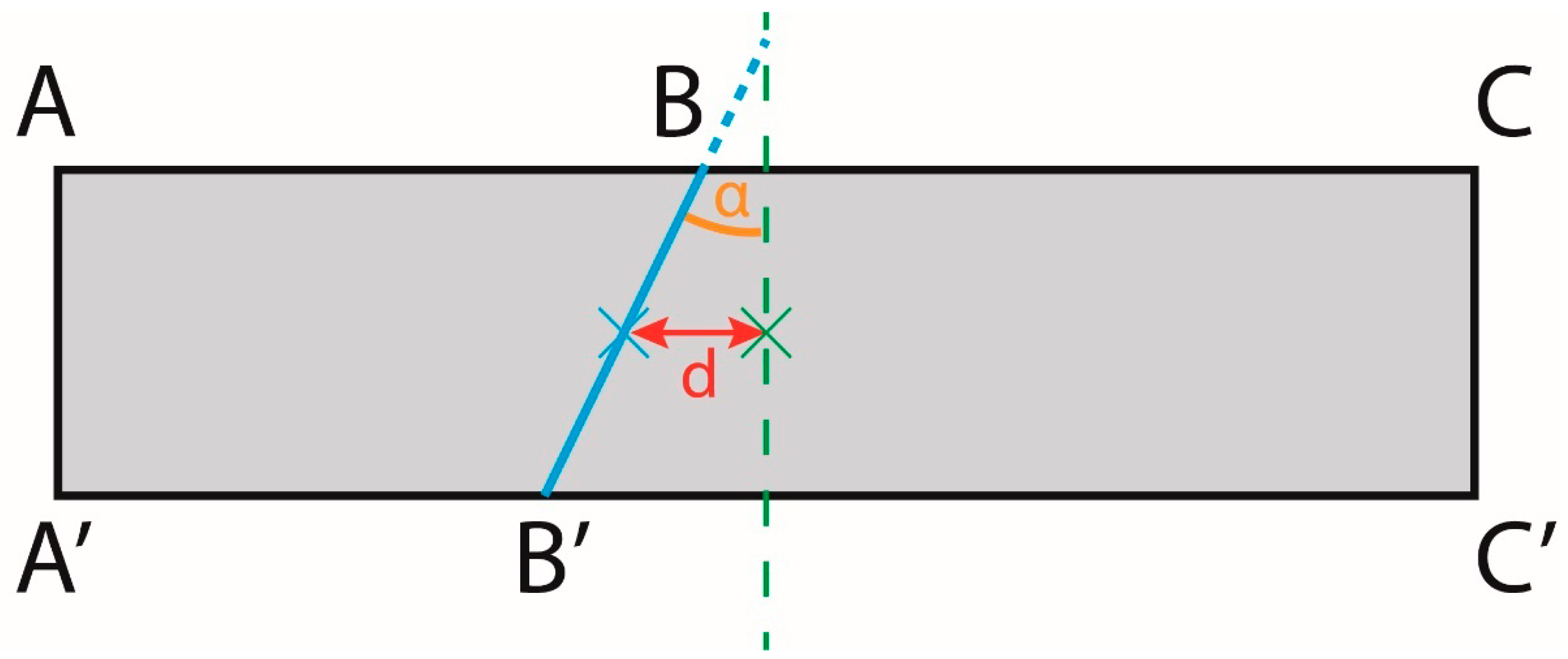
Appendix I
| Step of the Process | Weight Compared to Initial (%) | Height Compared to Initial (%) | Diameter Compared to Initial (%) | |
|---|---|---|---|---|
| All Samples | Uncompacted | Compacted | All Samples | |
| 1. Inoculation | 100.00 | 100.00 | 100.00 | |
| 2. Beginning of drying | 95.37 | 100.00 | 100.00 | |
| 3. Before cutting | 50.95 | 95.00 | / | |
| 4. Before post-growth treat. | 50.95 | 100.00 | 100.00 | 96.76 |
| 5. Before baking or drying | 51.90 | / | 50.14 | / |
| 6. Before mechanical test | 21.91 | 99.56 | 49.54 | 96.48 |
| Step of the Process | Weight Compared to Initial (%) | Height Compared to Initial (%) | Width Compared to Initial (%) | Length Compared to Initial (%) | ||||
|---|---|---|---|---|---|---|---|---|
| NC | C | NC | C | NC | C | NC | C | |
| Specimens with less than 10% contamination removed | ||||||||
| 1. Inoculation | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| 2. Beginning of drying | 78.82 | 85.21 | 111.90 | 99.93 | 97.65 | 98.40 | 98.50 | 99.13 |
| 3. Before post-growth treat. | 17.00 | 50.13 | 91.07 | 91.07 | 94.93 | / | 96.50 | / |
| 4. Before baking or drying | / | / | / | / | / | / | / | / |
| 5. Before cutting | / | 15.17 | 87.14 | 37.32 | / | 92.35 | / | 93.05 |
| All specimens | ||||||||
| 1. Inoculation | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| 2. Beginning of drying | 81.17 | 84.27 | 102.32 | 99.93 | 98.25 | 98.40 | 99.00 | 99.13 |
| 3. Before post-growth treat. | 37.65 | 50.13 | 91.96 | 91.07 | 94.93 | / | 96.50 | / |
| 4. Before baking or drying | / | / | / | / | / | / | / | / |
| 5. Before cutting | / | 15.17 | 90.71 | 37.32 | 95.07 | 92.35 | / | 93.05 |
References
- United Nations Environment Programme. 2021 Global Status Report for Buildings and Construction: Towards a Zero-Emission, Efficient and Resilient Buildings and Construction Sector; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- United States Environmental Protection Agency. Advancing Sustainable Materials Management: 2018 Fact Sheet; United States Environmental Protection Agency: Washington, DC, USA, 2020.
- Wadanambi, L.; Dubey, B.; Townsend, T. The Leaching of Lead from Lead-Based Paint in Landfill Environments. J. Hazard. Mater. 2008, 157, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q.; Courchesne, N.-M.D.; Duraj-Thatte, A.; Praveschotinunt, P.; Joshi, N.S. Engineered Living Materials: Prospects and Challenges for Using Biological Systems to Direct the Assembly of Smart Materials. Adv. Mater. 2018, 30, 1704847. [Google Scholar] [CrossRef]
- Houette, T.; Foresi, B.; Maurer, C.; Gruber, P. Growing Myceliated Facades-Manufacturing and Exposing Experimental Panels in a Facade Setting. In Proceedings of the Facade Tectonics World Congress 2020, 5–27 August 2020. [Google Scholar]
- Srubar, W.V. Engineered Living Materials: Taxonomies and Emerging Trends. Trends Biotechnol. 2020, 39, 574–583. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials From Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef]
- Yang, Z.J.; Zhang, F.; Still, B.; White, M.; Amstislavski, P. Physical and Mechanical Properties of Fungal Mycelium-Based Biofoam. J. Mater. Civ. Eng. 2017, 29. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, Physical and Chemical Characterisation of Mycelium-Based Composites with Different Types of Lignocellulosic Substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazvinian, A.; Farrokhsiar, P.; Vieira, F.; Pecchia, J.; Gursoy, B. Mycelium-Based Bio-Composites for Architecture: Assessing the Effects of Cultivation Factors on Compressive Strength. In Proceedings of the International Conference on Education and Research in Computer Aided Architectural Design in Europe, Porto, Portugal, 11–13 September 2019; Volume 2, pp. 505–514. [Google Scholar] [CrossRef]
- Vidholdová, Z.; Kormùthová, D.; Ždinský, J.I.; Lagaňa, R. Compressive Resistance of the Mycelium Composite. Ann. WULS For. Wood Technol. 2019, 107, 31–36. [Google Scholar] [CrossRef]
- Girometta, C.; Picco, A.M.; Baiguera, R.M.; Dondi, D.; Babbini, S.; Cartabia, M.; Pellegrini, M.; Savino, E. Physico-Mechanical and Thermodynamic Properties of Mycelium-Based Biocomposites: A Review. Sustainability 2019, 11, 281. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered Mycelium Composite Construction Materials from Fungal Biorefineries: A Critical Review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Lazaro Vasquez, E.S.; Vega, K. From Plastic to Biomaterials: Prototyping DIY Electronics with Mycelium. In Proceedings of the UbiComp/ISWC 2019-Adjunct Proceedings of the 2019 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2019 ACM International Symposium on Wearable Computers, ISWC, London, UK, 9 September 2019; Association for Computing Machinery: New York, NY, USA; pp. 308–311. [Google Scholar] [CrossRef]
- Lazaro Vasquez, E.S.; Vega, K. Myco-Accessories: Sustainable Wearables with Biodegradable Materials. In Proceedings of the 23rd International Symposium on Wearable Computers, ISWC, London, UK, 9 September 2019; Association for Computing Machinery: New York, NY, USA; pp. 306–311. [Google Scholar] [CrossRef]
- Silverman, J. Development and Testing of Mycelium-Based Composite Materials for Shoe Sole Applications; University of Delaware: Newark, DE, USA, 2018. [Google Scholar]
- Jones, M.; Bhat, T.; Huynh, T.; Kandare, E.; Yuen, R.; Wang, C.H.; John, S. Waste-Derived Low-Cost Mycelium Composite Construction Materials with Improved Fire Safety. Fire Mater. 2018, 42, 816–825. [Google Scholar] [CrossRef]
- Jones, M.; Bhat, T.; Kandare, E.; Thomas, A.; Joseph, P.; Dekiwadia, C.; Yuen, R.; John, S.; Ma, J.; Wang, C.-H. Thermal Degradation and Fire Properties of Fungal Mycelium and Mycelium-Biomass Composite Materials. Sci. Rep. 2018, 8, 17583. [Google Scholar] [CrossRef]
- Karana, E.; Blauwhoff, D.; Hultink, E.-J.; Camere, S. When the Material Grows: A Case Study on Designing (with) Mycelium-Based Materials. Int. J. Des. 2018, 12, 119–136. [Google Scholar]
- Luo, J.; Chen, X.; Crump, J.; Zhou, H.; Davies, D.G.; Zhou, G.; Zhang, N.; Jin, C. Interactions of Fungi with Concrete: Significant Importance for Bio-Based Self-Healing Concrete. Constr. Build. Mater. 2018, 164, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Abhijith, R.; Ashok, A.; Rejeesh, C.R. Sustainable Packaging Applications from Mycelium to Substitute Polystyrene: A Review. Mater. Today Proc. 2018, 5, 2139–2145. [Google Scholar] [CrossRef]
- Pelletier, M.G.; Holt, G.A.; Wanjura, J.D.; Bayer, E.; McIntyre, G. An Evaluation Study of Mycelium Based Acoustic Absorbers Grown on Agricultural By-Product Substrates. Ind. Crops Prod. 2013, 51, 480–485. [Google Scholar] [CrossRef]
- Atila, F. Effect of Different Substrate Disinfection Methods on the Production of Pleurotus Ostreatus. J. Agric. Stud. 2016, 4, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Inhabitat Phillip Ross Molds Fast-Growing Fungi Into Mushroom Building Bricks That Are Stronger Than Concrete. Available online: https://inhabitat.com/phillip-ross-molds-fast-growing-fungi-into-mushroom-building-bricks-that-are-stronger-than-concrete/ (accessed on 17 February 2019).
- Stott, R. Hy-Fi, the Organic Mushroom-Brick Tower Opens at MoMA’s PS1 Courtyard. Available online: https://www.archdaily.com/521266/hy-fi-the-organic-mushroom-brick-tower-opens-at-moma-s-ps1-courtyard (accessed on 17 February 2019).
- Heisel, F.; Schlesier, K.; Lee, J.; Rippmann, M.; Saeidi, N.; Javadian, A.; Nugroho Adi, R.; Hebel, D.; Block, P. Design of a Load-Bearing Mycelium Structure through Informed Structural Engineering. In Proceedings of the World Congress on Sustainable Technologies (WCST), Cambridge, UK, 11–14 December 2017; pp. 1–5. [Google Scholar]
- Archdaily This Pavillion Lives and Dies through Its Sustainable Agenda. Available online: https://www.archdaily.com/878519/this-pavillion-lives-and-dies-through-its-sustainable-agenda (accessed on 18 February 2019).
- Dessi-Olive, J. Monolithic Mycelium: Growing Vault Structures. In Proceedings of the 18th International Conference on Non-Conventional Materials and Technologies “Construction Materials & Technologies for Sustainability”, Nairobi, Kenya, 24–26 July 2019. [Google Scholar]
- Company New Heroes. The Growing Pavilion. Available online: https://thegrowingpavilion.com/ (accessed on 20 October 2020).
- Jones, M.; Huynh, T.T.; John, S. Inherent Species Characteristic Influence and Growth Performance Assessment for Mycelium Composite Applications Development of Microstrip Patch Antenna Strain Sensors for Wireless Structural Health Monitoring View Project Industrial Crops View Project. Adv. Mater. Lett. 2018, 9, 71–80. [Google Scholar] [CrossRef]
- Lelivelt, R.J.J. The Mechanical Possibilities of Mycelium Materials; Eidhoven University of Technology: Eidhoven, The Netherlands, 2015. [Google Scholar]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A.B. Fabrication Factors Influencing Mechanical, Moisture- and Water-Related Properties of Mycelium-Based Composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Attias, N.; Danai, O.; Abitbol, T.; Tarazi, E.; Ezov, N.; Pereman, I.; Grobman, Y.J. Mycelium Bio-Composites in Industrial Design and Architecture: Comparative Review and Experimental Analysis. J. Clean. Prod. 2020, 246, 119037. [Google Scholar] [CrossRef]
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium Composites: A Review of Engineering Characteristics and Growth Kinetics. J. Bionanoscience 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Ecovative Design. Mycocomposite. Available online: https://ecovativedesign.com/ (accessed on 27 November 2018).
- Chang, J.; Chan, P.L.; Xie, Y.; Ma, K.L.; Cheung, M.K.; Kwan, H.S. Modified Recipe to Inhibit Fruiting Body Formation for Living Fungal Biomaterial Manufacture. PLoS ONE 2019, 14, e0209812. [Google Scholar] [CrossRef] [PubMed]
- Lelivelt, R.J.J.; Lindner, G.; Teuffel, P.; Lamers, H. The Production Process and Compressive Strength of Mycelium-Based Materials. In Proceedings of the First International Conference on Bio-based Building Materials, Clermont-Ferrand, France, 22–25 June 2015; pp. 1–6. [Google Scholar]
- Appels, F.V.W.; Dijksterhuis, J.; Lukasiewicz, C.E.; Jansen, K.M.B.; Wösten, H.A.B.; Krijgsheld, P. Hydrophobin Gene Deletion and Environmental Growth Conditions Impact Mechanical Properties of Mycelium by Affecting the Density of the Material. Sci. Rep. 2018, 8, 4703. [Google Scholar] [CrossRef] [PubMed]
- Elsacker, E.; Vandelook, S.; Van Wylick, A.; Ruytinx, J.; De Laet, L.; Peeters, E. A Comprehensive Framework for the Production of Mycelium-Based Lignocellulosic Composites. Sci. Total Environ. 2020, 725, 138431. [Google Scholar] [CrossRef] [PubMed]
- Elsacker, E.; Søndergaard, A.; Van Wylick, A.; Peeters, E.; De Laet, L. Growing Living and Multifunctional Mycelium Composites for Large-Scale Formwork Applications Using Robotic Abrasive Wire-Cutting. Constr. Build. Mater. 2021, 283, 122732. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Atta, O.M.; Yang, G. Synthesis and Applications of Fungal Mycelium-Based Advanced Functional Materials. J. Bioresour. Bioprod. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Rigobello, A.; Ayres, P. Compressive Behaviour of Anisotropic Mycelium-Based Composites. Sci. Rep. 2022, 12, 6846. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Mechanical Behavior of Mycelium-Based Particulate Composites. J. Mater. Sci. 2018, 53, 16371–16382. [Google Scholar] [CrossRef]
- Tudryn, G.J.; Smith, L.C.; Freitag, J.; Bucinell, R.; Schadler, L.S. Processing and Morphology Impacts on Mechanical Properties of Fungal Based Biopolymer Composites. J. Polym. Environ. 2018, 26, 1473–1483. [Google Scholar] [CrossRef]
- Travaglini, S.; Noble, J.; Ross, P.G.; Dharan, C.K.H. Mycology Matrix Composites. In Proceedings of the 28th Annual Technical Conference of the American Society for Composites 2013, State College, PA, USA, 9–11 September 2013; Curran Associates, Inc.: Red Hook, NY, USA, 2013; pp. 517–535. [Google Scholar]
- Ashby, M.F.; Shercliff, H.; Cebon, D. Materials: Engineering, Science, Processing and Design, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2018; ISBN 9780081023778. [Google Scholar]
- Xia, X.C.; Chen, X.W.; Zhang, Z.; Chen, X.; Zhao, W.M.; Liao, B.; Hur, B. Effects of Porosity and Pore Size on the Compressive Properties of Closed-Cell Mg Alloy Foam. J. Magnes. Alloy. 2013, 1, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Morphology and Mechanics of Fungal Mycelium. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Walczyk, D.; McIntyre, G.; Bucinell, R.B. A New Approach to Manufacturing Biocomposite Sandwich Structures: Mycelium-Based Cores. In Proceedings of the ASME 2016 11th International Manufacturing Science and Engineering Conference, Volume 1: Processing, Blacksburg, VA, USA, 27 June–1 July 2016. [Google Scholar] [CrossRef]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms; Ten Speed Press: Berkeley, CA, USA, 1993; ISBN 1580081754. [Google Scholar]
- Stamets, P. Mycelium Running: How Mushrooms Can Help Save the World, 1st ed.; Ten Speed Press: Berkeley, CA, USA, 2005. [Google Scholar]
- Grimm, D.; Wösten, H.A.B. Mushroom Cultivation in the Circular Economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durr, L. Mycoremediation Project: Using Mycelium to Clean Up Diesel-Contaminated Soil in Orleans, California. 2016. Available online: https://static1.squarespace.com/static/61ad3dbc1635812fe1f11cae/t/61b975f2ef9ebd32bb073a9b/1639544319283/MycoremediationReport_FungaiaFarm_2016.pdf/ (accessed on 5 August 2020).
- ASTM D1037-12; Standard Test Methods for Evaluating Properties of Wood-Base Fiber and Particle Panel Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 2012. [CrossRef]
- ASTM D2166/D2166M-13; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. American Society for Testing and Materials: West Conshohocken, PA, USA, 2013. [CrossRef]
- ASTM D3501–94; Standard Test Methods for Wood-Based Structural Panels in Compression. American Society for Testing and Materials: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM C165-07; Standard Test Method for Measuring Compressive Properties of Thermal Insulations. American Society for Testing and Materials: West Conshohocken, PA, USA, 2017. [CrossRef]
- ASTM C303-10; Standard Test Method for Dimensions and Density of Preformed Block and Board–Type Thermal Insulation. American Society for Testing and Materials: West Conshohocken, PA, USA, 2002. [CrossRef]
- Watkinson, S.; Boddy, L.; Money, N. The Fungi, 3rd ed.; Academic Press: Waltham, MA, USA, 2015; ISBN 978-0-12-382034-1. [Google Scholar]
- Obodai, M.; Cleland-Okine, J.; Vowotor, K.A. Comparative Study on the Growth and Yield of Pleurotus Ostreatus Mushroom on Different Lignocellulosic By-Products. J. Ind. Microbiol. Biotechnol. 2003, 30, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.; Mackie, B.; Nothwehr, E. Nitrate and Phosphate Levels Positively Affect the Growth of Algae Species Found in Perry Pond. Tillers 2012, 4, 21–24. [Google Scholar]
- Smith, D.R.; King, K.W.; Williams, M.R. What Is Causing the Harmful Algal Blooms in Lake Erie? J. Soil Water Conserv. 2015, 70, 27A–29A. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, W.W.; Boyer, G.L. Health Impacts from Cyanobacteria Harmful Algae Blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef]
- Adey, W.H.; Kangas, P.C.; Mulbry, W. Algal Turf Scrubbing: Cleaning Surface Waters with Solar Energy While Producing a Biofuel. Bioscience 2011, 61, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Mulbry, W.; Kangas, P.; Kondrad, S. Toward Scrubbing the Bay: Nutrient Removal Using Small Algal Turf Scrubbers on Chesapeake Bay Tributaries. Ecol. Eng. 2010, 36, 536–541. [Google Scholar] [CrossRef]
- Camere, S.; Karana, E. Growing Materials for Product Design. In Proceedings of the EKSIG2017-International Conference on Experiential Knowledge and Emerging Materials, Delft, The Netherlands, 19–20 June 2017; pp. 101–115. [Google Scholar]
- Du, Z.Y.; Zienkiewicz, K.; Pol, N.V.; Ostrom, N.E.; Benning, C.; Bonito, G.M. Algal-Fungal Symbiosis Leads to Photosynthetic Mycelium. Elife 2019, 8, e47815. [Google Scholar] [CrossRef]
- Liber, J.A.; Bryson, A.E.; Bonito, G.; Du, Z.Y. Harvesting Microalgae for Food and Energy Products. Small Methods 2020, 4, 2000349. [Google Scholar] [CrossRef]
- Ginot, L.; Peyr, C.; Fontaine, A.; Cheymol, J.; Buisson, B.; Bellia, G.; Da Cruz, F.; Buisson, J. Dépistage du saturnisme infantile à partir de la recherche de plomb dans l’habitat: Une étude en région parisienne [Screening for Lead Poisoning in Children by Measuring Lead Levels in Housing: A Study of the Paris Region]. Rev. Epidemiol. Sante Publique 1995, 43, 477–484. [Google Scholar] [PubMed]
- Nduka, J.; Orisakwe, O.; Maduawguna, C. Lead Levels in Paint Flakes from Buildings in Nigeria: A Preliminary Study. Toxicol. Ind. Health 2008, 24, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Tehranifar, P.; Leighton, J.; Auchincloss, A.H.; Faciano, A.; Alper, H.; Paykin, A.; Wu, S. Immigration and Risk of Childhood Lead Poisoning: Findings from a Case-Control Study of New York City Children. Am. J. Public Health 2008, 98, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Reyes, N.L.; Wong, L.Y.; MacRoy, P.M.; Curtis, G.; Meyer, P.A.; Evens, A.; Brown, M.J. Identifying Housing That Poisons: A Critical Step in Eliminating Childhood Lead Poisoning. J. Public Health Manag. Pract. 2006, 12, 563–569. [Google Scholar] [CrossRef]
- Mattheck, C.; Sörensen, J.; Bethge, K. A Graphic Way for Notch Shape Optimization. WIT Trans. Ecol. Environ. 2006, 87, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, L.C.; Fox, A.M.; Jung, H.; Martin, E.G. Lead Exposure and Academic Achievement: Evidence from Childhood Lead Poisoning Prevention Efforts. J. Popul. Econ. 2019, 32, 179–218. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, A.N.; Mondal, R.; Kour, D.; Subrahmanyam, G.; Shabnam, A.A.; Khan, S.A.; Yadav, K.K.; Sharma, G.K.; Cabral-Pinto, M.; et al. Myco-Remediation: A Mechanistic Understanding of Contaminants Alleviation from Natural Environment and Future Prospect. Chemosphere 2021, 284, 131325. [Google Scholar] [CrossRef]
- Gadd, G.M. Fungi in Bioremediation; Gadd, G.M., Ed.; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Kumar, V.; Dwivedi, S.K. Mycoremediation of Heavy Metals: Processes, Mechanisms, and Affecting Factors. Environ. Sci. Pollut. Res. 2021, 28, 10375–10412. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar Stability in Soil: Meta-Analysis of Decomposition and Priming Effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Imhof, B.; Gruber, P. Built to Grow: Blending Architecture and Biology; Birkhäuser: Basel, Switzerland, 2015; ISBN 9783035609202. [Google Scholar]
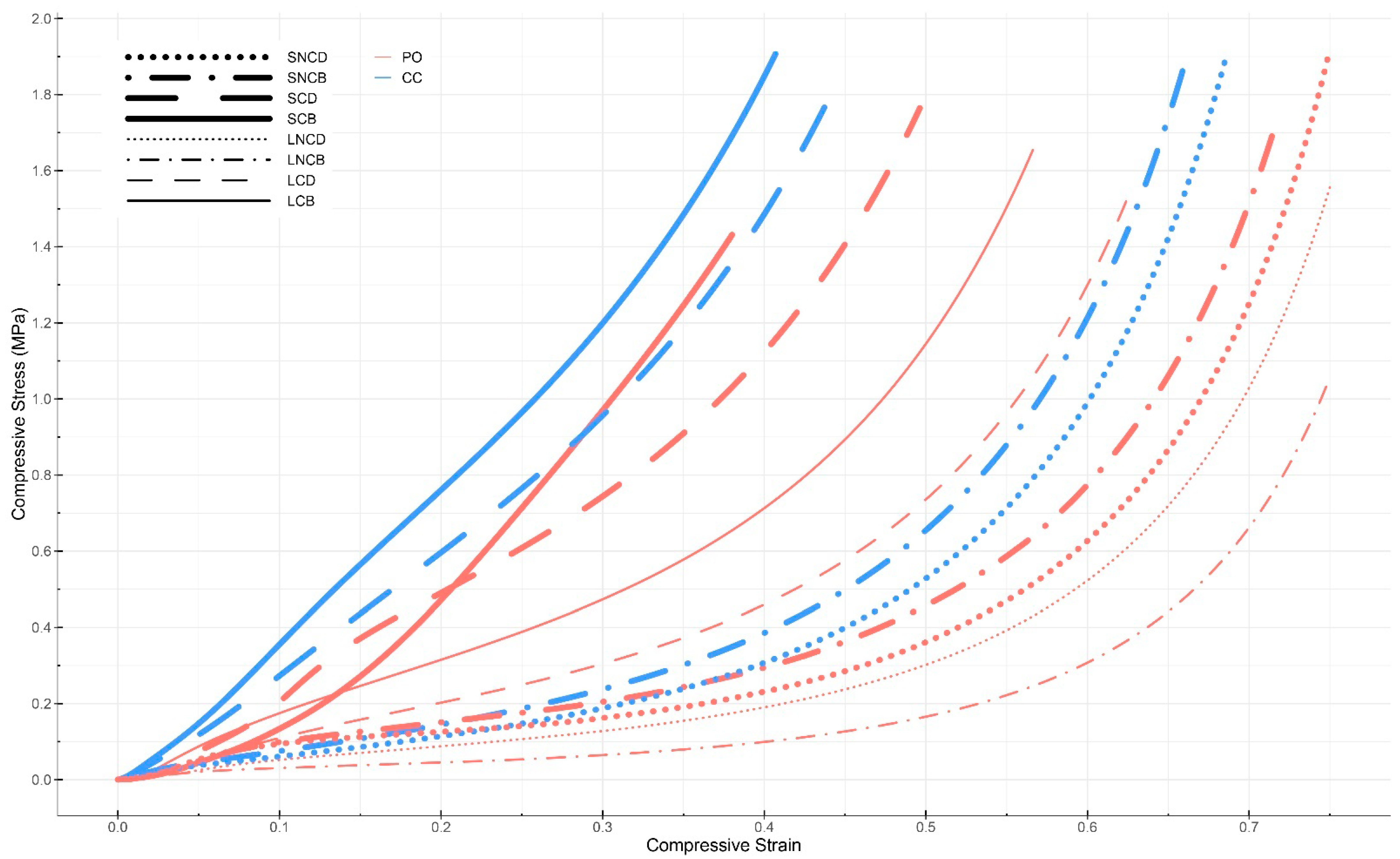


| Sample | Substrate Mixture | Compacted or Not | Dried or Baked |
|---|---|---|---|
| SNCD | Small (with fine particles) | Not compacted | Dried |
| SNCB | Baked | ||
| SCD | Compacted | Dried | |
| SCB | Baked | ||
| LNCD | Large (without fine particles) | Not compacted | Dried |
| LNCB | Baked | ||
| LCD | Compacted | Dried | |
| LCB | Baked |
| Sample | Sample Size | Relative Humidity (%) | Density (kg/m3) | Compressive Young’s Modulus (MPa) | ||
|---|---|---|---|---|---|---|
| Linear from 0.00 to 0.20 Strain | Linear from 0.19 to 0.21 Strain | Linear for Entire Test | ||||
| PO_SNCD | 3 | 9.23 ± 0.79 | 180.04 ± 8.54 | 0.63 ± 0.05 | 0.29 ± 0.03 | 2.56 ± 0.19 |
| PO_SNCB | 3 | 6.50 ± 0.32 | 186.13 ± 5.46 | 0.75 ± 0.13 | 0.51 ± 0.12 | 2.75 ± 0.52 |
| PO_SCD | 3 | 10.40 ± 1.10 | 258.93 ± 7.17 | 2.45 ± 0.34 | 2.31 ± 0.13 | 3.63 ± 0.14 |
| PO_SCB | 3 | 8.80 ± 1.09 | 283.07 ± 30.33 | 2.36 ± 0.79 | 4.55 ± 2.29 | 4.56 ± 0.73 |
| PO_LNCD | 3 | 7.78 ± 0.52 | 156.83 ± 5.45 | 0.44 ± 0.11 | 0.36 ± 0.11 | 2.08 ± 0.55 |
| PO_LNCB | 3 | 8.17 ± 1.12 | 136.22 ± 2.94 | 0.23 ± 0.01 | 0.15 ± 0.03 | 1.41 ± 0.16 |
| PO_LCD | 3 | 10.67 ± 0.34 | 188.88 ± 3.69 | 1.22 ± 0.30 | 1.08 ± 0.28 | 2.72 ± 0.29 |
| PO_LCB | 4 | 9.40 ± 0.90 | 217.46 ± 17.26 | 1.58 ± 0.24 | 1.40 ± 0.22 | 3.24 ± 0.21 |
| CC_SNCD | 10 | 6.51 ± 0.23 | 167.69 ± 3.83 | 0.57 ± 0.06 | 0.61 ± 0.07 | 3.17 ± 0.04 |
| CC_SNCB | 10 | 6.05 ± 0.23 | 175.79 ± 3.25 | 0.72 ± 0.07 | 0.78 ± 0.12 | 3.21 ± 0.05 |
| CC_SCD | 10 | 6.36 ± 0.45 | 264.55 ± 8.90 | 2.98 ± 0.30 | 3.24 ± 0.25 | 4.42 ± 0.17 |
| CC_SCB | 10 | 4.52 ± 0.97 | 270.21 ± 5.65 | 3.80 ± 0.17 | 3.93 ± 0.30 | 4.87 ± 0.17 |
| CC_LNCD | 0 | / | / | / | / | / |
| CC_LNCB | 0 | / | / | / | / | / |
| CC_LCD | 0 | / | / | / | / | / |
| CC_LCB | 0 | / | / | / | / | / |
| Sample | Height Deformation (%) | |||||
|---|---|---|---|---|---|---|
| Before Test 1 | After Test 1 | Before Test 2 | After Test 2 | 1 h after Test 2 | 1 Week after Test 2 | |
| PO_SNCD | 0.00 | 75.00 | 50.44 | 77.87 | 51.50 | 47.88 |
| PO_SNCB | 0.00 | 73.81 | 48.20 | 76.61 | 47.35 | 42.32 |
| PO_SCD | 0.00 | 50.39 | 21.01 | 51.94 | 21.95 | 18.06 |
| PO_SCB | 0.00 | 47.17 | 20.31 | 48.57 | 20.47 | 16.04 |
| PO_LNCD | 0.00 | 75.02 | / | / | 45.51 | 38.44 |
| PO_LNCB | 0.00 | 75.00 | 48.03 | 83.13 | 55.40 | 52.10 |
| PO_LCD | 0.00 | 61.15 | / | / | 29.80 | 23.91 |
| PO_LCB | 0.00 | 59.25 | 29.68 | 60.71 | 30.52 | 27.68 |
| Sample | Sample Size | Relative Humidity (%) | Density (kg/m3) | Elastic Modulus | Maximum Load (N) | Fracture Offset from Center (cm) | Fracture Angle (°) | |
|---|---|---|---|---|---|---|---|---|
| From Instron (MPa) | From ASTM D1037 (MPa) | |||||||
| PO_SNCD | 0 | / | / | / | / | / | / | / |
| PO_SNCB | 1 | 7.65 | 140.06 | / | 4.88 | 1.28 | 1.03 | 2.19 |
| PO_SCD | 6 | 6.03 ± 0.63 | 259.24 ± 17.86 | 31.66 ± 8.59 | 22.39 ± 8.41 | 3.62 ± 1.92 | 3.03 ± 1.86 | 3.60 ± 2.61 |
| PO_SCB | 0 | / | / | / | / | / | / | / |
| PO_LNCD | 2 | 5.68 ± 0.23 | 131.33 ± 4.36 | 6.37 ± 2.64 | 5.64 ± 3.07 | 0.64 ± 0.25 | 1.24 ± 0.34 | 13.35 ± 2.09 |
| PO_LNCB | 2 | 5.73 ± 0.13 | 123.63 ± 0.24 | 3.11 ± 0.29 | 2.46 ± 0.23 | 0.32 ± 0.08 | 0.81 ± 0.06 | 3.00 ± 2.24 |
| PO_LCD | 6 | 5.93 ± 0.56 | 235.32 ± 19.29 | 20.12 ± 17.44 | 18.46 ± 16.95 | 1.84 ± 1.48 | 4.29 ± 2.18 | 4.46 ± 3.23 |
| PO_LCB | 0 | / | / | / | / | / | / | / |
| PO_ISNCD | 5 | 5.73 ± 0.48 | 154.04 ± 4.73 | 8.91 ± 2.13 | 7.17 ± 1.60 | 0.90 ± 0.33 | 1.18 ± 0.58 | 4.27 ± 5.21 |
| PO_ISNCB | 3 | 5.65 ± 0.24 | 145.78 ± 1.77 | 6.08 ± 0.39 | 5.74 ± 0.58 | 0.50 ± 0.12 | 2.86 ± 1.51 | 6.84 ± 5.59 |
| PO_ISCD | 0 | / | / | / | / | / | / | / |
| PO_ISCB | 1 | 5.35 | 160.60 | 0.70 | 0.40 | 0.24 | 0.53 | 8.13 |
| PO_ILNCD | 3 | 5.40 ± 0.11 | 127.17 ± 11.70 | 3.10 ± 1.50 | 2.27 ± 1.16 | 0.24 ± 0.14 | 1.87 ± 0.99 | 7.88 ± 3.97 |
| PO_ILNCB | 2 | 5.70 ± 0.15 | 148.56 ± 1.37 | 5.45 ± 0.78 | 4.82 ± 0.68 | 0.49 ± 0.01 | 0.86 ± 0.56 | 8.00 ± 4.99 |
| PO_ILCD | 0 | / | / | / | / | / | / | / |
| PO_ILCB | 4 | 5.76 ± 0.51 | 286.67 ± 6.84 | 110.42 ± 40.73 | 106.08 ± 40.65 | 8.85 ± 2.58 | 0.94 ± 1.32 | 6.31 ± 5.19 |
| CC_SNCD | 12 | 5.62 ± 0.21 | 133.02 ± 4.83 | 1.58 ± 0.70 | 1.44 ± 0.72 | 0.19 ± 0.08 | 1.90 ± 1.49 | 0.51 ± 0.28 |
| CC_SNCB | 11 | 5.50 ± 0.31 | 132.49 ± 6.66 | 1.35 ± 0.55 | 1.16 ± 0.54 | 0.20 ± 0.13 | 2.46 ± 1.60 | 0.60 ± 0.52 |
| CC_SCD | 14 | 7.85 ± 0.72 | 239.52 ± 27.84 | 25.84 ± 12.69 | 22.34 ± 11.38 | 2.85 ± 1.25 | 1.16 ± 1.06 | 0.58 ± 0.51 |
| CC_SCB | 15 | 6.70 ± 0.30 | 291.66 ± 25.11 | 53.06 ± 25.14 | 48.39 ± 23.37 | 5.77 ± 2.26 | 1.27 ± 0.81 | 0.67 ± 0.56 |
| CC_LNCD | 0 | / | / | / | / | / | / | / |
| CC_LNCB | 0 | / | / | / | / | / | / | / |
| CC_LCD | 0 | / | / | / | / | / | / | / |
| CC_LCB | 0 | / | / | / | / | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houette, T.; Maurer, C.; Niewiarowski, R.; Gruber, P. Growth and Mechanical Characterization of Mycelium-Based Composites towards Future Bioremediation and Food Production in the Material Manufacturing Cycle. Biomimetics 2022, 7, 103. https://doi.org/10.3390/biomimetics7030103
Houette T, Maurer C, Niewiarowski R, Gruber P. Growth and Mechanical Characterization of Mycelium-Based Composites towards Future Bioremediation and Food Production in the Material Manufacturing Cycle. Biomimetics. 2022; 7(3):103. https://doi.org/10.3390/biomimetics7030103
Chicago/Turabian StyleHouette, Thibaut, Christopher Maurer, Remik Niewiarowski, and Petra Gruber. 2022. "Growth and Mechanical Characterization of Mycelium-Based Composites towards Future Bioremediation and Food Production in the Material Manufacturing Cycle" Biomimetics 7, no. 3: 103. https://doi.org/10.3390/biomimetics7030103
APA StyleHouette, T., Maurer, C., Niewiarowski, R., & Gruber, P. (2022). Growth and Mechanical Characterization of Mycelium-Based Composites towards Future Bioremediation and Food Production in the Material Manufacturing Cycle. Biomimetics, 7(3), 103. https://doi.org/10.3390/biomimetics7030103






