Effect of Cellulose–Chitosan Hybrid-Based Encapsulation on the Viability and Stability of Probiotics under Simulated Gastric Transit and in Kefir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Activation of Bacterial Culture
2.2. Preparation of Cellulose and Chitosan-Based (Cl-Ch) Capsules
2.3. Characterization of Beads
Zeta Potential Measurement
2.4. Encapsulation Efficiency
2.5. SEM
2.6. Release Study of Encapsulated Beads in Simulated Gastric Fluid
2.7. Release Study of Encapsulated Beads in Simulated Intestinal Fluid
2.8. Thermal Resistance
2.9. Product Development (Kefir)
2.9.1. Characterization of Kefir
2.9.2. Probiotic Enumeration
2.10. Statistical Analysis
3. Results and Discussion
3.1. Bead Size and Shape
3.2. Zeta Potential of Encapsulated Beads
3.3. Encapsulation Yield
3.4. Scanning Electron Microscopy (SEM)
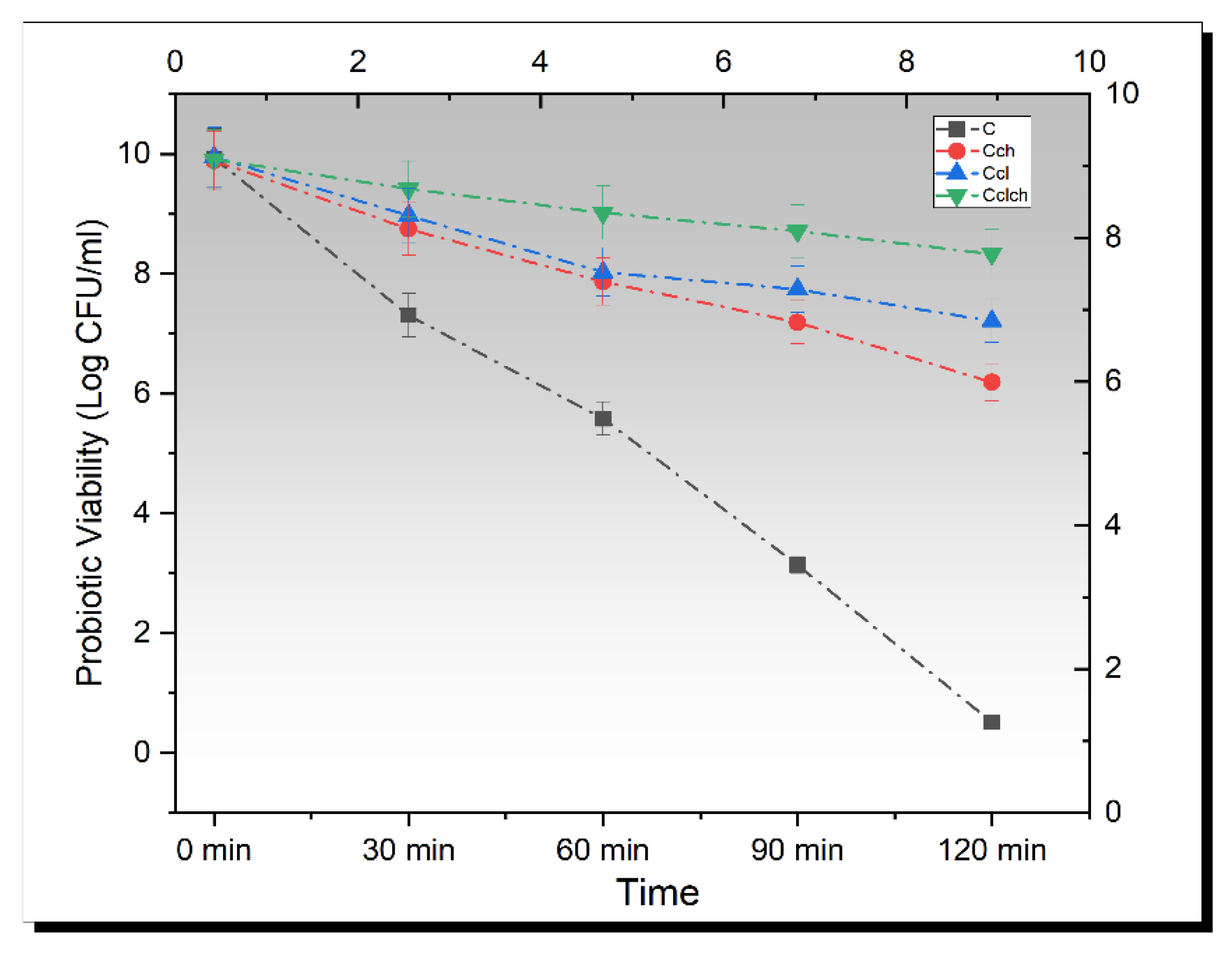
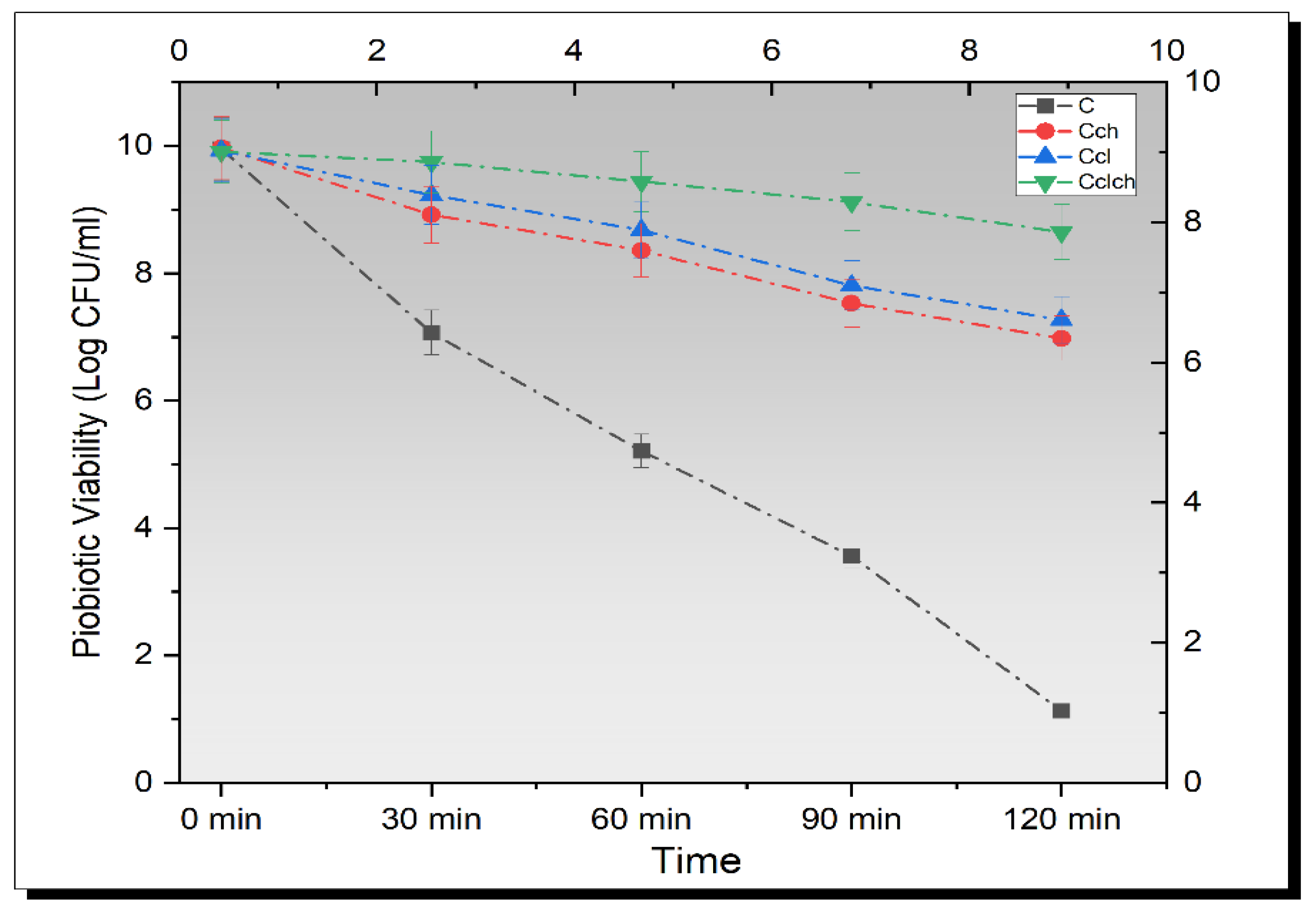
3.5. Release Study of Encapsulated Beads in Simulated Gastric Fluid
3.6. Release Study of Encapsulated Beads in Simulated Intestinal Fluid
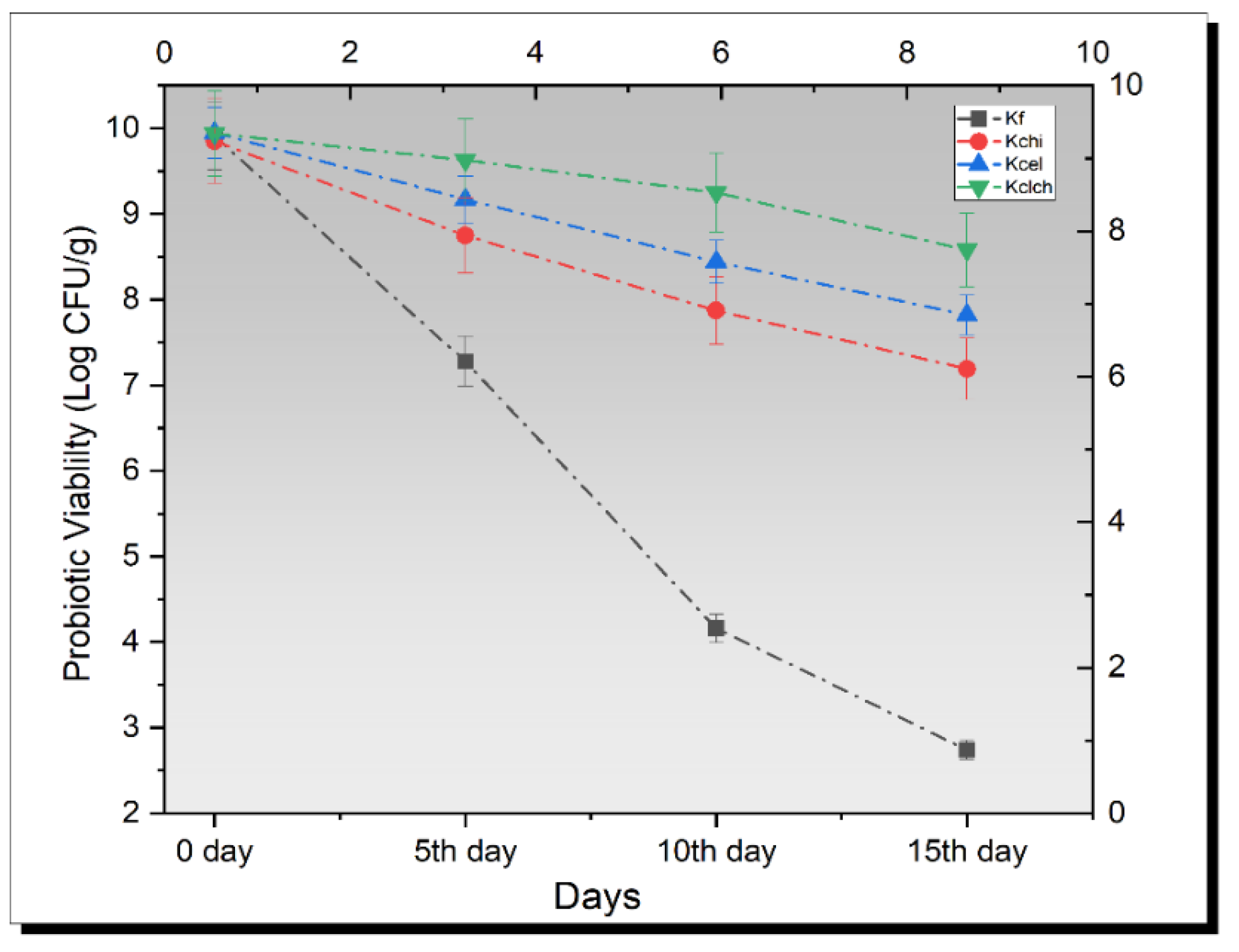
3.7. Probiotic Enumeration in Kefir
3.8. Thermal Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, S.; Prabha, K.; Sharanagat, V.S.; Mishra, V. Consumer awareness and willingness to purchase probiotic food and beverage products: A study of Sonipat district, Haryana. Br. Food J. 2020, 123, 2805–2817. [Google Scholar] [CrossRef]
- Kvakova, M.; Bertkova, I.; Stofilova, J.; Savidge, T.C. Co-encapsulated synbiotics and immobilized probiotics in human health and gut Microbiota modulation. Foods 2021, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.; Díaz-Castro, J.; López-Aliaga, I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Tremblay, A.; Fatani, A.; Ford, A.L.; Piano, A.; Nagulesapillai, V.; Auger, J.; MacPherson, C.W.; Christman, M.C.; Tompkins, T.A.; Dahl, W.J. Safety and effect of a low-and high-dose multi-strain probiotic supplement on microbiota in a general adult population: A randomized, double-blind, placebo-controlled study. J. Diet. Suppl. 2021, 18, 227–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, M.N.; Tagliapietra, B.L.; do Amaral Flores, V.; dos Santos Richards, N.S.P. In Vitro test to evaluate survival in the gastrointestinal tract of commercial probiotics. Curr. Res. Food Sci. 2021, 4, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Mbye, M.; Baig, M.A.; AbuQamar, S.F.; El-Tarabily, K.A.; Obaid, R.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Turner, M.S.; Shah, N.P.; Ayyash, M.M. Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1110–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jebbar, M.; Hickman-Lewis, K.; Cavalazzi, B.; Taubner, R.-S.; Rittmann, S.K.-M.; Antunes, A. Microbial diversity and biosignatures: An icy moons perspective. Space Sci. Rev. 2020, 216, 1–47. [Google Scholar] [CrossRef]
- Gholami, A.; Dabbaghmanesh, M.H.; Ghasemi, Y.; Talezadeh, P.; Koohpeyma, F.; Montazeri-Najafabady, N. Probiotics ameliorate pioglitazone-associated bone loss in diabetic rats. Diabetol. Metab. Syndr. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Stanisz, M.; Klapiszewski, Ł.; Jesionowski, T. Recent advances in the fabrication and application of biopolymer-based micro-and nanostructures: A comprehensive review. Chem. Eng. J. 2020, 397, 125409. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of bioactive compounds by “extrusion” technologies: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3100–3118. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Guo, Y.; Li, H.; Liu, X.; Fu, Y.; Ding, F. Recent advances in chitosan-based layer-by-layer biomaterials and their biomedical applications. Carbohydr. Polym. 2021, 271, 118427. [Google Scholar] [CrossRef] [PubMed]
- Güven, A.; Deveci, H.A.; Nur, G. The importance of kefir in healthy nutrition: Antioxidant and hypocholesterolemic effect. In Theory, Current Researches and New Trends/2021; IVPE: Cetinje, Montenegro, 2021; Volume 1. [Google Scholar]
- Afzaal, M.; Saeed, F.; Saeed, M.; Ahmed, A.; Ateeq, H.; Nadeem, M.T.; Tufail, T. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal conditions and in pasteurized grape juice. J. Food Process. Preserv. 2020, 44, e14346. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Tian, H.; Luo, X.; Liu, S. Encapsulation of Lactobacillus plantarum in cellulose based microgel with controlled release behavior and increased long-term storage stability. Carbohydr. Polym. 2019, 223, 115065. [Google Scholar] [CrossRef]
- Santos, M.A.; Machado, M.T. Coated alginate–chitosan particles to improve the stability of probiotic yeast. Int. J. Food Sci. Technol. 2021, 56, 2122–2131. [Google Scholar] [CrossRef]
- Yıldız-Akgül, F.; Yetişemiyen, A.; Şenel, E.; Yıldırım, Z. Microbiological, physicochemical, and sensory characteristics of kefir produced by secondary fermentation. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2018, 68, 201–213. [Google Scholar] [CrossRef]
- Ding, Y.; Song, C.; Gong, W.; Liu, L.; Wu, M.; Li, L.; Yao, J. Robust, sustainable, hierarchical multi-porous cellulose beads via pre-crosslinking strategy for efficient dye adsorption. Cellulose 2021, 28, 7227–7241. [Google Scholar] [CrossRef]
- Yasmin, I.; Saeed, M.; Pasha, I.; Zia, M.A. Development of whey protein concentrate-pectin-alginate based delivery system to improve survival of B. longum BL-05 in simulated gastrointestinal conditions. Probiotics Antimicrob. Proteins 2019, 11, 413–426. [Google Scholar] [CrossRef]
- De Wever, P.; Janssens, J.; Fardim, P. Fabrication of cellulose cryogel beads via room temperature dissolution in onium hydroxides. Carbohydr. Polym. Technol. Appl. 2022, 3, 100206. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Xu, J.; Qi, Y.; Zhao, N.; Fan, M. First insight into the probiotic properties of ten Streptococcus thermophilus strains based on in vitro conditions. Curr. Microbiol. 2020, 77, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, L.; Bao, X.; Yao, P. Curcumin, casein and soy polysaccharide ternary complex nanoparticles for enhanced dispersibility, stability and oral bioavailability of curcumin. Food Biosci. 2020, 35, 100569. [Google Scholar] [CrossRef]
- Sabikhi, L.; Babu, R.; Thompkinson, D.K.; Kapila, S. Resistance of microencapsulated Lactobacillus acidophilus LA1 to processing treatments and simulated gut conditions. Food Bioprocess Technol. 2010, 3, 586–593. [Google Scholar] [CrossRef]
- González-Sánchez, F.; Azaola, A.; Gutiérrez-López, G.F.; Hernández-Sánchez, H. Viability of microencapsulated Bifidobacterium animalis ssp. lactis BB12 in kefir during refrigerated storage. Int. J. Dairy Technol. 2010, 63, 431–436. [Google Scholar] [CrossRef]
- Li, W.; Luo, X.; Song, R.; Zhu, Y.; Li, B.; Liu, S. Porous cellulose microgel particle: A fascinating host for the encapsulation, protection, and delivery of lactobacillus plantarum. J. Agric. Food Chem. 2016, 64, 3430–3436. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Frutos, M.J. Effect of different types of encapsulation on the survival of Lactobacillus plantarum during storage with inulin and in vitro digestion. LWT Food Sci. Technol. 2015, 64, 824–828. [Google Scholar] [CrossRef]
- Chotiko, A.; Sathivel, S. Development of a combined low-methoxyl-pectin and rice-bran-extract delivery system to improve the viability of Lactobacillus plantarum under acid and bile conditions. LWT Food Sci. Technol. 2016, 66, 420–427. [Google Scholar] [CrossRef]
- Bove, P.; Gallone, A.; Russo, P.; Capozzi, V.; Albenzio, M.; Spano, G.; Fiocco, D. Probiotic features of Lactobacillus plantarum mutant strains. Appl. Microbiol. Biotechnol. 2012, 96, 431–441. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Ye, F.; Li, B.; Luo, X.; Liu, S. Probiotics in cellulose houses: Enhanced viability and targeted delivery of Lactobacillus plantarum. Food Hydrocoll. 2017, 62, 66–72. [Google Scholar] [CrossRef]
- Ji, Y.; Kim, H.; Park, H.; Lee, J.; Lee, H.; Shin, H.; Kim, B.; Franz, C.M.; Holzapfel, W.H. Functionality and safety of lactic bacterial strains from Korean kimchi. Food Control 2013, 31, 467–473. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B. Properties and applications of different probiotic delivery systems. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2012; pp. 541–594. [Google Scholar]
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G.R. Development of novel carboxymethyl cellulose/k-carrageenan blends as an enteric delivery vehicle for probiotic bacteria. Int. J. Biol. Macromol. 2017, 97, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Dinkçi, N.; Kesenkaş, H.; Korel, F.; Kınık, Ö. Inovativan pristup: Kefir na bazi mješavine kravljeg i zobenog mlijeka. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2015, 65, 177–186. [Google Scholar]
- Guzel-seydim, Z.; Wyffels, J.T.; Seydim, A.C.; Greene, A.K. Turkish kefir and kefir grains: Microbial enumeration and electron microscobic observation. Int. J. Dairy Technol. 2005, 58, 25–29. [Google Scholar] [CrossRef]
- Corcoran, B.; Stanton, C.; Fitzgerald, G.; Ross, R. Life under stress: The probiotic stress response and how it may be manipulated. Curr. Pharm. Des. 2008, 14, 1382–1399. [Google Scholar] [CrossRef]
- Lian, J.; Duan, X.; Ma, J.; Peng, P.; Kim, T.; Zheng, W. Hematite (α-Fe2O3) with various morphologies: Ionic liquid-assisted synthesis, formation mechanism, and properties. ACS Nano 2009, 3, 3749–3761. [Google Scholar] [CrossRef] [PubMed]


| Cell Type | Size (mm) | Encapsulation Efficiency (%) | Zeta Potential |
|---|---|---|---|
| Cch | 2.79 ± 0.16 | 78 ± 1.02 | −32 ± 0.03 mV |
| Ccl | 3.18 ± 0.15 | 80 ± 2.41 | −15 ± 0.09 mV |
| Cch-cl | 3.56 ± 1.12 | 87 ± 1.82 | −26 ± 0.07 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzaal, M.; Saeed, F.; Ateeq, H.; Shah, Y.A.; Hussain, M.; Javed, A.; Ikram, A.; Raza, M.A.; Nayik, G.A.; Alfarraj, S.; et al. Effect of Cellulose–Chitosan Hybrid-Based Encapsulation on the Viability and Stability of Probiotics under Simulated Gastric Transit and in Kefir. Biomimetics 2022, 7, 109. https://doi.org/10.3390/biomimetics7030109
Afzaal M, Saeed F, Ateeq H, Shah YA, Hussain M, Javed A, Ikram A, Raza MA, Nayik GA, Alfarraj S, et al. Effect of Cellulose–Chitosan Hybrid-Based Encapsulation on the Viability and Stability of Probiotics under Simulated Gastric Transit and in Kefir. Biomimetics. 2022; 7(3):109. https://doi.org/10.3390/biomimetics7030109
Chicago/Turabian StyleAfzaal, Muhammad, Farhan Saeed, Huda Ateeq, Yasir Abbas Shah, Muzzamal Hussain, Ahsan Javed, Ali Ikram, Muhammad Ahtisham Raza, Gulzar Ahmad Nayik, Saleh Alfarraj, and et al. 2022. "Effect of Cellulose–Chitosan Hybrid-Based Encapsulation on the Viability and Stability of Probiotics under Simulated Gastric Transit and in Kefir" Biomimetics 7, no. 3: 109. https://doi.org/10.3390/biomimetics7030109
APA StyleAfzaal, M., Saeed, F., Ateeq, H., Shah, Y. A., Hussain, M., Javed, A., Ikram, A., Raza, M. A., Nayik, G. A., Alfarraj, S., Ansari, M. J., & Karabagias, I. K. (2022). Effect of Cellulose–Chitosan Hybrid-Based Encapsulation on the Viability and Stability of Probiotics under Simulated Gastric Transit and in Kefir. Biomimetics, 7(3), 109. https://doi.org/10.3390/biomimetics7030109










