Emerging Treatment Options for Peanut Allergy
Abstract
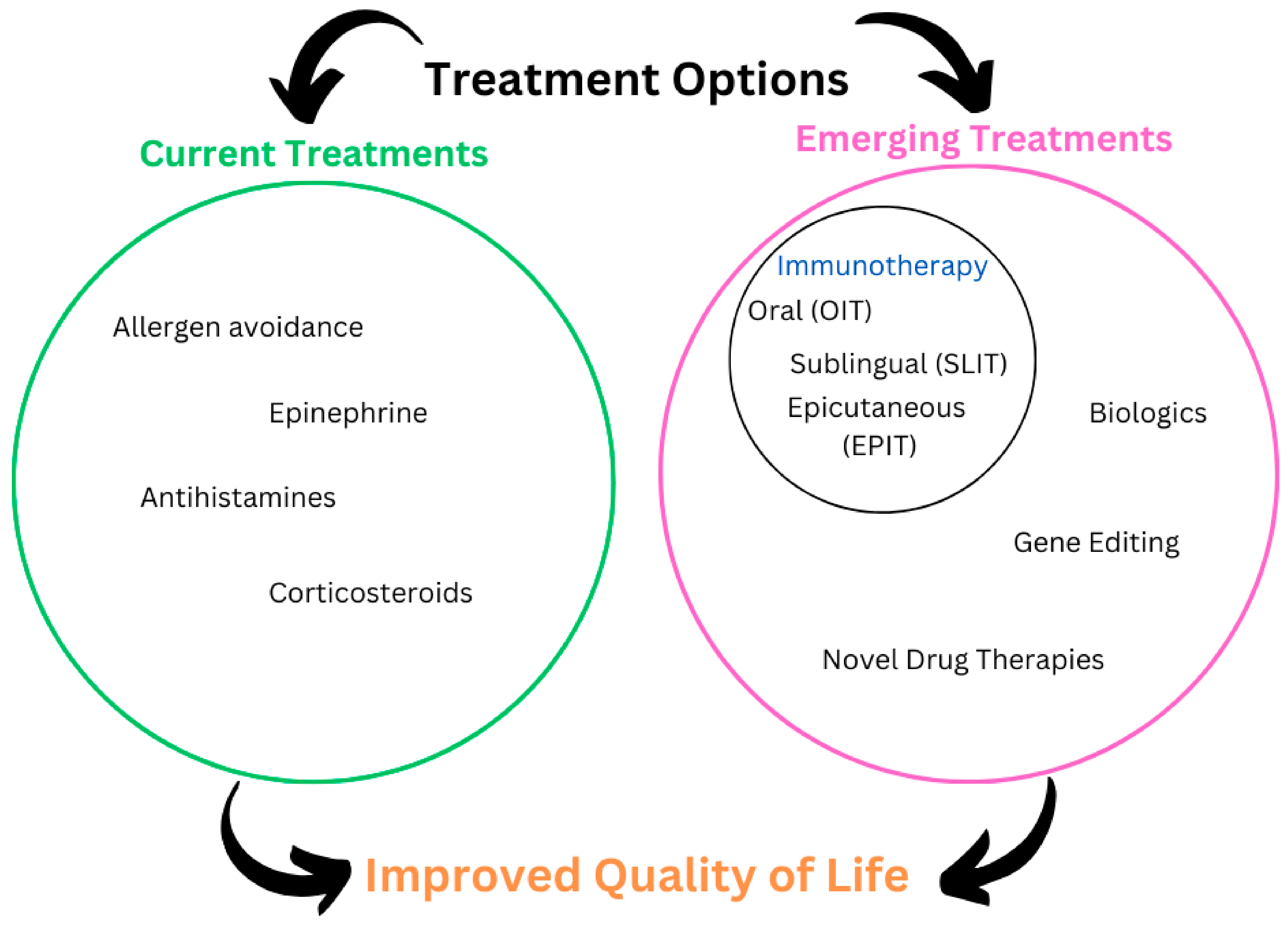
:1. Introduction
2. Current Treatment Options
3. Emerging Treatment Outcomes
3.1. Immunotherapy Approaches
3.1.1. Oral Immunotherapy (OIT)
3.1.2. Sublingual Immunotherapy (SLIT)
3.1.3. Epicutaneous Immunotherapy (EPIT)
3.2. Biologic Therapies
3.3. Gene Editing and CRISPR Technology
3.4. Novel Drug Therapies
Small Molecules
4. Challenges and Limitations in Emerging Treatments
Author Contributions
Funding
Conflicts of Interest
References
- Lange, L.; Klimek, L.; Beyer, K.; Blümchen, K.; Novak, N.; Hamelmann, E.; Bauer, A.; Merk, H.; Rabe, U.; Jung, K.; et al. White paper on peanut allergy—Part 1: Epidemiology, burden of disease, health economic aspects. Allergo J. Int. 2021, 30, 261. [Google Scholar] [CrossRef] [PubMed]
- Husain, Z.; Schwartz, R.A. Peanut allergy: An increasingly common life-threatening disorder. J. Am. Acad. Dermatol. 2012, 66, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Chan, E.S.; Sicherer, S. Peanut Allergy: New Advances and Ongoing Controversies. Pediatrics 2020, 145, e20192102. [Google Scholar] [CrossRef] [PubMed]
- Tsoumani, M.; Regent, L.; Warner, A.; Gallop, K.; Patel, R.; Ryan, R.; Vereda, A.; Acaster, S.; DunnGalvin, A.; Byrne, A. Allergy to Peanuts imPacting Emotions And Life (APPEAL): The impact of peanut allergy on children, teenagers, adults and caregivers in the UK and Ireland. PLoS ONE 2022, 17, e0262851. [Google Scholar] [CrossRef]
- Bublin, M.; Breiteneder, H. Developing Therapies for Peanut Allergy. Int. Arch. Allergy Immunol. 2014, 165, 179. [Google Scholar] [CrossRef]
- Cook, Q.S.; Kim, E.H. Update on peanut allergy: Prevention and immunotherapy. Allergy Asthma Proc. 2019, 40, 14–20. [Google Scholar] [CrossRef]
- Kim, E.H.; Patel, C.; Burks, A.W. Immunotherapy approaches for peanut allergy. Expert Rev. Clin. Immunol. 2020, 16, 167–174. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef]
- Du Toit, G.; Huffaker, M.F.; Radulovic, S.; Feeney, M.; Fisher, H.R.; Byron, M.; Dunaway, L.; Calatroni, A.; Johnson, M.; Foong, R.-X.; et al. Follow-up to Adolescence after Early Peanut Introduction for Allergy Prevention. NEJM Evid. 2024, 3, EVIDoa2300311. [Google Scholar] [CrossRef]
- Logan, K.; Bahnson, H.T.; Ylescupidez, A.; Beyer, K.; Bellach, J.; Campbell, D.E.; Craven, J.; Du Toit, G.; Clare Mills, E.N.; Perkin, M.R.; et al. Early introduction of peanut reduces peanut allergy across risk groups in pooled and causal inference analyses. Allergy 2023, 78, 1307–1318. [Google Scholar] [CrossRef]
- Togias, A.; Cooper, S.F.; Acebal, M.L.; Assa’ad, A.; Baker, J.R.; Beck, L.A.; Block, J.; Byrd-Bredbenner, C.; Chan, E.S.; Eichenfield, L.F.; et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. Ann. Allergy Asthma Immunol. 2017, 118, 166–173.e7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kanaley, M.; Negris, O.; Roach, A.; Bilaver, L. Understanding Precautionary Allergen Labeling (PAL) Preferences Among Food Allergy Stakeholders. J. Allergy Clin. Immunol. Pract. 2021, 9, 254–264.e1. [Google Scholar] [CrossRef] [PubMed]
- King, R.M.; Knibb, R.C.; Hourihane, J.O. Impact of peanut allergy on quality of life, stress and anxiety in the family. Allergy 2009, 64, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.M.; Roberts, M.C. Peanut allergy in children: Relationships to health-related quality of life, anxiety, and parental stress. Clin. Pediatr. 2011, 50, 1045–1051. [Google Scholar] [CrossRef]
- Dworaczyk, D.A.; Hunt, A.L.; Di Spirito, M.; Lor, M.; Dretchen, K.L.; Lamson, M.J.; Pollock, J.; Ward, T. A 13.2 mg epinephrine intranasal spray demonstrates comparable pharmacokinetics, pharmacodynamics, and safety to a 0.3 mg epinephrine autoinjector. J. Allergy Clin. Immunol. Glob. 2024, 3, 100200. [Google Scholar] [CrossRef]
- Navalpakam, A.; Thanaputkaiporn, N.; Poowuttikul, P. Management of Anaphylaxis. Immunol. Allergy Clin. N. Am. 2022, 42, 65–76. [Google Scholar] [CrossRef]
- Shaker, M.S.; Wallace, D.V.; Golden, D.B.K.; Oppenheimer, J.; Bernstein, J.A.; Campbell, R.L.; Dinakar, C.; Ellis, A.; Greenhawt, M.; Khan, D.A.; et al. Anaphylaxis—A 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J. Allergy Clin. Immunol. 2020, 145, 1082–1123. [Google Scholar] [CrossRef]
- Oppenheimer, J.J.; Nelson, H.S.; Bock, S.A.; Christensen, F.; Leung, D.Y. Treatment of peanut allergy with rush immunotherapy. J. Allergy Clin. Immunol. 1992, 90, 256–262. [Google Scholar] [CrossRef]
- Nelson, H.S.; Lahr, J.; Rule, R.; Bock, A.; Leung, D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J. Allergy Clin. Immunol. 1997, 99 Pt 1, 744–751. [Google Scholar] [CrossRef]
- Jones, S.M.; Kim, E.H.; Nadeau, K.C.; Nowak-Wegrzyn, A.; Wood, R.A.; Sampson, H.A.; Scurlock, A.M.; Chinthrajah, S.; Wang, J.; Pesek, R.D.; et al. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): A randomised placebo-controlled study. Lancet 2022, 399, 359–371. [Google Scholar] [CrossRef]
- Kim, E.H.; Keet, C.A.; Virkud, Y.V.; Chin, S.; Ye, P.; Penumarti, A.; Smeekens, J.; Guo, R.; Yue, X.; Li, Q.; et al. Open-label study of the efficacy, safety, and durability of peanut sublingual immunotherapy in peanut-allergic children. J. Allergy Clin. Immunol. 2023, 151, 1558–1565.e6. [Google Scholar] [CrossRef]
- Kim, E.H.; Bird, J.A.; Keet, C.A.; Virkud, Y.V.; Herlihy, L.; Ye, P.; Smeekens, J.M.; Guo, R.; Yue, X.; Penumarti, A.; et al. Desensitization and remission after peanut sublingual immunotherapy in 1- to 4-year-old peanut-allergic children: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2024, 153, 173–181.e10. [Google Scholar] [CrossRef]
- Kim, E.H.; Yang, L.; Ye, P.; Guo, R.; Li, Q.; Kulis, M.D.; Burks, A.W. Long-term sublingual immunotherapy for peanut allergy in children: Clinical and immunologic evidence of desensitization. J. Allergy Clin. Immunol. 2019, 144, 1320–1326.e1. [Google Scholar] [CrossRef]
- Narisety, S.D.; Frischmeyer-Guerrerio, P.A.; Keet, C.A.; Gorelik, M.; Schroeder, J.; Hamilton, R.G.; Wood, R.A. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J. Allergy Clin. Immunol. 2015, 135, 1275–1282.e6. [Google Scholar] [CrossRef] [PubMed]
- Keet, C.A.; Frischmeyer-Guerrerio, P.A.; Thyagarajan, A.; Schroeder, J.T.; Hamilton, R.G.; Boden, S.; Steele, P.; Driggers, S.; Burks, A.W.; Wood, R.A. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J. Allergy Clin. Immunol. 2012, 129, 448–455.e5. [Google Scholar] [CrossRef] [PubMed]
- Soller, L.; Abrams, E.M.; Carr, S.; Kapur, S.; Rex, G.A.; Leo, S.; McHenry, M.; Vander Leek, T.K.; Yeung, J.; Cook, V.E.; et al. First Real-World Effectiveness Analysis of Preschool Peanut Oral Immunotherapy. J. Allergy Clin. Immunol. Pract. 2021, 9, 1349–1356.e1. [Google Scholar] [CrossRef] [PubMed]
- PALISADE Group of Clinical Investigators. AR101 Oral Immunotherapy for Peanut Allergy. N. Engl. J. Med. 2018, 379, 1991–2001. [Google Scholar] [CrossRef]
- O’B Hourihane, J.; Beyer, K.; Abbas, A.; Fernández-Rivas, M.; Turner, P.J.; Blumchen, K.; Nilsson, C.; Ibáñez, M.D.; Deschildre, A.; Muraro, A.; et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): A multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Child Adolesc. Health 2020, 4, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.A.; Spergel, J.M.; Jones, S.M.; Rachid, R.; Assa’ad, A.H.; Wang, J.; Leonard, S.A.; Laubach, S.S.; Kim, E.H.; Vickery, B.P.; et al. Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. J. Allergy Clin. Immunol. Pract. 2018, 6, 476–485.e3. [Google Scholar] [CrossRef] [PubMed]
- Greenhawt, M.; Sindher, S.B.; Wang, J.; O’Sullivan, M.; du Toit, G.; Kim, E.H.; Albright, D.; Anvari, S.; Arends, N.; Arkwright, P.D.; et al. Phase 3 Trial of Epicutaneous Immunotherapy in Toddlers with Peanut Allergy. N. Engl. J. Med. 2023, 388, 1755–1766. [Google Scholar] [CrossRef]
- Sampson, H.A.; Shreffler, W.G.; Yang, W.H.; Sussman, G.L.; Brown-Whitehorn, T.F.; Nadeau, K.C.; Cheema, A.S.; Leonard, S.A.; Pongracic, J.A.; Sauvage-Delebarre, C.; et al. Effect of Varying Doses of Epicutaneous Immunotherapy vs Placebo on Reaction to Peanut Protein Exposure Among Patients with Peanut Sensitivity: A Randomized Clinical Trial. JAMA 2017, 318, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Pongracic, J.A.; Gagnon, R.; Sussman, G.; Siri, D.; Oriel, R.C.; Brown-Whitehorn, T.F.; Green, T.D.; Campbell, D.E.; Anvari, S.; Berger, W.E.; et al. Safety of Epicutaneous Immunotherapy in Peanut-Allergic Children: REALISE Randomized Clinical Trial Results. J. Allergy Clin. Immunol. Pract. 2022, 10, 1864–1873.e10. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.; Lieberman, J. Update on clinical research for food allergy treatment. Front. Allergy 2023, 4, 1154541. [Google Scholar] [CrossRef]
- Wood, R.A.; Togias, A.; Sicherer, S.H.; Shreffler, W.G.; Kim, E.H.; Jones, S.M.; Leung, D.Y.M.; Vickery, B.P.; Bird, J.A.; Spergel, J.M.; et al. Omalizumab, an anti-IgE monoclonal antibody that blocks the IgE receptor binding site, for the Treatment of Multiple Food Allergies. N. Engl. J. Med. 2024, 390, 889–899. [Google Scholar] [CrossRef] [PubMed]
- MacGinnitie, A.J.; Rachid, R.; Gragg, H.; Little, S.V.; Lakin, P.; Cianferoni, A.; Heimall, J.; Makhija, M.; Robison, R.; Chinthrajah, R.S.; et al. Omalizumab, an anti-IgE monoclonal antibody that blocks the IgE receptor binding site, facilitates rapid oral desensitization for peanut allergy. J. Allergy Clin. Immunol. 2017, 139, 873–881.e8. [Google Scholar] [CrossRef]
- Bajzik, V.; DeBerg, H.A.; Garabatos, N.; Rust, B.J.; Obrien, K.K.; Nguyen, Q.-A.; O’Rourke, C.; Smith, A.; Walker, A.H.; Quinn, C.; et al. Oral desensitization therapy for peanut allergy induces dynamic changes in peanut-specific immune responses. Allergy 2022, 77, 2534–2548. [Google Scholar] [CrossRef]
- Anagnostou, K.; Islam, S.; King, Y.; Foley, L.; Pasea, L.; Bond, S.; Palmer, C.; Deighton, J.; Ewan, P.; Clark, A. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): A phase 2 randomised controlled trial. Lancet 2014, 383, 1297–1304. [Google Scholar] [CrossRef]
- Reier-Nilsen, T.; Michelsen, M.M.; Lødrup Carlsen, K.C.; Carlsen, K.-H.; Mowinckel, P.; Nygaard, U.C.; Namork, E.; Borres, M.P.; Håland, G. Feasibility of desensitizing children highly allergic to peanut by high-dose oral immunotherapy. Allergy 2019, 74, 337–348. [Google Scholar] [CrossRef]
- Virkud, Y.V.; Burks, A.W.; Steele, P.H.; Edwards, L.J.; Berglund, J.P.; Jones, S.M.; Scurlock, A.M.; Perry, T.T.; Pesek, R.D.; Vickery, B.P. Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy. J. Allergy Clin. Immunol. 2017, 139, 882–888.e5. [Google Scholar] [CrossRef]
- Foti Randazzese, S.; Panasiti, I.; Caminiti, L.; Catamerò, F.; Landi, M.; De Filippo, M.; Votto, M.; Olcese, R.; Favuzza, F.; Giovannini, M.; et al. Current state and advances in desensitization for peanut allergy in pediatric age. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2024, 35, e14127. [Google Scholar] [CrossRef]
- Zhang, W.; Sindher, S.B.; Sampath, V.; Nadeau, K. Comparison of sublingual immunotherapy and oral immunotherapy in peanut allergy. Allergo J. Int. 2018, 27, 153. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Bird, J.A.; Kulis, M.; Laubach, S.; Pons, L.; Shreffler, W.; Steele, P.; Kamilaris, J.; Vickery, B.; Burks, A.W. Sublingual immunotherapy for peanut allergy: Clinical and immunologic evidence of desensitization. J. Allergy Clin. Immunol. 2011, 127, 640–646.e1. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M.; Burks, A.W.; Vickery, B.P.; Scurlock, A.M.; Wood, R.A.; Jones, S.M.; Sicherer, S.H.; Liu, A.H.; Stablein, D.; Henning, A.K.; et al. Sublingual immunotherapy for peanut allergy: A randomized, double-blind, placebo-controlled multicenter trial. J. Allergy Clin. Immunol. 2013, 131, 119–127.e7. [Google Scholar] [CrossRef] [PubMed]
- Burks, A.W.; Wood, R.A.; Jones, S.M.; Sicherer, S.H.; Fleischer, D.M.; Scurlock, A.M.; Vickery, B.P.; Liu, A.H.; Henning, A.K.; Lindblad, R.; et al. Sublingual immunotherapy for peanut allergy: Long-term follow-up of a randomized multicenter trial. J. Allergy Clin. Immunol. 2015, 135, 1240–1248.e3. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.J.; Vickery, B.P.; Kulis, M.D.; Kim, E.H.; Varshney, P.; Steele, P.; Kamilaris, J.; Hiegel, A.M.; Carlisle, S.K.; Smith, P.B.; et al. Sublingual versus oral immunotherapy for peanut-allergic children: A retrospective comparison. J. Allergy Clin. Immunol. 2013, 132, 476. [Google Scholar] [CrossRef]
- Jones, S.M.; Agbotounou, W.K.; Fleischer, D.M.; Burks, A.W.; Pesek, R.D.; Harris, M.W.; Martin, L.; Thebault, C.; Ruban, C.; Benhamou, P.-H. Safety of epicutaneous immunotherapy for the treatment of peanut allergy: A phase 1 study using the Viaskin patch. J. Allergy Clin. Immunol. 2016, 137, 1258–1261.e10. [Google Scholar] [CrossRef]
- Scurlock, A.M.; Burks, A.W.; Sicherer, S.H.; Leung, D.Y.M.; Kim, E.H.; Henning, A.K.; Dawson, P.; Lindblad, R.W.; Berin, M.C.; Cho, C.B.; et al. Epicutaneous immunotherapy for treatment of peanut allergy: Follow-up from the Consortium for Food Allergy Research. J. Allergy Clin. Immunol. 2020, 147, 992. [Google Scholar] [CrossRef]
- Fleischer, D.M.; Greenhawt, M.; Sussman, G.; Bégin, P.; Nowak-Wegrzyn, A.; Petroni, D.; Beyer, K.; Brown-Whitehorn, T.; Hebert, J.; Hourihane, J.O.; et al. Effect of Epicutaneous Immunotherapy vs Placebo on Reaction to Peanut Protein Ingestion Among Children with Peanut Allergy: The PEPITES Randomized Clinical Trial. JAMA 2019, 321, 946–955. [Google Scholar] [CrossRef]
- Jones, S.M.; Sicherer, S.H.; Burks, A.W.; Leung, D.Y.M.; Lindblad, R.W.; Dawson, P.; Henning, A.K.; Berin, M.C.; Chiang, D.; Vickery, B.P.; et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J. Allergy Clin. Immunol. 2017, 139, 1242–1252.e9. [Google Scholar] [CrossRef]
- Mondoulet, L.; Dioszeghy, V.; Ligouis, M.; Dhelft, V.; Puteaux, E.; Dupont, C.; Benhamou, P.-H. Epicutaneous Immunotherapy Compared with Sublingual Immunotherapy in Mice Sensitized to Pollen (Phleum pratense). ISRN Allergy 2012, 2012, 375735. [Google Scholar] [CrossRef]
- Bird, J.A.; Sánchez-Borges, M.; Ansotegui, I.J.; Ebisawa, M.; Martell, J.A.O. Skin as an immune organ and clinical applications of skin-based immunotherapy. World Allergy Organ. J. 2018, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.K.; Zhou, C.; Wu, M.X. Laser-facilitated epicutaneous immunotherapy to IgE-mediated allergy. J. Control. Release Off. J. Control. Release Soc. 2016, 235, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shah, D.; Kositratna, G.; Manstein, D.; Anderson, R.R.; Wu, M.X. Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology. J. Control. Release Off. J. Control. Release Soc. 2012, 159, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Tripp, C.H.; Voit, H.; An, A.; Seidl-Philipp, M.; Krapf, J.; Sigl, S.; Romani, N.; Del Frari, B.; Stoitzner, P. Laser-assisted epicutaneous immunization to target human skin dendritic cells. Exp. Dermatol. 2021, 30, 1279–1289. [Google Scholar] [CrossRef]
- Amani, H.; Shahbazi, M.-A.; D’Amico, C.; Fontana, F.; Abbaszadeh, S.; Santos, H.A. Microneedles for painless transdermal immunotherapeutic applications. J. Control. Release Off. J. Control. Release Soc. 2021, 330, 185–217. [Google Scholar] [CrossRef]
- Shakya, A.K.; Ingrole, R.S.J.; Joshi, G.; Uddin, M.J.; Anvari, S.; Davis, C.M.; Gill, H.S. Microneedles coated with peanut allergen enable desensitization of peanut sensitized mice. J. Control. Release Off. J. Control. Release Soc. 2019, 314, 38–47. [Google Scholar] [CrossRef]
- Landers, J.J.; Janczak, K.W.; Shakya, A.K.; Zarnitsyn, V.; Patel, S.R.; Baker, J.R.; Gill, H.S.; O’Konek, J.J. Targeted allergen-specific immunotherapy within the skin improves allergen delivery to induce desensitization to peanut. Immunotherapy 2022, 14, 539–552. [Google Scholar] [CrossRef]
- Brown-Whitehorn, T.F.; de Blay, F.; Spergel, J.M.; Green, T.D.; Peillon, A.; Sampson, H.A.; Campbell, D.E. Sustained unresponsiveness to peanut after long-term peanut epicutaneous immunotherapy. J. Allergy Clin. Immunol. Pract. 2021, 9, 524–526. [Google Scholar] [CrossRef]
- Fleischer, D.M.; Shreffler, W.G.; Campbell, D.E.; Green, T.D.; Anvari, S.; Assa’ad, A.; Bégin, P.; Beyer, K.; Bird, J.A.; Brown-Whitehorn, T.; et al. Long-term, open-label extension study of the efficacy and safety of epicutaneous immunotherapy for peanut allergy in children: PEOPLE 3-year results. J. Allergy Clin. Immunol. 2020, 146, 863–874. [Google Scholar] [CrossRef]
- Hong, T.S.; Hu, A.; Fahim, G.; Hermes-DeSantis, E.R. Emerging Therapies for Peanut Allergy. J. Pharm. Pract. 2022, 35, 289–297. [Google Scholar] [CrossRef]
- Zuberbier, T.; Wood, R.A.; Bindslev-Jensen, C.; Fiocchi, A.; Chinthrajah, R.S.; Worm, M.; Deschildre, A.; Fernandez-Rivas, M.; Santos, A.F.; Jaumont, X.; et al. Omalizumab, an anti-IgE monoclonal antibody that blocks the IgE receptor binding site, in IgE-Mediated Food Allergy: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2023, 11, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Stranks, A.J.; Minnicozzi, S.C.; Miller, S.J.; Burton, O.T.; Logsdon, S.L.; Spergel, J.M.; Nadeau, K.C.; Pongracic, J.A.; Umetsu, D.T.; Rachid, R.; et al. IgE blockade during food allergen ingestion enhances the induction of inhibitory IgG antibodies. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2019, 122, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Sindher, S.B.; Fiocchi, A.; Zuberbier, T.; Arasi, S.; Wood, R.A.; Chinthrajah, R.S. The Role of Biologics in the Treatment of Food Allergy. J. Allergy Clin. Immunol. Pract. 2024, 12, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, J.P.; Anderson, B.; Sindher, S.B. New biologics for food allergy. Curr. Opin. Allergy Clin. Immunol. 2024, 24, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hils, M.; Fischer, A.; Wölbing, F.; Biedermann, T.; Schnieke, A.; Fischer, K. Gene-edited pigs: A translational model for human food allergy against alpha-Gal and anaphylaxis. Front. Immunol. 2024, 15, 1358178. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z. CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Comput. Struct. Biotechnol. J. 2020, 18, 2401–2415. [Google Scholar] [CrossRef]
- Wang, M.; Schedel, M.; Gelfand, E.W. Gene editing in allergic diseases: Identification of novel pathways and impact of deleting allergen genes. J. Allergy Clin. Immunol. 2024, 154, 51–58. [Google Scholar] [CrossRef]
- Wang, M.; Strand, M.J.; Lanser, B.J.; Santos, C.; Bendelja, K.; Fish, J.; Esterl, E.A.; Ashino, S.; Abbott, J.K.; Knight, V.; et al. Expression and activation of the steroidogenic enzyme CYP11A1 is associated with IL-13 production in T cells from peanut allergic children. PLoS ONE 2020, 15, e0233563. [Google Scholar] [CrossRef]
- Dodo, H.W.; Konan, K.N.; Chen, F.C.; Egnin, M.; Viquez, O.M. Alleviating peanut allergy using genetic engineering: The silencing of the immunodominant allergen Ara h 2 leads to its significant reduction and a decrease in peanut allergenicity. Plant Biotechnol. J. 2008, 6, 135–145. [Google Scholar] [CrossRef]
- Wollenberg, A.; Ikeda, M.; Chu, C.-Y.; Eichenfield, L.F.; Seyger, M.M.B.; Prakash, A.; Angle, R.; Zhu, D.; Pontes, M.; Paller, A.S. Longer-term safety and efficacy of baricitinib for atopic dermatitis in pediatric patients 2 to <18 years old: A randomized clinical trial of extended treatment to 3.6 years. J. Dermatol. Treat. 2024, 35, 2411834. [Google Scholar] [CrossRef]
- Armario-Hita, J.C.; Pereyra-Rodriguez, J.J.; González-Quesada, A.; Herranz, P.; Suarez, R.; Galan-Gutiérrez, M.; Rodríguez-Serna, M.; Ortiz de Frutos, J.; Carrascosa, J.M.; Serra-Baldrich, E.; et al. Treatment of atopic dermatitis with abrocitinib in real practice in Spain: Efficacy and safety results from a 24-week multicenter study. Int. J. Dermatol. 2024, 63, e289–e295. [Google Scholar] [CrossRef] [PubMed]
- Zirpel, H.; Ludwig, R.J.; Olbrich, H.; Kridin, K.; Ständer, S.; Thaçi, D. Comparison of safety profile in patients with atopic dermatitis treated with dupilumab or conventional systemic treatment: Real world data from the US network. J. Dermatol. Treat. 2024, 35, 2421429. [Google Scholar] [CrossRef] [PubMed]
- Ireland, P.A.; Verheyden, M.; Jansson, N.; Sebaratnam, D.; Sullivan, J. Infection risk with JAK inhibitors in dermatoses: A meta-analysis. Int. J. Dermatol. 2024, 64, 24–36. [Google Scholar] [CrossRef]
- Perricone, C.; Pozzolo, R.D.; Cafaro, G.; Calvacchi, S.; Bruno, L.; Tromby, F.; Colangelo, A.; Gerli, R.; Bartoloni, E. Sudden improvement of alopecia universalis and psoriatic arthritis while receiving upadacitinib: A case-based review. Reumatismo 2024. [Google Scholar] [CrossRef] [PubMed]
| Treatment Approach | Description | Advantages | Limitations |
|---|---|---|---|
| Allergen Avoidance | Avoidance of peanuts and peanut-containing products. | Reduces risk of anaphylaxis. | Challenging due to inconsistent food labeling and risk of accidental exposure. |
| Epinephrine | Intramuscular or intranasal administration during anaphylaxis. | Definitive treatment for anaphylaxis. | Requires immediate access; burden of self-carrying; cost may be prohibitive. |
| Antihistamines and Corticosteroids | Adjunctive treatments for symptom relief post-epinephrine administration. | Relief of cutaneous symptoms and potential decrease in hospital stay. | Ineffective for acute anaphylaxis; cannot prevent biphasic reactions. |
| Immunotherapy Type | Delivery Method | Dose Range | Key Advantages | Common Side Effects | Challenges |
|---|---|---|---|---|---|
| OIT | Oral ingestion of allergen | Milligram-level doses | High efficacy; FDA-approved for peanut allergy. | Gastrointestinal symptoms; systemic reactions. | Psychological stress; long-term commitment. |
| SLIT | Allergen under the tongue | Micrograms to low milligrams | Fewer severe reactions; home dosing possible. | Oropharyngeal pruritus; mild systemic symptoms. | Lower efficacy compared to OIT. |
| EPIT | Allergen patch on skin | Microgram doses | Minimizes severe reactions; child-friendly. | Local site reactions (pruritus, redness). | Limited human studies; lower desensitization. |
| Treatment | Study/Trial | Number of Subjects | Efficacy Rate | Frequency of Side Effects |
|---|---|---|---|---|
| Oral Immunotherapy (OIT) | PALISADE Trial (Peanut Allergy Oral Immunotherapy Study of AR101 for Desensitization) | 496 | 67.2% tolerated 600 mg vs. 4% placebo | 95% had adverse events; 59.7% moderate; 4.3% severe |
| Sublingual Immunotherapy (SLIT) | PITS (Peanut Sublingual Immunotherapy | 50 randomized | 60% desensitization vs. 0% placebo; 48% remission vs. 0% placebo | Oropharyngeal itching more common in SLIT group; other adverse events similar |
| Epicutaneous Immunotherapy (EPIT) | EPITOPE Trial (Epicutaneous Treatment of Peanut Allergy in Toddlers) | 362 randomized | 67% response vs. 33.5% placebo | 100% adverse events (mostly mild); 1.6% treatment-related anaphylaxis |
| Biologic Agents (e.g., Omalizumab) | OUtMATCH Trial (Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen Oral Immunotherapy) | 177 randomized | 67% (peanut) | More injection-site reactions in omalizumab group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satnarine, T.; Xavier de Almeida, A.; Woody, M.; Banegas Carballo, K.; Chan, D.; Thompson, P.; Kleiner, G.; Gans, M. Emerging Treatment Options for Peanut Allergy. Allergies 2025, 5, 5. https://doi.org/10.3390/allergies5010005
Satnarine T, Xavier de Almeida A, Woody M, Banegas Carballo K, Chan D, Thompson P, Kleiner G, Gans M. Emerging Treatment Options for Peanut Allergy. Allergies. 2025; 5(1):5. https://doi.org/10.3390/allergies5010005
Chicago/Turabian StyleSatnarine, Travis, Alana Xavier de Almeida, Malaika Woody, Krisia Banegas Carballo, Diana Chan, Pytregay Thompson, Gary Kleiner, and Melissa Gans. 2025. "Emerging Treatment Options for Peanut Allergy" Allergies 5, no. 1: 5. https://doi.org/10.3390/allergies5010005
APA StyleSatnarine, T., Xavier de Almeida, A., Woody, M., Banegas Carballo, K., Chan, D., Thompson, P., Kleiner, G., & Gans, M. (2025). Emerging Treatment Options for Peanut Allergy. Allergies, 5(1), 5. https://doi.org/10.3390/allergies5010005






