Forkhead Box Protein P3 in the Immune System
Abstract
1. Introduction
2. Molecular Features of FOXP3
3. FOXP3 Expression in Tregs and Teffs
3.1. FOXP3 Expression in Tregs
3.2. Activation-Induced FOXP3 Expression in T Cells
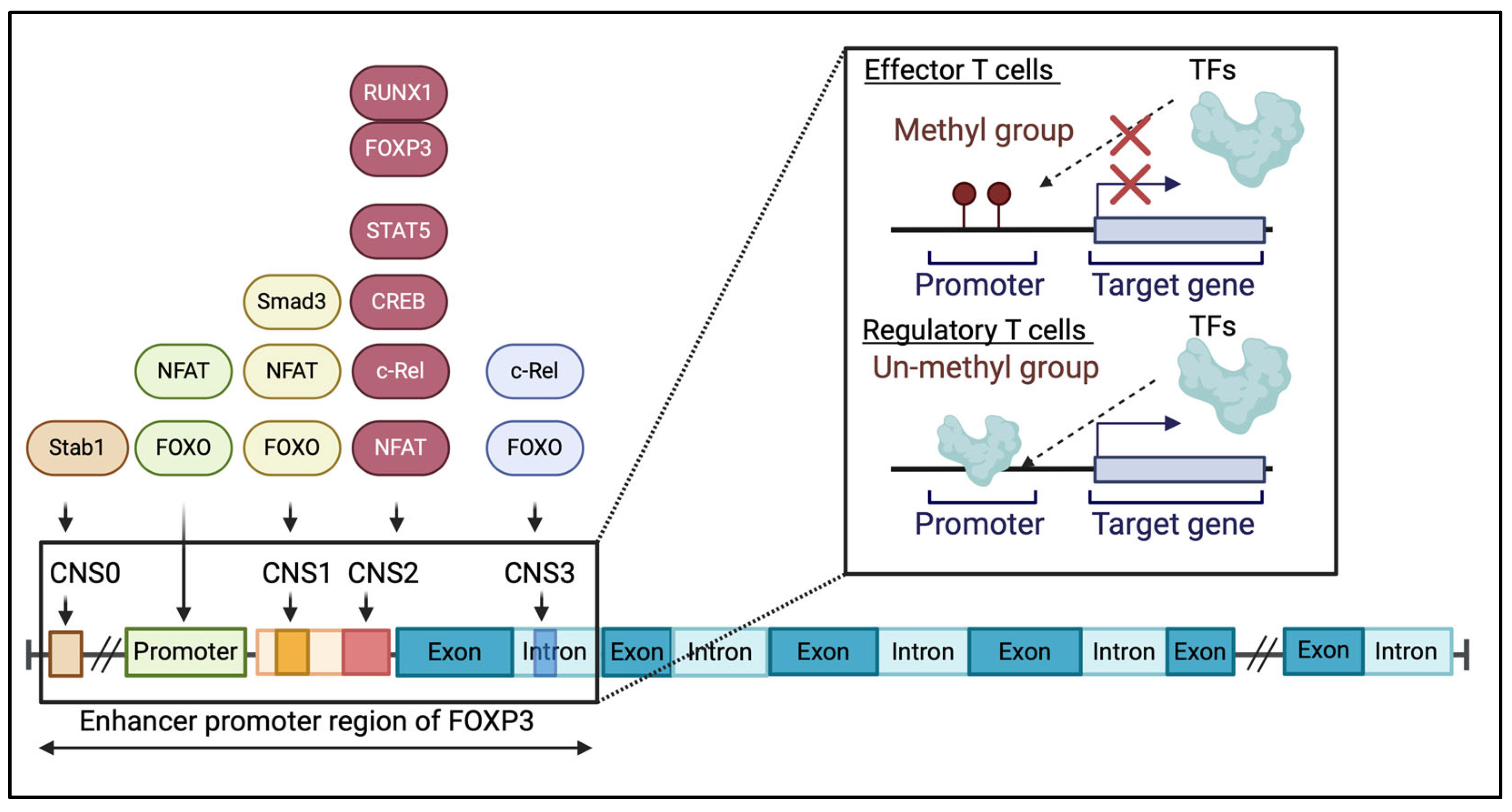
4. FOXP3 Gene Mutations Are Associated with IPEX Syndrome
5. Heterogenicity and Plasticity of FOXP3+ Tregs
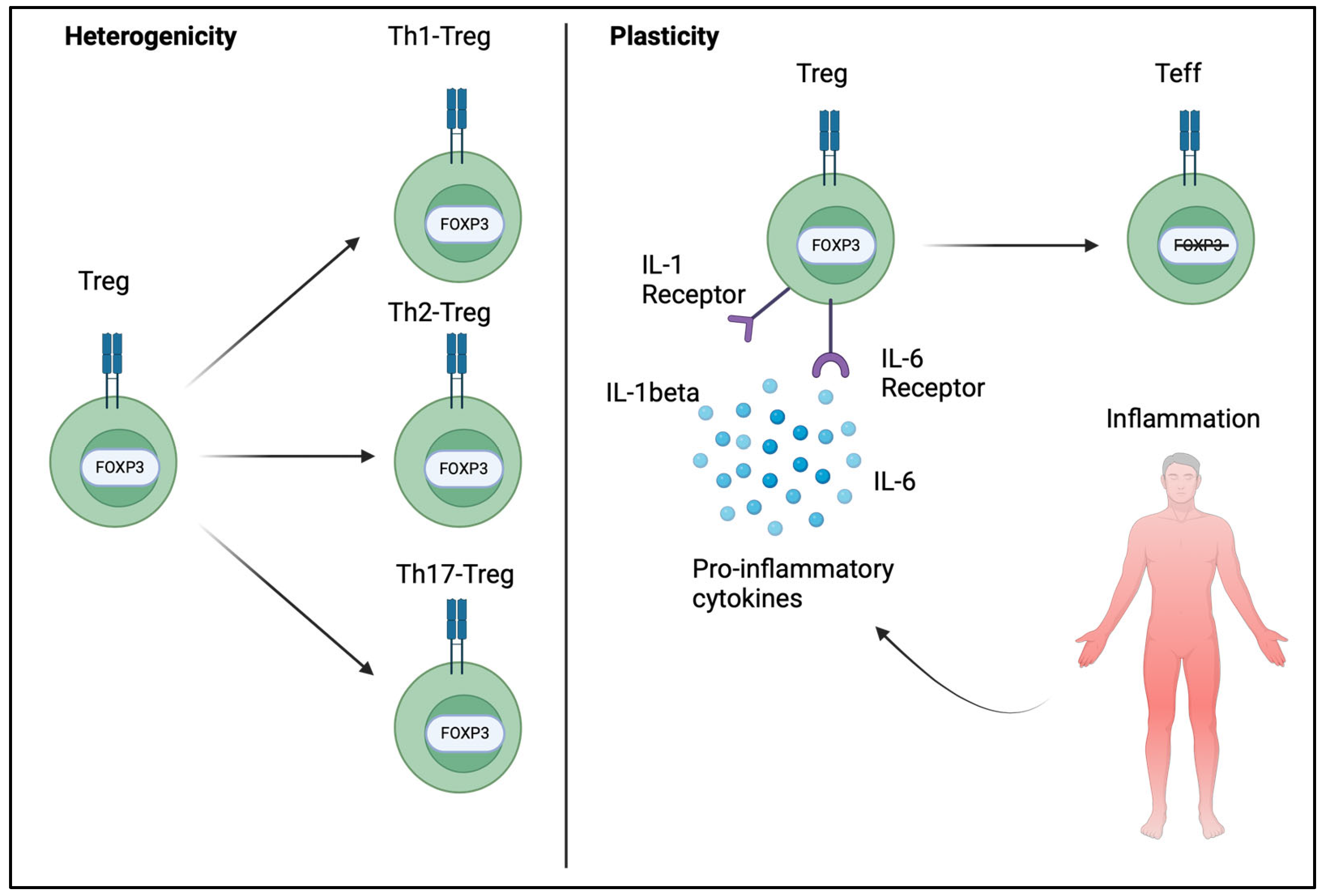
6. FOXP3’s Implications in Autoimmune Disorders
6.1. T1D
6.2. IBD
6.3. Multiple Sclerosis and Myasthenia Gravis
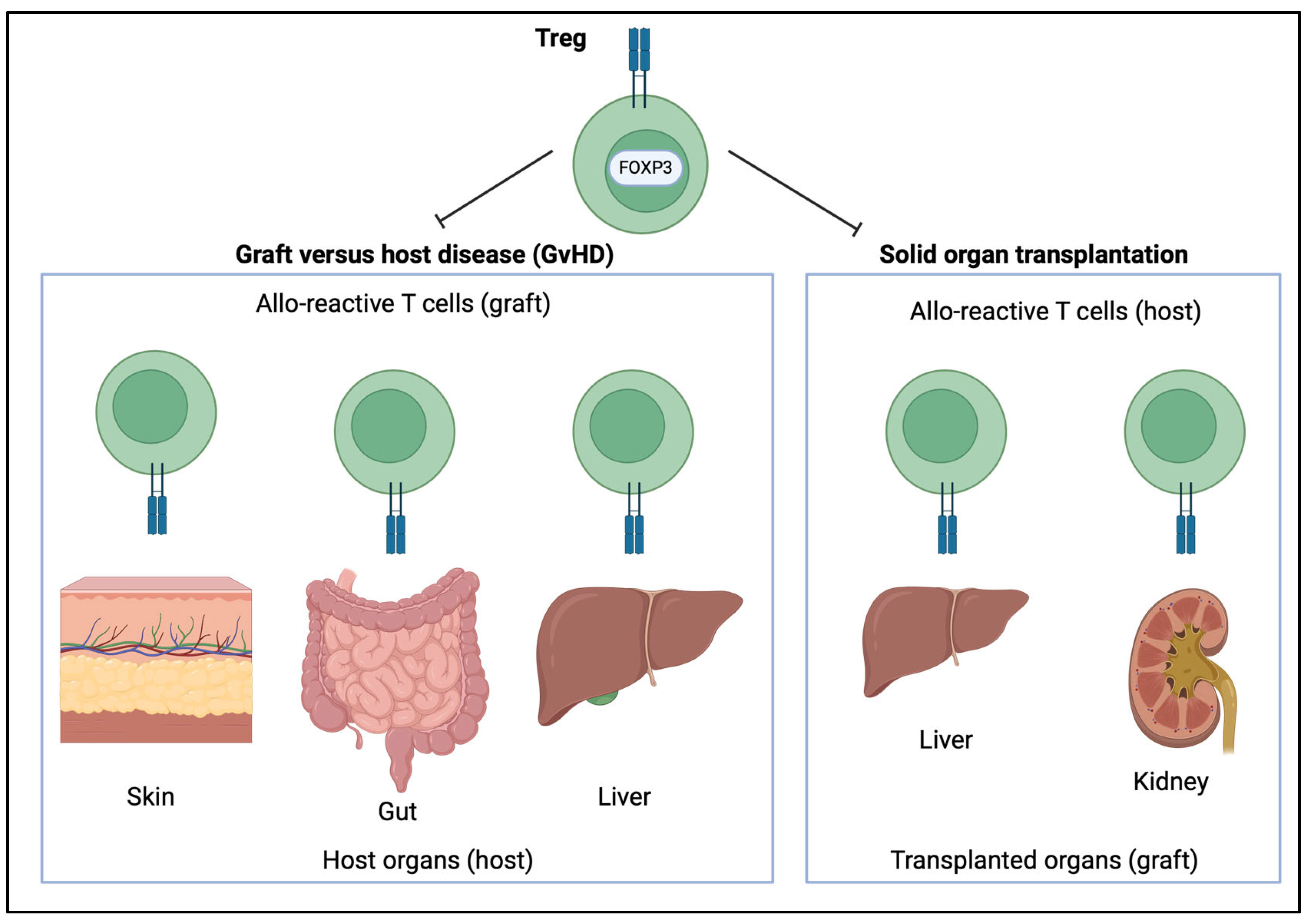
7. The Role of FOXP3 in Transplantation
7.1. The Role of FOXP3 in Hematopoietic Stem Cell Transplantation
7.2. The Role of FOXP3 in Solid Organ Transplantation
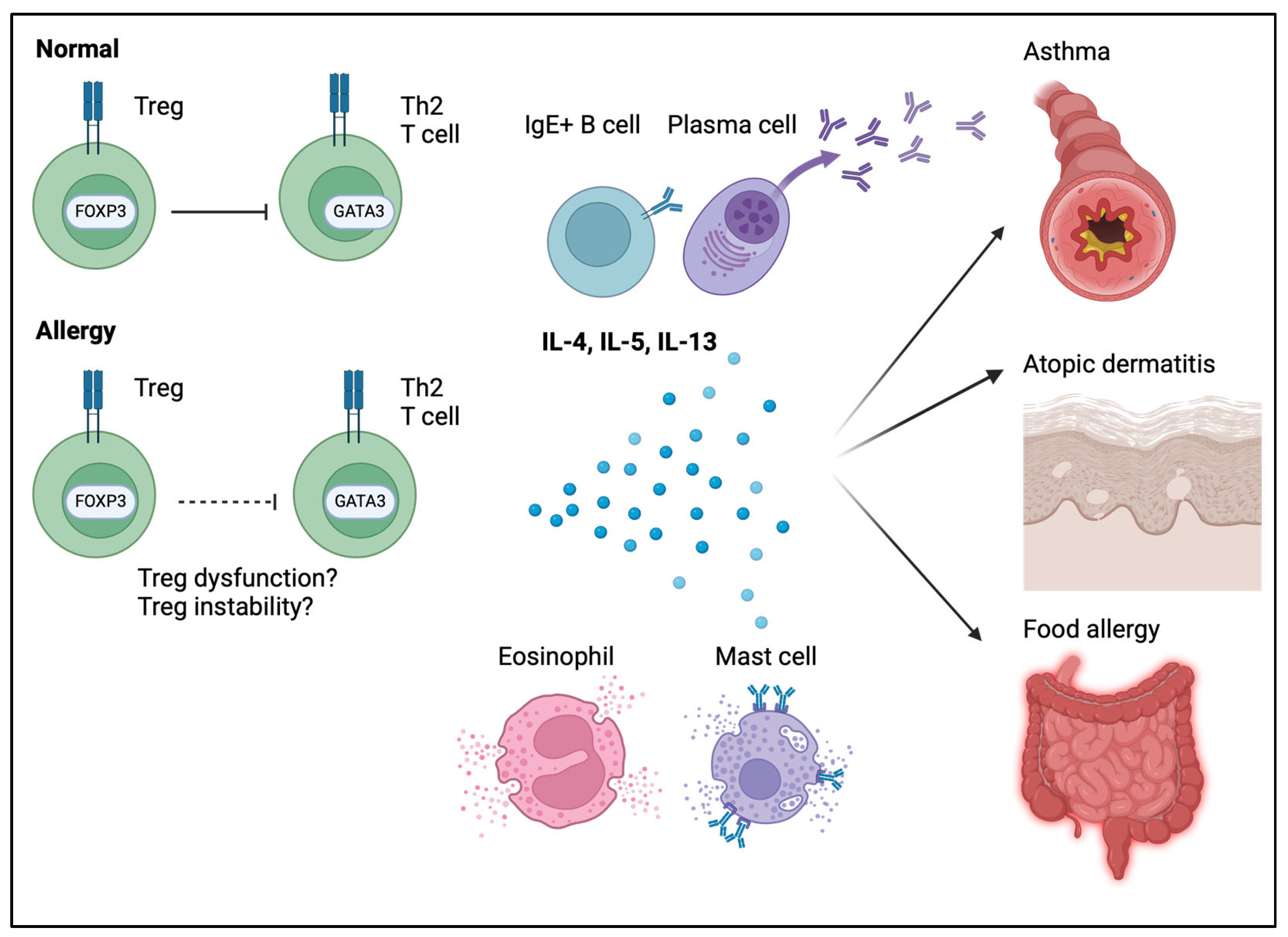
8. The Role of FOXP3 in Allergic Disease
8.1. The Role of Tregs in Food Allergies
8.2. The Role of Tregs in Various Allergic Diseases (Asthma, Atopic Dermatitis, and Urticaria)
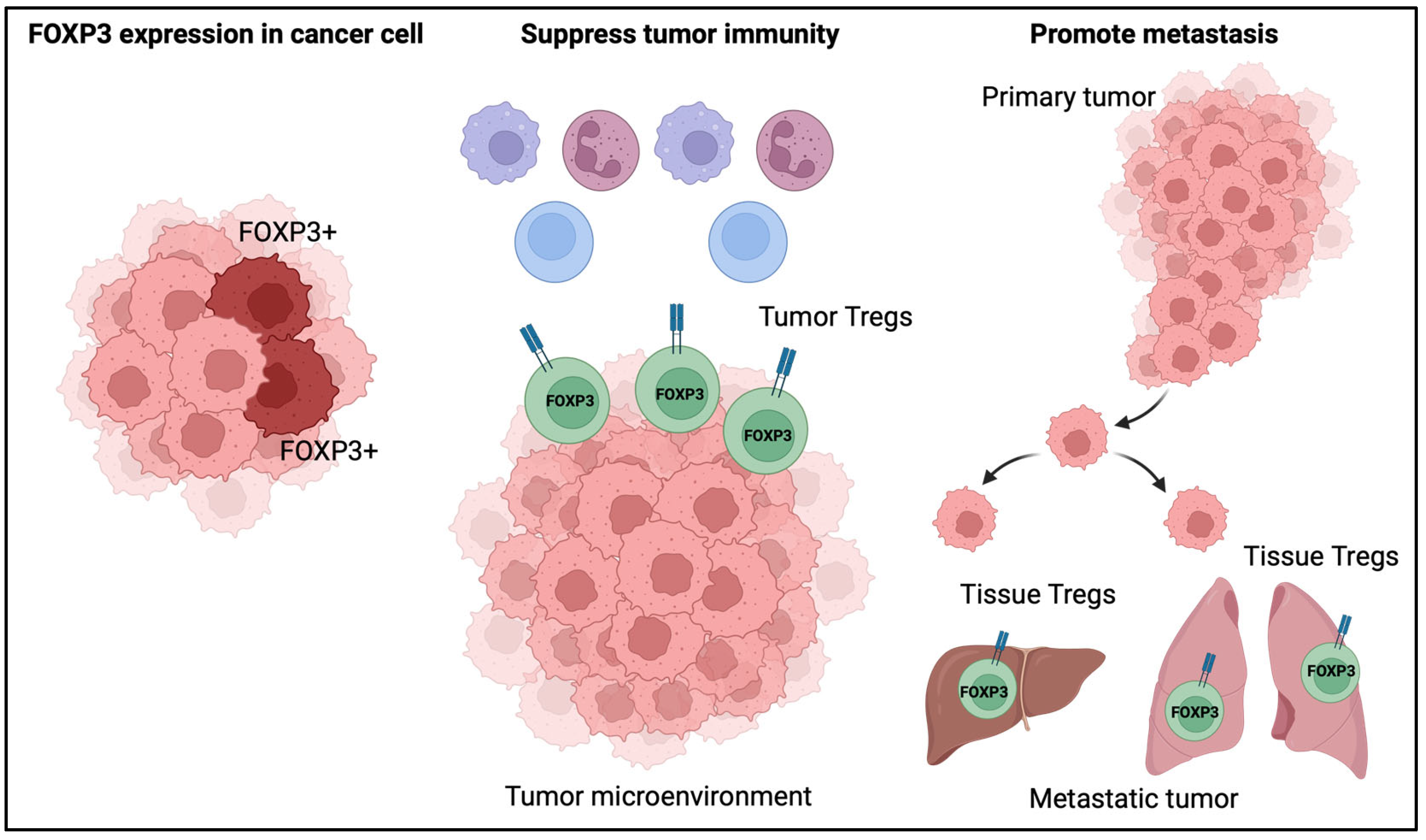
9. The Role of FOXP3 in Cancer
9.1. The Role of FOXP3 Expression in Cancer Cells
9.2. The Role of Tregs in the Tumor Microenvironment
9.3. The Role of Tregs in Tumor Metastasis
10. Treg Cell Therapy
10.1. Engineered Tregs
10.2. CAR-Treg
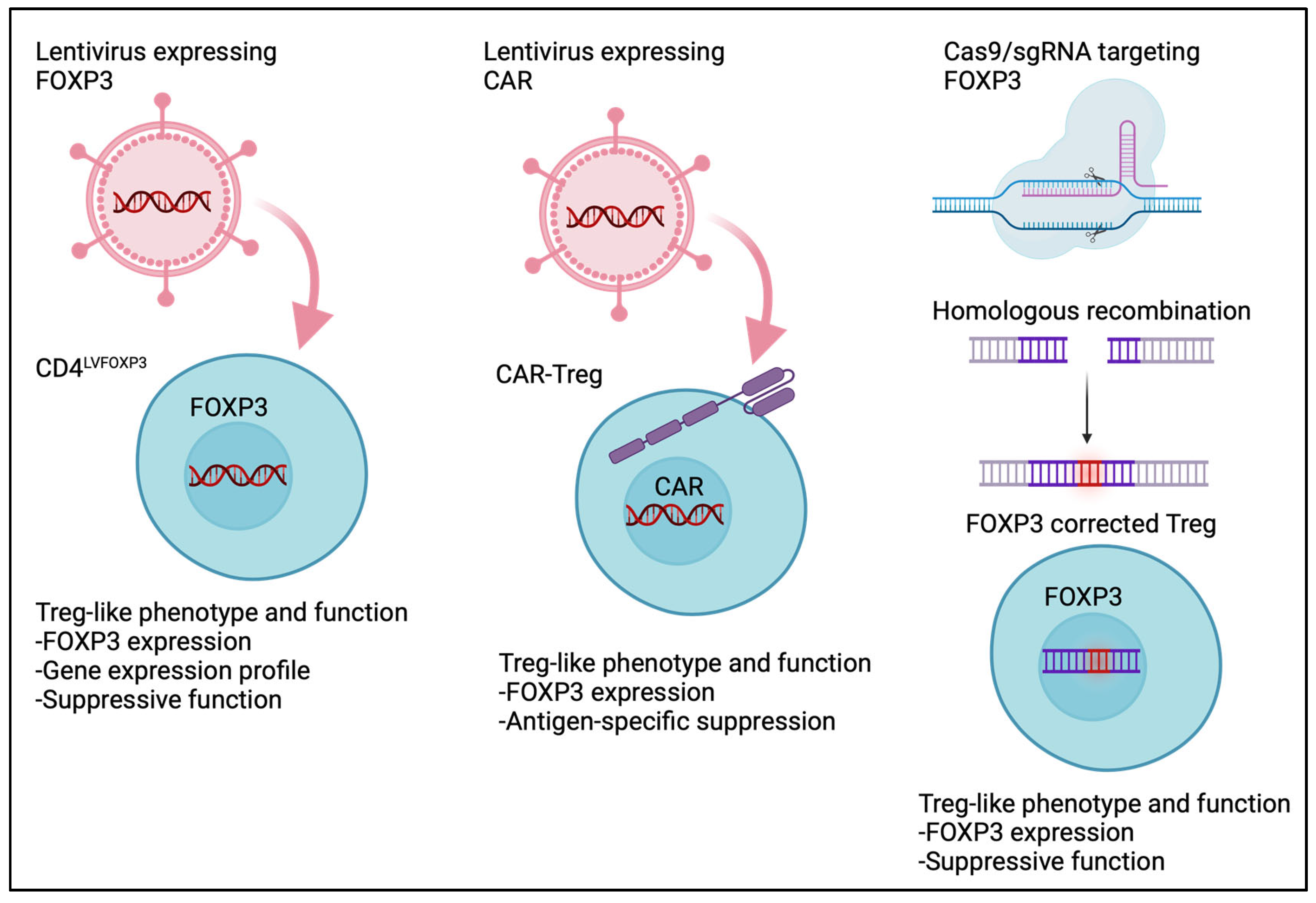
11. Ethical Issues in Treg-Based Therapy
12. Limitations of This Study
13. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef]
- Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef]
- Lam, E.W.; Brosens, J.J.; Gomes, A.R.; Koo, C.Y. Forkhead box proteins: Tuning forks for transcriptional harmony. Nat. Rev. Cancer 2013, 13, 482–495. [Google Scholar] [CrossRef]
- Song, X.; Li, B.; Xiao, Y.; Chen, C.; Wang, Q.; Liu, Y.; Berezov, A.; Xu, C.; Gao, Y.; Li, Z.; et al. Structural and biological features of FOXP3 dimerization relevant to regulatory T cell function. Cell Rep. 2012, 1, 665–675. [Google Scholar] [CrossRef]
- Zeng, W.P.; Sollars, V.E.; Belalcazar Adel, P. Domain requirements for the diverse immune regulatory functions of foxp3. Mol. Immunol. 2011, 48, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Bhairavabhotla, R.; Kim, Y.C.; Glass, D.D.; Escobar, T.M.; Patel, M.C.; Zahr, R.; Nguyen, C.K.; Kilaru, G.K.; Muljo, S.A.; Shevach, E.M. Transcriptome profiling of human FoxP3+ regulatory T cells. Hum. Immunol. 2016, 77, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Sadlon, T.J.; Wilkinson, B.G.; Pederson, S.; Brown, C.Y.; Bresatz, S.; Gargett, T.; Melville, E.L.; Peng, K.; D’Andrea, R.J.; Glonek, G.G.; et al. Genome-wide identification of human FOXP3 target genes in natural regulatory T cells. J. Immunol. 2010, 185, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Van der Veeken, J.; Glasner, A.; Zhong, Y.; Hu, W.; Wang, Z.M.; Bou-Puerto, R.; Charbonnier, L.M.; Chatila, T.A.; Leslie, C.S.; Rudensky, A.Y. The Transcription Factor Foxp3 Shapes Regulatory T Cell Identity by Tuning the Activity of trans-Acting Intermediaries. Immunity 2020, 53, 971–984 e975. [Google Scholar] [CrossRef]
- Delacher, M.; Simon, M.; Sanderink, L.; Hotz-Wagenblatt, A.; Wuttke, M.; Schambeck, K.; Schmidleithner, L.; Bittner, S.; Pant, A.; Ritter, U.; et al. Single-cell chromatin accessibility landscape identifies tissue repair program in human regulatory T cells. Immunity 2021, 54, 702–720 e717. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.L.; Mahmud, S.A.; Sjaastad, L.E.; Williams, J.B.; Spanier, J.A.; Simeonov, D.R.; Ruscher, R.; Huang, W.; Proekt, I.; Miller, C.N.; et al. Thymic regulatory T cells arise via two distinct developmental programs. Nat. Immunol. 2019, 20, 195–205. [Google Scholar] [CrossRef]
- Herppich, S.; Toker, A.; Pietzsch, B.; Kitagawa, Y.; Ohkura, N.; Miyao, T.; Floess, S.; Hori, S.; Sakaguchi, S.; Huehn, J. Dynamic Imprinting of the Treg Cell-Specific Epigenetic Signature in Developing Thymic Regulatory T Cells. Front. Immunol. 2019, 10, 2382. [Google Scholar] [CrossRef]
- Baron, U.; Werner, J.; Schildknecht, K.; Schulze, J.J.; Mulu, A.; Liebert, U.G.; Sack, U.; Speckmann, C.; Gossen, M.; Wong, R.J.; et al. Epigenetic immune cell counting in human blood samples for immunodiagnostics. Sci. Transl. Med. 2018, 10, eaan3508. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.E.; Crome, S.Q.; Crellin, N.K.; Passerini, L.; Steiner, T.S.; Bacchetta, R.; Roncarolo, M.G.; Levings, M.K. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007, 19, 345–354. [Google Scholar] [CrossRef]
- Wang, J.; Ioan-Facsinay, A.; van der Voort, E.I.; Huizinga, T.W.; Toes, R.E. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007, 37, 129–138. [Google Scholar] [CrossRef]
- Bennett, C.L.; Ochs, H.D. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr. Opin. Pediatr. 2001, 13, 533–538. [Google Scholar] [CrossRef]
- Chatila, T.A.; Blaeser, F.; Ho, N.; Lederman, H.M.; Voulgaropoulos, C.; Helms, C.; Bowcock, A.M. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Investig. 2000, 106, R75–R81. [Google Scholar] [CrossRef]
- Wildin, R.S.; Ramsdell, F.; Peake, J.; Faravelli, F.; Casanova, J.L.; Buist, N.; Levy-Lahad, E.; Mazzella, M.; Goulet, O.; Perroni, L.; et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001, 27, 18–20. [Google Scholar] [CrossRef]
- Powell, B.R.; Buist, N.R.; Stenzel, P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J. Pediatr. 1982, 100, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, R.; Barzaghi, F.; Roncarolo, M.G. From IPEX syndrome to FOXP3 mutation: A lesson on immune dysregulation. Ann. N. Y. Acad. Sci. 2018, 1417, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Barzaghi, F.; Amaya Hernandez, L.C.; Neven, B.; Ricci, S.; Kucuk, Z.Y.; Bleesing, J.J.; Nademi, Z.; Slatter, M.A.; Ulloa, E.R.; Shcherbina, A.; et al. Long-term follow-up of IPEX syndrome patients after different therapeutic strategies: An international multicenter retrospective study. J. Allergy Clin. Immunol. 2018, 141, 1036–1049 e1035. [Google Scholar] [CrossRef]
- Baxter, S.K.; Walsh, T.; Casadei, S.; Eckert, M.M.; Allenspach, E.J.; Hagin, D.; Segundo, G.; Lee, M.K.; Gulsuner, S.; Shirts, B.H.; et al. Molecular diagnosis of childhood immune dysregulation, polyendocrinopathy, and enteropathy, and implications for clinical management. J. Allergy Clin. Immunol. 2022, 149, 327–339. [Google Scholar] [CrossRef]
- Cepika, A.M.; Sato, Y.; Liu, J.M.; Uyeda, M.J.; Bacchetta, R.; Roncarolo, M.G. Tregopathies: Monogenic diseases resulting in regulatory T-cell deficiency. J. Allergy Clin. Immunol. 2018, 142, 1679–1695. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, C.; Wang, B.; Niu, Q.; Su, X.; Bai, Y.; Zhu, S.; Zhao, C.; Sun, Y.; Wang, J.; et al. Single-cell transcriptomic analysis reveals disparate effector differentiation pathways in human Treg compartment. Nat. Commun. 2021, 12, 3913. [Google Scholar] [CrossRef]
- Qiu, R.; Zhou, L.; Ma, Y.; Zhou, L.; Liang, T.; Shi, L.; Long, J.; Yuan, D. Regulatory T Cell Plasticity and Stability and Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2020, 58, 52–70. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Castillo, E.; Garcia-Rasilla, V.Y.; Garcia-Patino, M.G.; Licona-Limon, P. Stability and plasticity of regulatory T cells in health and disease. J. Leukoc. Biol. 2024, 116, 33–53. [Google Scholar] [CrossRef]
- Bjornvold, M.; Amundsen, S.S.; Stene, L.C.; Joner, G.; Dahl-Jorgensen, K.; Njolstad, P.R.; Ek, J.; Ascher, H.; Gudjonsdottir, A.H.; Lie, B.A.; et al. FOXP3 polymorphisms in type 1 diabetes and coeliac disease. J. Autoimmun. 2006, 27, 140–144. [Google Scholar] [CrossRef]
- Hull, C.M.; Peakman, M.; Tree, T.I.M. Regulatory T cell dysfunction in type 1 diabetes: What’s broken and how can we fix it? Diabetologia 2017, 60, 1839–1850. [Google Scholar] [CrossRef]
- Clough, J.N.; Omer, O.S.; Tasker, S.; Lord, G.M.; Irving, P.M. Regulatory T-cell therapy in Crohn’s disease: Challenges and advances. Gut 2020, 69, 942–952. [Google Scholar] [CrossRef]
- Zozulya, A.L.; Wiendl, H. The role of regulatory T cells in multiple sclerosis. Nat. Clin. Pract. Neurol. 2008, 4, 384–398. [Google Scholar] [CrossRef]
- Danikowski, K.M.; Jayaraman, S.; Prabhakar, B.S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J. Neuroinflammation 2017, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Komai, K.; Mise-Omata, S.; Iizuka-Koga, M.; Noguchi, Y.; Kondo, T.; Sakai, R.; Matsuo, K.; Nakayama, T.; Yoshie, O.; et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019, 565, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Flemming, A. NMDAR-directed CAAR T cells show promise for autoimmune encephalitis. Nat. Rev. Immunol. 2023, 23, 786. [Google Scholar] [CrossRef]
- Crunkhorn, S. CAAR T cells to treat encephalitis. Nat. Rev. Drug Discov. 2024, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Mielke, S.; Ahmadzadeh, M.; Kilical, Y.; Savani, B.N.; Zeilah, J.; Keyvanfar, K.; Montero, A.; Hensel, N.; Kurlander, R.; et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood 2006, 108, 1291–1297. [Google Scholar] [CrossRef]
- Meyer, E.H.; Laport, G.; Xie, B.J.; MacDonald, K.; Heydari, K.; Sahaf, B.; Tang, S.W.; Baker, J.; Armstrong, R.; Tate, K.; et al. Transplantation of donor grafts with defined ratio of conventional and regulatory T cells in HLA-matched recipients. JCI Insight 2019, 4, e127244. [Google Scholar] [CrossRef]
- Roncarolo, M.G.; Gregori, S.; Bacchetta, R.; Battaglia, M.; Gagliani, N. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity 2018, 49, 1004–1019. [Google Scholar] [CrossRef]
- Liu, J.M.; Chen, P.; Uyeda, M.J.; Cieniewicz, B.; Sayitoglu, E.C.; Thomas, B.C.; Sato, Y.; Bacchetta, R.; Cepika, A.M.; Roncarolo, M.G. Pre-clinical development and molecular characterization of an engineered type 1 regulatory T-cell product suitable for immunotherapy. Cytotherapy 2021, 23, 1017–1028. [Google Scholar] [CrossRef]
- Mfarrej, B.; Tresoldi, E.; Stabilini, A.; Paganelli, A.; Caldara, R.; Secchi, A.; Battaglia, M. Generation of donor-specific Tr1 cells to be used after kidney transplantation and definition of the timing of their in vivo infusion in the presence of immunosuppression. J. Transl. Med. 2017, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Truong, N.; Grossman, W.J.; Haribhai, D.; Williams, C.B.; Wang, J.; Martin, M.G.; Chatila, T.A. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J. Allergy Clin. Immunol. 2005, 116, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Narula, M.; Lakshmanan, U.; Borna, S.; Schulze, J.J.; Holmes, T.H.; Harre, N.; Kirkey, M.; Ramachandran, A.; Tagi, V.M.; Barzaghi, F.; et al. Epigenetic and immunological indicators of IPEX disease in subjects with FOXP3 gene mutation. J. Allergy Clin. Immunol. 2023, 151, 233–246 e210. [Google Scholar] [CrossRef]
- Van Gool, F.; Nguyen, M.L.T.; Mumbach, M.R.; Satpathy, A.T.; Rosenthal, W.L.; Giacometti, S.; Le, D.T.; Liu, W.; Brusko, T.M.; Anderson, M.S.; et al. A Mutation in the Transcription Factor Foxp3 Drives T Helper 2 Effector Function in Regulatory T Cells. Immunity 2019, 50, 362–377 e6. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Chatila, T.A. Regulatory T cells in allergic diseases. J. Allergy Clin. Immunol. 2016, 138, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, T.R.; Linane, A.; Moes, N.; Anover, S.; Mateo, V.; Rieux-Laucat, F.; Hermine, O.; Vijay, S.; Gambineri, E.; Cerf-Bensussan, N.; et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology 2007, 132, 1705–1717. [Google Scholar] [CrossRef]
- Syed, A.; Garcia, M.A.; Lyu, S.C.; Bucayu, R.; Kohli, A.; Ishida, S.; Berglund, J.P.; Tsai, M.; Maecker, H.; O’Riordan, G.; et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J. Allergy Clin. Immunol. 2014, 133, 500–510. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Cosenza, L.; Aitoro, R.; D’Argenio, V.; Del Monaco, V.; Di Scala, C.; Amoroso, A.; Di Costanzo, M.; Salvatore, F.; et al. Epigenetic features of FoxP3 in children with cow’s milk allergy. Clin. Epigenetics 2016, 8, 86. [Google Scholar] [CrossRef]
- Gu, Y.; Bartolome-Casado, R.; Xu, C.; Bertocchi, A.; Janney, A.; Heuberger, C.; Pearson, C.F.; Teichmann, S.A.; Thornton, E.E.; Powrie, F. Immune microniches shape intestinal T(reg) function. Nature 2024, 628, 854–862. [Google Scholar] [CrossRef]
- Provoost, S.; Maes, T.; Van Durme, Y.M.; Gevaert, P.; Bachert, C.; Schmidt-Weber, C.B.; Brusselle, G.G.; Joos, G.F.; Tournoy, K.G. Decreased FOXP3 protein expression in patients with asthma. Allergy 2009, 64, 1539–1546. [Google Scholar] [CrossRef]
- Roesner, L.M.; Floess, S.; Witte, T.; Olek, S.; Huehn, J.; Werfel, T. Foxp3(+) regulatory T cells are expanded in severe atopic dermatitis patients. Allergy 2015, 70, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, N.; Lehtimaki, S.; Lahl, K.; Savinko, T.; Lappetelainen, A.M.; Sparwasser, T.; Wolff, H.; Lauerma, A.; Alenius, H. Foxp3+ cells control Th2 responses in a murine model of atopic dermatitis. J. Investig. Dermatol. 2012, 132, 1672–1680. [Google Scholar] [CrossRef]
- Sun, R.S.; Sui, J.F.; Chen, X.H.; Ran, X.Z.; Yang, Z.F.; Guan, W.D.; Yang, T. Detection of CD4+ CD25+ FOXP3+ regulatory T cells in peripheral blood of patients with chronic autoimmune urticaria. Australas. J. Dermatol. 2011, 52, e15–e18. [Google Scholar] [CrossRef]
- Martin, F.; Ladoire, S.; Mignot, G.; Apetoh, L.; Ghiringhelli, F. Human FOXP3 and cancer. Oncogene 2010, 29, 4121–4129. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Seki, N.; Toh, U.; Hattori, S.; Kawahara, A.; Yamaguchi, T.; Koura, K.; Takahashi, R.; Otsuka, H.; Takahashi, H.; et al. FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol. Clin. Oncol. 2013, 1, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Liu, R.; Zhang, H.; Chang, X.; Liu, Y.; Wang, L.; Zheng, P.; Liu, Y. FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. J. Clin. Investig. 2007, 117, 3765–3773. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Saito, T.; Nishikawa, H.; Wada, H.; Nagano, Y.; Sugiyama, D.; Atarashi, K.; Maeda, Y.; Hamaguchi, M.; Ohkura, N.; Sato, E.; et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 2016, 22, 679–684. [Google Scholar] [CrossRef]
- Huppert, L.A.; Green, M.D.; Kim, L.; Chow, C.; Leyfman, Y.; Daud, A.I.; Lee, J.C. Tissue-specific Tregs in cancer metastasis: Opportunities for precision immunotherapy. Cell Mol. Immunol. 2022, 19, 33–45. [Google Scholar] [CrossRef]
- Shiri, A.M.; Fard-Aghaie, M.; Bedke, T.; Papazoglou, E.D.; Sabihi, M.; Zazara, D.E.; Zhang, S.; Lucke, J.; Seeger, P.; Evers, M.; et al. Foxp3 + Treg-derived IL-10 promotes colorectal cancer-derived lung metastasis. Sci. Rep. 2024, 14, 30483. [Google Scholar] [CrossRef]
- Kos, K.; Aslam, M.A.; van de Ven, R.; Wellenstein, M.D.; Pieters, W.; van Weverwijk, A.; Duits, D.E.M.; van Pul, K.; Hau, C.S.; Vrijland, K.; et al. Tumor-educated T(regs) drive organ-specific metastasis in breast cancer by impairing NK cells in the lymph node niche. Cell Rep. 2022, 38, 110447. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015, 7, 315ra189. [Google Scholar] [CrossRef]
- Ferreira, L.M.R.; Muller, Y.D.; Bluestone, J.A.; Tang, Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov. 2019, 18, 749–769. [Google Scholar] [CrossRef]
- Bluestone, J.A.; McKenzie, B.S.; Beilke, J.; Ramsdell, F. Opportunities for Treg cell therapy for the treatment of human disease. Front. Immunol. 2023, 14, 1166135. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Stabilini, A.; Migliavacca, B.; Horejs-Hoeck, J.; Kaupper, T.; Roncarolo, M.G. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 2006, 177, 8338–8347. [Google Scholar] [CrossRef]
- Fraser, H.; Safinia, N.; Grageda, N.; Thirkell, S.; Lowe, K.; Fry, L.J.; Scotta, C.; Hope, A.; Fisher, C.; Hilton, R.; et al. A Rapamycin-Based GMP-Compatible Process for the Isolation and Expansion of Regulatory T Cells for Clinical Trials. Mol. Ther. Methods Clin. Dev. 2018, 8, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Sawitzki, B.; Harden, P.N.; Reinke, P.; Moreau, A.; Hutchinson, J.A.; Game, D.S.; Tang, Q.; Guinan, E.C.; Battaglia, M.; Burlingham, W.J.; et al. Regulatory cell therapy in kidney transplantation (The ONE Study): A harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020, 395, 1627–1639. [Google Scholar] [CrossRef]
- Allan, S.E.; Alstad, A.N.; Merindol, N.; Crellin, N.K.; Amendola, M.; Bacchetta, R.; Naldini, L.; Roncarolo, M.G.; Soudeyns, H.; Levings, M.K. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol. Ther. 2008, 16, 194–202. [Google Scholar] [CrossRef]
- Passerini, L.; Rossi Mel, E.; Sartirana, C.; Fousteri, G.; Bondanza, A.; Naldini, L.; Roncarolo, M.G.; Bacchetta, R. CD4(+) T cells from IPEX patients convert into functional and stable regulatory T cells by FOXP3 gene transfer. Sci. Transl. Med. 2013, 5, 215ra174. [Google Scholar] [CrossRef]
- Sato, Y.; Passerini, L.; Piening, B.D.; Uyeda, M.J.; Goodwin, M.; Gregori, S.; Snyder, M.P.; Bertaina, A.; Roncarolo, M.G.; Bacchetta, R. Human-engineered Treg-like cells suppress FOXP3-deficient T cells but preserve adaptive immune responses in vivo. Clin. Transl. Immunol. 2020, 9, e1214. [Google Scholar] [CrossRef]
- Sato, Y.; Nathan, A.; Shipp, S.; Wright, J.F.; Tate, K.M.; Wani, P.; Roncarolo, M.G.; Bacchetta, R. A novel FOXP3 knockout-humanized mouse model for pre-clinical safety and efficacy evaluation of Treg-like cell products. Mol. Ther. Methods Clin. Dev. 2023, 31, 101150. [Google Scholar] [CrossRef]
- Borna, S.; Lee, E.; Sato, Y.; Bacchetta, R. Towards gene therapy for IPEX syndrome. Eur. J. Immunol. 2022, 52, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, M.; Lee, E.; Lakshmanan, U.; Shipp, S.; Froessl, L.; Barzaghi, F.; Passerini, L.; Narula, M.; Sheikali, A.; Lee, C.M.; et al. CRISPR-based gene editing enables FOXP3 gene repair in IPEX patient cells. Sci. Adv. 2020, 6, eaaz0571. [Google Scholar] [CrossRef] [PubMed]
- Honaker, Y.; Hubbard, N.; Xiang, Y.; Fisher, L.; Hagin, D.; Sommer, K.; Song, Y.; Yang, S.J.; Lopez, C.; Tappen, T.; et al. Gene editing to induce FOXP3 expression in human CD4(+) T cells leads to a stable regulatory phenotype and function. Sci. Transl. Med. 2020, 12, eaay6422. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.J.; Lin, D.T.S.; Gillies, J.K.; Uday, P.; Pesenacker, A.M.; Kobor, M.S.; Levings, M.K. Optimized CRISPR-mediated gene knockin reveals FOXP3-independent maintenance of human Treg identity. Cell Rep. 2021, 36, 109494. [Google Scholar] [CrossRef]
- Sato, Y.; Liu, J.; Lee, E.; Perriman, R.; Roncarolo, M.G.; Bacchetta, R. Co-Expression of FOXP3FL and FOXP3Delta2 Isoforms Is Required for Optimal Treg-Like Cell Phenotypes and Suppressive Function. Front. Immunol. 2021, 12, 752394. [Google Scholar] [CrossRef]
- McCullough, M.J.; Tune, M.K.; Cabrera, J.C.; Torres-Castillo, J.; He, M.; Feng, Y.; Doerschuk, C.M.; Dang, H.; Beltran, A.S.; Hagan, R.S.; et al. Characterization of the MT-2 Treg-like cell line in the presence and absence of forkhead box P3 (FOXP3). Immunol. Cell Biol. 2024, 102, 211–224. [Google Scholar] [CrossRef]
- Lam, A.J.; Haque, M.; Ward-Hartstonge, K.A.; Uday, P.; Wardell, C.M.; Gillies, J.K.; Speck, M.; Mojibian, M.; Klein Geltink, R.I.; Levings, M.K. PTEN is required for human Treg suppression of costimulation in vitro. Eur. J. Immunol. 2022, 52, 1482–1497. [Google Scholar] [CrossRef]
- Sato, Y.; Osada, E.; Manome, Y. Non-canonical NFKB signaling endows suppressive function through FOXP3-dependent regulatory T cell program. Heliyon 2023, 9, e22911. [Google Scholar] [CrossRef]
- MacDonald, K.G.; Hoeppli, R.E.; Huang, Q.; Gillies, J.; Luciani, D.S.; Orban, P.C.; Broady, R.; Levings, M.K. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Investig. 2016, 126, 1413–1424. [Google Scholar] [CrossRef]
- MacDonald, K.N.; Piret, J.M.; Levings, M.K. Methods to manufacture regulatory T cells for cell therapy. Clin. Exp. Immunol. 2019, 197, 52–63. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.N.; Ivison, S.; Hippen, K.L.; Hoeppli, R.E.; Hall, M.; Zheng, G.; Dijke, I.E.; Aklabi, M.A.; Freed, D.H.; Rebeyka, I.; et al. Cryopreservation timing is a critical process parameter in a thymic regulatory T-cell therapy manufacturing protocol. Cytotherapy 2019, 21, 1216–1233. [Google Scholar] [CrossRef] [PubMed]
- Dawson, N.A.; Lamarche, C.; Hoeppli, R.E.; Bergqvist, P.; Fung, V.C.; McIver, E.; Huang, Q.; Gillies, J.; Speck, M.; Orban, P.C.; et al. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight 2019, 4, e123672. [Google Scholar] [CrossRef] [PubMed]
- Safety & Tolerability Study of Chimeric Antigen Receptor T-Reg Cell Therapy in Living Donor Renal Transplant Recipients (STEADFAST). Available online: https://clinicaltrials.gov/study/NCT04817774 (accessed on 26 March 2021).
- Pierini, A.; Iliopoulou, B.P.; Peiris, H.; Perez-Cruz, M.; Baker, J.; Hsu, K.; Gu, X.; Zheng, P.P.; Erkers, T.; Tang, S.W.; et al. T cells expressing chimeric antigen receptor promote immune tolerance. JCI Insight 2017, 2, e92865. [Google Scholar] [CrossRef]
- Rui, X.; Alvarez Calderon, F.; Wobma, H.; Gerdemann, U.; Albanese, A.; Cagnin, L.; McGuckin, C.; Michaelis, K.A.; Naqvi, K.; Blazar, B.R.; et al. Human OX40L-CAR-T(regs) target activated antigen-presenting cells and control T cell alloreactivity. Sci. Transl. Med. 2024, 16, eadj9331. [Google Scholar] [CrossRef] [PubMed]
- Cargill, S.S.; Eidsvik, A.; Lamm, M. Updating the ethical guidance for gene and cell therapy research participation. Mol. Ther. 2021, 29, 2394–2395. [Google Scholar] [CrossRef]
- Rosenzwajg, M.; Lorenzon, R.; Cacoub, P.; Pham, H.P.; Pitoiset, F.; El Soufi, K.; C, R.I.; Bernard, C.; Aractingi, S.; Banneville, B.; et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 2019, 78, 209–217. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y. Forkhead Box Protein P3 in the Immune System. Allergies 2025, 5, 6. https://doi.org/10.3390/allergies5010006
Sato Y. Forkhead Box Protein P3 in the Immune System. Allergies. 2025; 5(1):6. https://doi.org/10.3390/allergies5010006
Chicago/Turabian StyleSato, Yohei. 2025. "Forkhead Box Protein P3 in the Immune System" Allergies 5, no. 1: 6. https://doi.org/10.3390/allergies5010006
APA StyleSato, Y. (2025). Forkhead Box Protein P3 in the Immune System. Allergies, 5(1), 6. https://doi.org/10.3390/allergies5010006




