2.1. System Overview
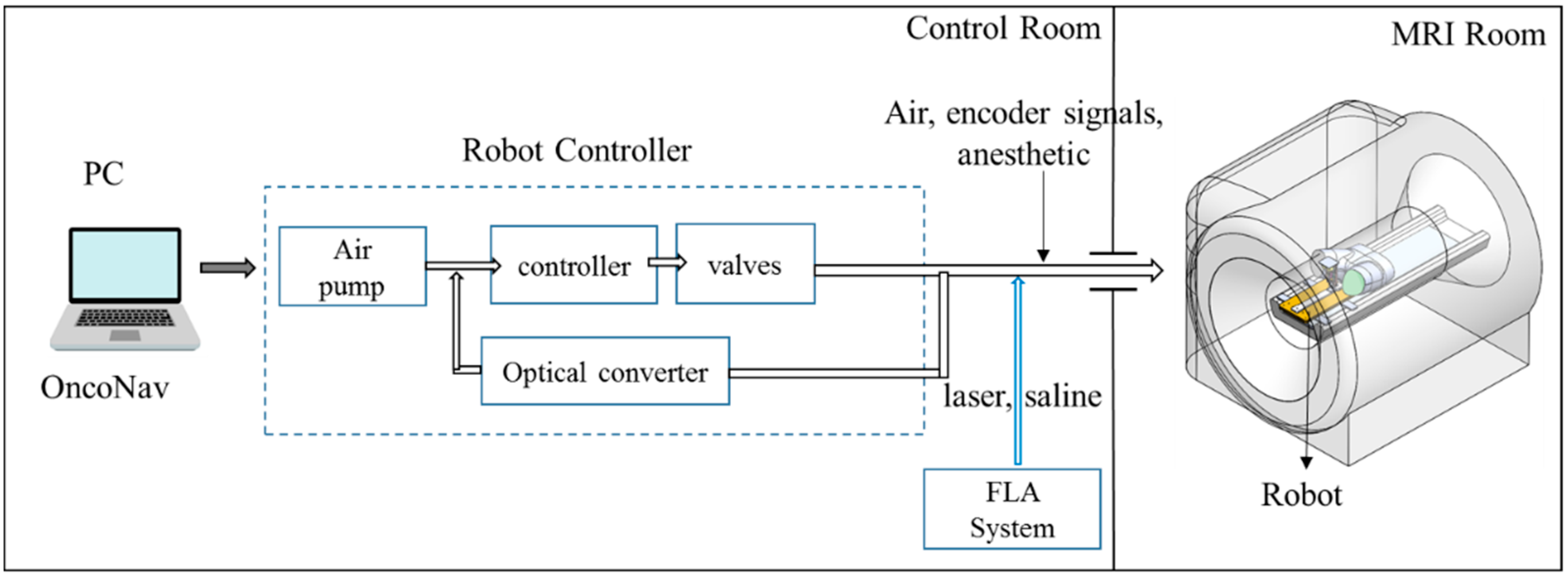
A MRI-guided robot assisted focal laser ablation system consisted of the following four components (
Figure 2): (1)
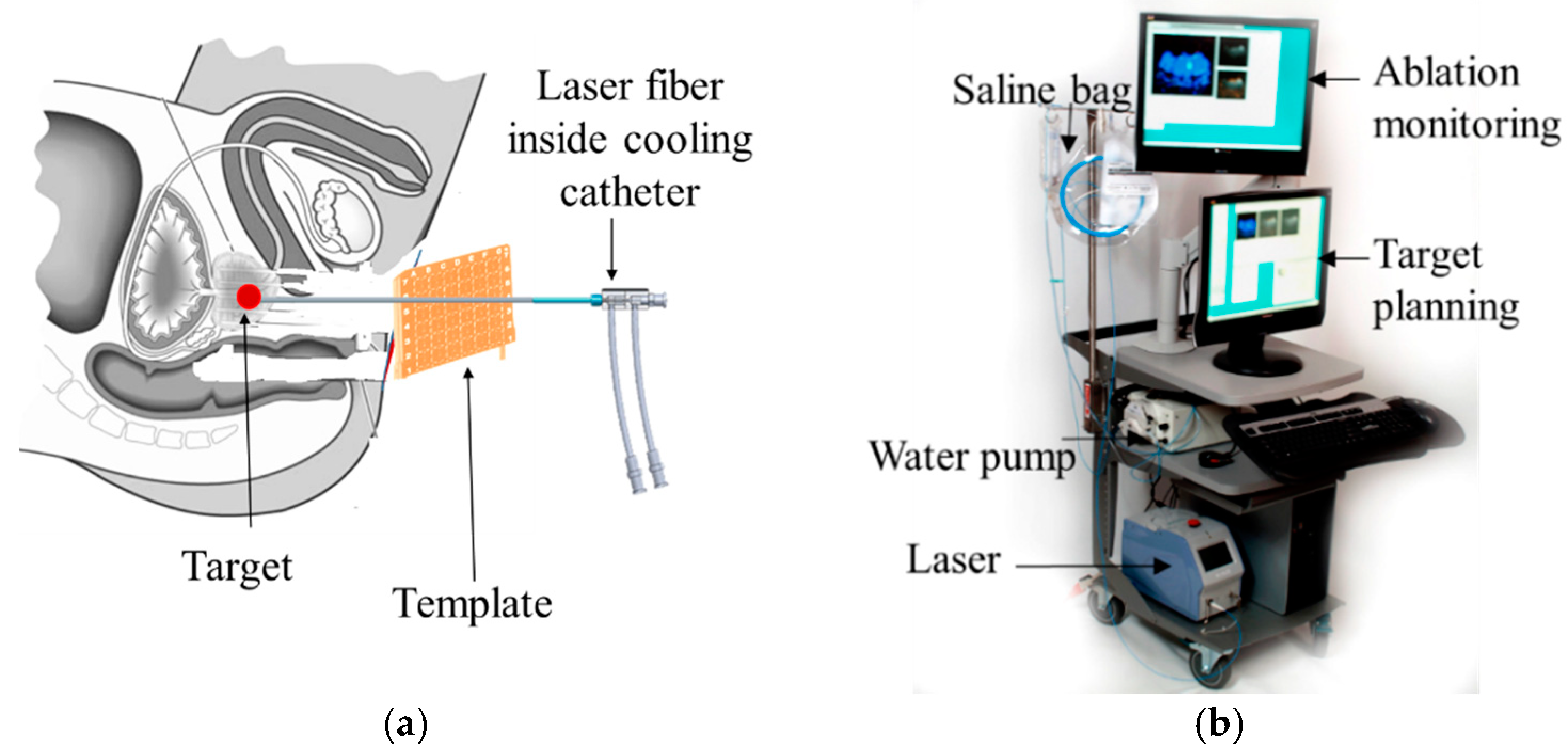
robotic system, which included the custom robot, robot controller hardware, and an air pump; (2) standard 3T MRI, for navigation, targeting, and temperature monitoring; (3) custom navigation, planning, and controller software, multi-focal laser ablation software and ablation monitoring all embedded in a custom-developed software called “OncoNav”, which runs on a PC; and (4) standard laser ablation system including the laser generator, optical fibers, laser sheath, foot pedals, a cooling catheter, and a water pump. A MR-compatible monitor facilitates in room review navigation, monitoring, and verification.
To minimize the number of components that were required to be MR compatible, the robot controller hardware, the air pump, the PC that runs OncoNav, the laser generator, and the cooling pump, were all placed in the control room. Only the robot and the laser sheath were placed on the MRI table inside the MRI room. A cable bundle that included air tubes and optical fibers for robot encoders, the optical fibers for the laser ablation, the cooling water tubes, and anesthesiology tube (during in vivo study) were all passed through a conduit tube (waveguide) that connects the controller room to the MRI room. The following sections outline details of the different components.
2.2. The Robotic System
The robotic system included the robot hardware, the controller hardware and software, and an air pump.
2.2.1. Robot
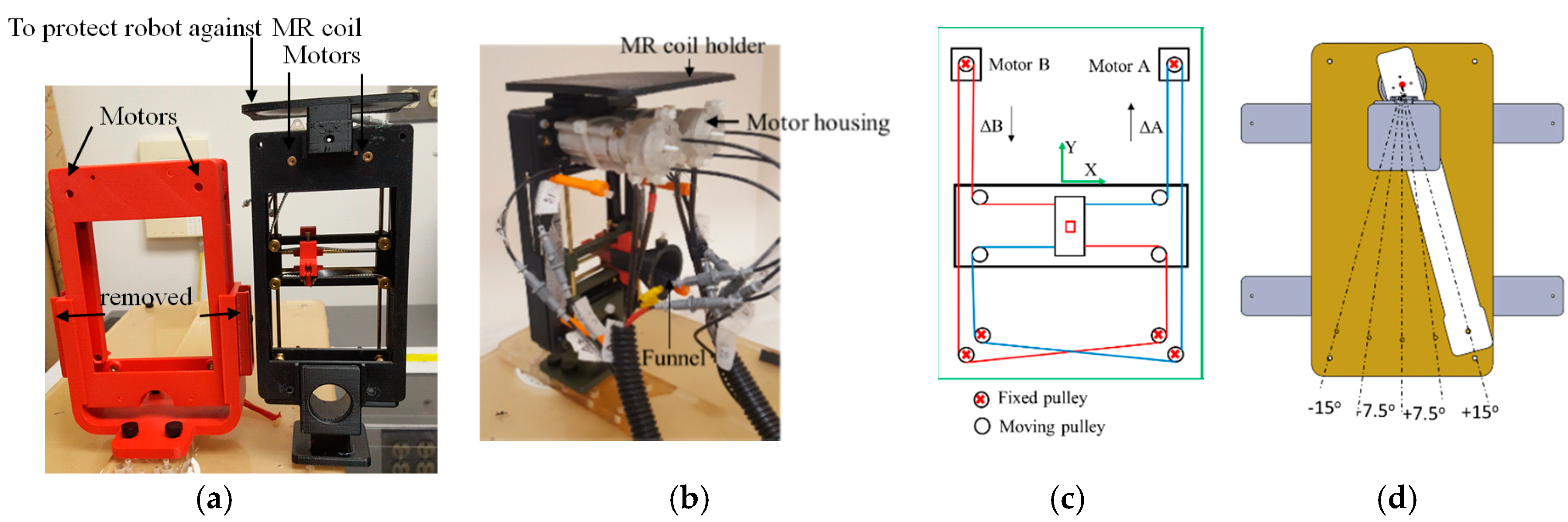
The robot is shown in
Figure 3a and it included a template mimicking CoreXY stage [
13] shown in
Figure 3c. The CoreXY stage guided the needle automatically toward a target selected by the physician from the preoperative MR image, and the physician then inserted the needle manually, thereby the physician was in a full control of the procedure while avoiding the burden of needle guidance. In the current design, the width of the robot was reduced by 40 mm (from 130 to 90 mm) in comparison to the previous design [
12]; however, the height was the same as the original design (180 mm). Despite downsizing the robot’s footprint, the overall robot workspace was kept the same as the previous design (53 mm × 90 mm). The desired workspace is based on a grid template which is used during prostate biopsy or brachytherapy (typically 65 × 65 mm).
To achieve this, we did the following: (1) removed the motors shafts toward each other. (2) We then use new pneumatic motors that are more compact. For this purpose, we redesigned and 3D printed the housing of the air motor (
Figure 3b). (3) removed the guide rail that supports the MR coil holder and moved it to the top (
Figure 3a). Then, we 3D printed all components, and rebuilt the robot. The robot kinematics however is still the same. The robot had two translational DoF, left-right and up-down. Two custom designed MRI-conditional pneumatic motors with built-in optical encoders were mounted on the top of the CoreXY frame to actuate the template [
13]. As illustrated in
Figure 3c, the template position was coupled with the rotational position of the two motors, which can be expressed by the following equation:
where Δ
X and Δ
Y are the displacement of the template, respectively, and Δ
A and Δ
B are the motor rotational position.
Compared to the motors reported in [
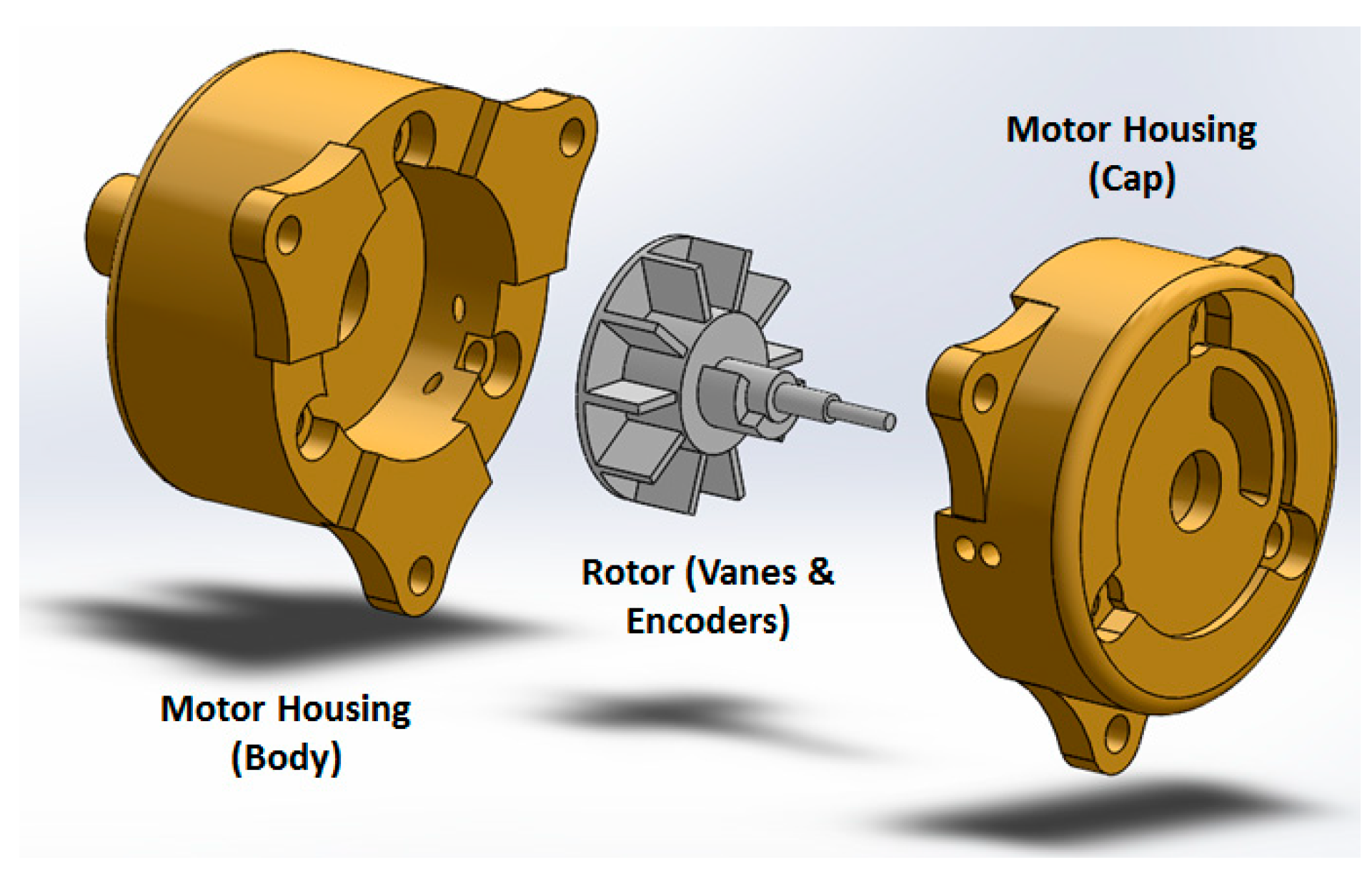
14], two upgrades have been applied: (1) the motor has become more compact; and (2) the number of transmitters/receivers for optical encoding has increased from one pair to two, for redundant measurements and optimized accuracy. On the external of the of the motor housing (cap and body), extensions were added to allow the motor casing to be assembled independently of affixation to the gearbox and robot, assuring the robustness of the motor as an independent unit. Motor encoding was improved by integrating a pair of interruption-based fiber optic lines, which allowed quadrature encoding and a higher degree of reliability for the encoding. The cap was extended to allow a pair of stacked fiber optic cables to be inserted, while an extension on the rotor supported the asymmetric interrupters, which blocked transmission of the optical signal.
Figure 4 shows the motor CAD design.
Two sets of belts, red and blue lines in
Figure 3c, transfer the motor rotational motion to template translational DoF. To move the template in the X-direction, the motor rotational motion should be the same in both direction and magnitude. Similarly, template Y-direction motion can be produced by running the two motors in the opposite direction with the same magnitude. Moving one motor could generate the template diagonal motion in ±45°, as can be seen in Equation (1). The robot can be easily mounted on the holder (
Figure 3a) which is attached to a rotating arm which is pivoted to the robot base plate using a ceramic bearing (
Figure 3d). The rotating arm DoF pivots the robot about the approximate location of the prostate, providing 0°, ±7.5° and ±15° relative to 0° parallel to the axis of the bore. This permits the targeting of tumors obstructed by the pubic bone, which may occur in a straight needle trajectory scenario for the right or left lateral part of the prostate. The rotating arm is manually moved and is not actuated as it is used occasionally. Although not clinically significant, the end-effector speed is 3 mm/s.
Most of the robot components were made out of plastic (P430 ABS plus, StrataSys, Eden Prairie, MN, USA, MN—tensile strength: 33 MPa), using a 3D printer (StrataSys, Eden Prairie, MN, USA). The robot also included brass rods and belt guides. The shaft of the motor was made from brass to increase strength under the load applied by the timing belt. Six sets of planetary gear boxes (Tamiya, Shizuoka, Japan) were utilized for each air-motor to reduce the speed and increase the torque. A removable plate was considered at the top of the CoreXY robot to protect the motors from the pressure applied by the imaging coil. The robot and rotating arm was installed on a double layer of 0.375” thick acrylic sheet.
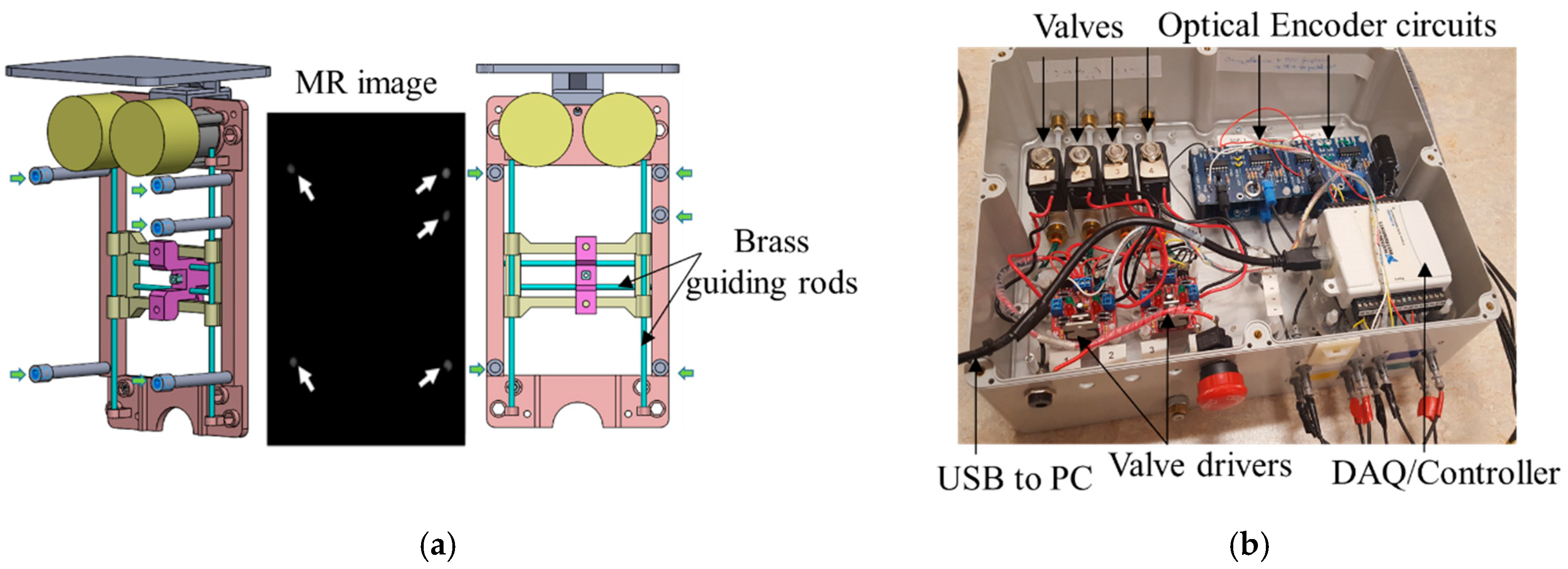
The robot was registered to the MRI coordinate system by MR visible markers as showed in
Figure 5a.
Five fiducial markers (D = 6 mm, Beekley Inc., Bristol, CT, USA) were precisely located at the predefined positions on the transverse plane of the robot. All markers are located on a single plane because the robot end-effector only moves in a plane parallel to this plane. Prior to navigation and planning, high-resolution scanning was conducted to locate the fiducial markers’ positions in the imaging coordinate system. The fiducial markers were located 50 mm away to avoid any potential interference with the brass guiding rod. Since the fiducial markers’ positions in the robot coordinate and MR coordinate were known, registration could be accomplished with the rigid transformation method. The basic principle of this algorithm can be written as the following equation:
where
A was the marker position in the robot frame,
B was the marker position in the MR image frame,
R was the rotation matrix, and
t was the translation vector. In this study, we chose the Landmark-Least Squares Method to get the optimal
R and
t [
15]. Although three markers is theoretically sufficient to define
R and
t, we chose 5 to increase the accuracy. It should be noted that the registration needs to be updated after each time that the rotating arm is rotated.
2.2.2. Controller
Figure 5b shows the controller box, which contained 4 solenoid valves (two per motor–1/4 Inch Electric Air Water Solenoid Valve, WIC Valve, San Jose, CA, USA) that were controlled by a 4 custom developed driver. Two custom-developed circuit boards interpreted the signal coming from the optical encoders to close the control loop. The controller box also had 4 air tubes and 8 optical fibers attached to it. The valves connecting to the 4 air tubes are normally closed, and opened when a command came from the controller. A PC-based measurement and control system (NI USB 6009, National Instrument, Austin, TX, USA) was used both as a DAQ system and a controller, thus enabling the PC to receive the position signals from encoders, compute the control law, and send commands to the valves. A 2.0-Gal. air pump (2010ALFC Ultra Quiet, California Air Tools, San Diego, CA, USA) was used, and the air pressure was set to 100 psi. An 8 m long air tube connected the air pump to the robot controller. Lastly, a safety stop button was implemented for an emergency.
The controller architecture was achieved via the proportional integral (PI) algorithm. The robot inverse kinematics (Equation (1)) calculated the desired motor motion (θ𝑑) according to the relationship between the moving frame current position and the desired position. The custom designed optical encoder provided motor rotational position (θ) to the control loop. Based on the difference between θ𝑑 and θ, the PI controller applied a constant voltage to power the pneumatic valves, which regulated the pneumatic air flow supplied to the motor. This continued until the error falls within a certain threshold at which point, the voltage dropped to zero.
The robot controller software was originally implemented in Labview (National Instrument, Austin, TX, USA). The new version implements in C++, in order to use the navigation and planning software in OncoNav to directly command the robot. An existing API was utilized to enable communication of the visual studio and the DAQ/controller hardware.
2.3. OncoNav Is a Software Platform Used for Navigation, Robot Control, and Ablation Multi-Focal Planning and Monitoring
Targeting: Once the registration was done, the suspicious target identified by an expert radiologist from a pre-operative image was localized on the intra-operative image by the operator. The coordinates of the target in MR were converted to the robot’s coordinate system and were sent to the robot’s controller. The robot moved to the target and waited for the user to insert the needle to the depth calculated by the software.
Iterative Treatment Planning: Prior to ablation, the optimal treatment plan was calculated by minimizing the number of catheter insertions to cover the entire tumor volume. The Visualase
TM Laser Ablation System was used in this study. Ablation size was limited to be within 5–8 mm around the catheter, which required multiple ablations to treat a large tumor. The laser ablation zone has the shape of an elongated ellipsoid, which can be formulated as follows:
where
p and
q (normally
q >
p) are semi-axes lengths. The size of the ablation zone (
x,
y and
z parameters in Equation (3)) is a function of ablation time. Since the prostate does not have major blood vessels, the heat sink effect is small, making the ablation zone highly predictable.
The estimated ablation result through the intraoperative temperature map can be used to update the original treatment plan. The treatment plan was iteratively updated until all the residual tumor was covered. The target was obtained by subtracting the estimated model from the preoperative tumor model after each ablation. The spatial information of the target can be derived from the T2w volumetric image and registered to the robot coordinate frame, which was used for robot joint space motion calculation. Once the needle was inserted to the desired position, another T2w scan was performed to verify its position with respect to the target. Reinsertion or repositioning is performed sequentially until the tumor is fully treated, defined by tumor target margin coverage.
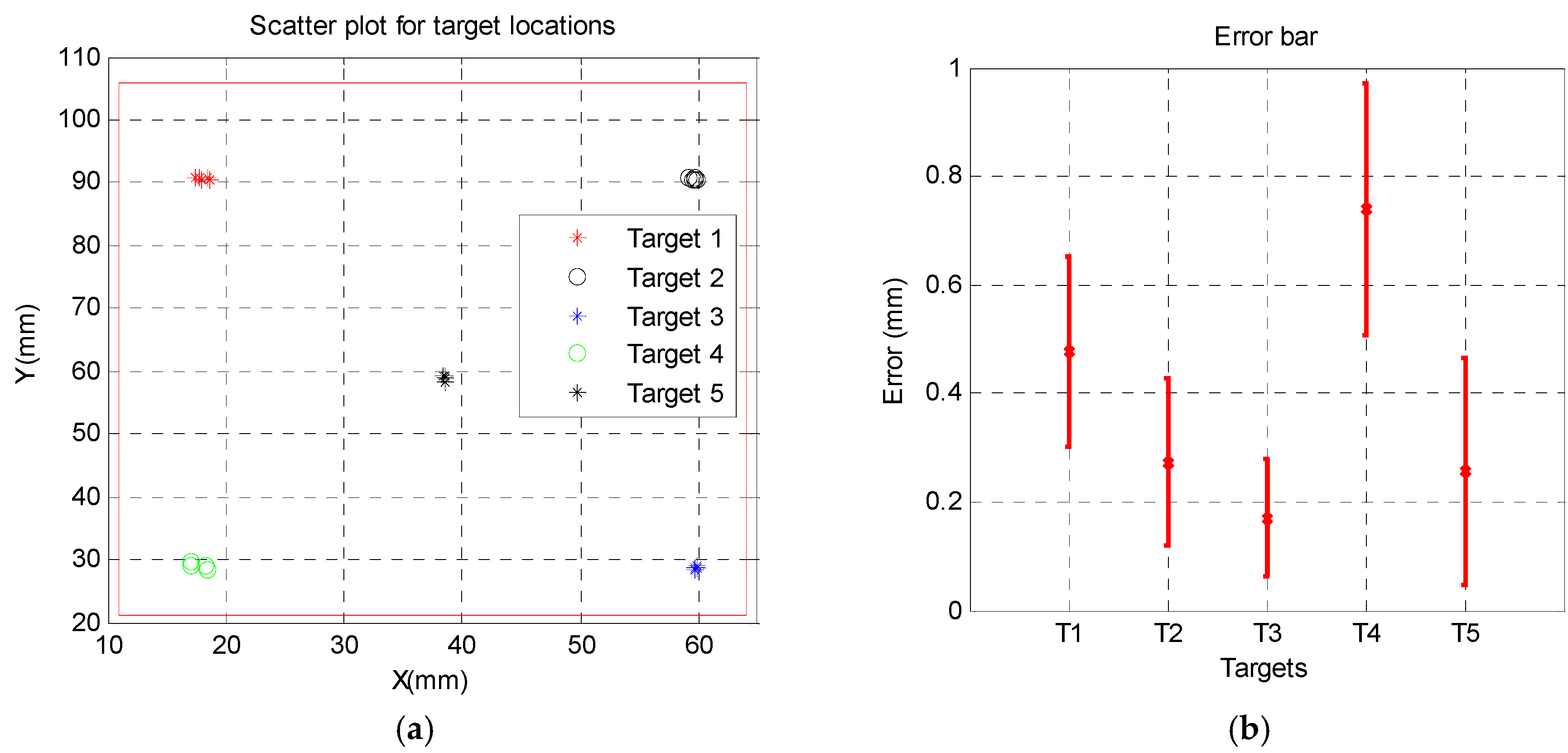
2.5. Experiments
Free space Accuracy was tested before testing the new robot in gantry. There were 5 targets, 4 at the corners and one in the center and it was targeted 5 times starting from 1 to 5.
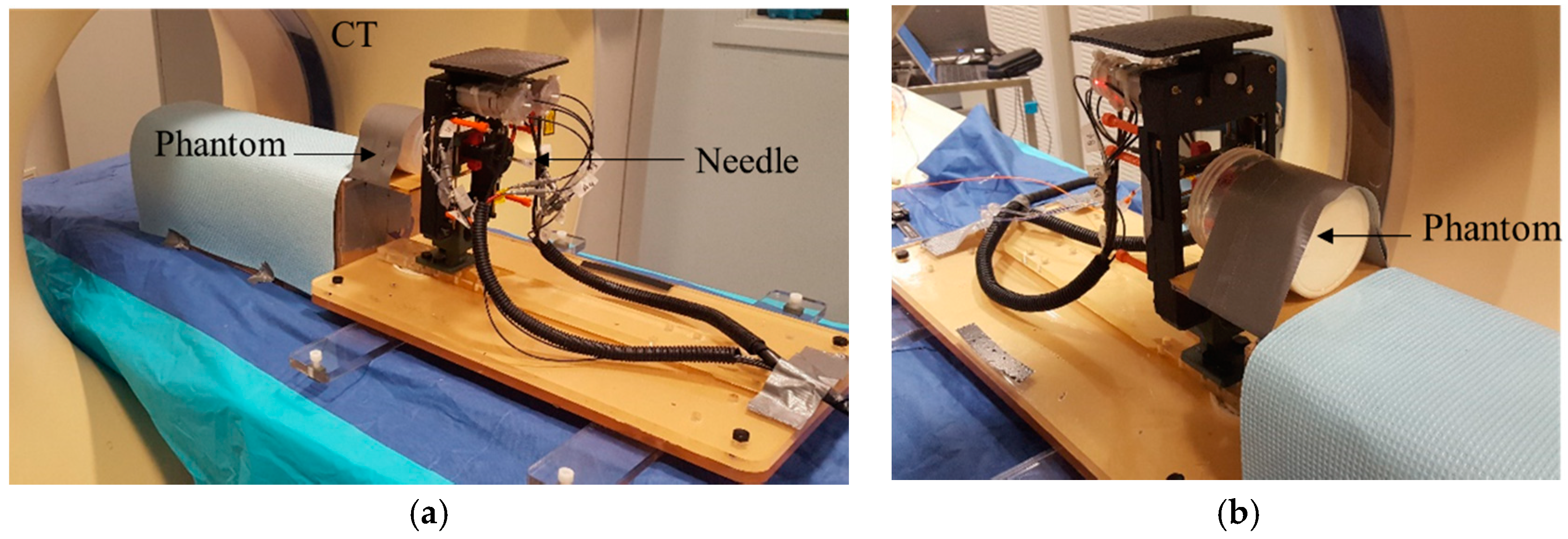
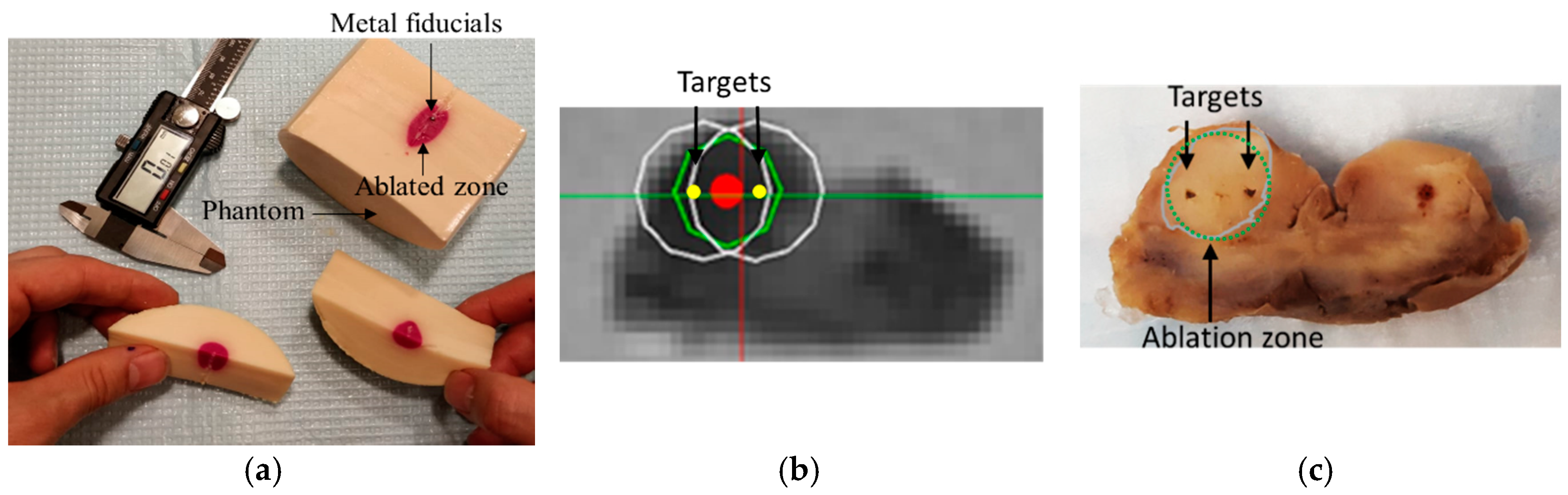
A Phantom Study under CT guidance was performed to study the entire workflow, the robot’s accuracy, and the ablation planning and surgical workflow. The system was initially tested prior to the MRI experiment in the CT room.
Figure 6 shows the experimental setup.
An acrylamide thermochromic gel phantom was made inside a cylindrical container (D = 100 mm, L = 120 mm). Details of how to make the acrylamide gel can be found in [
16]; briefly, an aqueous solution of 40% (
w/
v) acrylamide/bis-acrylamide (VWR Int. Bridgeport, NJ, USA) with feed ratio (acrylamide to bis-acrylamide) of 19:1 (52.8 mL) was mixed under magnetic stirring with ammonium persulfate (APS, 0.46 g, Sigma Aldrich, St. Louis, MO, USA) to initiate polymerization and degassed, de-ionized water (231.1 mL). This was followed by the addition of Kromagen Magenta MB60-NH concentrate (15.1 mL, TCR Hallcrest, Glenview, IL, USA). Finally, N,N,N′,N′-tetramethylethylenediamine (0.46 mL, Sigma Aldrich, St. Louis, MO, USA) was added as catalyst. The final solution was immediately transferred to a cylindrical container with desired size and kept in a cold room (4 °C) overnight. If polymerization occurs at room temperature, the temperature of the phantom material rises due to the heat of the polymerization process [
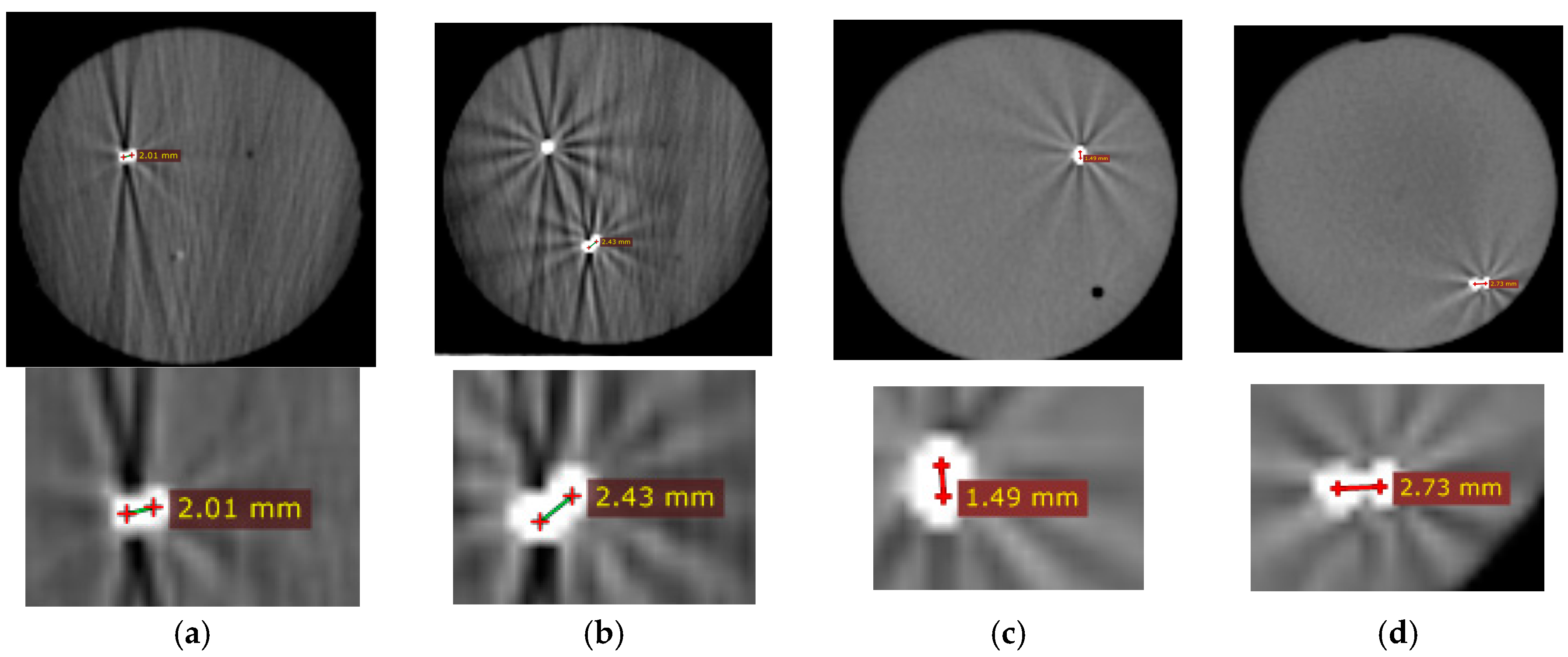
17] thereby imparting unwanted color change to the phantom. Therefore, it is important to allow the phantom material to slowly polymerize at 4 °C, to avoid undesired and confounding color changes. The gel changed its color from pale yellow to pink when heated above 55 °C. There were 4 spherical metal markers embedded inside the phantom at 4 different locations and depths. Two of the markers were 2 mm in diameter and the other two had the diameter of 0.5 mm. The phantom was secured to the table to minimize motion artifact.
Ex vivo study under MRI guidance: A whole prostate gland (Science Care Inc., Philadelphia, PA, USA) was ordered after screening a few potential candidates. The specimen was from a pre de-identified 63 year old Caucasian donor who died of metastatic prostate cancer as the secondary cause of death (and metastatic lung cancer as the primary cause), with a body mass index (BMI) = 21.7. The subject reportedly received chemotherapy within 1–3 months prior to death. The gland was taken out within 5 days of postmortem, formalin fixed, and was shipped with seminal vesicles to help with anatomic orientation. Extra cleaning of tissue was performed in house by an experienced urologist, to remove unnecessary surrounding tissues such as the bladder wall, rectal wall, and penis muscular tissue.
Then, the whole prostate specimen was placed inside a 60 × 60 × 80 mm plastic box, with the rectal wall touching the base of the box. The acrylamide tissue mimicking gel phantom was made and poured into the box to fixate the prostate, thus eliminating potential motion artifact during MRI scanning. To avoid heating of the prostate tissue as the phantom gel set, the casting process was done by immersing the box into ice water.
The ex vivo phantom was scanned with a Philips Achieva 3T scanner (Amsterdam, the Netherland) with an endorectal coil placed under the specimen. An mpMRI prostate scan (including T1w and T2w and ADC map) with a 3 mm slice thickness was acquired from the ex vivo tissue. The suspicious location was selected by an expert radiologist in T2w image.
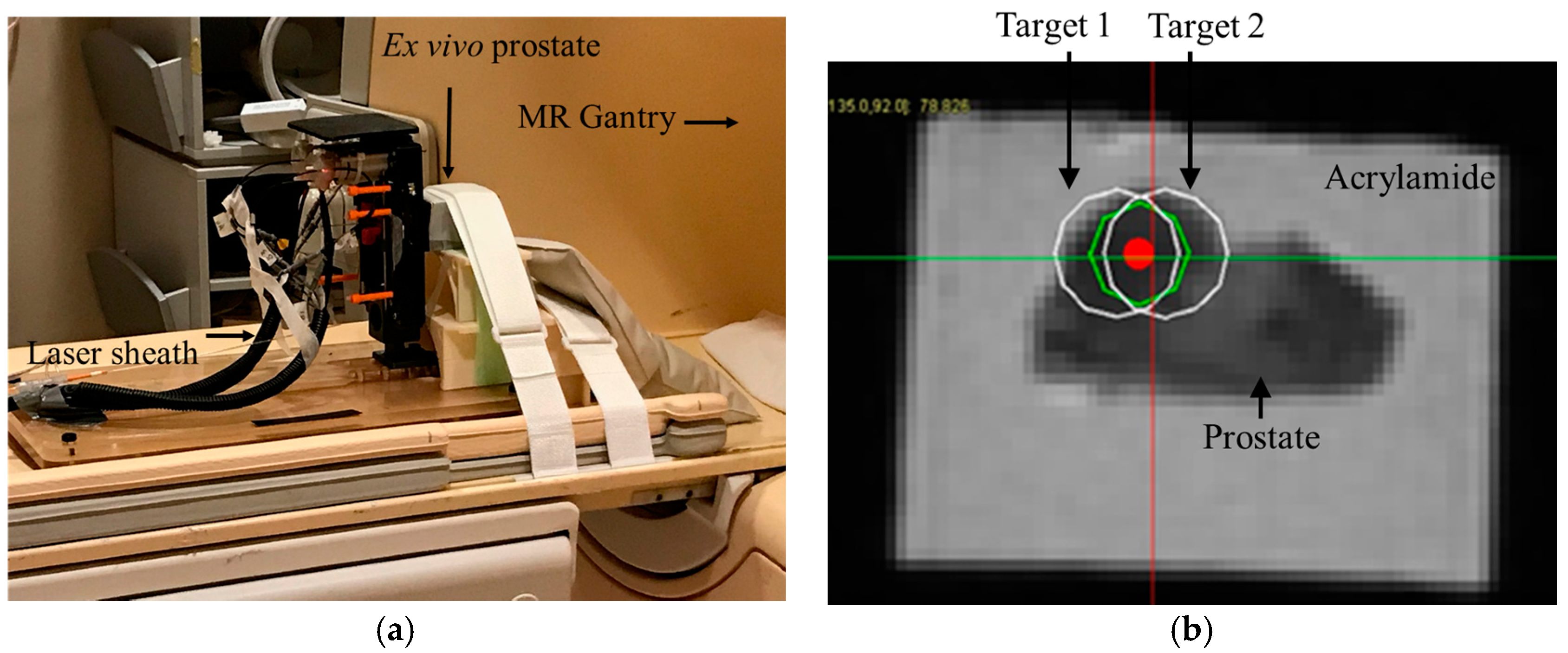
Next, an opening was created on one of the faces of the casing of the phantom to allow transperineal needle access during MRI ablation experiment. The phantom was secured in front of the robot as shown in
Figure 7a.
During the in-gantry experiment, the robot was initially scanned and registration was performed using the same method as described earlier. Then, a high resolution T2w image was acquired. The ablation plan was then superimposed on the T2w image.
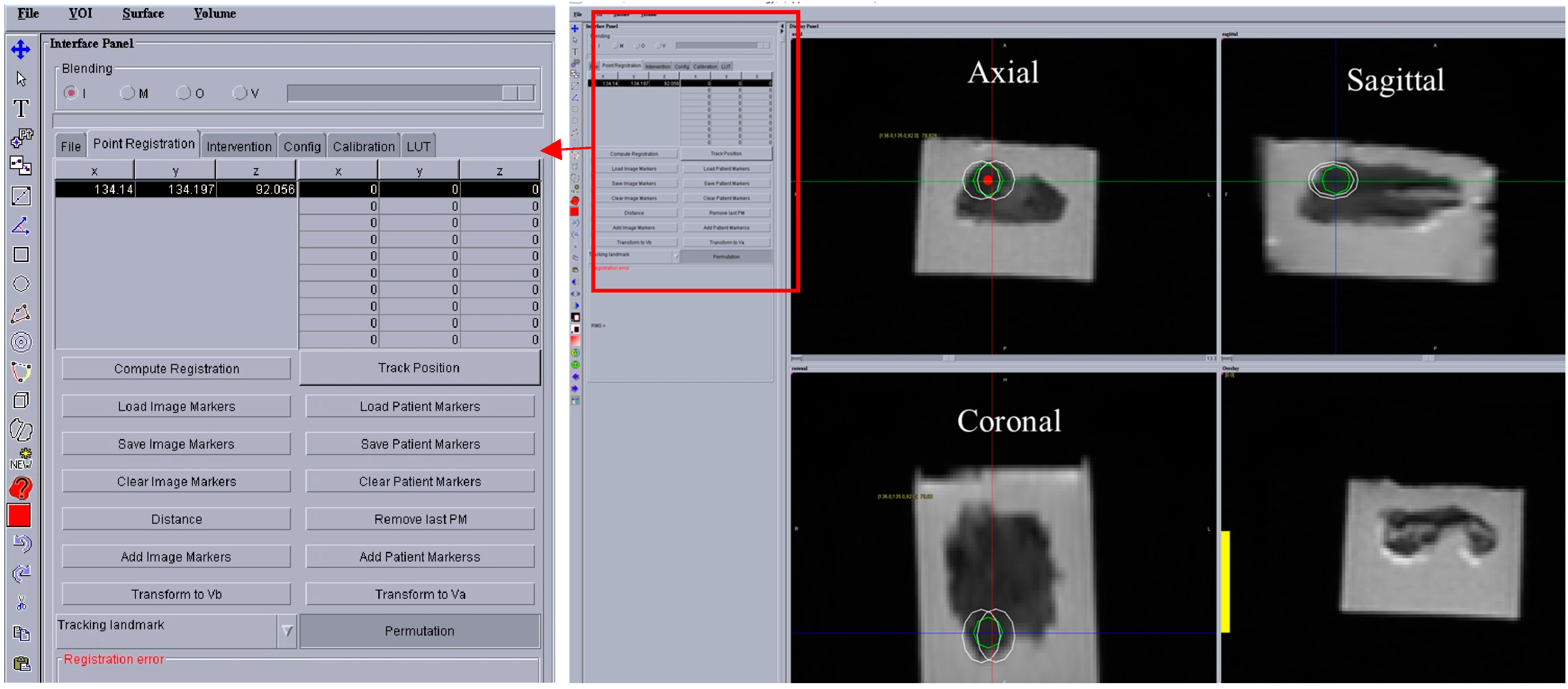
Figure 8 shows a screenshot of the OncoNav with ablation plan. Since the ablation area was relatively large, the software suggested the minimum number of ablations which still covered the whole area with some safety margin [
18] to be two.
Figure 7b shows a close-up view of the Axial T2w image with superimposed ablation plan. Each planned ablation zone was an ellipsoid with 19 mm long axis and 14 mm short axis.
Next, the robot was commanded to targets 1 and 2, respectively. An MRI compatible needle with a plastic sheath (Medtronic, Minneapolis, MN, USA) was inserted to the depth calculated by the robot controller. The needle was removed while the plastic sheath was left behind. Next, the saline cooled catheter was inserted 5 mm ahead of the required depth (per recommendation of the manual) and the target was ablated at 15 W for 90 s. A confirmation T2w image was acquired. Finally, the ex vivo prostate was removed and sliced for gross observation.