3D Printing Endobronchial Models for Surgical Training and Simulation
Abstract
1. Introduction
2. Materials and Methods
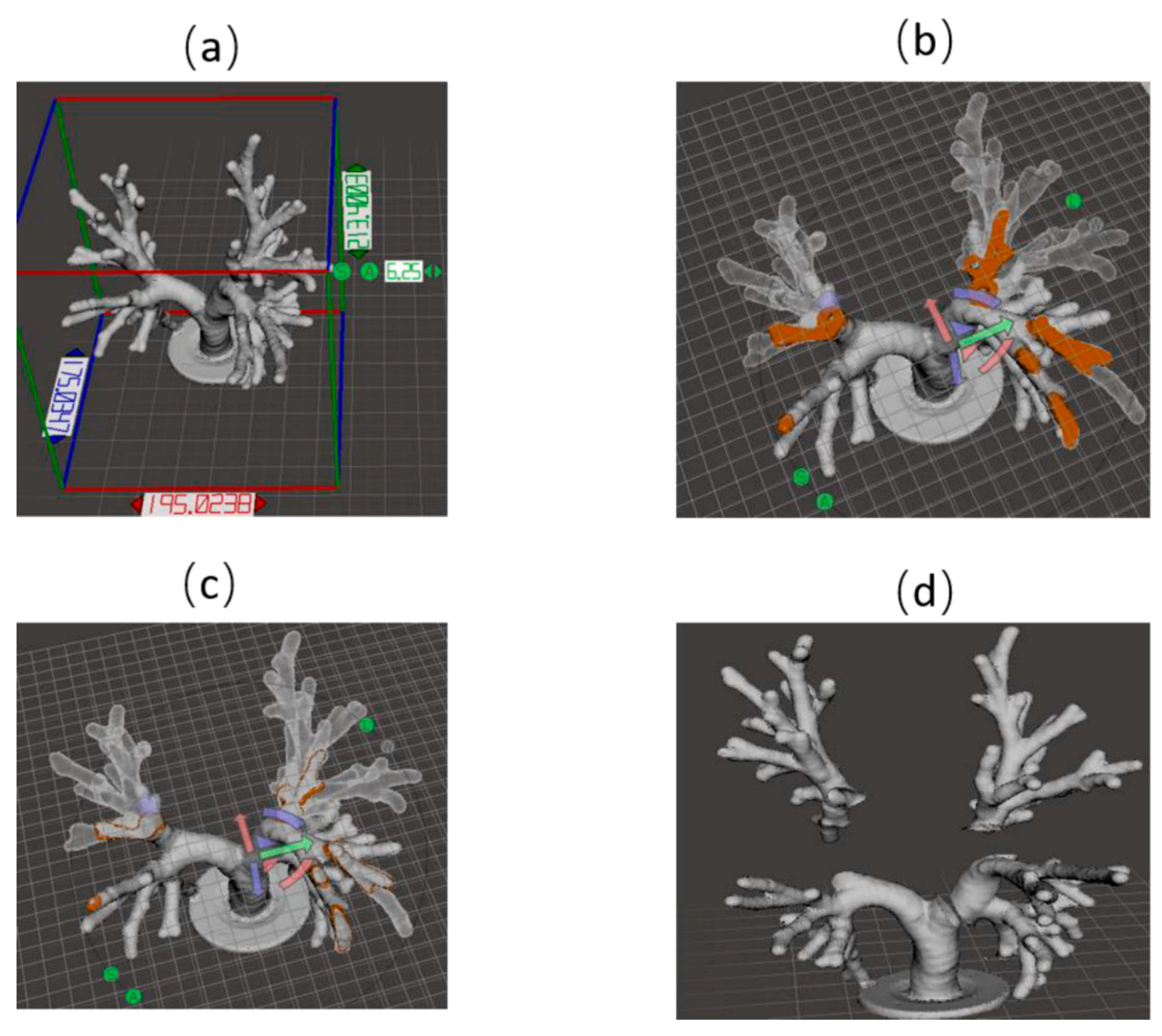
2.1. 3D Model Design
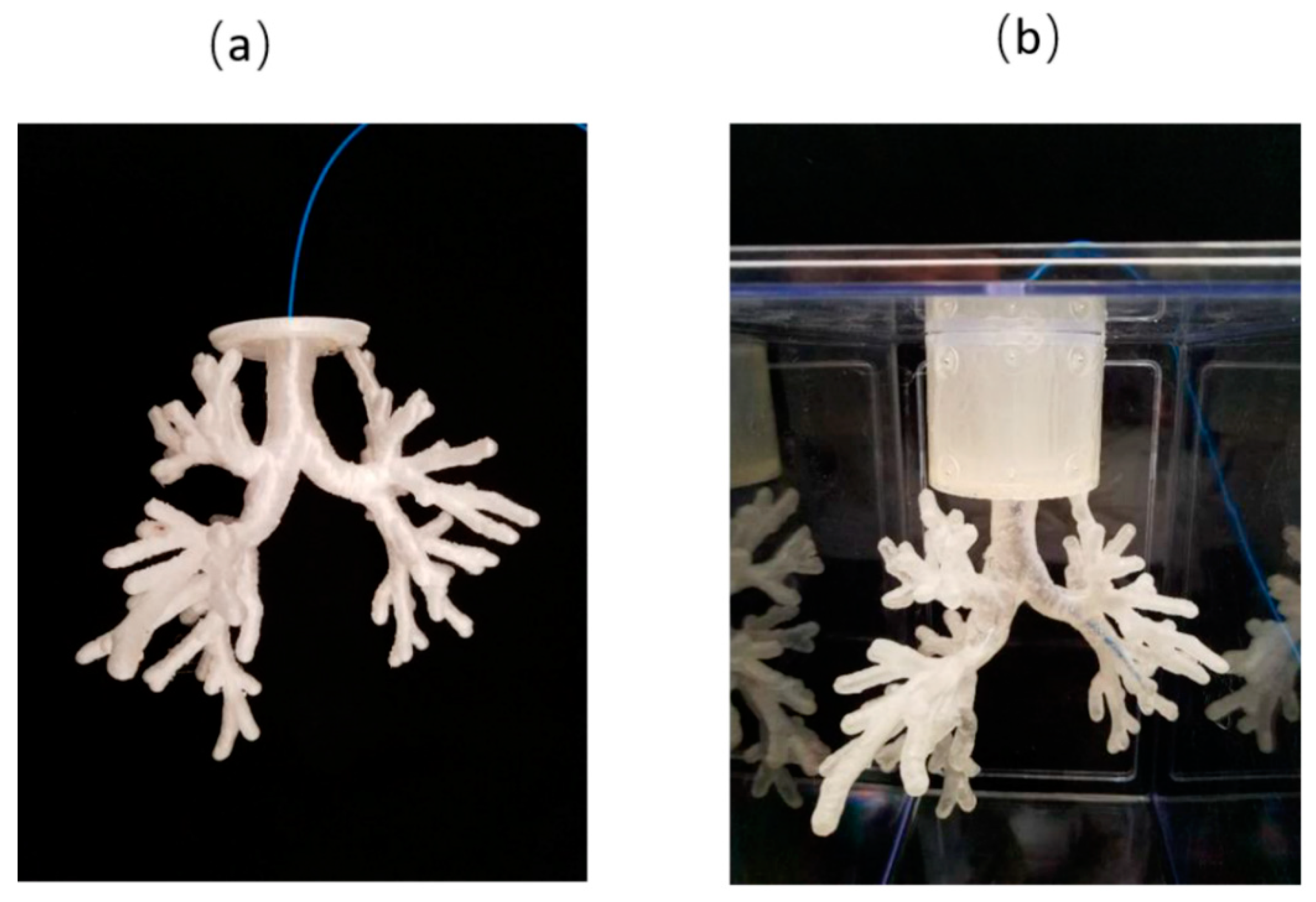
2.2. 3D Printing
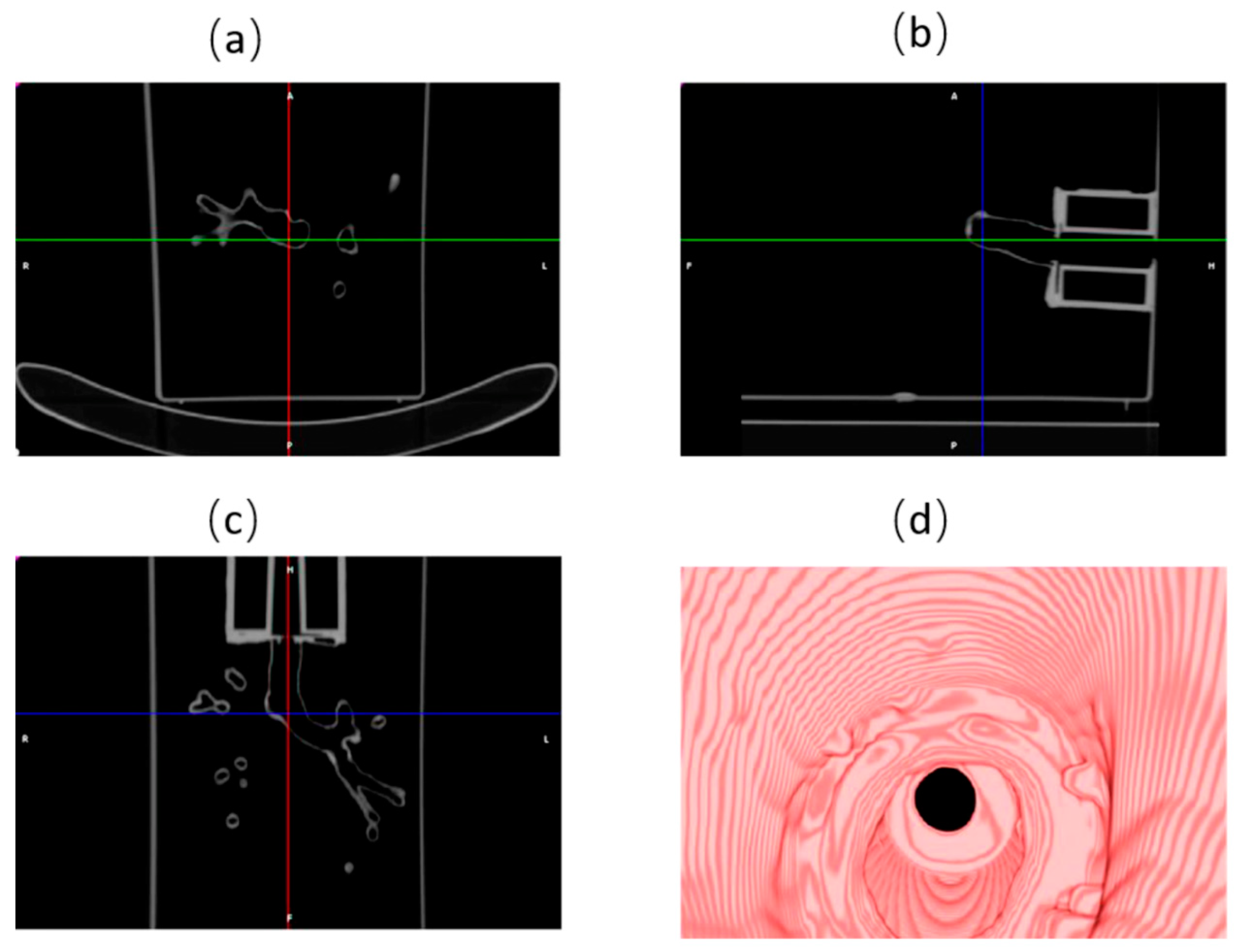
2.3. Phantom Performance Test
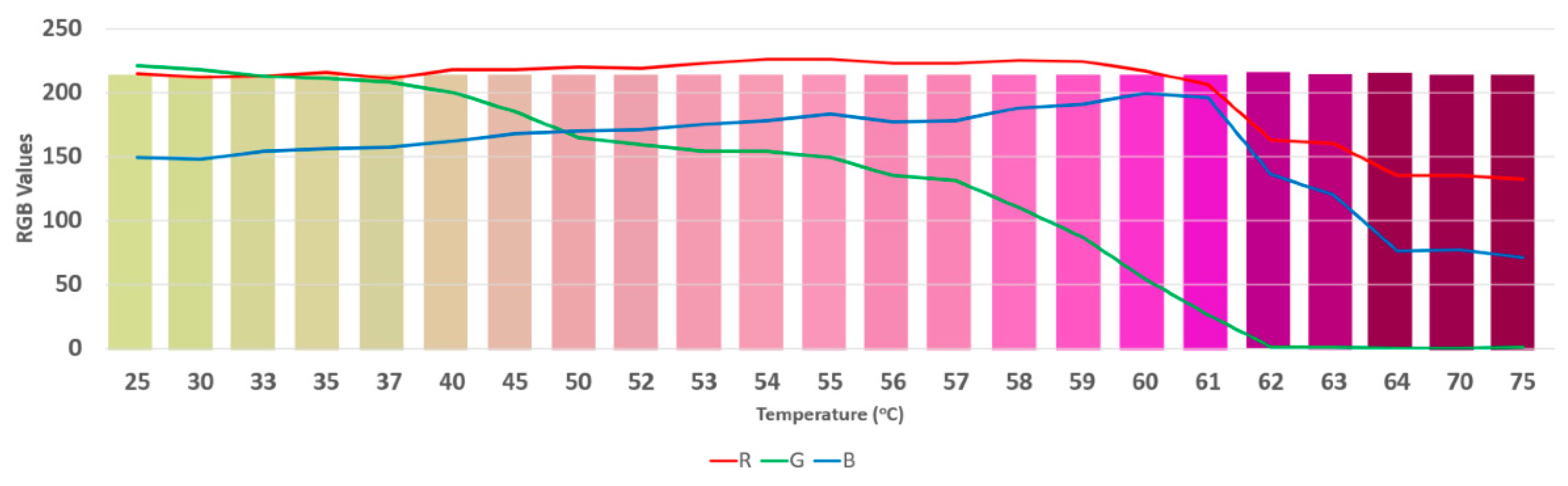
2.3.1. Localization Test
2.3.2. Ablation Test
3. Results
3.1. Lung Airway Phantom
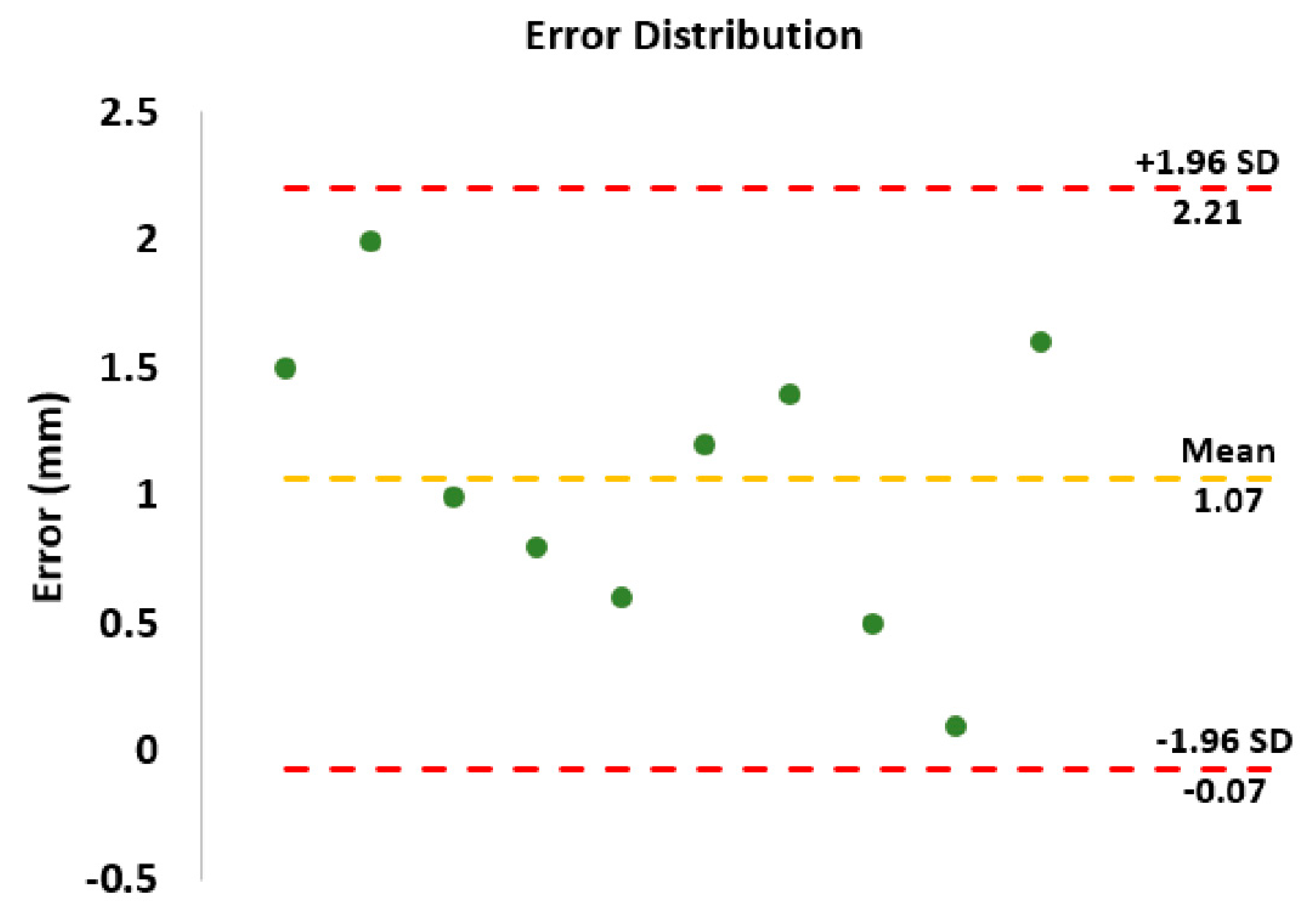
3.2. Phantom Performance Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Uneri, A.; Nithiananthan, S.; Schafer, S.; Otake, Y.; Stayman, J.W.; Kleinszig, G.; Sussman, M.S.; Prince, J.L.; Siewerdsen, J.H. Deformable registration of the inflated and deflated lung in cone-beam CT-guided thoracic surgery: Initial investigation of a combined model-and image-driven approach. Med. Phys. 2013, 40, 017501. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Traub, J.; Hautmann, H.; Ahmadian, A.; Navab, N. Fiducial-free registration procedure for navigated bronchoscopy. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2007. MICCAI 2007. Lecture Notes in Computer Science; Ayache, N., Ourselin, S., Maeder, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4791, pp. 475–482. [Google Scholar]
- Xue, Z.; Wong, K.; Wong, S.T. Joint registration and segmentation of serial lung CT images for image-guided lung cancer diagnosis and therapy. Comput. Med. Imaging Graph. 2010, 34, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Lung Cancer, 2017. Available online: https://www.cdc.gov/cancer/lung/ (accessed on 16 September).
- American Cancer Society. How Common is Lung Cancer? 2017. Available online: https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html (accessed on 16 September 2018).
- He, T.; Xue, Z.; Lu, K.; Valdivia y Alvarado, M.; Wong, K.K.; Xie, W.; Wong, S.T. A minimally invasive multimodality image-guided (MIMIG) system for peripheral lung cancer intervention and diagnosis. Comput. Med. Imaging Graph. 2012, 36, 345–355. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Lung Cancer and Smoking, 2017. Available online: https://www.fda.gov/tobaccoproducts/publichealtheducation/healthinformation/ucm468635.htm (accessed on 16 September).
- U.S. National Library of Medicine. Lung Cancer, 2017. Available online: https://medlineplus.gov/lungcancer.html (accessed on 16 September 2018).
- Potti, A.; Mukherjee, S.; Petersen, R.; Dressman, H.K.; Bild, A.; Koontz, J.; Kratzke, R.; Watson, M.A.; Kelley, M.; Ginsburg, G.S.; et al. A genomic strategy to refine prognosis in early-stage non–small-cell lung cancer. N. Engl. J. Med. 2006, 355, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Steinfort, D.P.; Liew, D.; Conron, M.; Hutchinson, A.F.; Irving, L.B. Cost-benefit of minimally invasive staging of non-small cell lung cancer: A decision tree sensitivity analysis. J. Thorac. Oncol. 2010, 5, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.M.; Haidegger, T.; Birkfellner, W.; Cleary, K.; Peters, T.M.; Maier-Hein, L. Electromagnetic tracking in medicine—A review of technology, validation, and applications. IEEE Trans. Med. Imaging 2014, 33, 1702–1725. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.J.; Zhang, H.; Durrani, A.; Glossop, N.; Ranjan, S.; Lindisch, D.; Levy, E.; Banovac, F.; Borgert, J.; Krueger, S.; et al. Navigation with electromagnetic tracking for interventional radiology procedures: A feasibility study. J. Vasc. Interv. Radiol. 2005, 16, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Suh, T.S.; Lee, D.S. Development of a deformable lung phantom for the evaluation of deformable registration. J. Appl. Clin. Med. Phys. 2010, 11, 281–286. [Google Scholar] [CrossRef]
- Kairn, T.; Crowe, S.B.; Markwell, T. Use of 3D printed materials as tissue-equivalent phantoms. In IFMBE Proceedings, Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Toronto, Canada, 7–12 June 2015; Jaffray, D.A., Ed.; Springer: Cham, Switzerland, 2015; Volume 51, pp. 728–731. [Google Scholar]
- Followill, D.S.; Evans, D.R.; Cherry, C.; Molineu, A.; Fisher, G.; Hanson, W.F.; Ibbott, G.S. Design, development, and implementation of the radiological physics center’s pelvis and thorax anthropomorphic quality assurance phantoms. Med. Phys. 2007, 34, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Bui, J.T.; Gaba, R.C.; Knuttinen, M.G.; Omene, B.O.; Shon, A.; Martinez, B.K.; Owens, C.A. Microwave lung ablation complicated by bronchocutaneous fistula: Case report and literature review. In Seminars in Interventional Radiology; Thieme Medical Publishers: Stuttgart, Germany, 2011; Volume 28, p. 152. [Google Scholar]
- Carrafiello, G.; Mangini, M.; Fontana, F.; Di Massa, A.; Ierardi, A.M.; Cotta, E.; Piacentino, F.; Cardim, L.N.; Pellegrino, C.; Fugazzola, C. Complications of microwave and radiofrequency lung ablation: Personal experience and review of the literature. Radiol. Med. 2012, 117, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.M.; Beauchamp, R.D.; Evers, B.M.; Mattox, K.L. Textbook of Surgery, 19th ed.; Elsevier: Philadelphia, PA, USA, 2012; pp. 1182–1226. [Google Scholar]
- Mikhail, A.S.; Negussie, A.H.; Graham, C.; Mathew, M.; Wood, B.J.; Partanen, A. Evaluation of a tissue-mimicking thermochromic phantom for radiofrequency ablation. Med. Phys. 2016, 43, 4304. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Thiruvalluvan, K.; Krzeminski, L.; Moore, W.H.; Xu, Z.; Liang, Z. CT-guided robotic needle biopsy of lung nodules with respiratory motion–experimental system and preliminary test. Int. J. Med. Robot. Comput. Assist. Surg. 2013, 9, 317–330. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Lulzbot | Form 2 |
|---|---|---|
| Printing Time | 40 h | 30 h |
| Amount of material/money | 300 g/$15.6 | 200 mL/$29.8 |
| Resolution | 0.2 mm | 0.05 mm |
| Surface | Rough | Smooth |
| Transparency | Opaque | Completely transparent |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Xu, S.; Wood, B.J.; Tse, Z.T.H. 3D Printing Endobronchial Models for Surgical Training and Simulation. J. Imaging 2018, 4, 135. https://doi.org/10.3390/jimaging4110135
Zhao Z, Xu S, Wood BJ, Tse ZTH. 3D Printing Endobronchial Models for Surgical Training and Simulation. Journal of Imaging. 2018; 4(11):135. https://doi.org/10.3390/jimaging4110135
Chicago/Turabian StyleZhao, Zhuo, Sheng Xu, Bradford J. Wood, and Zion Tsz Ho Tse. 2018. "3D Printing Endobronchial Models for Surgical Training and Simulation" Journal of Imaging 4, no. 11: 135. https://doi.org/10.3390/jimaging4110135
APA StyleZhao, Z., Xu, S., Wood, B. J., & Tse, Z. T. H. (2018). 3D Printing Endobronchial Models for Surgical Training and Simulation. Journal of Imaging, 4(11), 135. https://doi.org/10.3390/jimaging4110135





