Age Determination of Blood-Stained Fingerprints Using Visible Wavelength Reflectance Hyperspectral Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Blood-Stained Fingerprints
2.2. HSI System
2.3. Hyperspectral Reflectance Image Acquisition and Pre-Processing
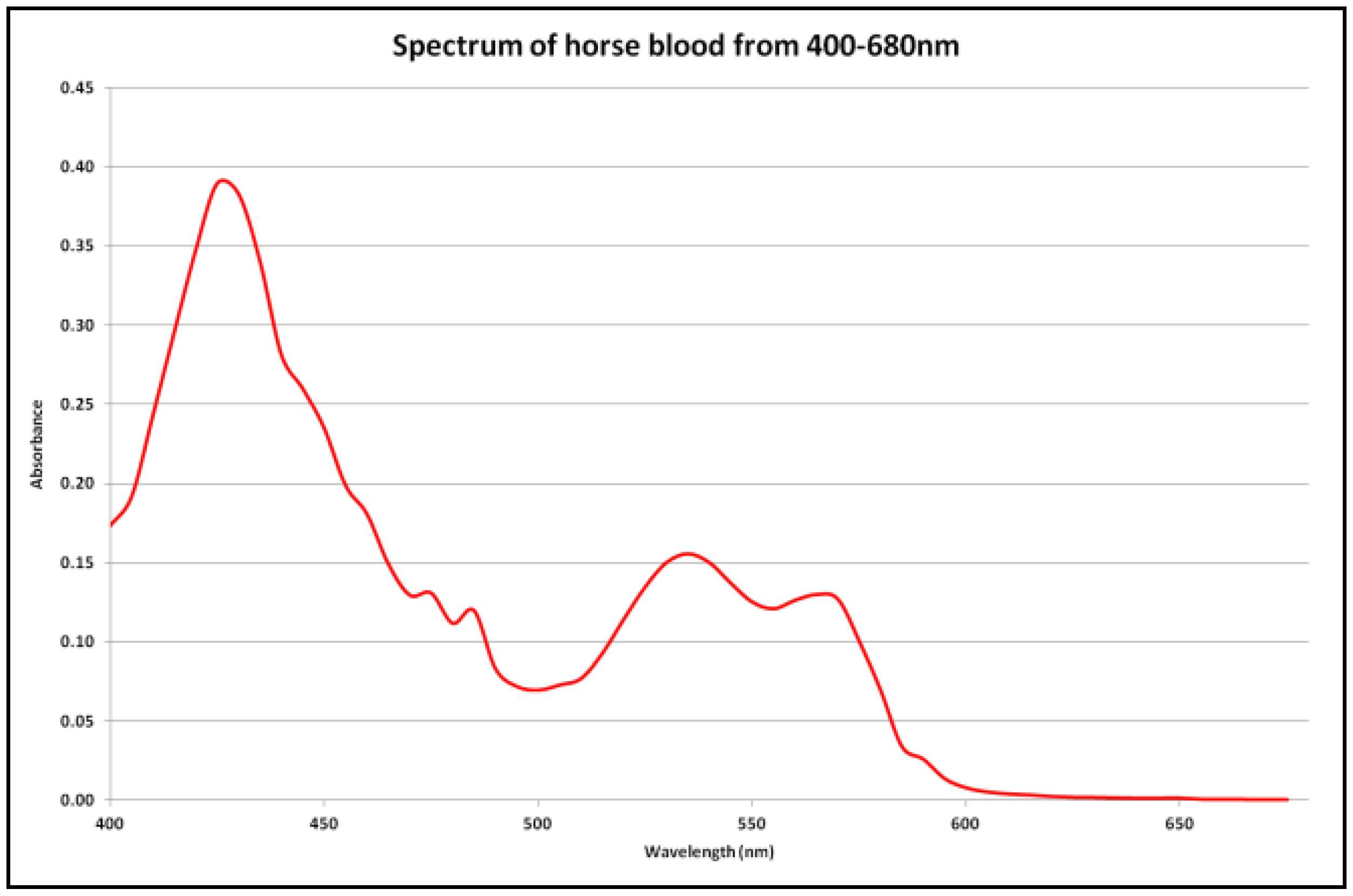
2.4. Criteria for the Identification of Blood Stains
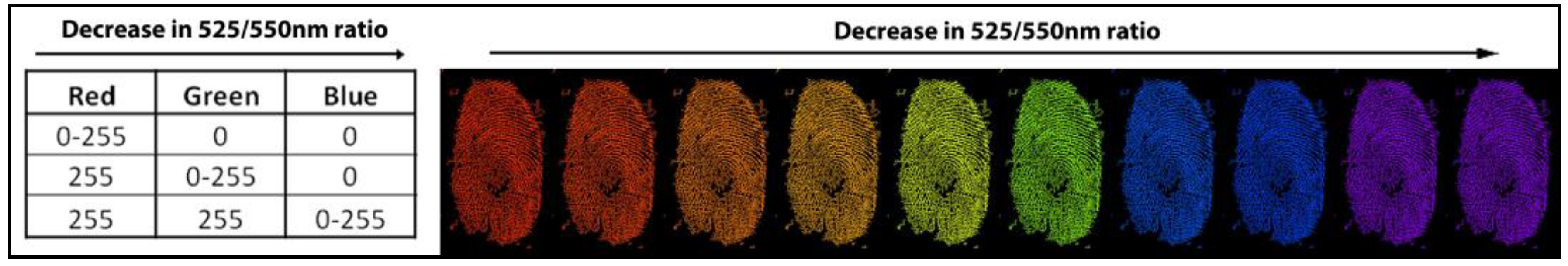
2.5. Age Determination Methodology for Blood-Stained Fingerprints
2.6. Digital Single Lens Reflex (DSLR) Setup
2.7. Age Estimation Intervals
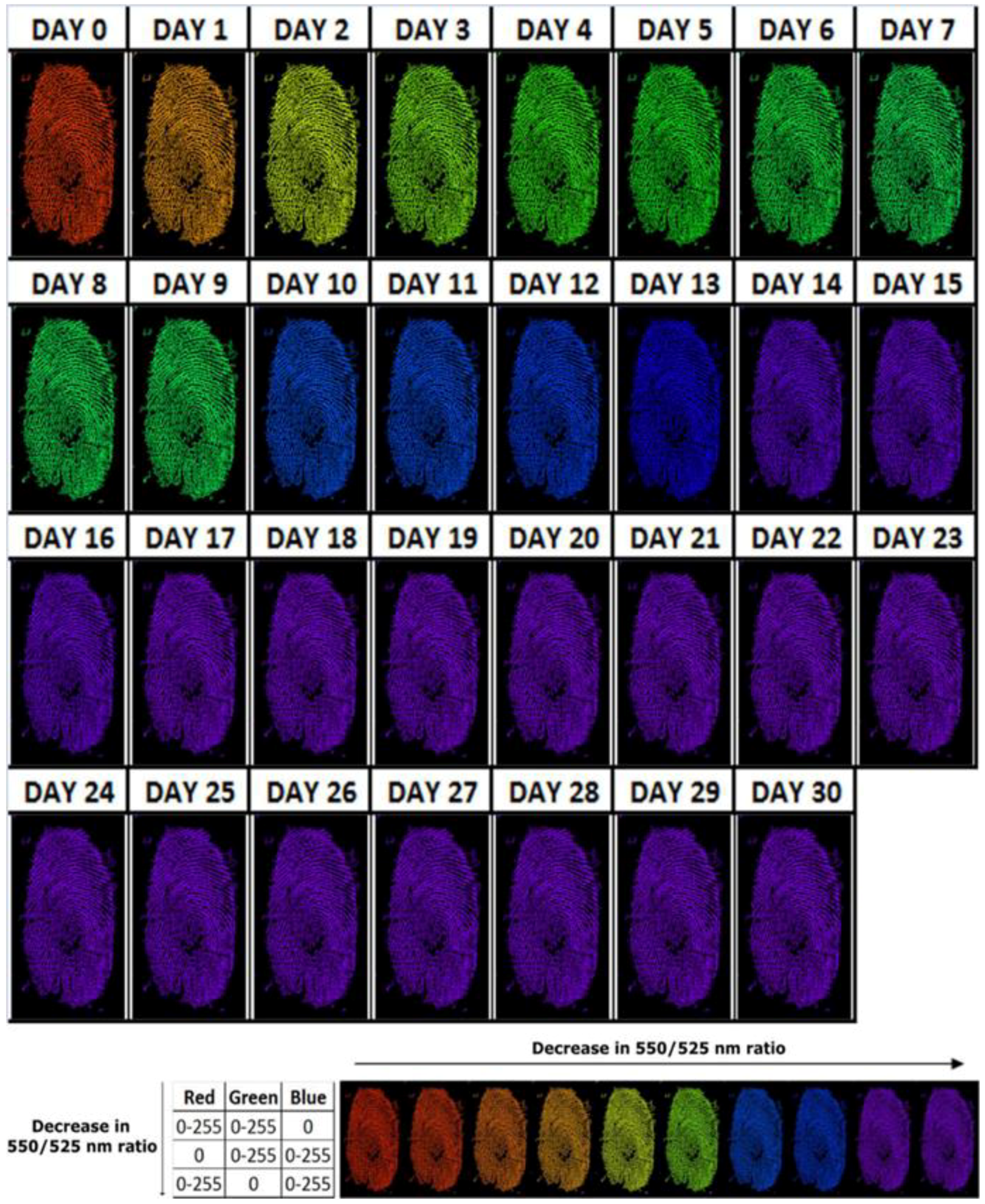
2.7.1. Aging of Blood-Stained Fingerprints over 30 Days
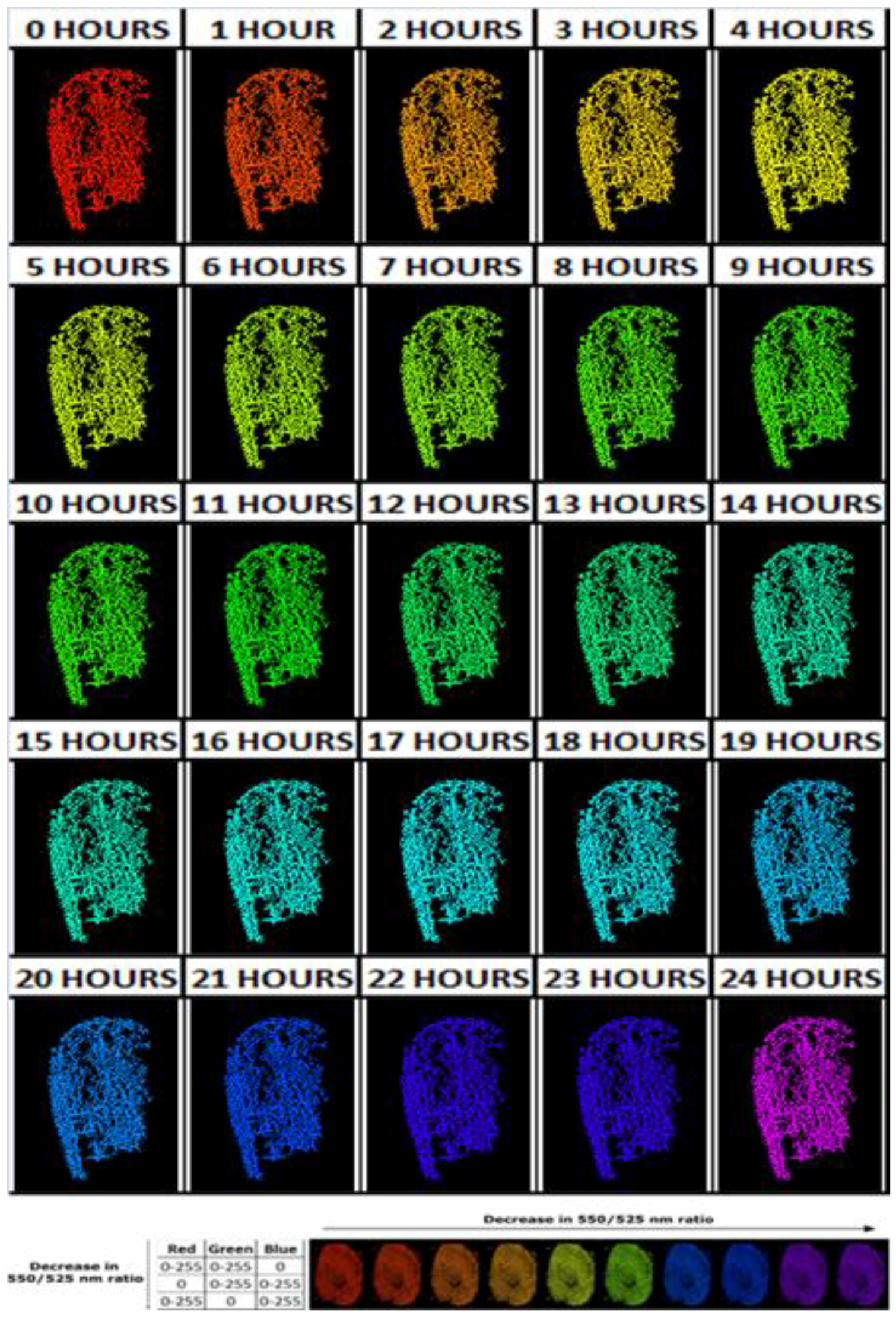
2.7.2. Aging of Blood-Stained Fingerprints over 24 h
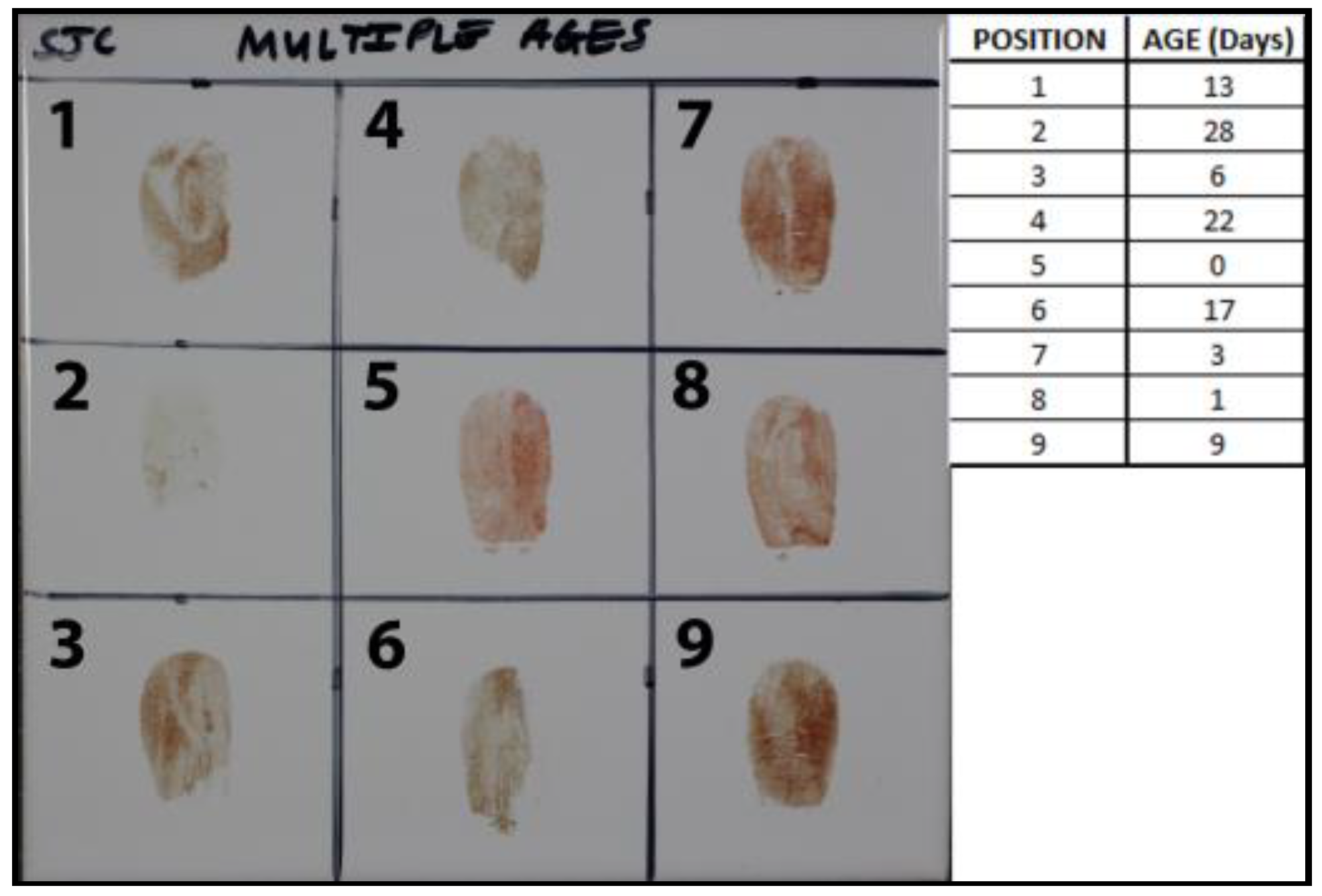
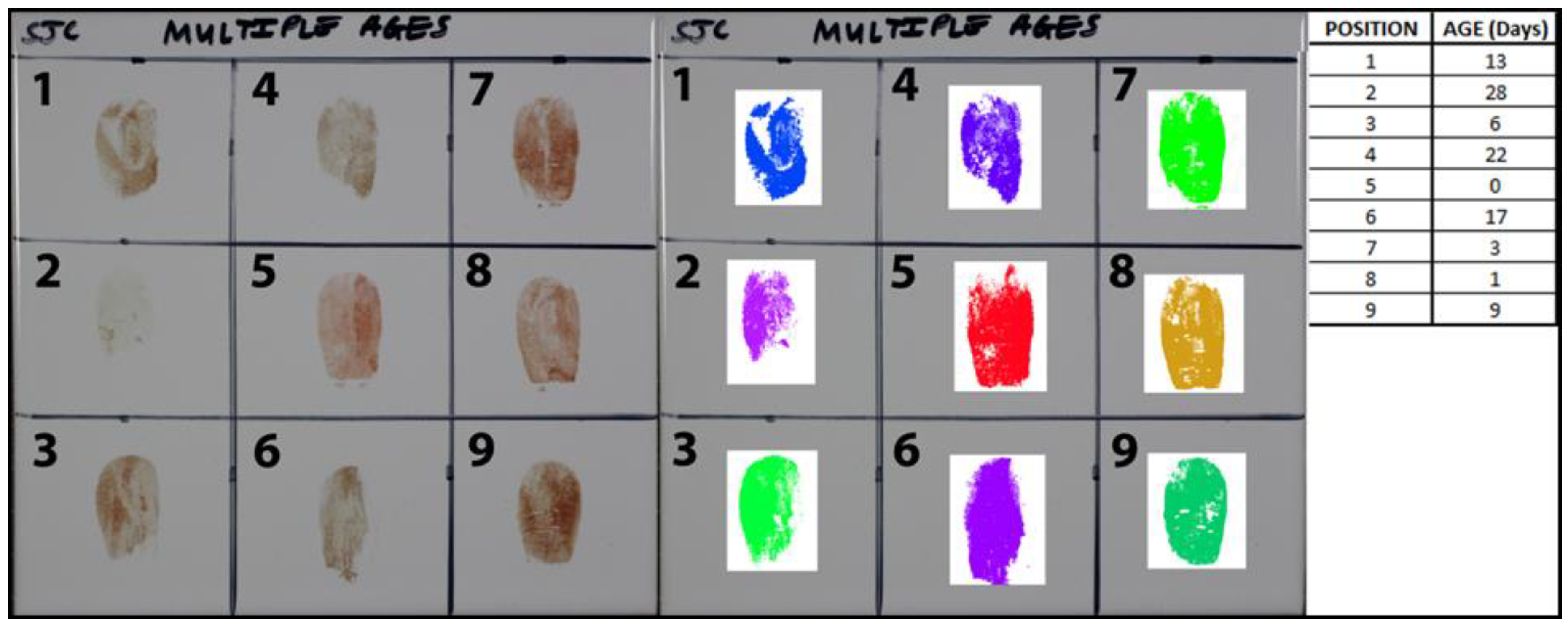
2.7.3. Age Estimations of Nine Blood-Stained Fingerprints
3. Results
3.1. Aging of Blood-Stained Fingerprints over 30 Days
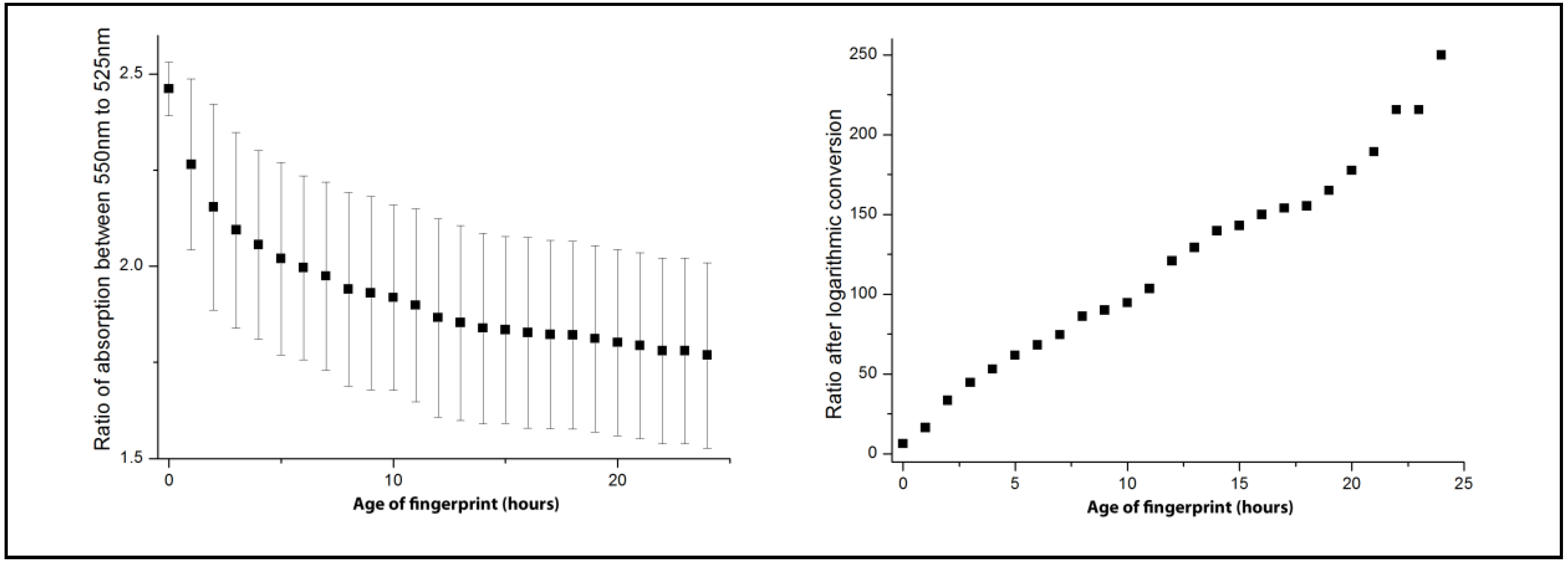
3.2. Aging of Blood-Stained Fingerprints over 24 h
3.3. Age Estimations of Nine Blood-Stained Fingerprints
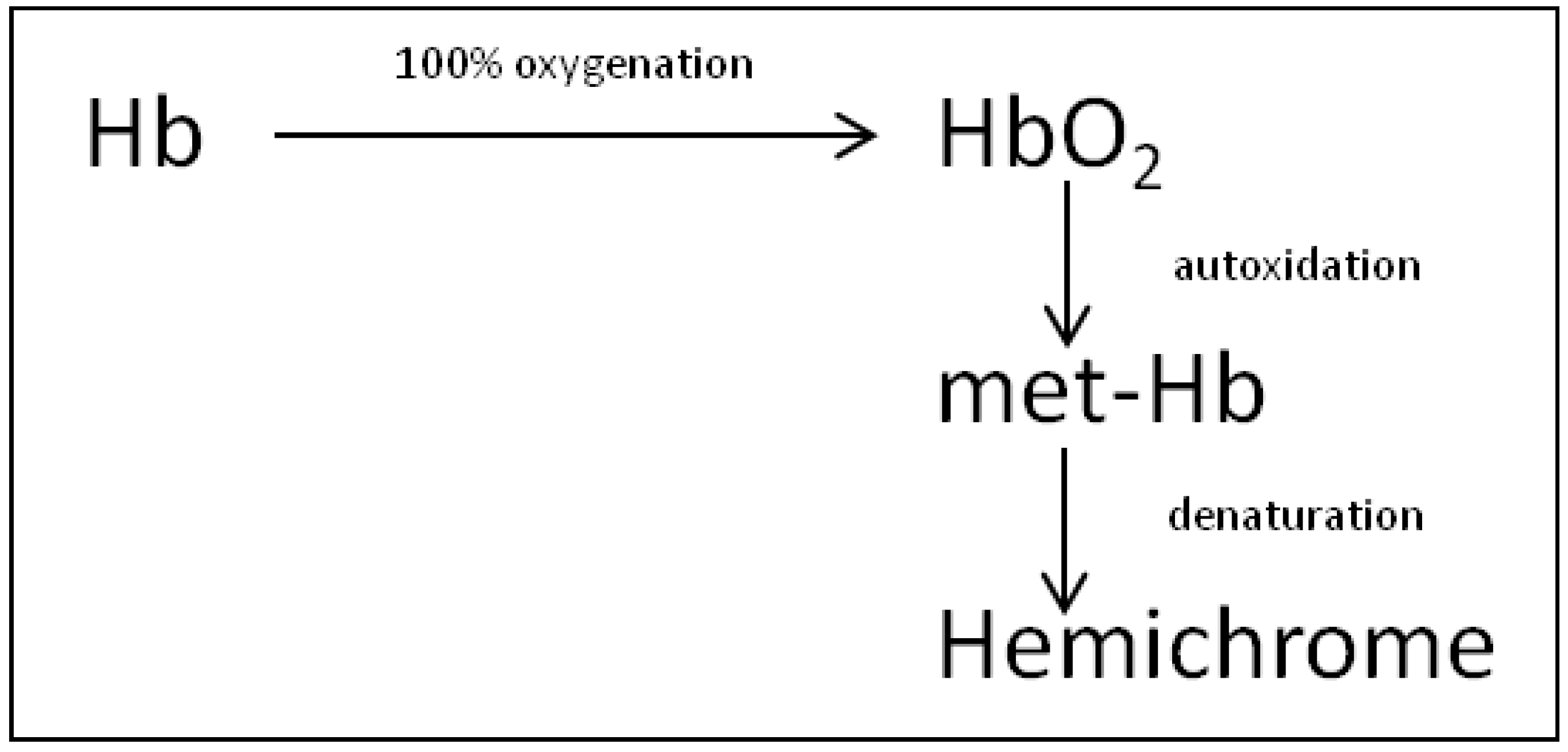
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finnis, J.; Lewis, J.; Davidson, A. Comparison of methods for visualizing blood on dark surfaces. Sci. Justice 2013, 53, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Home Office CAST. Fingerprint Sourcebook, Chapter 3, 3.1 Acid Dyes, 1st ed.; Home Office: London, UK, 2013.
- Li, B.; Beveridge, P.; O’Hare, W.T.; Islam, M. The application of visible wavelength reflectance hyperspectral imaging for the detection and identification of blood stains. Sci. Justice 2014, 54, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Passi, N.; Garg, R.K.; Yadav, M.; Singh, R.S.; Kharoshah, M.A. Effect of luminol and bleaching agent on the serological and DNA analysis from bloodstain. Egypt. J. Forensic Sci. 2012, 2, 54–61. [Google Scholar] [CrossRef]
- Anderson, S.; Howard, B.; Hobbs, G.R.; Bishop, C.P. A method for determining the age of a bloodstain. Forensic Sci. Int. 2005, 148, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bremmer, R.H.; Nadort, A.; van Leeuwen, T.G.; van Gemert, M.J.C.; Aalders, M.C.G. Age estimation of blood stains by hemoglobin derivative determination using reflectance spectroscopy. Forensic Sci. Int. 2011, 206, 166–171. [Google Scholar] [CrossRef] [PubMed]
- de Wael, K.; Lepot, L.; Gason, F.; Gilbert, B. In search of blood—Detection of minute particles using spectroscopic methods. Forensic Sci. Int. 2008, 180, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.C.; Hsieh, H.S.; Li, T.; Linacre, A.; Lee, J.C. Forensic Applications of Infrared Imaging for the Detection and Recording of Latent Evidence. J. Forensic Sci. 2007, 52, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, G.; Sikirzhytski, V.; Lednev, I.K. Circumventing substrate interference in the Raman spectroscopic identification of blood stains. Forensic Sci. Int. 2013, 231, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Stoilovic, M. Detection of semen and blood stains using polilight as a light source. Forensic Sci. Int. 1991, 51, 289–296. [Google Scholar] [CrossRef]
- Strasser, S.; Zink, A.; Kada, G.; Hinterdorfer, P.; Peschel, O.; Heckl, W.M.; Nerlich, A.G.; Thalhammer, S. Age determination of blood spots in forensic medicine by force spectroscopy. Forensic Sci. Int. 2007, 170, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Turrina, S.; Filippini, G.; Atzei, R.; Zaglia, E.; de Leo, D. Validation studies of rapid stain identification-blood (RSID-blood) kit in forensic caseworks. Forensic Sci. Int. Genet. Suppl. Ser. 2008, 1, 74–75. [Google Scholar] [CrossRef]
- Wawryk, J.; Odell, M. Fluorescent identification of biological and other stains on skin by the use of alternative light sources. J. Clin. Forensic Med. 2005, 12, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Cadd, S.; Islam, M.; Manson, P.; Bleay, S. Fingerprint composition and aging: A literature review. Sci. Justice 2015. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.; Anderson, T. Criminal Evidence: Principles and Cases, 7th ed.; Cengage Learning: Belmont, CA, USA, 2009. [Google Scholar]
- Adebsi, S. Fingerprint Studies—The Recent Challenges And Advancements: A Literary View. Internet J. Boil. Anthr. 2008, 2, 1–9. [Google Scholar]
- Midkiff, C. Lifetime of a Latent Print How Long? Can You Tell? J. Forensic Identif. 1993, 43, 386–396. [Google Scholar]
- Bremmer, R.H.; de Bruin, K.G.; van Gemert, M.J.C.; van Leeuwen, T.G.; Aalders, M.C.G. Forensic quest for age determination of bloodstains. Forensic Sci. Int. 2012, 216, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schwarzacher, D. Determination of the age of bloodstains. Am. J. Police Sci. 1930. [Google Scholar] [CrossRef]
- Matsuoka, T.; Taguchi, T.; Okuda, J. Estimation of bloodstain age by rapid determinations of oxyhemoglobin by use of oxygen electrode and total hemoglobin. Boil. Pharm. Bull. 1995, 18, 1031–1035. [Google Scholar] [CrossRef]
- Bauer, M.; Polzin, S.; Patzelt, D. Quantification of RNA degradation by semi-quantitative duplex and competitive RT-PCR: A possible indicator of the age of bloodstains? Forensic Sci. Int. 2003, 138, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M. RNA in forensic science. Forensic Sci. Int. Genet. 2007, 1, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Virkler, K.; Lednev, I.K. Analysis of body fluids for forensic purposes: From laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci. Int. 2009, 188, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Tsuchiya, K.; Abe, S.; Takiguchi, Y.; Kubo, S.; Sakurai, H. Estimation of the age of human bloodstains by electron paramagnetic resonance spectroscopy: Long-term controlled experiment on the effects of environmental factors. Forensic Sci. Int. 2005, 152, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D. Use of reflectance measurements in assessing the colour changes of ageing bloodstains. Nature 1960, 20, 688–689. [Google Scholar] [CrossRef]
- Kind, S.S.; Patterson, D.; Owen, G.W. Estimation of the age of dried blood stains by a spectrophotometric method. Forensic Sci. 1972, 1, 27–54. [Google Scholar] [CrossRef]
- Blazek, V.; Lins, G. Spectroscopic age determination of blood stains: New technical aspects. Acta Med. Leg. Et Soc. 1982, 32, 613–616. [Google Scholar]
- Li, B.; Beveridge, P.; O’Hare, W.T.; Islam, M. The estimation of the age of a blood stain using reflectance spectroscopy with a microspectrophotometer, spectral pre-processing and linear discriminant analysis. Forensic Sci. Int. 2011, 212, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Janchaysang, S.; Sumriddetchkajorn, S.; Buranasiri, P. Tunable filter-based multispectral imaging for detection of blood stains on construction material substrates Part 1: Developing blood stain discrimination criteria. Appl. Opt. 2012, 51, 6984–6996. [Google Scholar] [CrossRef] [PubMed]
- Janchaysang, S.; Sumriddetchkajorn, S.; Buranasiri, P. Tunable filter-based multispectral imaging for detection of blood stains on construction material substrates Part 2: Realization of rapid blood stain detection. Appl. Opt. 2013, 52, 4898–4910. [Google Scholar] [CrossRef] [PubMed]
- Edelman, G.J.; Gaston, E.; van Leeuwen, T.G.; Cullen, P.J.; Aalders, M.C.G. Hyperspectral imaging for non-contact analysis of forensic traces. Forensic Sci. Int. 2012, 223, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Beveridge, P.; O’Hare, W.T.; Islam, M. The age estimation of blood stains up to 30 days old using visible wavelength hyperspectral image analysis and linear discriminant analysis. Sci. Justice 2013, 53, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Cadd, S.; Li, B.; Beveridge, P.; O’Hare, W.T.; Campbell, A.; Islam, M. Non-contact detection and identification of blood stained fingerprints using visible wavelength reflectance hyperspectral imaging: Part 1. Sci. Justice 2016, 56, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Cadd, S.; Li, B.; Beveridge, P.; O’Hare, W.T.; Campbell, A.; Islam, M. Non-contact detection and identification of blood stained fingerprints using visible wavelength reflectance hyperspectral imaging: Part II effectiveness on a range of substrates. Sci. Justice 2016, 56, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bremmer, R.H.; de Bruin, D.M.; de Joode, M.; jan Buma, W.; van Leeuwen, T.G.; Aalders, M.C.G. Biphasic oxidation of oxy-hemoglobin in bloodstains. PLoS ONE 2011, 6, e21845. [Google Scholar] [CrossRef] [PubMed]
- Langford, M.; Fox, A.; Smith, R.S. Langford’s Basic Photography, 8th ed.; Focal Press: Oxford, UK, 2009. [Google Scholar]
- Cadd, S.; Bleay, S.; Sears, V. Evaluation of the solvent black 3 fingermark enhancement reagent: Part 2—Investigation of the optimum formulation and application parameters. Sci. Justice 2013, 53, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.; Charles, Y.; Elliott, J.; Jickells, S. Changes in the lipid composition of latent fingerprint residue with time after deposition on a surface. Forensic Sci. Int. 2005, 154, 224–239. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadd, S.; Li, B.; Beveridge, P.; O'Hare, W.T.; Islam, M. Age Determination of Blood-Stained Fingerprints Using Visible Wavelength Reflectance Hyperspectral Imaging. J. Imaging 2018, 4, 141. https://doi.org/10.3390/jimaging4120141
Cadd S, Li B, Beveridge P, O'Hare WT, Islam M. Age Determination of Blood-Stained Fingerprints Using Visible Wavelength Reflectance Hyperspectral Imaging. Journal of Imaging. 2018; 4(12):141. https://doi.org/10.3390/jimaging4120141
Chicago/Turabian StyleCadd, Samuel, Bo Li, Peter Beveridge, William T. O'Hare, and Meez Islam. 2018. "Age Determination of Blood-Stained Fingerprints Using Visible Wavelength Reflectance Hyperspectral Imaging" Journal of Imaging 4, no. 12: 141. https://doi.org/10.3390/jimaging4120141
APA StyleCadd, S., Li, B., Beveridge, P., O'Hare, W. T., & Islam, M. (2018). Age Determination of Blood-Stained Fingerprints Using Visible Wavelength Reflectance Hyperspectral Imaging. Journal of Imaging, 4(12), 141. https://doi.org/10.3390/jimaging4120141






