Abstract
The increasing significance of batteries in the 21st century and the challenges posed by the anticipated surge in end-of-life batteries, particularly within the European context, are examined in this study. Forecasts predict a notable escalation in battery waste, necessitating a focus on the recycling of black mass (BM)—a complex and hazardous byproduct of the battery recycling process. Employing systematic analysis, this research investigates the hazardous nature of BM derived from various battery types. The study underscores the urgent need for definitive legislative classification of BM’s hazardous properties (HPs), in accordance with European regulations. This comprehensive examination of BM’s HPs contributes significantly to the understanding of BM recycling complexities, proving essential for industry stakeholders and guiding future developments in this field. Additionally, the study explores innovative technologies and strategies that could improve recycling efficiency and reduce associated risks. A pivotal finding of this investigation is the inherently hazardous nature of BM, leading to the recommendation that BM should be classified at a minimum under the “HP3—Flammable” category. This discovery underscores the critical need for stringent management protocols and robust regulatory frameworks to address the burgeoning challenge of battery waste in Europe.
1. Introduction
The onset of the 21st century marks a significant escalation in the importance of batteries, a trend anticipated to intensify in the ensuing years. This escalation is not merely a reflection of technological progress but also indicates a fundamental shift in global energy dynamics. With the imminent battery boom, it becomes imperative to comprehend its widespread implications, especially concerning sustainability and resource management. Forecasts for the global battery market suggest a rapid expansion, with projections pointing to a market value exceeding 400 billion dollars and a total size reaching 4.7 TWh by 2030 [1]. This growth, predominantly propelled by the mobility sector, not only underscores an economic opportunity but also presents a series of challenges and responsibilities. A critical challenge in this context is the adaptation of the entire supply chain to accommodate this exponential growth. The battery industry, while flourishing, confronts a crucial limitation in the sourcing of raw materials, raising both availability and ethical concerns [2]. Therefore, a shift towards more sustainable, circular strategies is essential. These strategies encompass extending battery life, planning for their second life, and, most importantly, enhancing recycling processes [3]. Projected data for 2030 highlight an expected increase in end-of-life batteries across Europe, estimated to be around 264.000t. This figure presents a significant challenge when compared to the current recycling infrastructure, capable of handling only about 80.000t [4]. A crucial aspect of this challenge is the processing of black mass (BM), a complex powder byproduct, difficult to recycle. It results from initial processing stages such as disassembly and pre-treatment, including mechanical size reduction, thermal processes, and density-based separation. This complexity leads to a critical shortfall in efficient and cost-effective technologies for subsequent recycling steps [5]. The difficulties in recycling BM exacerbate the existing imbalance between the demand for and the capacity of recycling solutions. Consequently, bridging this gap requires a comprehensive approach that goes beyond logistical and financial strategies. It calls for an integrated response that combines political will and scientific efforts, working in synergy to develop a sustainable and efficient battery recycling ecosystem. Considering that BM accounts for 40 to 50% of a battery’s total weight [6], projections for 2030 underscore a significant challenge in Europe with the management of an estimated 105.000t to 132.000t of this material. The current practice of exporting BM to countries with advanced recycling facilities, while effective in handling the material, leads to a loss of potential revenue for Europe, as BM contains valuable elements like Cobalt and Lithium [7]. This exportation trend stands in contrast to the goals of the Critical Raw Materials Act [8], which aims to reduce Europe’s reliance on imported raw materials. Additionally, the BM, despite its free transborder movement, is potentially hazardous. The composition of BM includes substances that are classified as hazardous under multiple EU regulations, starting from the Classification, Labeling, and Packaging (CLP) Regulation (EU) No. 1272/2008 [9], which adheres to the United Nations’ Globally Harmonized System (GHS) for the classification and labeling of chemicals. Additionally, these substances are regulated under REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) Regulation (EU) No. 1907/2006, which is directly applicable in all EU member states [10]. Furthermore, these substances have been identified as hazardous by producers and importers in their notifications to the European Chemicals Agency (ECHA). It is noteworthy that these sources offer slightly different labeling and classifications, with the notifications typically indicating a more extensive range of hazardous aspects. Classifying BM as hazardous waste could potentially restrict its export beyond European borders, thereby ensuring the retention of valuable metals and non-metals within Europe [11]. This approach aligns with the Battery Regulation (EU) 2023/1542 [12], which aims to foster a sustainable internal market by setting specific targets and threshold [13]. Among these, by 2027, the regulation mandates a 50% recovery rate for lithium, escalating to 80% by 2031. For cobalt, copper, lead, and nickel, the targets are set at 90% recovery by 2027, rising to 95% by 2031 [12]. In addressing these challenges, the European Parliament has introduced an amendment, P9_TA (2023)0325, to the proposal for the European Critical Raw Materials Act Regulation. The amendment specifically adds paragraph 7a to Article 25, calling for the development of dedicated waste codes for lithium-ion batteries and their intermediate waste streams, with a particular focus on BM [14].
The primary research objective focuses on a systematic and comprehensive analysis of BM, as sourced from a diverse range of battery types. This involves an extensive collection of BM characterizations available in the current scientific literature, assessing their hazardousness in line with the criteria outlined in Regulation (EU) No. 1357/2014, which defines waste classifications as hazardous and non-hazardous, based on the hazardous properties of the substances they contain [15]. This research objective aims to address the notable lack of specific studies dedicated to this topic and to provide orientation and methodologies for the interested stakeholders. In the dynamic field of battery recycling, the second research question targets the identification of advanced technologies and solutions specifically addressing hazardous elements highlighted in the initial research findings. This inquiry is crucial for guiding investments towards comprehensive solutions, not just interim measures. While the industry eagerly anticipates the development of batteries that are efficient, cost-effective, high-performing, and 100% recyclable with minimal effort, aspiring to reach Technology Readiness Level (TRL) 9, it is also imperative to address current challenges. This balance ensures that in striving for future technological breakthroughs, the immediate issues in battery recycling are not overlooked but are effectively resolved with targeted and strategic approaches.
2. Materials and Methods
2.1. PRISMA Methodology
In conducting this systematic literature review (SLR), the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology is meticulously adhered to [16]. PRISMA provides a structured framework for gathering, evaluating, and synthesizing scientific evidence, ensuring the transparency and reproducibility of the research process. The initial stage involved a comprehensive literature search. This search was conducted in two main scientific databases: Scopus and Science Direct. The search, conducted on 02 December 2023, used the following string: “black mass” AND (“recycling” OR “management” OR “characterization” OR “Europe” OR “hazardous” OR “regulation” OR “legislation” OR “sustainability” OR “waste” OR “technologies”). This allowed us to collect 1045 potentially useful papers for this SLR. Once collected and stored in files (.res format) containing the basic information of each paper and the related abstracts, they were uploaded to the Rayyan platform to facilitate the Appraisal step [17]. This phase opens with the definition of the eligibility criteria to be used for the preliminary inclusion and exclusion of the papers, which are as follows:
- Inclusion of peer-reviewed research articles, conference papers, and possibly government or industry reports. Exclusion of non-peer-reviewed sources like blog posts, news articles, or opinion pieces.
- Studies specifically focusing on BM in battery recycling, its characterization, management strategies, or related environmental and hazardous aspects.
- Inclusion of research articles published in English.
- Date Range of last 15 years (since 2008).
Through the eligibility criteria, it was possible to proceed to the operational phase of selecting the papers that constitute the basis of the SLR in the Rayyan platform, following the procedure proposed by the PRISMA 2020 flow chart (Figure 1).
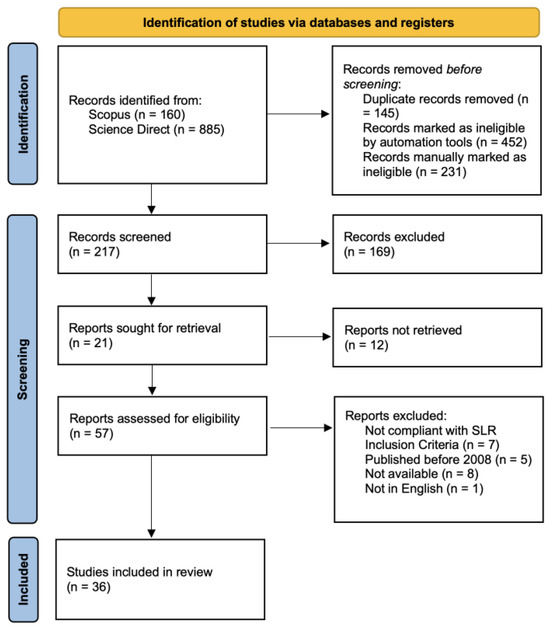
Figure 1.
PRISMA 2020 flow diagram illustrating the selection process for research papers.
At the conclusion of the screening phase, a total of 36 research papers were selected for inclusion in this SLR. Among these, a significant 52.8% were published in the year 2023, followed by 19.4% in 2022, and 13.9% in 2021. To synthesize the findings of this sub-chapter, Table 1 is presented, which serves as a comprehensive repository of the characterizations gathered from the literature, expressed in weight percentage (wt.%). The elements identified in the study are as follows: lithium (Li), cobalt (Co), nickel (Ni), manganese (Mn), aluminum (Al), iron (Fe), carbon (C), cadmium (Cd), potassium (K), titanium (Ti), silicon (Si), calcium (Ca), magnesium (Mg), copper (Cu), zinc (Zn), lead (Pb), phosphorus (P), fluorine (F), sodium (Na), tin (Sn), arsenic (As), and silver (Ag). This table systematically categorizes the content of various substances found in BM, expressed in percentage terms. It also delineates the initial types of batteries from which the BM is derived, classified into distinct categories: lithium-ion batteries (LIBs), nickel–cadmium (Ni-Cd), nickel–metal hydride (Ni-MH), and two separate groups of zinc/manganese batteries—zinc/manganese carbon (Zn/Mn Mn-C) and zinc/manganese (Zn/Mn). The rationale for these distinct categorizations stems from the unique chemical compositions and recycling challenges associated with each battery type:

Table 1.
Characterization of black mass (BM) samples detailed in the scientific literature, expressed as percentages of constituent elements (wt.%).
- Ni-Cd vs. Ni-MH: These batteries are separated due to their differing electrode materials and associated environmental impacts. Ni-Cd batteries contain cadmium, a toxic heavy metal, requiring specific handling and recycling processes. In contrast, Ni-MH batteries, while also based on nickel, replace cadmium with a metal hydride, altering for the composition of the resultant BM.
- Zn/Mn Mn-C vs. Zn/Mn: The division between these Zinc/Manganese battery types is based on their distinct internal chemistries. Zinc/manganese carbon (Mn-C) batteries, traditionally known as alkaline batteries, typically incorporate added carbon, affecting their chemical profile and recycling process. In contrast, standard Zinc/Manganese batteries, referred to as zinc–carbon batteries, present a different composition, influencing the nature of their BM and are commonly used in household applications.
2.2. Black Mass Hazardousness Classification
The methodology employed to classify the hazardousness of BM in this study is multifaceted and robust, incorporating various regulatory frameworks and a comprehensive database analysis. The initial step involved attributing hazard statement codes (HSCs) to the elements detected in BM. These codes, as defined by the CLP Regulation, REACH, and company notifications, identify specific types of hazards. For instance, lithium is assigned the HSC “H260—Water-react. 1” by the CLP, indicating that it releases flammable gases upon contact with water, which may ignite spontaneously. The HSCs associated with the various elements present in BM are comprehensively detailed in Table 2. In addressing the complexity of BM, which contains elements in a variety of compounds and mixtures, the classification process focused on the most fundamental compound form of each element. This methodology was adopted to ensure a precise assessment of the potential hazards, recognizing that the inherent risk of each element can significantly vary depending on its chemical state within the BM.

Table 2.
Hazard statement codes (HSCs) of the BM elements defined by the CLP Regulation, REACH, and company notifications.
Subsequently, EU Regulation No. 1357/2014 was consulted. This regulation assigns hazard categories to substances based on the presence or exceedance of certain percentages of substances with specific HSCs. Following this, a cross-referencing of the data was conducted. This process involved excluding hazard categories where the substances present in the BM did not contain the required HSCs and analyzing the remaining relevant hazard categories. Table 3 delineates the criteria used to assign hazardous properties (HP) for waste classification, detailing the elements of BM analyzed. For HP2—Oxidizing and HP3—Flammable, the mere presence of specific substances is the determining factor. Other HPs require quantifiable thresholds for each HSC, with some thresholds pertaining to individual substances (Indicated as “Ind.” in the table), such as HP5—Specific Target Organ Toxicity (STOT). Conversely, certain thresholds are cumulative, applying to the total sum of substances within a particular HSC category.

Table 3.
EU Regulation No. 1357/2014 hazardousness criteria with corresponding BM elements.
The final stage of the methodology involved a detailed analysis of results from two distinct perspectives. It encompassed an evaluation of individual BM samples, assessing both their minimum and average values, and the construction of theoretical category-specific BM (CSBM). This theoretical construction is based on the original battery types, considering the average values across different BM samples within a category, such as Mixed LIBs, as well as the lowest values observed in these samples. If a substance’s minimum value is zero in any category, it is excluded from that CSBM composition (Table 4). Three distinct scenarios were considered for a multifaceted hazard assessment:

Table 4.
Average (Av.) and minimum (Min.) category-specific BM (CSBM) compositions (wt.%).
- Scenario One: Focused exclusively on the CLP Regulation classifications, this scenario adheres to the GHS criteria and the labeling rules agreed upon by the United Nations.
- Scenario Two: This intermediate scenario broadens the scope by incorporating HSCs from both CLP and REACH classifications, thus expanding the regulatory purview.
- Scenario Three: The most expansive scenario, it compiles all classifications, including those by manufacturers and importers, to reveal the full potential of HPs associated with the BM. This comprehensive view is inclusive of extra-European legislative considerations and provides the most extensive hazard potential profile.
This structured scenario analysis allows for a discerning and layered evaluation of BM hazards, distinguishing the fundamental HP classification from the extended potential risks across varying regulatory frameworks. The approach underpins a thorough appraisal of the BM’s intrinsic and potential hazards within the battery recycling milieu.
3. Results
In the results chapter, an intricate analysis was conducted for each HP as defined in the EU Regulation No. 1357/2014. This analysis cross-referenced the conditions of the regulation with the attributed HSCs in the three scenarios outlined in the methodology. The analysis was applied to both individual BM samples and CSBM, assessing their minimum and average values. The findings are summarized in two distinct tables for each scenario, outlining the classification of BM as hazardous waste:
- Individual Sample Compatibility with HPs: The first table presents the compatibility of individual samples with each HP, categorized by battery type. Each value represents the percentage of samples in a battery category adhering to a specific HP, ranging from 0% (in green), indicating no sample falls under the category, to 100% (in red), denoting all samples fit the category. These percentages are displayed on a color scale to visually represent the increasing likelihood of BM from a specific battery category falling under the respective HP.
- CSBM Analysis: The second table focuses on CSBM, considering the average and minimum values as described in Table 4. It uses a simple “Y” (Yes) in red to indicate classification under a specific HP, or “N” (No) in green when it does not fall under that HP.
3.1. Scenario One: CLP Regulation Classifications
Scenario One is instrumental in understanding which HPs the BM should be classified under, as it solely considers the substance classification provided by the CLP Regulation. A comprehensive overview, synthesized in Table 5 and Table 6, reveals that all samples are classifiable under HP3—Flammable due to the presence of Li, K, Na, and Ca, even in the CSBM constructed with the lowest values among the samples. Two other notable categories are HP4—Irritant and HP8—Corrosive. The BM derived from LIBs and nickel-based batteries shows a significant presence of elements with the H314 code (Skin corr. 1A and 1B) exceeding 1%. Specifically, Li in the former manifests a fluctuating presence ranging from 1.65% to 9.73%, whereas K in the latter is observed within a narrower band of 2.3% to 2.8%. It is noteworthy that all LIBs samples that do not fall under HP4—Irritant have a percentage of Li above 5%, classifying them in the HP8—Corrosive category. Furthermore, nearly all the LIBs and nickel-based BM samples are classified as HP7—Carcinogenic due to more than 0.1% presence of Co. Indeed, with the exception of Sample 12, Co is present in quantities ranging from a minimum of 0.17% in Sample 17 to a maximum of 32.3% in Sample 18. These samples are also classified under HP10—Toxic for reproduction and HP11—Mutagenic criteria, requiring the presence of Co to be equal to or exceed 0.3% and 1%, respectively, thus excluding Sample 17 as well. The analysis reveals that BM originating from LIBs is potentially classified under nine distinct HPs, in contrast to zinc-based BMs, which may be categorized under two HPs. This distinction arises from the LIBs BM samples containing adequate amounts of Li, Co, and F. For BMs from Zn/Mn Mn-C and Zn/Mn, the presence of K and Na results in a definitive classification of the former as HP8—Corrosive, while the latter is likely to be classified under HP4—Irritant.

Table 5.
Hazardousness classification of Individual BM samples under Scenario One, ranging from 0% (No involvement) to 100% (All BM samples falls under a specific HP).

Table 6.
Consolidated hazardousness classification for CSBM in Scenario One, denoted by “Y” (Red) for “Yes”, indicating classification under specific HP, and “N” (Green) for “No” involvement.
3.2. Scenario Two: CLP Regulation and REACH Classifications
Scenario Two extends the analysis to encompass REACH classification, marking a significant divergence from Scenario One (Table 7 and Table 8). Beyond reinforcing the HPs identified earlier, this scenario introduces additional categories. This results in the attribution of additional HPs to the BM from LIBs and nickel-based batteries. In HP5—STOT, BM is classified due to the presence of Ni and Cd at or above 1%, recognized as H372—STOT RE 1, and/or Co at 10% classified as H373—STOT RE 2 under REACH. For HP6—Acute Toxicity, while P and As remain under the limits for H300—Acute Tox. 2 (Oral) and H301—Acute Tox. 3 (Oral), Co exceeds 25% for H302—Acute Tox. 4 (Oral), F surpasses 0.1% for H330—Acute Tox. 1 (Inhal.), and the cumulative presence of Co, Cd, P, and F crosses 0.5% for H330—Acute Tox. 2 (Inhal.). Furthermore, the addition of Ni as H317—Skin Sens. 1 by REACH leads to almost all LIBs and nickel-based samples falling under HP13—Sensitizing. Ni’s classification by REACH as H350—Carc. 1A and 1B and H3512—Carc. 2 specifically impacts Zn/Mn Mn-C batteries in terms of HP7—Carcinogenic. In summary, for LIBs, 10 HP categories are identified, with only one at 100% but with the others showing very high percentages. Ni-Cd and Ni-MH batteries are classified under eight categories, all achieving 100% (alternating between HP4 and HP8 for exceeding 5%). Zn/Mn Mn-C fall under three categories, all at 100%, and Zn/Mn under two. Consequently, under CLP and REACH regulations, Zn/Mn batteries emerge as the least hazardous, although they should still be classified as HP3—Flammable based on the literature samples analyzed.

Table 7.
Hazardousness classification of individual BM samples under Scenario Two, ranging from 0% (No involvement) to 100% (All BM samples falls under a specific HP).

Table 8.
Consolidated hazardousness classification for CSBM in Scenario Two, denoted by “Y” (Red) for “Yes”, indicating classification under specific HP, and “N” (Green) for “No” involvement.
3.3. Scenario Three: CLP Regulation, REACH, and Notification Classifications
Scenario Three is as critical as its predecessors because it incorporates notifications that fall outside European regulations yet signal the presence of potential additional hazards in BM substances (Table 9 and Table 10). These notifications call for actions to be taken or, at the very least, for a careful evaluation of the reasons behind their issuance. In this scenario, BM from LIBs-based batteries has achieved 100% classification in six categories: HP3—Flammable, HP4—Irritant, HP5—STOT, HP6—Acute Toxicity, HP8—Corrosive, and HP11—Mutagenic. Categories HP7—Carcinogenic, HP10—Toxic for reproduction, and HP13—Sensitizing are near 100% due to specific samples, such as Sample 12, which lacks Co and has high levels of Si, and Sample 17, which is rich in C and Fe. For nickel-based BM, there is an increase in percentages for HP8—Corrosive, while the other HPs remain largely unchanged. Notable changes are apparent in Zn/Mn Mn-C and Zn/Mn categories. Both reach 100% classification in every involved HP, escalating from three to eight and from two to six categories, respectively, falling under HP5—STOT, HP6—Acute Toxicity, HP10—Toxic for reproduction, and HP11—Mutagenic.

Table 9.
Hazardousness classification of individual BM samples under Scenario Three, ranging from 0% (No involvement) to 100% (All BM samples falls under a specific HP).

Table 10.
Consolidated hazardousness classification for CSBM in Scenario Three, denoted by “Y” (Red) for “Yes”, indicating classification under specific HP, and “N” (Green) for “No” involvement.
4. Discussion
A pivotal aspect of this analysis is the comparison of CSBM across different scenarios, with a particular focus on Table 10, which contrasts the minimum values in Scenario One and the average values in Scenario Three for CSBM. Scenario One serves as the baseline of our analysis. Here, the classification is conservative, primarily driven by the flammability risk due to the presence of elements like Li, K, Na, and Ca, which directly contribute to HP3—Flammable. In this baseline scenario, other substances such as F play a role in HP4—Irritant and HP8—Corrosive, while Co influences HP7—Carcinogenic; HP10—Toxic for reproduction; and HP11—Mutagenic. In contrast, Scenario Three is recognized as the most precautionary, given that it considers a wider array of properties. By incorporating average values in each CSBM, Scenario Three takes into account the international concern related to BM elements, revealing a broadened hazard profile. The final line of Table 11 contemplates a generic BM classification based on HPs that appear across all CSBMs. This reveals that, within the baseline scenario, HP3—Flammable is the sole HP involved, dictated by the aforementioned elements.

Table 11.
Range of HPs classification of CSBM and generic BM.
To address the flammability issue associated with BM during the recycling processes, the risk mitigation strategies proposed by [38] can be considered. In this work, the author examines various methods to reduce the fire hazard during the preliminary stages of the recycling process. First, discharging the spent batteries is crucial to prevent sparks or explosions during dismantling. This is achieved mainly through three methods: electrolytic discharge using salt solutions, ohmic discharge using an external circuit, and cryogenic discharge with liquid nitrogen or in a vacuum atmosphere. Each of these methods has specific advantages and disadvantages, such as the use of alkali salts to prevent corrosion in electrolytic discharge, or the limited effectiveness and high costs of cryogenic discharge. Second, for industrial-scale recycling, the method of shredding the batteries in a protected environment is often preferred to reduce the fire risk. Techniques such as the use of water sprays or nitrogen gas, CO2 atmospheres, or inert gases like argon or nitrogen during shredding have been implemented to prevent the oxidation of lithium and other reactive elements in the batteries. Further studies have indicated additional significant innovative pre-treatment that can be integrated to enhance both the safety and efficiency of the process. The significance of crusher grid size and pre-treatment temperature in the processing of BM is highlighted by Wilke et al. [39]. It has been observed that finer grid sizes and optimized pre-treatment temperatures can effectively minimize the formation of fine, potentially flammable particles. This approach not only contributes to a reduction in flammability risks but also facilitates the management of particle size distribution, crucial for subsequent processing stages. Punt et al. [40] focus on the method of discharging batteries using a 5% NaCl solution. This technique serves a dual purpose: it mitigates the risk of hazardous reactions inherent in the recycling process and enhances the recovery efficiency of valuable metals. The reduction of reactive and flammable components within the BM through this method renders the material safer for handling and further processing. The concept of mechanical activation, particularly through milling, as a means to influence the reactivity and reducibility of BM is introduced by Babanejad et al. [29]. This process not only advances the energy efficiency of the overall recycling procedure but also impacts the particle size, leading to a more controlled reduction process. The alteration in particle size brought about by mechanical activation plays a pivotal role in managing the flammability risks associated with BM. Moreover, integrating machine learning techniques in the sorting phase can significantly enhance the precision of battery component classification, thereby aiding in the selection of optimal pre-treatment methods and reducing the risk of flammability [41,42]. The preceding sections have provided an in-depth analysis of pretreatment strategies essential for the conditioning of BM from LIBs. These strategies facilitate the separation of materials and enhance the reactivity of the input for recycling processes. Moving forward from pretreatment to the recycling of BM, a detailed synopsis is presented in Table 12, in which a diverse array of BM recycling methodologies, characterized by their distinct operational principles, recovery efficiencies, and inherent limitations. The TRL of each technology were determined through a comprehensive evaluation process. This process included reviewing academic literature and comparing the studies with established TRL definitions outlined by the European Commission [43]. Key factors considered in this evaluation were the development stage, operational data, and technology maturity indicators of each methodology.

Table 12.
Overview of technologies for recycling BM.
The construction of a European infrastructure capable of efficiently managing the current and future amount of BM becomes a compelling economic proposition. In light of Europe’s strategic imperative to internalize BM recycling processes, a combined technical and economic analysis of the prevailing technologies is crucial. This analysis must weigh the operational maturity and recovery efficacy of each process against its economic viability and alignment with sustainable development objectives. Emerging recycling technologies, such as direct leaching in H2SO4 with a dry digestion method [23], acid leaching using molasses [26], and selective leaching using formic acid and hydrogen peroxide [28], are distinguished by their high-efficiency rates, achieving up to 100% lithium recovery. These methods signify considerable strides in material recuperation from expended LIBs. However, the economic feasibility of such high-efficiency technologies must be gauged considering the significant initial capital investment, operational expenditure, and maintenance requisites. The fiscal implications of achieving such high recovery rates must justify the financial and environmental costs incurred. Furthermore, innovative processes like electrochemical junction transfer (ETJ) [44] and early-stage lithium recovery (ESLR) using supercritical CO2 leaching [25], despite being at the vanguard of physicochemical innovation, require enhancements in energy efficiency and CO2 emission mitigation. These enhancements are essential to ensure their economic and environmental sustainability. The operational complexities of technologies like carbothermic reduction and hydrochlorination [27] and electro-assisted leaching [49], while promising in recovery rates, necessitate rigorous management of materials and fine-tuning of process conditions. The economic implications of these processes must account for their maintenance intensity and the requirement for continuous adaptation to evolving battery chemistries. The mechanochemical treatment and acid leaching approach [45], although less energy-demanding, highlights the necessity for process flexibility in the face of persistent impurities. This underscores the fact that the economic viability of recycling technologies is contingent upon the specific composition of the battery materials being processed. Methods such as solvent extraction for Zn and Mn recovery [48] and the exploitation of organic solvents [46], positioned at TRL 5-6, represent a critical juncture in the transition from experimental to scalable operations. These processes must maintain a delicate balance between the elevated operational temperatures required and the imperative for optimization to be economically viable. Lastly, the scalable direct recycling of cathode BM [50] promotes a recycling process that adheres to circularity principles. This not only ensures high recovery rates but also maintains the functional integrity of the material, offering a sustainable trajectory for recycling that could have a profound economic impact.
5. Conclusions
The present study has delved into the multifaceted and pressing issue of BM recycling from batteries, an area growing in urgency alongside the expected increase in end-of-life batteries. The research has unveiled the inherently complex and hazardous character of BM, as deduced from the interplay of various regulations within the European framework that govern the categorization and labeling of substances and waste. A pivotal finding of this inquiry is the indispensable need for definitive legislative action to classify the hazardous nature of BM, and in this regard, the study contributes significantly by establishing that BM should be classified, at a minimum, under the HP3 due to its flammability, while also acknowledging its relevance to a broader spectrum of HPs.
Addressing the identified hazards, the study concurrently casts light on the emergence of innovative technologies and methodologies that promise to mitigate these risks while bolstering the efficiency of BM recycling—answering the second research question. These advancements lend credence to the notion that while BM recycling presents formidable challenges, they are surmountable with the development and application of such technologies, which are vital to revolutionizing BM handling and enhancing the safety and efficacy of recycling practices.
While this research has imparted significant insights, it also delineates avenues for further investigation. The limited sample size, while illustrative, invites more comprehensive future studies employing the robust methodology established here to deepen the understanding of BM recycling across a more extensive range of BM types and compositions.
The study also acknowledges the complexity introduced by the variability of BM composition across different battery types. This diversity, though challenging, offers fertile ground for further research into an array of recycling methods, potentially yielding more nuanced and efficient recycling strategies that accommodate the vast spectrum of BM characteristics.
As Europe faces an influx of battery waste and an accompanying rise in BM volume, this study identifies an urgent need to appraise the continent’s current recycling capacities and to pinpoint existing gaps. Addressing these deficiencies calls for an integrated approach in investing and evaluating the innovative technologies explored in preceding chapters. Notably, those technologies at a higher TRL—such as acid leaching using molasses, scalable direct recycling, and direct selective leaching of lithium—showcase operational maturity and a high degree of material recovery efficiency, suggesting their potential for scalable and economically viable applications.
Future research endeavors should endeavor to perform a detailed economic feasibility analysis of these technologies, considering their potential for industrial-scale application, the time required for technological maturation, cost implications, and the possible revenues from the recovered materials. Such an approach is essential to assure that the recycling processes are economically appealing and environmentally prudent, attracting investment and fostering entrepreneurial interest.
The conducted study establishes a pivotal foundation for future advancements in BM recycling. Bridging scientific inquiry with legislative frameworks, it marks a significant stride towards responsible and effective management in the battery recycling domain. This investigation not only tackles the immediate complexities but also establishes a solid base for future scientific and regulatory developments, which are vital for navigating the environmental challenges ahead.
Author Contributions
Conceptualization, M.G. and E.M.M.; methodology, M.G. and E.M.M.; validation, E.M.M. and M.M.; formal analysis, F.T.; investigation, M.G. and F.T.; resources, E.M.M.; data curation, M.G.; writing—original draft preparation, M.G.; writing—review and editing, E.M.M., M.M. and F.T.; visualization, M.G.; supervision, M.M.; project administration, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Al | Aluminum |
| As | Arsenic |
| BM | Black Mass |
| Cd | Cadmium |
| Ca | Calcium |
| C | Carbon |
| CSBM | Category-Specific Black Mass |
| CLP | Classification, Labeling, and Packaging |
| Co | Cobalt |
| Cu | Copper |
| DES | Deep Eutectic Solvents |
| ESLR | Early-stage Lithium Recovery |
| ECHA | European Chemicals Agency |
| EC | European Commission |
| ETJ | Electrolytic Junction Transfer |
| EU | European Union |
| F | Fluorine |
| GHS | Globally Harmonized System |
| HSC | Harmonized System Codes |
| HP | Hazardous Properties |
| IL | Ionic Liquids |
| Fe | Iron |
| Pb | Lead |
| LIB | Lithium-Ion Batteries |
| LCO | Lithium Cobalt Oxide |
| Mg | Magnesium |
| Mn | Manganese |
| Ni | Nickel |
| NMC | Nickel Manganese Cobalt |
| P | Phosphorus |
| K | Potassium |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| Si | Silicon |
| Ag | Silver |
| Na | Sodium |
| STOT | Specific Target Organ Toxicity |
| SLR | Systematic Literature Review |
| TRL | Technology Readiness Level |
| Sn | Tin |
| Ti | Titanium |
| Zn | Zinc |
References
- McKinsey Co.; Global Battery Alliance. Battery 2030: Resilient, Sustainable, and Circular Battery Demand Is Growing—And So Is the Need for. 2023. Available online: https://www.mckinsey.com/industries/automotive-and-assembly/our-insights/battery-2030-resilient-sustainable-and-circular (accessed on 29 January 2024).
- Dai, Q.; Kelly, J.C.; Gaines, L.; Wang, M. Life cycle analysis of lithium-ion batteries for automotive applications. Batteries 2019, 5, 48. [Google Scholar] [CrossRef]
- Bhuyan, A.; Tripathy, A.; Padhy, R.K.; Gautam, A. Evaluating the lithium-ion battery recycling industry in an emerging economy: A multi-stakeholder and multi-criteria decision-making approach. J. Clean. Prod. 2022, 331, 130007. [Google Scholar] [CrossRef]
- Motus, E. Strategy&, and Politecnico Milano, Il Riciclo Delle Batterie Dei Veicoli Elettrici @ 2050: Scenari Evolutivi e Tecnologie Abilitanti. 2023. Available online: https://www.motus-e.org/wp-content/uploads/2023/03/Motus-E_PwCS_PoliMi_Il-riciclo-delle-batterie-dei-veicoli-elettrici-@2050-scenari-evolutivi-e-tecnologie-abilitanti.pdf (accessed on 29 January 2024).
- Donnelly, L.; Pirrie, D.; Power, M.; Corfe, I.; Kuva, J.; Lukkari, S.; Lahaye, Y.; Liu, X.; Dehaine, Q.; Jolis, E.M.; et al. The Recycling of End-of-Life Lithium-Ion Batteries and the Phase Characterisation of Black Mass. Recycling 2023, 8, 59. [Google Scholar] [CrossRef]
- AquaMetals. What Exactly Is Lithium Battery ‘Black Mass’? Available online: https://aquametals.com/recyclopedia/what-exactly-is-black-mass/ (accessed on 29 January 2024).
- Dadé, M.; Wallmach, T.; Laugier, O. Detailed Microparticle Analyses Providing Process Relevant Chemical and Microtextural Insights into the Black Mass. Minerals 2022, 12, 119. [Google Scholar] [CrossRef]
- European Commission. COM(2023) 160 Final. 2023. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:903d35cc-c4a2-11ed-a05c-01aa75ed71a1.0001.02/DOC_1&format=PDF (accessed on 29 January 2024).
- European Commission. Regulation (EC) No 1272/2008. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272 (accessed on 29 January 2024).
- European Commission. Regulation (EC) No 1907/2006. 2006. Available online: https://eur-lex.europa.eu/eli/reg/2006/1907/2023-08-06 (accessed on 29 January 2024).
- Frédéric, S. EU Urged to Restrict Export of Black Mass from Used Electric Vehicles, Euractive. Available online: https://www.euractiv.com/section/circular-materials/news/eu-urged-to-restrict-export-of-black-mass-from-used-electric-vehicles/ (accessed on 29 January 2024).
- European Commission. Regulation (EU) No 2023/1542. 2023. Available online: https://eur-lex.europa.eu/eli/reg/2023/1542/oj (accessed on 29 January 2024).
- Melin, H.E.; Rajaeifar, M.A.; Ku, A.Y.; Kendall, A.; Harper, G.; Heidrich, O. Global implications of the EU battery regulation. Science 2021, 373, 384–387. [Google Scholar] [CrossRef]
- European Parliament. P9_TA(2023)0325. Available online: https://www.europarl.europa.eu/doceo/document/TA-9-2023-0325_EN.pdf (accessed on 29 January 2024).
- European Commission. Regulation (EU) No 1357/2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014R1357 (accessed on 29 January 2024).
- Mengist, W.; Soromessa, T.; Legese, G. Method for conducting systematic literature review and meta-analysis for environmental science research. MethodsX 2020, 7, 100777. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Marsili, F. Evaluating software tools to conduct systematic reviews: A feature analysis and user survey. Form@ Re-Open J. Form. Rete 2021, 21, 124–140. [Google Scholar] [CrossRef]
- Mousa, E.; Hu, X.; Ånnhagen, L.; Ye, G.; Cornelio, A.; Fahimi, A.; Bontempi, E.; Frontera, P.; Badenhorst, C.; Santos, A.C.; et al. Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries. Sustainability 2023, 15, 15. [Google Scholar] [CrossRef]
- Stallmeister, C.; Friedrich, B. Influence of Flow-Gas Composition on Reaction Products of Thermally Treated NMC Battery Black Mass. Metals 2023, 13, 923. [Google Scholar] [CrossRef]
- Babanejad, S.; Ahmed, H.; Andersson, C.; Samuelsson, C.; Lennartsson, A.; Hall, B.; Arnerlöf, L. High-Temperature Behavior of Spent Li-Ion Battery Black Mass in Inert Atmosphere. J. Sustain. Metall. 2022, 8, 566–581. [Google Scholar] [CrossRef]
- Vassura, I.; Morselli, L.; Bernardi, E.; Passarini, F. Chemical characterisation of spent rechargeable batteries. Waste Manag. 2009, 29, 2332–2335. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, M.; Vonderstein, C.; Dertmann, C.; Klimko, J.; Oráč, D.; Miškufová, A.; Havlík, T.; Friedrich, B. A combined pyro-and hydrometallurgical approach to recycle pyrolyzed lithium-ion battery black mass part 1: Production of lithium concentrates in an electric arc furnace. Metals 2020, 10, 1069. [Google Scholar] [CrossRef]
- Klimko, J.; Oráč, D.; Miškufová, A.; Vonderstein, C.; Dertmann, C.; Sommerfeld, M.; Friedrich, B.; Havlík, T. A combined pyro-and hydrometallurgical approach to recycle pyrolyzed lithium-ion battery black mass part 2: Lithium recovery from li enriched slag—Thermodynamic study, kinetic study, and dry digestion. Metals 2020, 10, 1558. [Google Scholar] [CrossRef]
- Gerold, E.; Lerchbammer, R.; Antrekowitsch, H. Evaluation of the Influence Exerted by Increased Silicon Contents on the Leaching Behavior of NMC-Based Black Mass. Metals 2023, 13, 785. [Google Scholar] [CrossRef]
- Schwich, L.; Schubert, T.; Friedrich, B. Early-stage recovery of lithium from tailored thermal conditioned black mass part i: Mobilizing lithium via supercritical co2-carbonation. Metals 2021, 11, 177. [Google Scholar] [CrossRef]
- Amalia, D.; Singh, P.; Zhang, W.; Nikoloski, A.N. The Effect of a Molasses Reductant on Acetic Acid Leaching of Black Mass from Mechanically Treated Spent Lithium-Ion Cylindrical Batteries. Sustainability 2023, 15, 13171. [Google Scholar] [CrossRef]
- Makuza, B.; Yu, D.; Huang, Z.; Guo, X.; Tian, Q.; Zhang, K.; Zhang, B.; Liu, P. Synergetic carbothermic reduction and selective hydrochlorination of spent Li-ion batteries black mass towards enhanced metal recovery. J. Clean. Prod. 2023, 386, 135831. [Google Scholar] [CrossRef]
- Zhao, T.; Marthi, R.; Mahandra, H.; Chae, S.; Traversy, M.; Sadri, F.; Choi, Y.; Ghahreman, A. Direct selective leaching of lithium from industrial-grade black mass of waste lithium-ion batteries containing LiFePO4 cathodes. Waste Manag. 2023, 171, 134–142. [Google Scholar] [CrossRef]
- Babanejad, S.; Ahmed, H.; Andersson, C.; Heikkinen, E.P. Mechanical Activation-Assisted Recovery of Valuable Metals from Black Mass in the Form of Fe/Cu Alloys. J. Sustain. Metall. 2023, 9, 522–536. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Domańska, U. Separation of cobalt, lithium and nickel from the ‘black mass’ of waste Li-ion batteries by ionic liquids, DESs and organophosphorous-based acids extraction. J. Mol. Liq. 2021, 343, 117694. [Google Scholar] [CrossRef]
- Biswas, J.; Ulmala, S.; Wan, X.; Partinen, J.; Lundström, M.; Jokilaakso, A. Selective Sulfation Roasting for Cobalt and Lithium Extraction from Industrial LCO-Rich Spent Black Mass. Metals 2023, 13, 358. [Google Scholar] [CrossRef]
- Hazotte, C.; Leclerc, N.; Diliberto, S.; Meux, E.; Lapicque, F. End-of-life nickel-cadmium accumulators: Characterization of electrode materials and industrial Black Mass. Environ. Technol. 2015, 36, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Lach, J.; Wróbel, K.; Domańska, U. New method for recovery of nickel and cadmium from the black mass of spent Ni-Cd batteries by solvent extraction. J. Mol. Liq. 2022, 357, 119087. [Google Scholar] [CrossRef]
- Romo, L.A.; López-Fernández, A.; García-Díaz, I.; Fernández, P.; Urbieta, A.; López, F.A. From spent alkaline batteries to ZnxMn3−xO4 by a hydrometallurgical route: Synthesis and characterization. RSC Adv. 2018, 8, 33496–33505. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, M.K.; Jung, S.C.; Jung, H.Y.; Kim, H.; Park, Y.K. Effect of palladium on the black mass-based catalyst prepared from spent Zn/Mn alkaline batteries for catalytic combustion of volatile organic compounds. Chemosphere 2021, 276, 130209. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Jung, S.C.; Jung, H.Y.; Khan, M.A.; Jeon, B.H.; Kim, S.C. The use of black mass in spent primary battery as an oxidative catalyst for removal of volatile organic compounds. J. Ind. Eng. Chem. 2022, 114, 323–330. [Google Scholar] [CrossRef]
- Park, Y.K.; Shim, W.G.; Jung, S.C.; Jung, H.Y.; Kim, S.C. Catalytic removal of volatile organic compounds using black mass from spent batteries. Korean J. Chem. Eng. 2022, 39, 161–166. [Google Scholar] [CrossRef]
- Premathilake, D.S.; Junior, A.B.B.; Tenório, J.A.S.; Espinosa, D.C.R.; Vaccari, M. Designing of a Decentralized Pretreatment Line for EOL-LIBs Based on Recent Literature of LIB Recycling for Black Mass. Metals 2023, 13, 374. [Google Scholar] [CrossRef]
- Wilke, C.; Werner, D.M.; Kaas, A.; Peuker, U.A. Influence of the Crusher Settings and a Thermal Pre-Treatment on the Properties of the Fine Fraction (Black Mass) from Mechanical Lithium-Ion Battery Recycling. Batteries 2023, 9, 514. [Google Scholar] [CrossRef]
- Punt, T.; Bradshaw, S.M.; van Wyk, P.; Akdogan, G. The Efficiency of Black Mass Preparation by Discharge and Alkaline Leaching for LIB Recycling. Minerals 2022, 12, 753. [Google Scholar] [CrossRef]
- Ran, A.; Liang, Z.; Chen, S.; Cheng, M.; Sun, C.; Ma, F.; Wang, K.; Li, B.; Zhou, G.; Zhang, X.; et al. Fast Clustering of Retired Lithium-Ion Batteries for Secondary Life with a Two-Step Learning Method. ACS Energy Lett. 2022, 7, 3817–3825. [Google Scholar] [CrossRef]
- Tao, S.; Liu, H.; Sun, C.; Ji, H.; Ji, G.; Han, Z.; Gao, R.; Ma, J.; Ma, R.; Chen, Y.; et al. Collaborative and privacy-preserving retired battery sorting for profitable direct recycling via federated machine learning. Nat. Commun. 2023, 14, 8032. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Work Programme 2023–2024. 2022. Available online: https://ec.europa.eu/info/funding-tenders/opportunities/docs/2021-2027/horizon/wp-call/2023-2024/wp-13-general-annexes_horizon-2023-2024_en.pdf (accessed on 29 January 2024).
- Guyot, E.; Boulanger, C.; Lecuire, J.M. Leaching optimization of battery black mass for lithium recovery by Electrochemical Junction Transfer (ETJ) technology. Chem. Eng. Trans. 2014, 41, 67–72. [Google Scholar] [CrossRef]
- Porvali, A.; Mäkelä, T.; Bachér, J. Observations on the Leaching of Milled Black Mass with Additives. J. Sustain. Metall. 2023, 9, 816–825. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Dimachki, Z.; Bryant, W.; Zhang, J.; Biniaz, P.; Holl, M.M.B.; Pozo-Gonzalo, C.; Banerjee, P.C. Removal of polyvinylidene fluoride binder and other organics for enhancing the leaching efficiency of lithium and cobalt from black mass. J. Environ. Manag. 2023, 343, 118205. [Google Scholar] [CrossRef]
- Vieceli, N.; Benjamasutin, P.; Promphan, R.; Hellström, P.; Paulsson, M.; Petranikova, M. Recycling of Lithium-Ion Batteries: Effect of Hydrogen Peroxide and a Dosing Method on the Leaching of LCO, NMC Oxides, and Industrial Black Mass. ACS Sustain. Chem. Eng. 2023, 11, 9662–9673. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Lach, J.; Wrobel, K.; Domańska, U. Recovery of zinc and manganese from ‘black mass’ of waste Zn-MnO2 alkaline batteries by solvent extraction technique with ionic liquids, DESs and organophosphorous-based acids. J. Mol. Liq. 2021, 338, 116590. [Google Scholar] [CrossRef]
- Hazotte, C.; Meux, E.; Leclerc, N.; Lapicque, F. Electroassisted leaching of black mass solids from Ni-Cd batteries for metal recovery: Investigation of transport and transfer phenomena coupled to reactions. Chem. Eng. Process. Process Intensif. 2015, 96, 83–93. [Google Scholar] [CrossRef]
- Gupta, V.; Yu, X.; Gao, H.; Brooks, C.; Li, W.; Chen, Z. Scalable Direct Recycling of Cathode Black Mass from Spent Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2203093. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).