Sustainable Valorization of Brewer’s Spent Grain via Submerged Fermentation Using Talaromyces stollii for Laccase and Phenolic Compounds Production
Abstract
1. Introduction
2. Results and Discussion
2.1. BSG Characterization
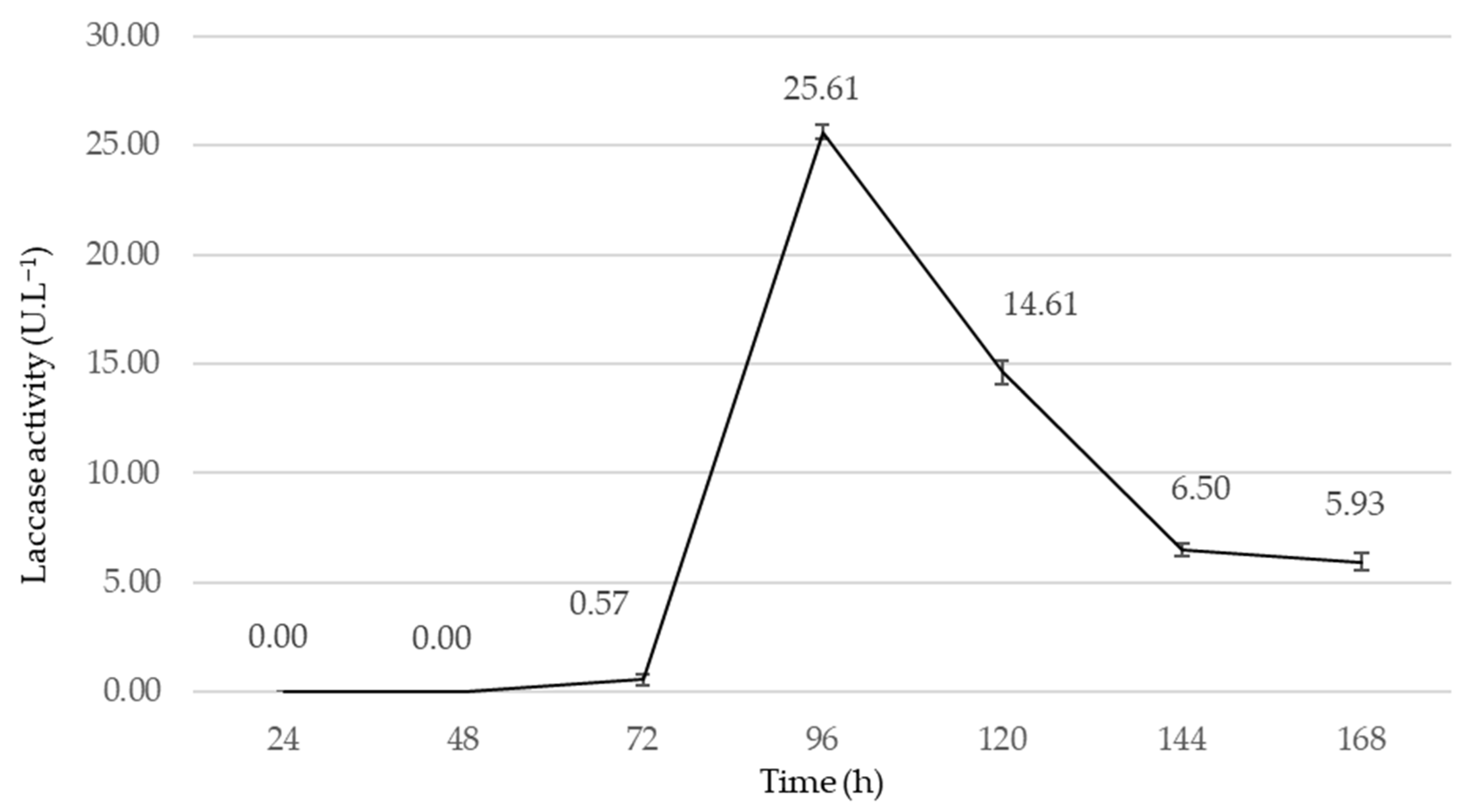
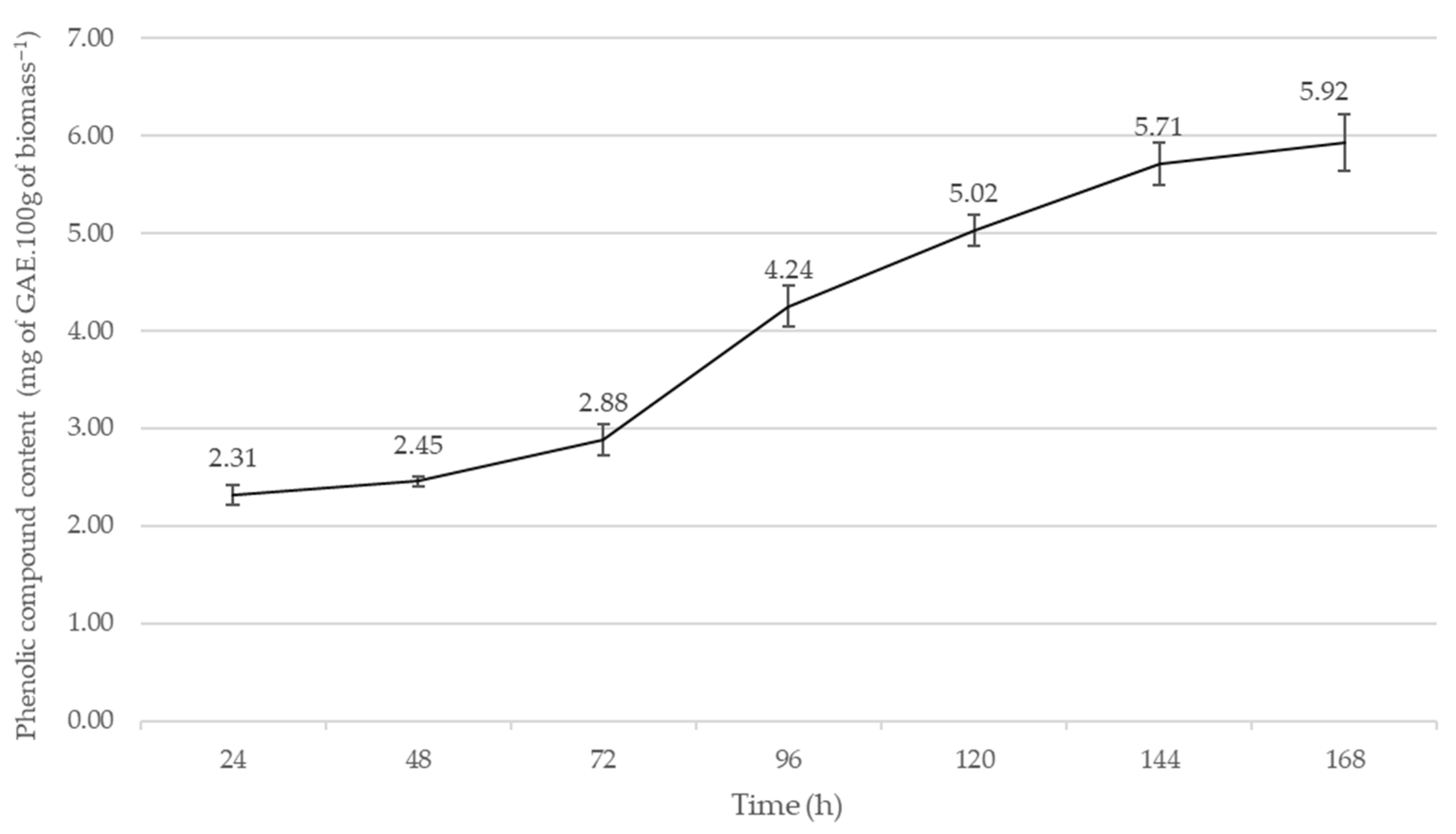
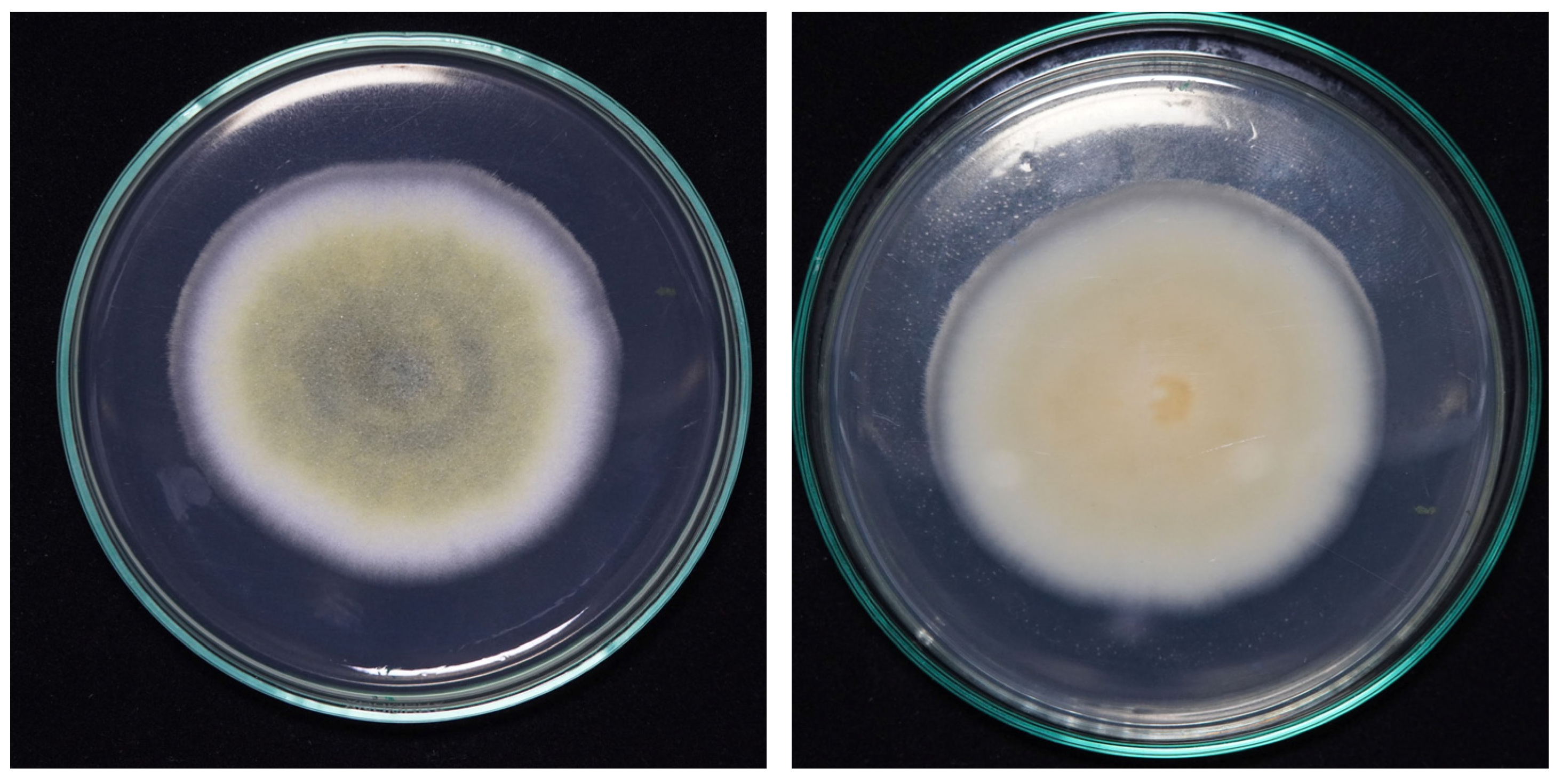
2.2. Preliminary Submerged Fermentations (PSmF)
2.3. Submerged Fermentation with Supplemented Medium
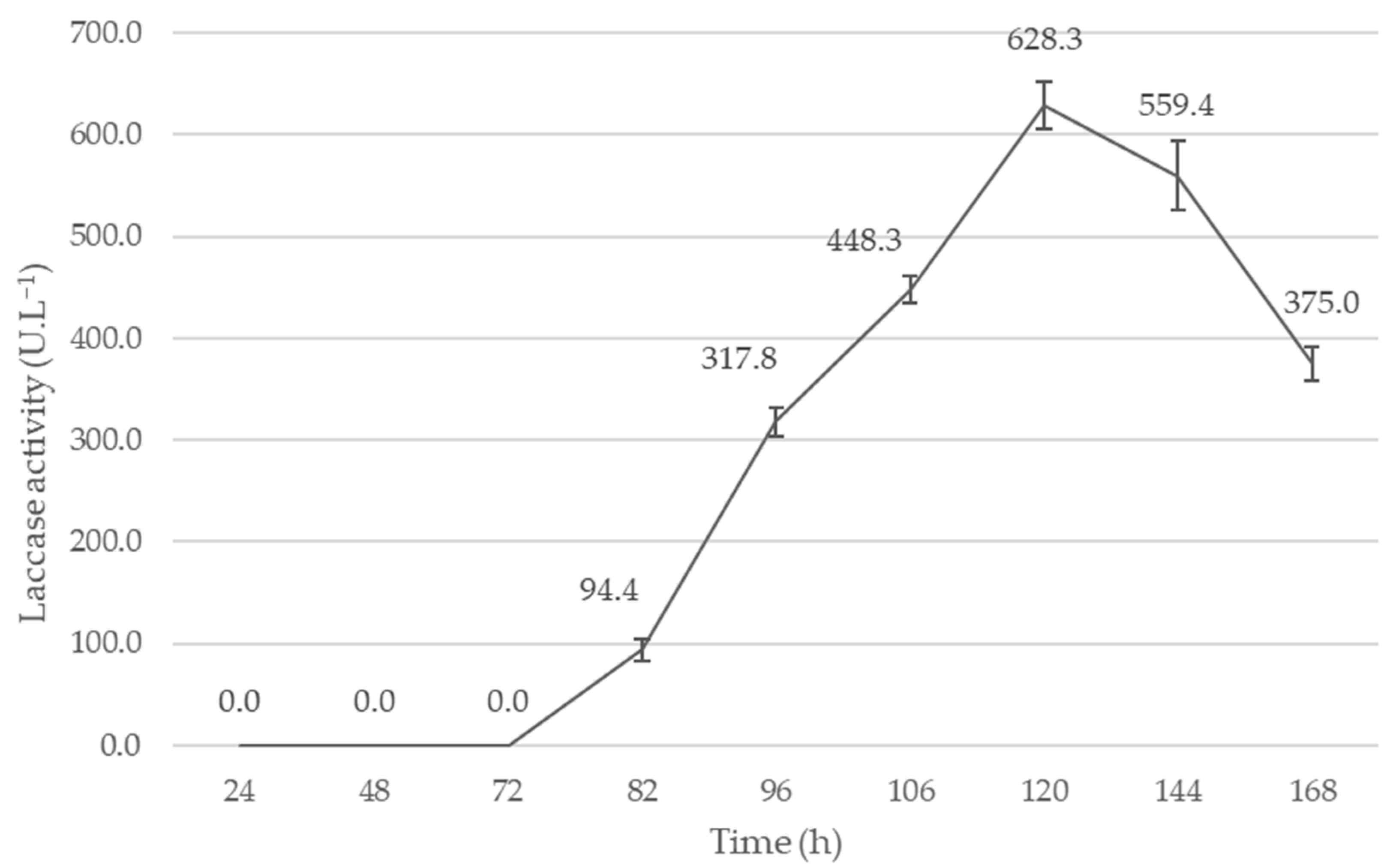
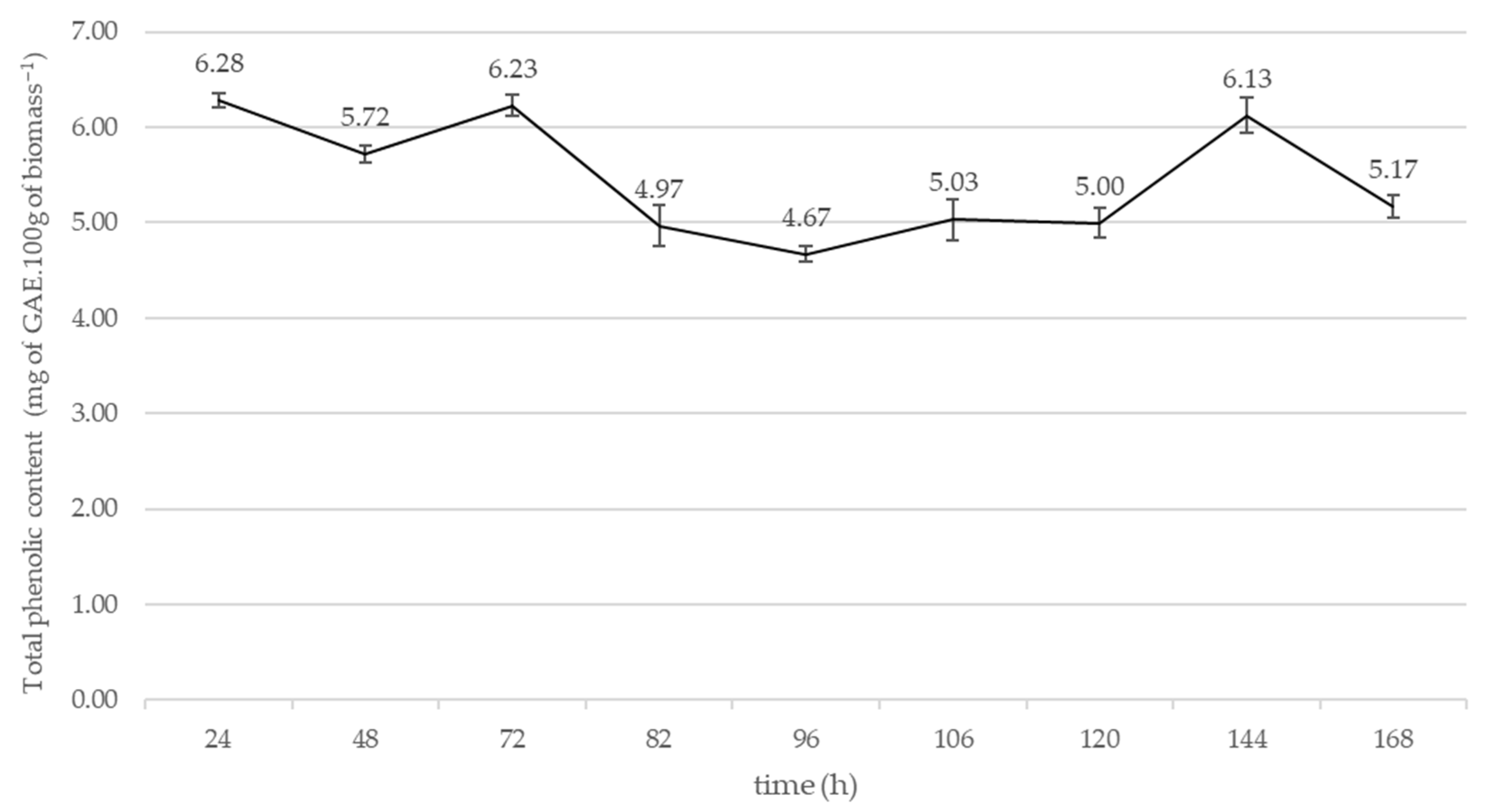
2.4. Submerged Fermentation with Copper and Manganese Inducers
3. Materials and Methods
3.1. BSG Characterization
3.1.1. Determination of Moisture Content
3.1.2. Determination of Crude Protein Content
3.1.3. Determination of Extractives Content
3.1.4. Determination of Cellulose, Hemicellulose, Lignin, and Ash Content
3.2. Microorganism
3.3. Spore Suspension
3.4. Preliminary Submerged Fermentations
3.5. Submerged Fermentations with Enriched Medium
3.6. Submerged Fermentations with Inducers
3.7. Determination of Laccase Activity
3.8. Determination of Total Phenolic Content
3.9. Determination of the Presence of Copper and Manganese
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSG | Brewers’ Spent Grain |
| Lac | Laccase |
| TPC | Total Phenolic Content |
| GAE | Gallic Acid Equivalent |
| SmF | Submerged Fermentation |
| PSmF | Preliminary Submerged Fermentation |
| XRF | X-Ray Fluorescence |
References
- Agrawal, D.; Gopaliya, D.; Willoughby, N.; Khare, S.K.; Kumar, V. Recycling Potential of Brewer’s Spent Grains for Circular Biorefineries. Curr. Opin. Green Sustain. Chem. 2023, 40, 100748. [Google Scholar] [CrossRef]
- Statista. Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 24 July 2025).
- Fortune Business Insights. Available online: https://www.fortunebusinessinsights.com/beer-market-102489 (accessed on 24 July 2025).
- Sibono, L.; Tronci, S.; Grosso, M.; Hajrizaj, R.; Errico, M. Brewer’s Spent Grain to Bioethanol Through a Hybrid Saccharification and Fermentation Process. Chem. Eng. Trans. 2023, 99, 7–12. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Olanrewaju, O.A.; Usman, M.A.; Adeosun, S.O. Lignin from Brewers’ Spent Grain: Structural and Thermal Evaluations. Polymers 2023, 15, 2346. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Budroni, M.; Zara, S.; Mannazzu, I.; Fancello, F.; Zara, G. The Role of Microorganisms on Biotransformation of Brewers’ Spent Grain. Appl. Microbiol. Biotechnol. 2020, 104, 8661–8678. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Boeira Cataneo, C.; Caliari, V.; Valdemiro Gonzaga, L.; Marta Kuskoski, E.; Fett, R. Antioxidant activity and phenolic content of agricultural by-products from wine production. Semina Cienc. Agrar. 2008, 29, 93–102. [Google Scholar]
- Mafra, E. Brasil Mostra Que é um País Cada vez Mais Cervejeiro, Forbes Agro, 1 Set. 2022. Available online: https://forbes.com.br/forbesagro/2022/09/brasil-mostra-que-e-um-pais-cada-vez-mais-cervejeiro (accessed on 23 July 2023).
- Cervbrasil. Anuário da Cerveja. Ministério da Agricultura, Pecuária e Abastecimento. 2024. Available online: http://www.cervbrasil.org.br/novo_site/anuario/ (accessed on 23 July 2025).
- IBGE—Instituto Brasileiro de Geografia e Estatística. Produção de Cevada. 2021. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/cevada/br (accessed on 19 July 2023).
- Gałązka, A.; Jankiewicz, U.; Orzechowski, S. The Role of Ligninolytic Enzymes in Sustainable Agriculture: Applications and Challenges. Agronomy 2025, 15, 451. [Google Scholar] [CrossRef]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of Lignocellulose Using Ligninolytic Enzymes from White-Rot Fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef] [PubMed]
- Chattaraj, S.; Mitra, D.; Ganguly, A.; Thatoi, H.; Das Mohapatra, P.K. A Critical Review on the Biotechnological Potential of Brewers’ Waste: Challenges and Future Alternatives. Curr. Res. Microb. Sci. 2024, 6, 100228. [Google Scholar] [CrossRef]
- United Nations. 17 Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 24 July 2025).
- Guo, H.; Zhao, Y.; Chang, J.-S.; Lee, D.-J. Enzymes and Enzymatic Mechanisms in Enzymatic Degradation of Lignocellulosic Biomass: A Mini-Review. Bioresour. Technol. 2023, 367, 128252. [Google Scholar] [CrossRef]
- Volf, I.; Popa, V.I. Integrated Processing of Biomass Resources for Fine Chemical Obtaining: Polyphenols. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018; pp. 113–160. [Google Scholar] [CrossRef]
- Chu, H.-Y.I.; Miri, T.; Onyeaka, H. Valorization of Bioactive Compounds Extracted from Brewer’s Spent Grain (BSG) for Sustainable Food Waste Recycling. Sustainability 2025, 17, 2477. [Google Scholar] [CrossRef]
- Arellano, T. Exploring the Supply Chain for Brewery’s by-Product Recycling: A Study on a Potential Alternative for a Brewery’s by-Product Valorization. Master’s Thesis, Université de Liège, Liège, Belgium, 2023. [Google Scholar]
- Tišma, M.; Jurić, A.; Bucić-Kojić, A.; Panjičko, M.; Planinić, M. Biovalorization of Brewers’ Spent Grain for the Production of Laccase and Polyphenols: Biovalorization of Brewers’ Spent Grain. J. Inst. Brew. 2018, 124, 182–186. [Google Scholar] [CrossRef]
- Wagner, E.; Ceriani Nakamurakare, E.; Robles, C.A.; Carmaran, C.C.; Rojas, N.L. An Unexplored Enzymatic Source for Brewer’s Spent Grain Valorization: The Role of Wood-Substrate Beetle-Associated Fungi and Endophytes. Biomass Bioenergy 2025, 197, 107788. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, J.; Wang, K.; Gong, C.; Liu, D.; Gou, F.; Liu, D.; Kong, Z.; Hao, J.; Sun, W.; et al. Transcriptomic Analysis of Two Ascomycete Fungi on the Utilization of Tobacco Biomass and Identification of Key Pectinases in Polysaccharides Deconstruction. Environ. Technol. Innov. 2025, 38, 104191. [Google Scholar] [CrossRef]
- Egbewale, S.O.; Kumar, A.; Mokoena, M.P.; Olaniran, A.O. Mycotransformation of Anthracene by Indigenous Trichoderma Lixii and Talaromyces Pinophilus Isolates: Insights into the Metabolic Pathways, Enzyme Profiles and Acute Toxicity. Biodegradation 2025, 36, 51. [Google Scholar] [CrossRef]
- Dos Santos, P.M.; Baruque, J.R.; De Souza Lira, R.K.; Leite, S.G.F.; Do Nascimento, R.P.; Borges, C.P.; Wojcieszak, R.; Itabaiana, I. Corn Cob as a Green Support for Laccase Immobilization—Application on Decolorization of Remazol Brilliant Blue R. Int. J. Mol. Sci. 2022, 23, 9363. [Google Scholar] [CrossRef] [PubMed]
- Aza, P.; Camarero, S. Fungal Laccases: Fundamentals, Engineering and Classification Update. Biomolecules 2023, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Eugenio, M.E.; Tomás-Pejó, E. Laccases as Versatile Enzymes: From Industrial Uses to Novel Applications. J. Chem. Technol. Biotechnol. 2020, 95, 481–494. [Google Scholar] [CrossRef]
- De Salas, F.; Aza, P.; Gilabert, J.F.; Santiago, G.; Kilic, S.; Sener, M.E.; Vind, J.; Guallar, V.; Martínez, A.T.; Camarero, S. Engineering of a Fungal Laccase to Develop a Robust, Versatile and Highly-Expressed Biocatalyst for Sustainable Chemistry. Green Chem. 2019, 21, 5374–5385. [Google Scholar] [CrossRef]
- Pazarlıoǧlu, N.K.; Sariişik, M.; Telefoncu, A. Laccase: Production by Trametes Versicolor and Application to Denim Washing. Process Biochem. 2005, 40, 1673–1678. [Google Scholar] [CrossRef]
- Vittorio, O.; Cojoc, M.; Curcio, M.; Spizzirri, U.G.; Hampel, S.; Nicoletta, F.P.; Iemma, F.; Dubrovska, A.; Kavallaris, M.; Cirillo, G. Polyphenol Conjugates by Immobilized Laccase: The Green Synthesis of Dextran-Catechin. Macro Chem. Phys. 2016, 217, 1488–1492. [Google Scholar] [CrossRef]
- Mayolo-Deloisa, K.; González-González, M.; Rito-Palomares, M. Laccases in Food Industry: Bioprocessing, Potential Industrial and Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 8, 222. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Uses of Laccases in the Food Industry. Enzym. Res. 2010, 2010, 918761. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Skwarek, E.; Pawlik, A. Potential of Laccase as a Tool for Biodegradation of Wastewater Micropollutants. Water 2023, 15, 3770. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Šelo, G.; Zelić, B.; Planinić, M.; Tišma, M. Recovery of Phenolic Acid and Enzyme Production from Corn Silage Biologically Treated by Trametes Versicolor. Appl. Biochem. Biotechnol. 2017, 181, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.; Clifton, P. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, F.F.; De Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Massardi, M.M.; Massini, R.M.M.; Silva, D.D.J. Caracterização química do bagaço de malte e avaliação do seu potencial para obtenção de produtos de valor agregado. J. Eng. Exact Sci. 2020, 6, 83–91. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Kanauchi, O.; Mitsuyama, K.; Araki, Y. Development of a Functional Germinated Barley Foodstuff from Brewer’s Spent Grain for the Treatment of Ulcerative Colitis. J. Am. Soc. Brew. Chem. 2001, 59, 59–62. [Google Scholar] [CrossRef]
- Pedro Silva, J.; Sousa, S.; Rodrigues, J.; Antunes, H.; Porter, J.J.; Gonçalves, I.; Ferreira-Dias, S. Adsorption of Acid Orange 7 Dye in Aqueous Solutions by Spent Brewery Grains. Sep. Purif. Technol. 2004, 40, 309–315. [Google Scholar] [CrossRef]
- Mainali, K.; Yadav, M.P.; Sharma, B.K.; Sarker, M.I.; Ngo, H.; Hotchkiss, A.; Simon, S. Isolation and Characterization of the Physiochemical Properties of Brewer’s Spent Grain. Agriculture 2024, 15, 47. [Google Scholar] [CrossRef]
- Qin, F.; Johansen, A.Z.; Mussatto, S.I. Evaluation of Different Pretreatment Strategies for Protein Extraction from Brewer’s Spent Grains. Ind. Crops Prod. 2018, 125, 443–453. [Google Scholar] [CrossRef]
- Langenaeken, N.A.; De Schepper, C.F.; De Schutter, D.P.; Courtin, C.M. Carbohydrate Content and Structure during Malting and Brewing: A Mass Balance Study: Carbohydrates throughout Malting and Brewing. J. Inst. Brew. 2020, 126, 253–262. [Google Scholar] [CrossRef]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; Del Nozal, M.J. Variability of Brewer’s Spent Grain within a Brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Silva, V.E.; Silva, A.C.; Pereira, R.C.; Camargo, P.B.D.; Silva, B.P.C.; Barral, U.M.; Mendonça Filho, C.V. Composição lignocelulósica e isótopica da vegetação e da matéria orgânica do solo de uma turfeira tropical: I—Composição florística, fitomassa e acúmulo de carbono. Rev. Bras. Ciênc. Solo 2013, 37, 121–133. [Google Scholar] [CrossRef]
- Forootanfar, H.; Faramarzi, M.A.; Shahverdi, A.R.; Yazdi, M.T. Purification and Biochemical Characterization of Extracellular Laccase from the Ascomycete Paraconiothyrium Variabile. Bioresour. Technol. 2011, 102, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.I.; Shimizu, E.; Zapata, P.D.; Villalba, L.L. Copper Inducing Effect on Laccase Production of White Rot Fungi Native from Misiones (Argentina). Enzym. Microb. Technol. 2010, 46, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.A.; Seviour, R.J.; Schmid, F. Growth of Filamentous Fungi in Submerged Culture: Problems and Possible Solutions. Crit. Rev. Biotechnol. 2000, 20, 17–48. [Google Scholar] [CrossRef]
- De Oliveira, F.; Ferreira, L.C.; Neto, Á.B.; Simas Teixeira, M.F.; De Carvalho Santos Ebinuma, V. Biosynthesis of Natural Colorant by Talaromyces Amestolkiae: Mycelium Accumulation and Colorant Formation in Incubator Shaker and in Bioreactor. Biochem. Eng. J. 2020, 161, 107694. [Google Scholar] [CrossRef]
- Silva, L.C.; Kupski, L.; Beserra Da Silva De Souza, S.; Bolanho Barros, B.C. Influence of Fermentation Conditions by Rhizopus Oryzae on the Release of Phenolic Compounds, Composition, and Properties of Brewer’s Spent Grain. Food Biosci. 2024, 61, 104591. [Google Scholar] [CrossRef]
- Mollea, C.; Bosco, F. Solid-State Fermentation of Brewery Spent Grains to Enhance Biomolecule Extraction. Separations 2025, 12, 58. [Google Scholar] [CrossRef]
- Othman, A.M.; Mahmoud, M.; Abdelraof, M.; Abdel Karim, G.S.A.; Elsayed, A.M. Enhancement of Laccase Production from a Newly Isolated Trichoderma Harzianum S7113 Using Submerged Fermentation: Optimization of Production Medium via Central Composite Design and Its Application for Hydroquinone Degradation. Int. J. Biol. Macromol. 2021, 192, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Chakroun, H.; Mechichi, T.; Martinez, M.J.; Dhouib, A.; Sayadi, S. Purification and Characterization of a Novel Laccase from the Ascomycete Trichoderma Atroviride: Application on Bioremediation of Phenolic Compounds. Process Biochem. 2010, 45, 507–513. [Google Scholar] [CrossRef]
- Santos, S.F.D.M.; Macedo, G.R.D.; Silva, F.L.H.D.; Souza, R.L.A.D.; Pinto, G.A.S. Aplicação da metodologia de superfície de resposta no estudo da produção e extração da poligalacturonase. Quím. Nova 2008, 31, 1973–1978. [Google Scholar] [CrossRef][Green Version]
- França, E.D.S.; De Souza, A.F.; Rodríguez, D.M.; De Paula, N.Z.; Neves, A.G.D.; Cardoso, K.B.B.; Campos-Takaki, G.M.D.; De Lima, M.A.B.; Porto, A.L.F. Valorization of Spent Coffee Grounds as a Substrate for Fungal Laccase Production and Biosorbents for Textile Dye Decolorization. Fermentation 2025, 11, 396. [Google Scholar] [CrossRef]
- Da Costa Maia, I.; Thomaz Dos Santos D’Almeida, C.; Guimarães Freire, D.M.; d’Avila Costa Cavalcanti, E.; Cameron, L.C.; Furtado Dias, J.; Simões Larraz Ferreira, M. Effect of Solid-State Fermentation over the Release of Phenolic Compounds from Brewer’s Spent Grain Revealed by UPLC-MSE. LWT 2020, 133, 110136. [Google Scholar] [CrossRef]
- Wong, D.W.S. Structure and Action Mechanism of Ligninolytic Enzymes. Appl Biochem Biotechnol 2009, 157, 174–209. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic Enzymes and Its Mechanisms for Degradation of Lignocellulosic Waste in Environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Nigam, S.; Pandey, A. Biotechnological Potential of Brewing Industry By-Products. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Springer: Dordrecht, The Netherlands, 2009; pp. 313–326. [Google Scholar] [CrossRef]
- Jin, Z.; Lan, Y.; Ohm, J.-B.; Gillespie, J.; Schwarz, P.; Chen, B. Physicochemical Composition, Fermentable Sugars, Free Amino Acids, Phenolics, and Minerals in Brewers’ Spent Grains Obtained from Craft Brewing Operations. J. Cereal Sci. 2022, 104, 103413. [Google Scholar] [CrossRef]
- Nielsen, S.S. Moisture Content Determination. In Food Analysis Laboratory Manual; Food Science Text Series; Springer: Cham, Switzerland, 2017; pp. 105–115. [Google Scholar] [CrossRef]
- Thiex, N.J.; Manson, M.; Andersson, S.; Persson, J. Determination of crude protein in animal feed, forage, grain, and oilseeds by using block digestion with a copper catalyst and steam distillation into boric acid: Collaborative study. J. AOAC Int. 2002, 85, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); Issue Date 7/17/2005; Technical Report NREL/TP-510-42619; Laboratory Analytical Procedure (LAP) of NREL: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; Laboratory Analytical Procedure (LAP) of NREL: Golden, CO, USA, 2008. [Google Scholar]
- Dos Santos, P.M. Valorização do Bagaço de Malte Como Fonte de Obtenção de Compostos Fenólicos por Fermentação Submersa e Recuperação por Membranas. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2022. [Google Scholar]
- Venkatachalam, M.; Shum-Chéong-Sing, A.; Dufossé, L.; Fouillaud, M. Statistical Optimization of the Physico-Chemical Parameters for Pigment Production in Submerged Fermentation of Talaromyces Albobiverticillius 30548. Microorganisms 2020, 8, 711. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Samson, R.A. Polyphasic Taxonomy of the Genus Talaromyces. Stud. Mycol. 2014, 78, 175–341. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, J.P.; Morales-Oyervides, L.; Giuffrida, D.; Dufossé, L.; Montañez, J.C. Production of Pigments under Submerged Culture through Repeated Batch Fermentation of Immobilized Talaromyces Atroroseus GH2. Fermentation 2023, 9, 171. [Google Scholar] [CrossRef]
- Lorenzo, M.; Moldes, D.; Sanromán, M.Á. Effect of Heavy Metals on the Production of Several Laccase Isoenzymes by Trametes Versicolor and on Their Ability to Decolourise Dyes. Chemosphere 2006, 63, 912–917. [Google Scholar] [CrossRef]
- Agrawal, K.; Verma, P. Laccase: Addressing the Ambivalence Associated with the Calculation of Enzyme Activity. 3 Biotech 2019, 9, 365. [Google Scholar] [CrossRef]
- Silveira, M.; Oster, A.; Moura, C.F.H.; Silva, E.d.O.; Silva, L.M.A.; Sousa, A.E.D. Protocolos para Avaliação das Características Físicas e Físico-Químicas dos Compostos Bioativos e Atividade Antioxidante do Pedúnculo do Caju. EMBRAPA. 2018. Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1090475 (accessed on 25 July 2025).
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid Microplate High-Throughput Methodology for Assessment of Folin-Ciocalteu Reducing Capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef]
- Wierzba, S.; Rajfur, M.; Nabrdalik, M.; Kłos, A. Assessment of the Influence of Counter Ions on Biosorption of Copper Cations in Brewer’s Spent Grain—Waste Product Generated during Beer Brewing Process. Microchem. J. 2019, 145, 196–203. [Google Scholar] [CrossRef]
- Shobande, O.A.; Tiwari, A.K.; Ogbeifun, L.; Trabelsi, N. Demystifying Circular Economy and Inclusive Green Growth for Promoting Energy Transition and Carbon Neutrality in Europe. Struct. Change Econ. Dyn. 2024, 70, 666–681. [Google Scholar] [CrossRef]
| Components | % (% Dry wt) |
|---|---|
| Cellulose | 14.74 ± 1.04 |
| Hemicellulose | 22.13 ± 0.41 |
| Lignin | 15.13 ± 1.47 |
| Ash | 5.36 ± 0.57 |
| Crude Protein | 20.54 ± 0.28 |
| Potassium/Phosphate Buffer (mM) | K (g·L−1) * | P (g·L−1) * | Lac (U·L−1) * |
|---|---|---|---|
| 25 | 1.03 | 0.77 | 782.5 ± 29.1 |
| 50 | 2.06 | 1.54 | 983.3 ± 28.3 |
| 100 | 4.13 | 3.09 | 1535 ± 151.6 |
| Cu2+ (g·L−1) | Mn2+ (mM) | Lac (U·L−1) |
|---|---|---|
| 0.025 | 0 | 992.5 ± 99.1 |
| 0.05 | 0 | 879.1 ± 25.8 |
| 0.075 | 0 | 291.6 ± 62.2 |
| 0 | 1 | 775 ± 46.6 |
| 0 | 2 | 665 ± 30.1 |
| 0 | 3 | 80 ± 8 |
| 0.025 | 1 | 0 |
| 0.05 | 2 | 0 |
| 0.075 | 3 | 0 |
| Component | wt % |
|---|---|
| Mn2+ | 39.0016 |
| Cu2+ | 60.9984 |
| Experiment | Cu2+ (g·L−1) | Mn2+ (mM) | Buffer Concentration (mM) |
|---|---|---|---|
| 1 | 0.025 | 0 | 25 |
| 2 | 0.05 | 0 | 50 |
| 3 | 0.075 | 0 | 100 |
| 4 | 0 | 1 | 25 |
| 5 | 0 | 2 | 50 |
| 6 | 0 | 3 | 100 |
| 7 | 0.025 | 1 | 25 |
| 8 | 0.05 | 2 | 50 |
| 9 | 0.075 | 3 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, E.C.S.; Nascimento, A.C.B.d.; Nascimento, R.P.d.; Itabaiana, I., Jr. Sustainable Valorization of Brewer’s Spent Grain via Submerged Fermentation Using Talaromyces stollii for Laccase and Phenolic Compounds Production. Recycling 2025, 10, 166. https://doi.org/10.3390/recycling10040166
Lima ECS, Nascimento ACBd, Nascimento RPd, Itabaiana I Jr. Sustainable Valorization of Brewer’s Spent Grain via Submerged Fermentation Using Talaromyces stollii for Laccase and Phenolic Compounds Production. Recycling. 2025; 10(4):166. https://doi.org/10.3390/recycling10040166
Chicago/Turabian StyleLima, Eric Coelho S., Ana Caroline B. do Nascimento, Rodrigo P. do Nascimento, and Ivaldo Itabaiana, Jr. 2025. "Sustainable Valorization of Brewer’s Spent Grain via Submerged Fermentation Using Talaromyces stollii for Laccase and Phenolic Compounds Production" Recycling 10, no. 4: 166. https://doi.org/10.3390/recycling10040166
APA StyleLima, E. C. S., Nascimento, A. C. B. d., Nascimento, R. P. d., & Itabaiana, I., Jr. (2025). Sustainable Valorization of Brewer’s Spent Grain via Submerged Fermentation Using Talaromyces stollii for Laccase and Phenolic Compounds Production. Recycling, 10(4), 166. https://doi.org/10.3390/recycling10040166







