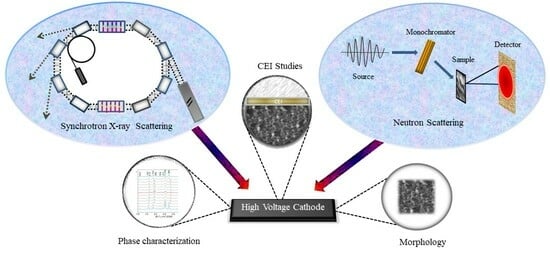
Understanding High-Voltage Behavior of Sodium-Ion Battery Cathode Materials Using Synchrotron X-ray and Neutron Techniques: A Review
Abstract
:1. Introduction
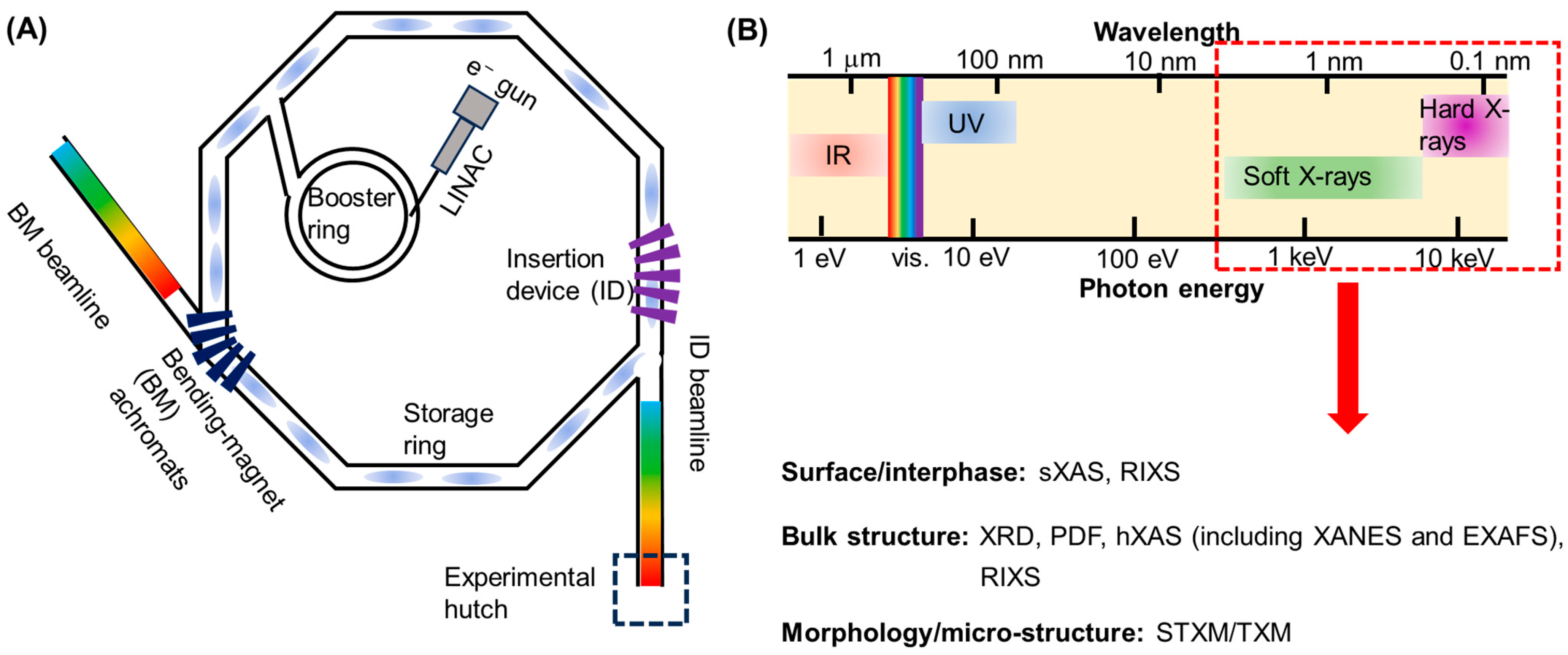
2. Brief Overview of Synchrotron Radiation
3. Brief Overview of Neutron Technology
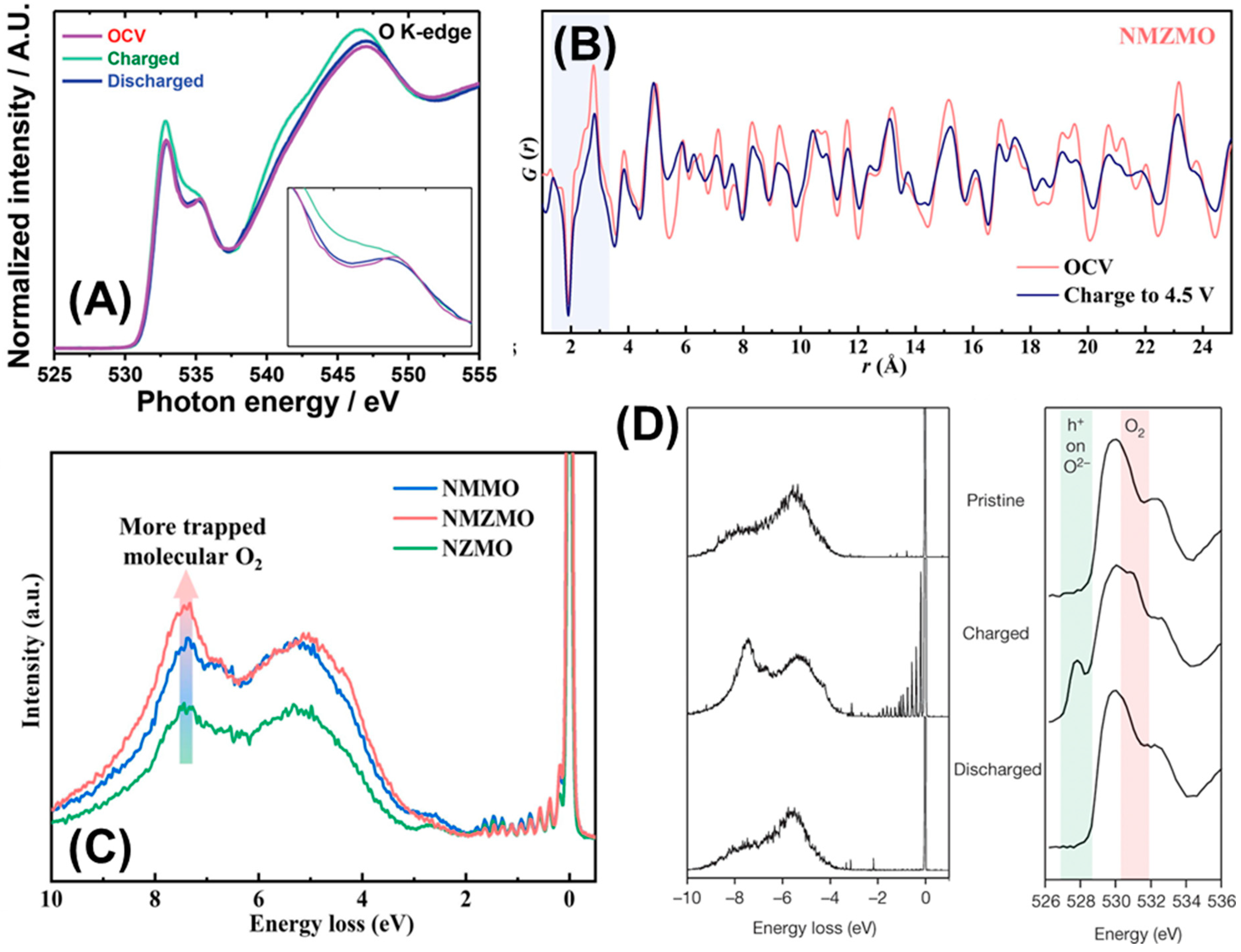
4. Layered Oxide Cathode Materials
5. Polyanion Compounds
6. Prussian Blue Analogue and Organic Materials
7. Conclusions and Perspectives
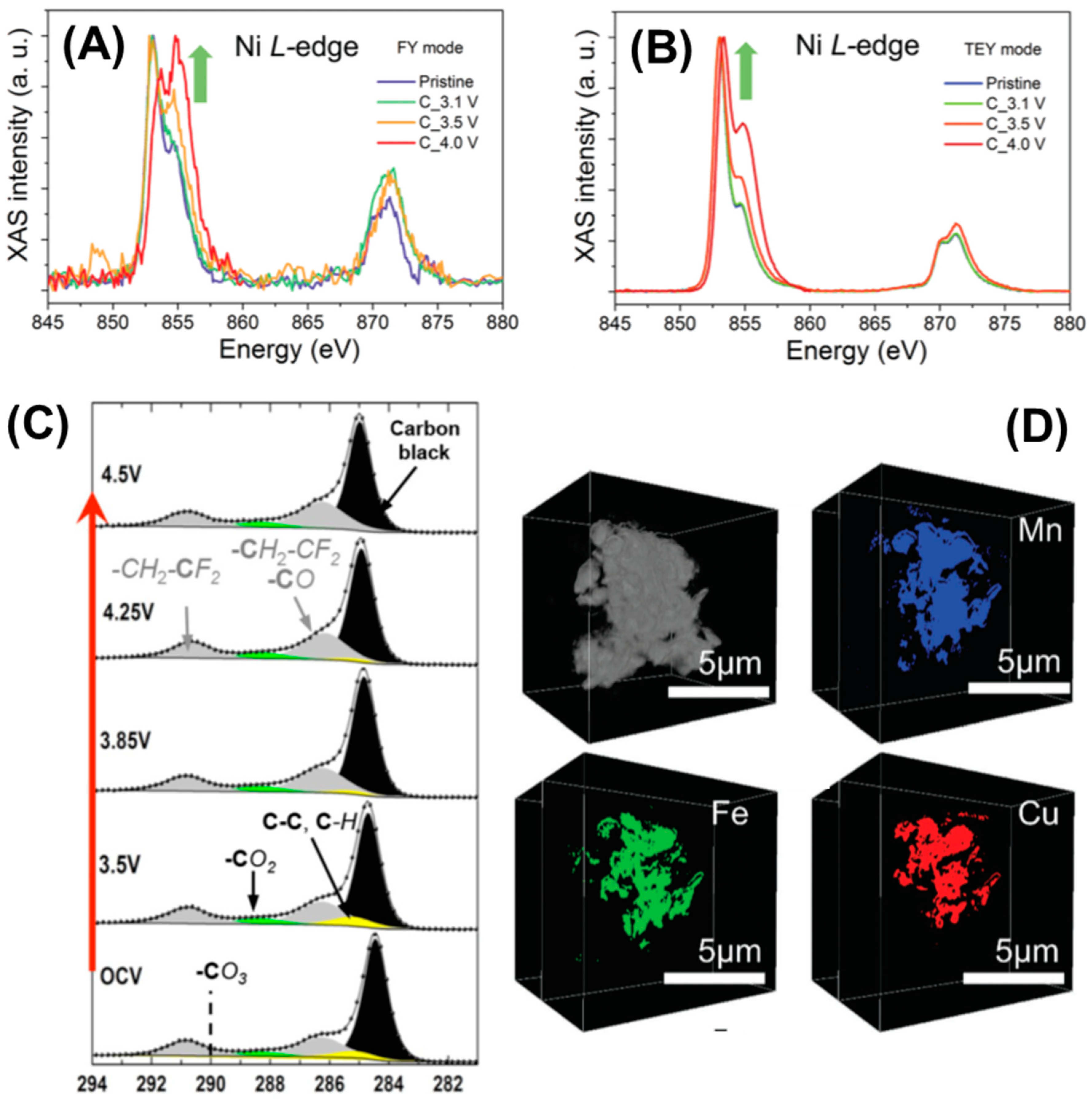
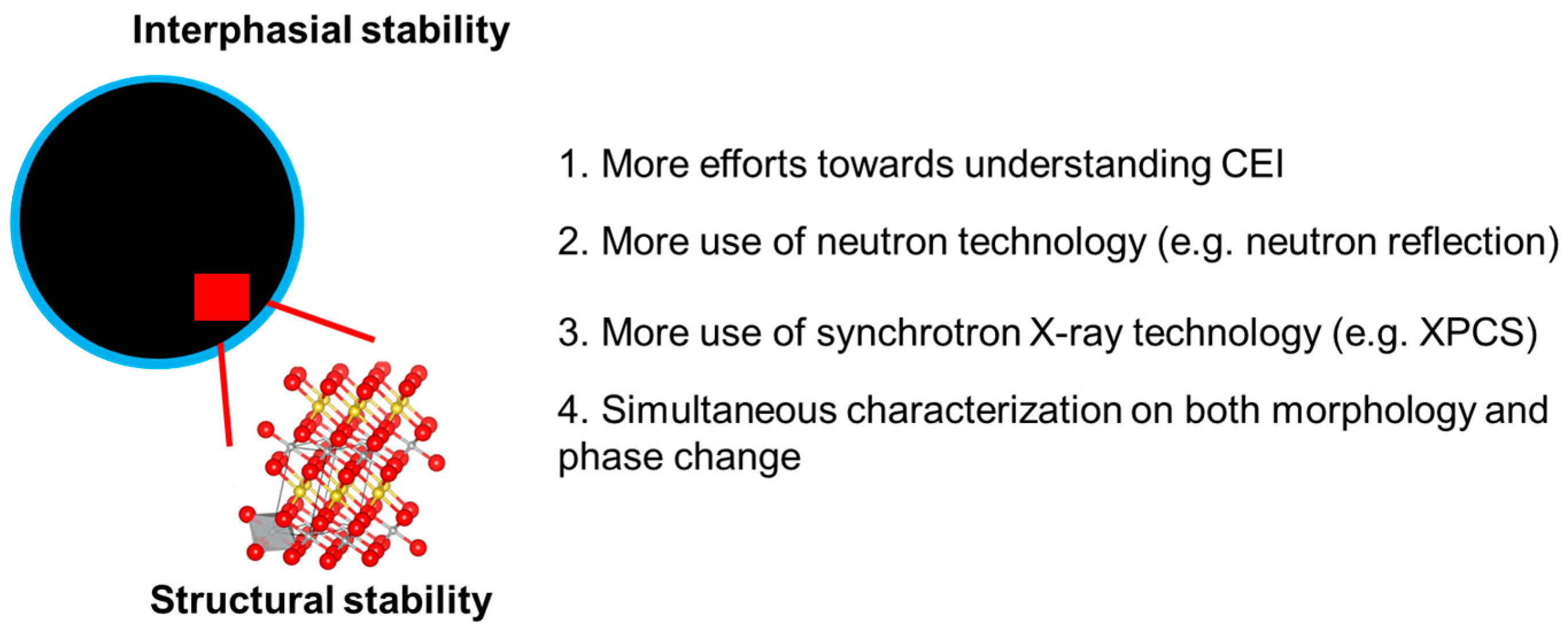
- Investigating CEI in high-voltage polyanion-type cathode: Investigations into the CEI of polyanion-type cathode materials have attracted substantial interest. However, most of the reported results in this area are from lab-based characterizations, which suggests that there is plenty of scope for applying synchrotron X-ray and neutron scattering techniques. As a successful example, the use of synchrotron-based soft XAS with both FY and TEY mode was able to reveal the transition metal activity in the CEI of layered transition metal oxide cathodes. More similar advanced techniques are encouraged for application in the investigation of the CEI of high-voltage polyanion-type cathodes to obtain more insights with the hope of extending cell lifetime;
- Extending neutron scattering techniques: In the research area of high-voltage SIB cathode materials, ND has been widely used to study the bulk structure of layered transition metal oxide cathodes and polyanion-type cathodes. There remains wide scope for using neutron scattering techniques in CEI studies. SANS is a powerful tool for obtaining the size and shape information of materials at the nanometer scale which has been successfully used in studying the chemical nature, morphology, and size evolution of the solid–electrolyte interphase (SEI) in LIBs [117,118]. Neutron reflection testing is able to probe the structure and kinetics at the materials’ interphase by detecting the reflected intensity as a function of angle, which has been used to study the structure and composition gradients of SEI in LIBs [119,120]. These successful examples from LIBs suggest the possibility of involving these neutron-scattering-based techniques in SIB CEI studies from different perspectives;
- Exploring new techniques: To accelerate the understanding of the SIB cathode’s behavior at high voltage, more synchrotron-X-ray- and neutron-based complementary techniques are needed to obtain a more complete picture of the material’s structure, dynamics, and electrochemical behavior. For example, the introduction of dark-field X-ray microscopy [121] could complement TXM in characterizing material morphology by enhancing contrast with selective detection of scattered X-rays. Especially, dark-field X-ray microscopy [121] is highly sensitive to surface features, which could provide valuable information about the interphase morphology. Due to the sensitivity to the dynamic behavior of nanoscale features, the use of X-ray photon correlation spectroscopy (XPCS) could provide information about the movement and fluctuations of nanoparticles and crystal domains [122,123]. This is important for understanding working ion diffusion, phase transitions, and structural heterogeneities or defects on the nanosecond to microsecond timescales;
- Bridging materials characterizations to electrode performance: Although there have been substantial efforts towards understanding high-voltage SIB cathode materials using synchrotron X-ray and neutron scattering techniques, it is challenging to directly link the materials characterization results with the electrode, as well as cell device performance partially due to the disconnection between morphology and phase characterization. One finds it is difficult to perform transmission microscopy and diffraction for the same batch of particles to obtain morphology and phase change information simultaneously. It would be ideal if we could collect morphology and structural phase information for a controllable number of particles at the same time in the near future. By studying the behavior of these particles statistically, one could establish a model to predict the electrode performance with the aid of artificial intelligence, which could also guide the selection of the materials synthesis methodology.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Yuan, B.; Sun, Q.; Wennersten, R. Application of Energy Storage in Integrated Energy Systems—A Solution to Fluctuation and Uncertainty of Renewable Energy. J. Energy Storage 2022, 52, 104812. [Google Scholar] [CrossRef]
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A Comprehensive Review of Stationary Energy Storage Devices for Large Scale Renewable Energy Sources Grid Integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Xu, J. Critical Review on Cathode–Electrolyte Interphase toward High-Voltage Cathodes for Li-Ion Batteries. Nano-Micro Lett. 2022, 14, 166. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Pollard, T.P.; Li, Q.; Tan, S.; Hou, S.; Wan, H.; Chen, F.; He, H.; Hu, E.; et al. Electrolyte Design for Li-Ion Batteries under Extreme Operating Conditions. Nature 2023, 614, 694–700. [Google Scholar] [CrossRef]
- Ray, A.; Saruhan, B. Application of Ionic Liquids for Batteries and Supercapacitors. Materials 2021, 14, 2942. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cormier, M.; Zhang, N.; Inglis, J.; Li, J.; Dahn, J.R. Is Cobalt Needed in Ni-Rich Positive Electrode Materials for Lithium Ion Batteries? J. Electrochem. Soc. 2019, 166, A429. [Google Scholar] [CrossRef]
- Zhang, N.; Zaker, N.; Li, H.; Liu, A.; Inglis, J.; Jing, L.; Li, J.; Li, Y.; Botton, G.A.; Dahn, J.R. Cobalt-Free Nickel-Rich Positive Electrode Materials with a Core–Shell Structure. Chem. Mater. 2019, 31, 10150–10160. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-Ion Batteries: Present and Future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, Z.; Khaleel, M.A.; Belharouak, I. Materials and Engineering Endeavors Towards Practical Sodium-Ion Batteries. Energy Storage Mater. 2020, 25, 520–536. [Google Scholar] [CrossRef]
- Fang, C.; Huang, Y.; Zhang, W.; Han, J.; Deng, Z.; Cao, Y.; Yang, H. Routes to High Energy Cathodes of Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501727. [Google Scholar] [CrossRef]
- Lei, Z.-Q.; Guo, Y.-J.; Wang, E.-H.; He, W.-H.; Zhang, Y.-Y.; Xin, S.; Yin, Y.-X.; Guo, Y.-G. Layered Oxide Cathode-Electrolyte Interface Towards Na-Ion Batteries: Advances and Perspectives. Chem. Asian J. 2022, 17, e202200213. [Google Scholar] [CrossRef] [PubMed]
- Boivin, E.; House, R.A.; Marie, J.-J.; Bruce, P.G. Controlling Iron Versus Oxygen Redox in the Layered Cathode Na0.67fe0.5mn0.5o2: Mitigating Voltage and Capacity Fade by Mg Substitution. Adv. Energy Mater. 2022, 12, 2200702. [Google Scholar] [CrossRef]
- Clément, R.J.; Bruce, P.G.; Grey, C.P. Review—Manganese-Based P2-Type Transition Metal Oxides as Sodium-Ion Battery Cathode Materials. J. Electrochem. Soc. 2015, 162, A2589. [Google Scholar] [CrossRef]
- Li, Z.; Kong, W.; Yu, Y.; Zhang, J.; Wong, D.; Xu, Z.; Chen, Z.; Schulz, C.; Bartkowiak, M.; Liu, X. Tuning Bulk O2 and Nonbonding Oxygen State for Reversible Anionic Redox Chemistry in P2-Layered Cathodes. Angew. Chem. Int. Ed. 2022, 61, e202115552. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gao, X.; Chen, J.; Gao, J.; Yin, S.; Zhang, S.; Ji, X. Reversible OP4 Phase in P2–Na2/3Ni1/3Mn2/3O2 Sodium Ion Cathode. J. Power Sources 2021, 508, 230324. [Google Scholar] [CrossRef]
- Liu, J.; Didier, C.; Sale, M.; Sharma, N.; Guo, Z.; Peterson, V.K.; Ling, C.D. Elucidation of the High-Voltage Phase in the Layered Sodium Ion Battery Cathode Material P3–Na0.5Ni0.25Mn0.75O2. J. Mater. Chem. A 2020, 8, 21151–21162. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Wen, T.; Chen, L.; Pu, X.; Chen, Z. Advances in Vanadium-Redoxed Polyanions for High-Voltage Sodium-Ion Batteries. Batteries 2023, 9, 56. [Google Scholar] [CrossRef]
- Subramanian, Y.; Oh, W.; Choi, W.; Lee, H.; Jeong, M.; Thangavel, R.; Yoon, W.-S. Optimizing High Voltage Na3V2(PO4)2F3 Cathode for Achieving High Rate Sodium-Ion Batteries with Long Cycle Life. Chem. Eng. J. 2021, 403, 126291. [Google Scholar] [CrossRef]
- Berlanga, C.; Monterrubio, I.; Armand, M.; Rojo, T.; Galceran, M.; Casas-Cabanas, M. Cost-Effective Synthesis of Triphylite-NaFePO4 Cathode: A Zero-Waste Process. ACS Sustain. Chem. Eng. 2020, 8, 725–730. [Google Scholar] [CrossRef]
- Tang, W.; Song, X.; Du, Y.; Peng, C.; Lin, M.; Xi, S.; Tian, B.; Zheng, J.; Wu, Y.; Pan, F.; et al. High-Performance NaFePO4 Formed by Aqueous Ion-Exchange and Its Mechanism for Advanced Sodium Ion Batteries. J. Mater. Chem. A 2016, 4, 4882–4892. [Google Scholar] [CrossRef]
- Dong, J.; Xiao, J.; Yu, Y.; Wang, J.; Chen, F.; Wang, S.; Zhang, L.; Ren, N.; Pan, B.; Chen, C. Electronic Structure Regulation of Na2FePO4F Cathode toward Superior High-Rate and High-Temperature Sodium-Ion Batteries. Energy Storage Mater. 2022, 45, 851–860. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.; Wang, S.; Wang, X.; Xie, Y.; Hu, J.; Lai, Y.; Zhang, Z. Scalable Synthesis of the Na2FePO4F Cathode through an Economical and Reliable Approach for Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 11798–11806. [Google Scholar] [CrossRef]
- Barpanda, P.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.-C.; Yamada, Y.; Yamada, A. Na2FeP2O7: A Safe Cathode for Rechargeable Sodium-Ion Batteries. Chem. Mater. 2013, 25, 3480–3487. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lim, C.H.; Kim, J.-H.; Kim, D.K. Na2FeP2O7 as a Positive Electrode Material for Rechargeable Aqueous Sodium-Ion Batteries. RSC Adv. 2014, 4, 9799–9802. [Google Scholar] [CrossRef]
- Plewa, A.; Kulka, A.; Baster, D.; Molenda, J. An Alluaudite Compounds Na2Fe2(SO4)3 Vs. Na2.5Fe1.75(SO4)3 as Earth Abundant Cathode Materials for Na-Ion Batteries. Solid State Ion. 2019, 335, 15–22. [Google Scholar] [CrossRef]
- Shishkin, M.; Sato, H. Ab Initio Study of Stability of Na2Fe2(SO4)3, a High Potential Na-Ion Battery Cathode Material. J. Phys. Chem. C 2017, 121, 20067–20074. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Zhang, W.; Li, Z.; Ji, X.; Miao, L.; Yuan, L.; Hu, X.; Huang, Y. Sodium Storage in Na-Rich NaxFeFe(CN)6 Nanocubes. Nano Energy 2015, 12, 386–393. [Google Scholar] [CrossRef]
- Wang, Y.P.; Hou, B.P.; Cao, X.R.; Wu, S.Q.; Zhu, Z.Z. Structural Evolution, Redox Mechanism, and Ionic Diffusion in Rhombohedral Na2FeFe(CN)6 for Sodium-Ion Batteries: First-Principles Calculations. J. Electrochem. Soc. 2022, 169, 010525. [Google Scholar] [CrossRef]
- Xiao, P.; Song, J.; Wang, L.; Goodenough, J.B.; Henkelman, G. Theoretical Study of the Structural Evolution of a Na2FeMn(CN)6 Cathode Upon Na Intercalation. Chem. Mater. 2015, 27, 3763–3768. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Jiang, Y. Synthesis of Na2Mn0.8Fe0.2Fe(CN)6 and Its Application as Cathode Material of Sodium Ion Battery. IOP Conf. Ser. Earth Environ. Sci. 2021, 621, 012061. [Google Scholar] [CrossRef]
- Ma, X.-H.; Wei, Y.-Y.; Wu, Y.-D.; Wang, J.; Jia, W.; Zhou, J.-H.; Zi, Z.-F.; Dai, J.-M. High Crystalline Na2Ni[Fe(CN)6] Particles for a High-Stability and Low-Temperature Sodium-Ion Batteries Cathode. Electrochim. Acta 2019, 297, 392–397. [Google Scholar] [CrossRef]
- Su, X.; Wang, Y.; Zhou, J.; Gu, S.; Li, J.; Zhang, S. Operando Spectroscopic Identification of Active Sites in NiFe Prussian Blue Analogues as Electrocatalysts: Activation of Oxygen Atoms for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 11286–11292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, Y.; Liu, X.; Yang, Z.; He, X.; Li, L.; Qiao, Y.; Chen, W.; Zeng, R.; Wang, Y.; et al. Organic Cathode Materials for Sodium-Ion Batteries: From Fundamental Research to Potential Commercial Application. Adv. Funct. Mater. 2022, 32, 2107718. [Google Scholar] [CrossRef]
- Delmas, C.; Fouassier, C.; Hagenmuller, P. Structural Classification and Properties of the Layered Oxides. Phys. B Phys. C 1980, 99, 81–85. [Google Scholar] [CrossRef]
- Peng, B.; Sun, Z.; Jiao, S.; Wang, G.; Zhang, G. Electrochemical Performance Optimization of Layered P2-Type Na0.67MnO2 through Simultaneous Mn-Site Doping and Nanostructure Engineering. Batter. Supercaps 2020, 3, 147–154. [Google Scholar] [CrossRef]
- Guo, J.-Z.; Gu, Z.-Y.; Du, M.; Zhao, X.-X.; Wang, X.-T.; Wu, X.-L. Emerging Characterization Techniques for Delving Polyanion-Type Cathode Materials of Sodium-Ion Batteries. Mater. Today 2023, 66, 221–244. [Google Scholar] [CrossRef]
- You, Y.; Manthiram, A. Progress in High-Voltage Cathode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1701785. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Liu, Q.; Wang, J.; Chou, S.; Liu, H.; Dou, S. Prussian Blue Analogues for Sodium-Ion Batteries: Past, Present, and Future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Tang, Y.; Jia, C.; Ji, X.; Wang, H. Understanding the Sodium Storage Mechanisms of Organic Electrodes in Sodium Ion Batteries: Issues and Solutions. Energy Environ. Sci. 2020, 13, 1568–1592. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, K.; Chen, J. Recent Advances and Prospects of Cathode Materials for Sodium-Ion Batteries. Adv. Mater. 2015, 27, 5343–5364. [Google Scholar] [CrossRef]
- Xiao, J.; Li, X.; Tang, K.; Wang, D.; Long, M.; Gao, H.; Chen, W.; Liu, C.; Liu, H.; Wang, G. Recent Progress of Emerging Cathode Materials for Sodium Ion Batteries. Mater. Chem. Front. 2021, 5, 3735–3764. [Google Scholar] [CrossRef]
- Lin, F.; Liu, Y.; Yu, X.; Cheng, L.; Singer, A.; Shpyrko, O.G.; Xin, H.L.; Tamura, N.; Tian, C.; Weng, T.-C.; et al. Synchrotron X-Ray Analytical Techniques for Studying Materials Electrochemistry in Rechargeable Batteries. Chem. Rev. 2017, 117, 13123–13186. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chou, S.-L.; Dou, S.-X. Understanding Challenges of Cathode Materials for Sodium-Ion Batteries Using Synchrotron-Based X-Ray Absorption Spectroscopy. Batter. Supercaps 2019, 2, 842–851. [Google Scholar] [CrossRef]
- Gu, Q.; Kimpton, J.A.; Brand, H.E.A.; Wang, Z.; Chou, S. Solving Key Challenges in Battery Research Using in Situ Synchrotron and Neutron Techniques. Adv. Energy Mater. 2017, 7, 1602831. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, Y.; Li, Y.; Jiang, L.; Rong, X.; Hu, Y.-S.; Li, H.; Chen, L. Novel Methods for Sodium-Ion Battery Materials. Small Methods 2017, 1, 1600063. [Google Scholar] [CrossRef]
- Shah, A.R.; Shutt, R.R.C.; Smith, K.; Hack, J.; Neville, T.P.; Headen, T.F.; Brett, D.J.L.; Howard, C.A.; Miller, T.S.; Cullen, P.L. Neutron Studies of Na-Ion Battery Materials. J. Phys. Mater. 2021, 4, 042008. [Google Scholar] [CrossRef]
- Pérez, G.E.; Brittain, J.M.; McClelland, I.; Hull, S.; Jones, M.O.; Playford, H.Y.; Cussen, S.A.; Baker, P.J.; Reynolds, E.M. Neutron and Muon Characterisation Techniques for Battery Materials. J. Mater. Chem. A 2023, 11, 10493–10531. [Google Scholar] [CrossRef]
- Zhao, E.; Wang, H.; Yin, W.; He, L.; Ke, Y.; Wang, F.; Zhao, J. Spatiotemporal-Scale Neutron Studies on Lithium-Ion Batteries and Beyond. Appl. Phys. Lett. 2022, 121, 110501. [Google Scholar] [CrossRef]
- Ren, Y.; Zuo, X. Synchrotron X-Ray and Neutron Diffraction, Total Scattering, and Small-Angle Scattering Techniques for Rechargeable Battery Research. Small Methods 2018, 2, 1800064. [Google Scholar] [CrossRef]
- Delmas, C.; Braconnier, J.-J.; Fouassier, C.; Hagenmuller, P. Electrochemical Intercalation of Sodium in NaxCoO2 Bronzes. Solid State Ion. 1981, 3-4, 165–169. [Google Scholar] [CrossRef]
- Gupta, P.; Pushpakanth, S.; Haider, M.A.; Basu, S. Understanding the Design of Cathode Materials for Na-Ion Batteries. ACS Omega 2022, 7, 5605–5614. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kan, W.H.; Ling, C.D. Insights into the High Voltage Layered Oxide Cathode Materials in Sodium-Ion Batteries: Structural Evolution and Anion Redox. J. Power Sources 2021, 481, 229139. [Google Scholar] [CrossRef]
- Kang, W.; Yu, D.Y.W.; Lee, P.-K.; Zhang, Z.; Bian, H.; Li, W.; Ng, T.-W.; Zhang, W.; Lee, C.-S. P2-Type NaxCu0.15Ni0.20Mn0.65O2 Cathodes with High Voltage for High-Power and Long-Life Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 31661–31668. [Google Scholar] [CrossRef]
- Wu, X.; Guo, J.; Wang, D.; Zhong, G.; McDonald, M.J.; Yang, Y. P2-Type Na0.66Ni0.33–XZnxMn0.67O2 as New High-Voltage Cathode Materials for Sodium-Ion Batteries. J. Power Sources 2015, 281, 18–26. [Google Scholar] [CrossRef]
- Xu, G.-L.; Amine, R.; Xu, Y.-F.; Liu, J.; Gim, J.; Ma, T.; Ren, Y.; Sun, C.-J.; Liu, Y.; Zhang, X.; et al. Insights into the Structural Effects of Layered Cathode Materials for High Voltage Sodium-Ion Batteries. Energy Environ. Sci. 2017, 10, 1677–1693. [Google Scholar] [CrossRef]
- Wang, K.; Wan, H.; Yan, P.; Chen, X.; Fu, J.; Liu, Z.; Deng, H.; Gao, F.; Sui, M. Dopant Segregation Boosting High-Voltage Cyclability of Layered Cathode for Sodium Ion Batteries. Adv. Mater. 2019, 31, 1904816. [Google Scholar] [CrossRef]
- Konarov, A.; Kim, H.J.; Jo, J.-H.; Voronina, N.; Lee, Y.; Bakenov, Z.; Kim, J.; Myung, S.-T. High-Voltage Oxygen-Redox-Based Cathode for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 2001111. [Google Scholar] [CrossRef]
- Matsui, M.; Mizukoshi, F.; Hasegawa, H.; Imanishi, N. Ca-Substituted P3-Type NaxNi1/3Mn1/3Co1/3O2 as a Potential High Voltage Cathode Active Material for Sodium-Ion Batteries. J. Power Sources 2021, 485, 229346. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, X.; Li, H.; Zhang, X.; He, P.; Guo, S.; Zhou, H. Suppressed the High-Voltage Phase Transition of P2-Type Oxide Cathode for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 14848–14853. [Google Scholar] [CrossRef]
- Pei, Q.; Lu, M.; Liu, Z.; Li, D.; Rao, X.; Liu, X.; Zhong, S. Improving the Na0.67Ni0.33Mn0.67O2 Cathode Material for High-Voltage Cyclability Via Ti/Cu Codoping for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 1953–1962. [Google Scholar] [CrossRef]
- Bhange, D.S.; Ali, G.; Kim, D.-H.; Anang, D.A.; Shin, T.J.; Kim, M.-G.; Kang, Y.-M.; Chung, K.Y.; Nam, K.-W. Honeycomb-Layer Structured Na3Ni2BiO6 as a High Voltage and Long Life Cathode Material for Sodium-Ion Batteries. J. Mater. Chem. A 2017, 5, 1300–1310. [Google Scholar] [CrossRef]
- Tapia-Ruiz, N.; Dose, W.M.; Sharma, N.; Chen, H.; Heath, J.; Somerville, J.W.; Maitra, U.; Islam, M.S.; Bruce, P.G. High Voltage Structural Evolution and Enhanced Na-Ion Diffusion in P2-Na2/3Ni1/3−XMgxMn2/3O2 (0 ≤ X ≤ 0.2) Cathodes from Diffraction, Electrochemical and Ab Initio Studies. Energy Environ. Sci. 2018, 11, 1470–1479. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Zhang, X.; Ren, Y.; Zuo, P.; Yin, G.; Wang, J. Unravelling the Origin of Irreversible Capacity Loss in NaNiO2 for High Voltage Sodium Ion Batteries. Nano Energy 2017, 34, 215–223. [Google Scholar] [CrossRef]
- Jeong, M.; Lee, H.; Yoon, J.; Yoon, W.-S. O3-Type NaNi1/3Fe1/3Mn1/3O2 Layered Cathode for Na-Ion Batteries: Structural Evolution and Redox Mechanism Upon Na (De) Intercalation. J. Power Sources 2019, 439, 227064. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, Z.; Luo, W.; Hu, Z.; Liu, G.; Zhang, L.; Huang, Y. Vitalization of P2–Na2/3Ni1/3Mn2/3O2 at High-Voltage Cyclability Via Combined Structural Modulation for Sodium-Ion Batteries. Energy Storage Mater. 2020, 29, 182–189. [Google Scholar] [CrossRef]
- Xu, J.; Lee, D.H.; Clément, R.J.; Yu, X.; Leskes, M.; Pell, A.J.; Pintacuda, G.; Yang, X.-Q.; Grey, C.P.; Meng, Y.S. Identifying the Critical Role of Li Substitution in P2–Nax[LiyNizMn1–Y–Z]O2 (0 < X, Y, Z < 1) Intercalation Cathode Materials for High-Energy Na-Ion Batteries. Chem. Mater. 2014, 26, 1260–1269. [Google Scholar]
- Liu, K.; Tan, S.; Moon, J.; Jafta, C.J.; Li, C.; Kobayashi, T.; Dai, S. Insights into the Enhanced Cycle and Rate Performances of the F-Substituted P2-Type Oxide Cathodes for Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000135. [Google Scholar] [CrossRef]
- Clément, R.J.; Billaud, J.; Robert Armstrong, A.; Singh, G.; Rojo, T.; Bruce, P.G.; Grey, C.P. Structurally Stable Mg-Doped P2-Na2/3Mn1−YMgyO2 Sodium-Ion Battery Cathodes with High Rate Performance: Insights from Electrochemical, NMR and Diffraction Studies. Energy Environ. Sci. 2016, 9, 3240–3251. [Google Scholar] [CrossRef]
- Zhou, D.; Ning, D.; Wang, J.; Liu, J.; Zhang, G.; Xiao, Y.; Zheng, J.; Li, Y.; Li, J.; Liu, X. Clarification of Underneath Capacity Loss for O3-Type Ni, Co Free Layered Cathodes at High Voltage for Sodium Ion Batteries. J. Energy Chem. 2023, 77, 479–486. [Google Scholar] [CrossRef]
- Park, J.-H.; Ko, I.-H.; Lee, J.; Park, S.; Kim, D.; Yu, S.-H.; Sung, Y.-E. Anionic Redox Reactions in Cathodes for Sodium-Ion Batteries. ChemElectroChem 2021, 8, 625–643. [Google Scholar] [CrossRef]
- Voronina, N.; Shin, M.Y.; Kim, H.J.; Yaqoob, N.; Guillon, O.; Song, S.H.; Myung, S.T. Hysteresis-Suppressed Reversible Oxygen-Redox Cathodes for Sodium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2103939. [Google Scholar] [CrossRef]
- Yu, Y.; Ning, D.; Li, Q.; Franz, A.; Zheng, L.; Zhang, N.; Ren, G.; Schumacher, G.; Liu, X. Revealing the Anionic Redox Chemistry in O3-Type Layered Oxide Cathode for Sodium-Ion Batteries. Energy Storage Mater. 2021, 38, 130–140. [Google Scholar] [CrossRef]
- Ji, H.; Ji, W.; Xue, H.; Chen, G.; Qi, R.; Huang, Z.; Fang, H.; Chu, M.; Liu, L.; Ma, Z.; et al. Synergistic Activation of Anionic Redox Via Cosubstitution to Construct High-Capacity Layered Oxide Cathode Materials for Sodium-Ion Batteries. Sci. Bull. 2023, 68, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Hu, E.; Liu, J.; Zhang, Y.; Yang, X.-Q.; Nanda, J.; Huq, A.; Page, K. A Novel P3-Type Na2/3Mg1/3Mn2/3O2 as High Capacity Sodium-Ion Cathode Using Reversible Oxygen Redox. J. Mater. Chem. A 2019, 7, 1491–1498. [Google Scholar] [CrossRef]
- Rong, X.; Liu, J.; Hu, E.; Liu, Y.; Wang, Y.; Wu, J.; Yu, X.; Page, K.; Hu, Y.-S.; Yang, W.; et al. Structure-Induced Reversible Anionic Redox Activity in Na Layered Oxide Cathode. Joule 2018, 2, 125–140. [Google Scholar] [CrossRef]
- House, R.A.; Maitra, U.; Pérez-Osorio, M.A.; Lozano, J.G.; Jin, L.; Somerville, J.W.; Duda, L.C.; Nag, A.; Walters, A.; Zhou, K.-J.; et al. Superstructure Control of First-Cycle Voltage Hysteresis in Oxygen-Redox Cathodes. Nature 2020, 577, 502–508. [Google Scholar] [CrossRef]
- Mu, L.; Feng, X.; Kou, R.; Zhang, Y.; Guo, H.; Tian, C.; Sun, C.; Du, X.; Nordlund, D.; Xin, H.L.; et al. Deciphering the Cathode–Electrolyte Interfacial Chemistry in Sodium Layered Cathode Materials. Adv. Energy Mater. 2018, 8, 1801975. [Google Scholar] [CrossRef]
- Song, J.; Xiao, B.; Lin, Y.; Xu, K.; Li, X. Interphases in Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703082. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Lin, F.; Nordlund, D.; Markus, I.M.; Weng, T.-C.; Xin, H.L.; Doeff, M.M. Profiling the Nanoscale Gradient in Stoichiometric Layered Cathode Particles for Lithium-Ion Batteries. Energy Environ. Sci. 2014, 7, 3077–3085. [Google Scholar] [CrossRef]
- Mu, L.; Rahman, M.M.; Zhang, Y.; Feng, X.; Du, X.-W.; Nordlund, D.; Lin, F. Surface Transformation by a “Cocktail” Solvent Enables Stable Cathode Materials for Sodium Ion Batteries. J. Mater. Chem. A 2018, 6, 2758–2766. [Google Scholar] [CrossRef]
- Doubaji, S.; Philippe, B.; Saadoune, I.; Gorgoi, M.; Gustafsson, T.; Solhy, A.; Valvo, M.; Rensmo, H.; Edström, K. Passivation Layer and Cathodic Redox Reactions in Sodium-Ion Batteries Probed by Haxpes. ChemSusChem 2016, 9, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Xu, Y.; Cheng, H.; Shi, Q.; Kou, R.; Mu, L.; Liu, Q.; Xia, S.; Xiao, X.; Sun, C.-J.; et al. Empowering Multicomponent Cathode Materials for Sodium Ion Batteries by Exploring Three-Dimensional Compositional Heterogeneities. Energy Environ. Sci. 2018, 11, 2496–2508. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Y.; Sun, F.; Osenberg, M.; Hilger, A.; Manke, I.; Cao, R.; Dou, S.X.; Fan, H.J. Designing Solvated Double-Layer Polymer Electrolytes with Molecular Interactions Mediated Stable Interfaces for Sodium Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202219000. [Google Scholar] [CrossRef]
- Manthiram, A.; Goodenough, J.B. Lithium Insertion into Fe2(SO4)3 Frameworks. J. Power Sources 1989, 26, 403–408. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Q.; Xiao, L.; Rong, Y.; Liu, Y.; Chen, Z.; Ai, X.; Cao, Y.; Yang, H.; Xie, J.; et al. A Fully Sodiated Navopo4 with Layered Structure for High-Voltage and Long-Lifespan Sodium-Ion Batteries. Chem 2018, 4, 1167–1180. [Google Scholar] [CrossRef]
- Deng, L.; Yu, F.-D.; Xia, Y.; Jiang, Y.-S.; Sui, X.-L.; Zhao, L.; Meng, X.-H.; Que, L.-F.; Wang, Z.-B. Stabilizing Fluorine to Achieve High-Voltage and Ultra-Stable Na3V2(PO4)2F3 Cathode for Sodium Ion Batteries. Nano Energy 2021, 82, 105659. [Google Scholar] [CrossRef]
- Song, T.; Yao, W.; Kiadkhunthod, P.; Zheng, Y.; Wu, N.; Zhou, X.; Tunmee, S.; Sattayaporn, S.; Tang, Y. A Low-Cost and Environmentally Friendly Mixed Polyanionic Cathode for Sodium-Ion Storage. Angew. Chem. Int. Ed. 2020, 59, 740–745. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, W.; Zhu, J.; Xia, F.; Zhao, H.; Tian, W.; Wang, T.; Zhang, Y.; Zhang, S.; Mu, S. Fe-Rich Pyrophosphate with Prolonged High-Voltage-Plateaus and Suppressed Voltage Decay as Sodium-Ion Battery Cathode. Nano Energy 2023, 116, 108822. [Google Scholar] [CrossRef]
- Tong, S.; Pan, H.; Liu, H.; Zhang, X.; Liu, X.; Jia, M.; Kang, Y.; Yuan, Y.; Du, X.; Yan, X. Titanium Doping Induced the Suppression of Irreversible Phase Transformation at High Voltage for V-Based Phosphate Cathodes of Na-Ion Batteries. ChemSusChem 2023, 16, e202300244. [Google Scholar] [CrossRef]
- Gao, H.; Seymour, I.D.; Xin, S.; Xue, L.; Henkelman, G.; Goodenough, J.B. Na3MnZr(PO4)3: A High-Voltage Cathode for Sodium Batteries. J. Am. Chem. Soc. 2018, 140, 18192–18199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, H.; Zhang, Z.; Xu, M.; Lai, Y.; Chou, S.-L. Full Activation of Mn4+/Mn3+ Redox in Na4MnCr(PO4)3 as a High-Voltage and High-Rate Cathode Material for Sodium-Ion Batteries. Small 2020, 16, 2001524. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hua, W.; Xiao, J.; Cortie, D.; Guo, X.; Wang, E.; Dou, S.X. Development and Investigation of a Nasicon-Type High-Voltage Cathode Material for High-Power Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Seo, D.-H.; Shi, T.; Chen, S.; Tian, Y.; Kim, H.; Ceder, G. A High-Energy Nasicon-Type Cathode Material for Na-Ion Batteries. Adv. Energy Mater. 2020, 10, 1903968. [Google Scholar] [CrossRef]
- Nishimura, S.-I.; Suzuki, Y.; Lu, J.; Torii, S.; Kamiyama, T.; Yamada, A. High-Temperature Neutron and X-Ray Diffraction Study of Fast Sodium Transport in Alluaudite-Type Sodium Iron Sulfate. Chem. Mater. 2016, 28, 2393–2399. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Zhao, X.; He, L.; Liu, H.; Song, Y.; Sun, S.; Li, Q.; Xing, X.; Chen, J. A Novel Nasicon-Type Na4MnCr(PO4)3 Demonstrating the Energy Density Record of Phosphate Cathodes for Sodium-Ion Batteries. Adv. Mater. 2020, 32, 1906348. [Google Scholar] [CrossRef]
- Buryak, N.S.; Anishchenko, D.V.; Levin, E.E.; Ryazantsev, S.V.; Martin-Diaconescu, V.; Zakharkin, M.V.; Nikitina, V.A.; Antipov, E.V. High-Voltage Structural Evolution and Its Kinetic Consequences for the Na4MnV(PO4)3 Sodium-Ion Battery Cathode Material. J. Power Sources 2022, 518, 230769. [Google Scholar] [CrossRef]
- Kawai, K.; Zhao, W.; Nishimura, S.-I.; Yamada, A. High-Voltage Cr4+/Cr3+ Redox Couple in Polyanion Compounds. ACS Appl. Energy Mater. 2018, 1, 928–931. [Google Scholar] [CrossRef]
- Qin, B.; Zarrabeitia, M.; Hoefling, A.; Jusys, Z.; Liu, X.; Tübke, J.; Behm, R.J.; Cui, G.; Varzi, A.; Passerini, S. A Unique Polymer-Inorganic Cathode-Electrolyte-Interphase (Cei) Boosts High-Performance Na3V2(PO4)2F3 Batteries in Ether Electrolytes. J. Power Sources 2023, 560, 232630. [Google Scholar] [CrossRef]
- He, J.; Tao, T.; Yang, F.; Sun, Z. Optimizing the Electrolyte Systems for Na3(VO1−XPO4)2F1+2x (0≤X≤1) Cathode and Understanding Their Interfacial Chemistries Towards High-Rate Sodium-Ion Batteries. ChemSusChem 2022, 15, e202102522. [Google Scholar] [CrossRef]
- He, J.; Tao, T.; Yang, F.; Sun, Z. Unravelling Li+ Intercalation Mechanism and Cathode Electrolyte Interphase of Na3V2(PO4)3 and Na3(VOPO4)2F Cathode as Robust Framework Towards High-Performance Lithium-Ion Batteries. ChemSusChem 2022, 15, e202200817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Shao, T.; Li, X.; Chen, G.; Liu, H.; Ma, Z.-F. Mg2+-Doping Constructed a Continuous and Homogeneous Cathode-Electrolyte Interphase Film on Na3.12Fe2.44(P2O7)2 with Superior and Stable High-Temperature Performance for Sodium-Ion Storage. ACS Appl. Mater. Interfaces 2022, 14, 14253–14263. [Google Scholar] [CrossRef]
- Ihsan-Ul-Haq, M.; Cui, J.; Mubarak, N.; Xu, M.; Shen, X.; Luo, Z.; Huang, B.; Kim, J.-K. Revealing Cathode–Electrolyte Interface on Flower-Shaped Na3V2(PO4)3/C Cathode through Cryogenic Electron Microscopy. Adv. Energy Sustain. Res. 2021, 2, 2100072. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Kim, H.; Kang, K.; Choi, N.-S. Highly Stable Linear Carbonate-Containing Electrolytes with Fluoroethylene Carbonate for High-Performance Cathodes in Sodium-Ion Batteries. J. Power Sources 2016, 320, 49–58. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Z.; Deng, Z.; Li, X.; Wang, B.; Chen, X.; Ong, S.P. Water Contributes to Higher Energy Density and Cycling Stability of Prussian Blue Analogue Cathodes for Aqueous Sodium-Ion Batteries. Chem. Mater. 2019, 31, 5933–5942. [Google Scholar] [CrossRef]
- Jeong, G.S.; Jung, K.H.; Choi, S.; Kim, K.C. Electrochemical Characteristics of Cyanoquinones as Organic Cathodes for High-Potential Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 11328–11336. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Mazumder, A.; Dilip, P.S.; Hariharan, M.; Shaijumon, M.M. Synergistically Improving the Stability and Operating Potential of Organic Cathodes for Sodium-Ion Battery. Batter. Supercaps 2023, 6, e202300111. [Google Scholar] [CrossRef]
- Xie, B.; Zuo, P.; Wang, L.; Wang, J.; Huo, H.; He, M.; Shu, J.; Li, H.; Lou, S.; Yin, G. Achieving Long-Life Prussian Blue Analogue Cathode for Na-Ion Batteries Via Triple-Cation Lattice Substitution and Coordinated Water Capture. Nano Energy 2019, 61, 201–210. [Google Scholar] [CrossRef]
- Sottmann, J.; Bernal, F.L.M.; Yusenko, K.V.; Herrmann, M.; Emerich, H.; Wragg, D.S.; Margadonna, S. In Operando Synchrotron Xrd/Xas Investigation of Sodium Insertion into the Prussian Blue Analogue Cathode Material Na1.32Mn[Fe(CN)6]0.83·Z H2O. Electrochim. Acta 2016, 200, 305–313. [Google Scholar] [CrossRef]
- Seok, J.; Yu, S.-H.; Abruña, H.D. Operando Synchrotron-Based X-Ray Study of Prussian Blue and Its Analogue as Cathode Materials for Sodium-Ion Batteries. J. Phys. Chem. C 2020, 124, 16332–16337. [Google Scholar] [CrossRef]
- Wang, W.; Gang, Y.; Peng, J.; Hu, Z.; Yan, Z.; Lai, W.; Zhu, Y.; Appadoo, D.; Ye, M.; Cao, Y.; et al. Effect of Eliminating Water in Prussian Blue Cathode for Sodium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2111727. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, J.; Huang, L.; Ou, M.; Fan, C.; Wei, P.; Peng, J.; Liu, Y.; Qiu, Y.; Sun, X.; et al. Structure Distortion Induced Monoclinic Nickel Hexacyanoferrate as High-Performance Cathode for Na-Ion Batteries. Adv. Energy Mater. 2019, 9, 1803158. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Wang, J.; Li, L.; Lai, W.; Yang, Q.; Zhang, B.; Li, X.; Du, Y.; Liu, H.; et al. Processing Rusty Metals into Versatile Prussian Blue for Sustainable Energy Storage. Adv. Energy Mater. 2021, 11, 2102356. [Google Scholar] [CrossRef]
- Nielsen, I.; Dzodan, D.; Ojwang, D.O.; Henry, P.F.; Ulander, A.; Ek, G.; Häggström, L.; Ericsson, T.; Boström, H.L.B.; Brant, W.R. Water Driven Phase Transitions in Prussian White Cathode Materials. J. Phys. Energy 2022, 4, 044012. [Google Scholar] [CrossRef]
- Kuan, H.-C.; Luu, N.T.H.; Ivanov, A.S.; Chen, T.-H.; Popovs, I.; Lee, J.-C.; Kaveevivitchai, W. A Nitrogen- and Carbonyl-Rich Conjugated Small-Molecule Organic Cathode for High-Performance Sodium-Ion Batteries. J. Mater. Chem. A 2022, 10, 16249–16257. [Google Scholar] [CrossRef]
- Shimizu, T.; Mameuda, T.; Toshima, H.; Akiyoshi, R.; Kamakura, Y.; Wakamatsu, K.; Tanaka, D.; Yoshikawa, H. Application of Porous Coordination Polymer Containing Aromatic Azo Linkers as Cathode-Active Materials in Sodium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 5191–5198. [Google Scholar] [CrossRef]
- Bridges, C.A.; Sun, X.-G.; Zhao, J.; Paranthaman, M.P.; Dai, S. In Situ Observation of Solid Electrolyte Interphase Formation in Ordered Mesoporous Hard Carbon by Small-Angle Neutron Scattering. J. Phys. Chem. C 2012, 116, 7701–7711. [Google Scholar] [CrossRef]
- Sacci, R.L.; Bañuelos, J.L.; Veith, G.M.; Littrell, K.C.; Cheng, Y.Q.; Wildgruber, C.U.; Jones, L.L.; Ramirez-Cuesta, A.J.; Rother, G.; Dudney, N.J. Structure of Spontaneously Formed Solid-Electrolyte Interphase on Lithiated Graphite Determined Using Small-Angle Neutron Scattering. J. Phys. Chem. C 2015, 119, 9816–9823. [Google Scholar] [CrossRef]
- Owejan, J.E.; Owejan, J.P.; DeCaluwe, S.C.; Dura, J.A. Solid Electrolyte Interphase in Li-Ion Batteries: Evolving Structures Measured in Situ by Neutron Reflectometry. Chem. Mater. 2012, 24, 2133–2140. [Google Scholar] [CrossRef]
- Kawaura, H.; Harada, M.; Kondo, Y.; Kondo, H.; Suganuma, Y.; Takahashi, N.; Sugiyama, J.; Seno, Y.; Yamada, N.L. Operando Measurement of Solid Electrolyte Interphase Formation at Working Electrode of Li-Ion Battery by Time-Slicing Neutron Reflectometry. ACS Appl. Mater. Interfaces 2016, 8, 9540–9544. [Google Scholar] [CrossRef]
- Simons, H.; King, A.; Ludwig, W.; Detlefs, C.; Pantleon, W.; Schmidt, S.; Stöhr, F.; Snigireva, I.; Snigirev, A.; Poulsen, H.F. Dark-Field X-Ray Microscopy for Multiscale Structural Characterization. Nat. Commun. 2015, 6, 6098. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Jiang, Z.; Lurio, L.B. X-Ray Photon Correlation Spectroscopy Studies of Surfaces and Thin Films. Adv. Mater. 2014, 26, 7764–7785. [Google Scholar] [CrossRef] [PubMed]
- Shpyrko, O. X-ray Photon Correlation Spectroscopy. J. Synchrotron Radiat. 2014, 21, 1057–1064. [Google Scholar] [CrossRef] [PubMed]



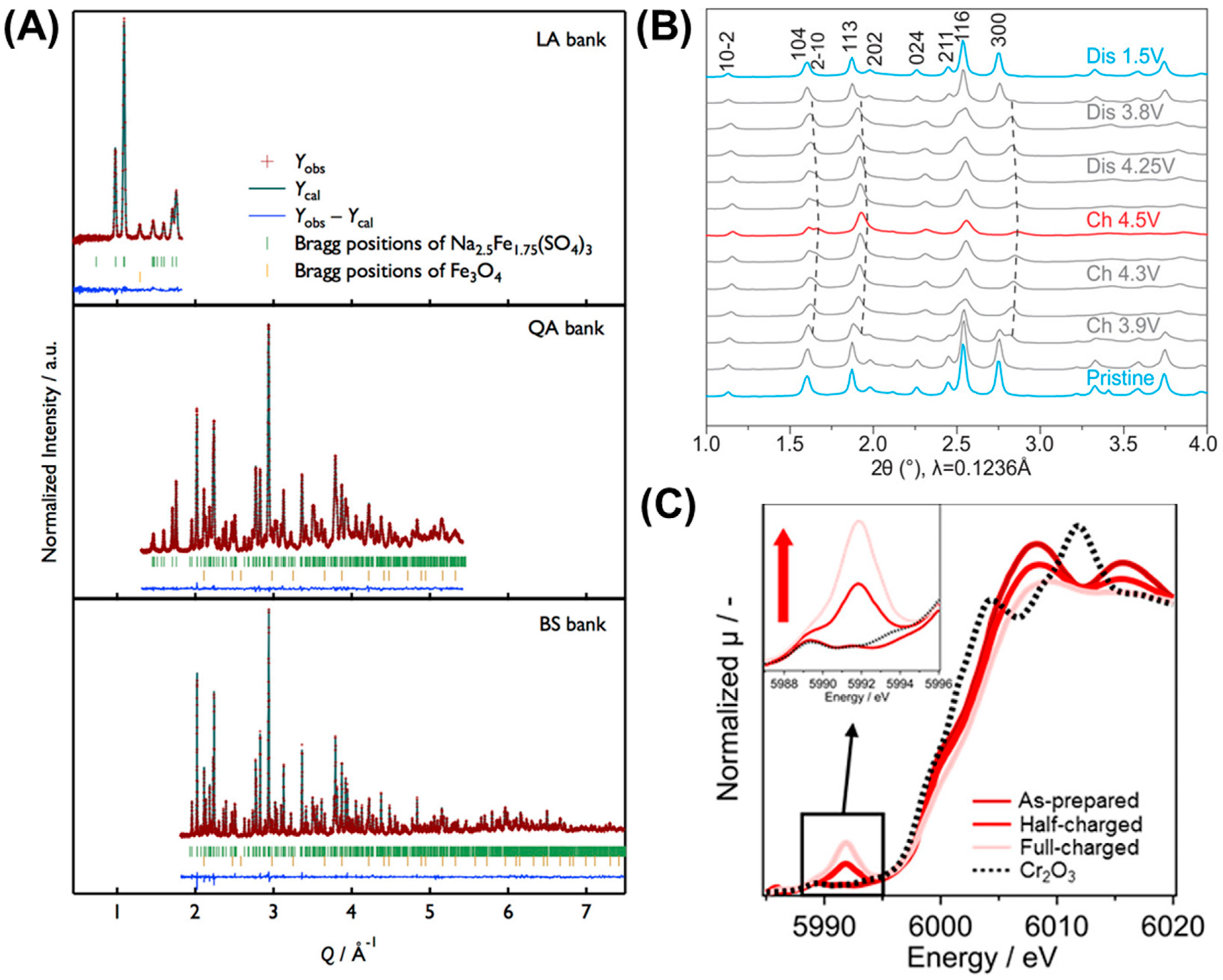


| Material | Electrolyte | Temperature | Cycling Rate | Voltage Range | Cycling No. Until 80% Capacity Retention | Ref. |
|---|---|---|---|---|---|---|
| Na0.5Cu0.15Ni0.2Mn0.65O2/Na | 1M NaClO4 in PC + 5%FEC | Room temperature | 1000 mA/g | 3–4.2 V | 1000 | [53] |
| Na0.66Ni0.26Zn0.07Mn0.67O2/Na | 1M NaClO4 in PC + 2%FEC | Room temperature | 12 mA/g | 2–4.4 V | 30 | [54] |
| NaxNi1/3Co1/3Mn1/3O2/Na | 1M NaPF6 in PC + 2%FEC | Room temperature | 0.5C | 2–4.4 V | 100 | [55] |
| Na0.67Ni0.23Mn0.67Mg0.1O2/Na | 1M NaClO4 in PC + 5%FEC | Room temperature | 0.1C | 2–4.5 V | 100 | [56] |
| Na2/3[(Ni0.5Zn0.5)0.3Mn0.7]O2/Na | 0.5M NaPF6 in PC + 2%FEC | Room temperature | 0.1C | 2.3–4.6 V | >200 | [57] |
| Na0.6-xCaxNi1/3Mn1/3Co1/3O2/Na | 1M NaPF6 in EC/DEC 1/1 + 3%FEC | Room temperature | 200 mA/g | 2.5–4.2 V | ~100 | [58] |
| Ru-substituted Na0.6MnO2/hard carbon | 1M NaClO4 in PC + 5%FEC | Room temperature | 100 mA/g | 1.5–4.4 V | 50 | [59] |
| Na0.67Ni0.19Cu0.14Mn0.52Ti0.15O2/Na | 1M NaClO4 in EC/DEC 1/1 + 5%FEC | Room temperature | 0.1C | 2–4.5 V | 100 | [60] |
| Material | Electrolyte | Temperature | Cycling Rate | Voltage Range | Cycling No. Until 80% Capacity Retention | Ref. |
|---|---|---|---|---|---|---|
| NaVOPO4//Na | 1M NaClO4 in EC/DEC 1/1 | Room temperature | 0.5C | 2–4.2 V | 1000 | [86] |
| Na3V2(PO4)2F3/Na | 1M NaClO4 in EC/DEC 1/1 + 5%FEC | Room temperature | 10C | 2.5–4.5 V | >1000 | [87] |
| Na2Fe(C2O4)SO4⋅H2O/Na | 1M NaClO4 in PC + 3%FEC | Room temperature | 0.2C | 1.7–4.2 V | 500 | [88] |
| Na1.4Fe1.3P2O7/Na | 1M NaClO4 in EC/PC 1/1 + 5%FEC | Room temperature | 1C | 1.5–4.2 V | 650 | [89] |
| Na2VTi(PO4)2F3/Na | NaPF6 in PC/EC/DMC 1/1/1 | Room temperature | 0.1C | 3–4.4 V | >100 | [90] |
| Na3MnZr(PO4)3/Na | 1M NaClO4 in PC/FEC 9/1 | Room temperature | 0.5C | 2.5–4.2 V | >500 | [91] |
| Na4MnCr(PO4)3/Na | 1M NaClO4 in PC/FEC 9/1 | Room temperature | 10C | 1.5–4.3 V | >500 | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shipitsyn, V.; Jayakumar, R.; Zuo, W.; Sun, B.; Ma, L. Understanding High-Voltage Behavior of Sodium-Ion Battery Cathode Materials Using Synchrotron X-ray and Neutron Techniques: A Review. Batteries 2023, 9, 461. https://doi.org/10.3390/batteries9090461
Shipitsyn V, Jayakumar R, Zuo W, Sun B, Ma L. Understanding High-Voltage Behavior of Sodium-Ion Battery Cathode Materials Using Synchrotron X-ray and Neutron Techniques: A Review. Batteries. 2023; 9(9):461. https://doi.org/10.3390/batteries9090461
Chicago/Turabian StyleShipitsyn, Vadim, Rishivandhiga Jayakumar, Wenhua Zuo, Bing Sun, and Lin Ma. 2023. "Understanding High-Voltage Behavior of Sodium-Ion Battery Cathode Materials Using Synchrotron X-ray and Neutron Techniques: A Review" Batteries 9, no. 9: 461. https://doi.org/10.3390/batteries9090461
APA StyleShipitsyn, V., Jayakumar, R., Zuo, W., Sun, B., & Ma, L. (2023). Understanding High-Voltage Behavior of Sodium-Ion Battery Cathode Materials Using Synchrotron X-ray and Neutron Techniques: A Review. Batteries, 9(9), 461. https://doi.org/10.3390/batteries9090461