Functional Surface Coating to Enhance the Stability of LiNi0.6Mn0.2Co0.2O2
Abstract
:1. Introduction
2. Materials and Methods
3. Results
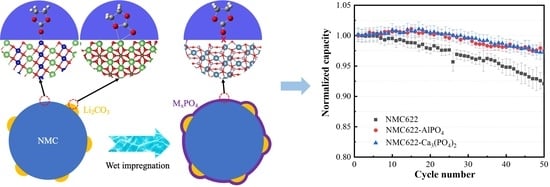
3.1. Surface Modification of NMC622
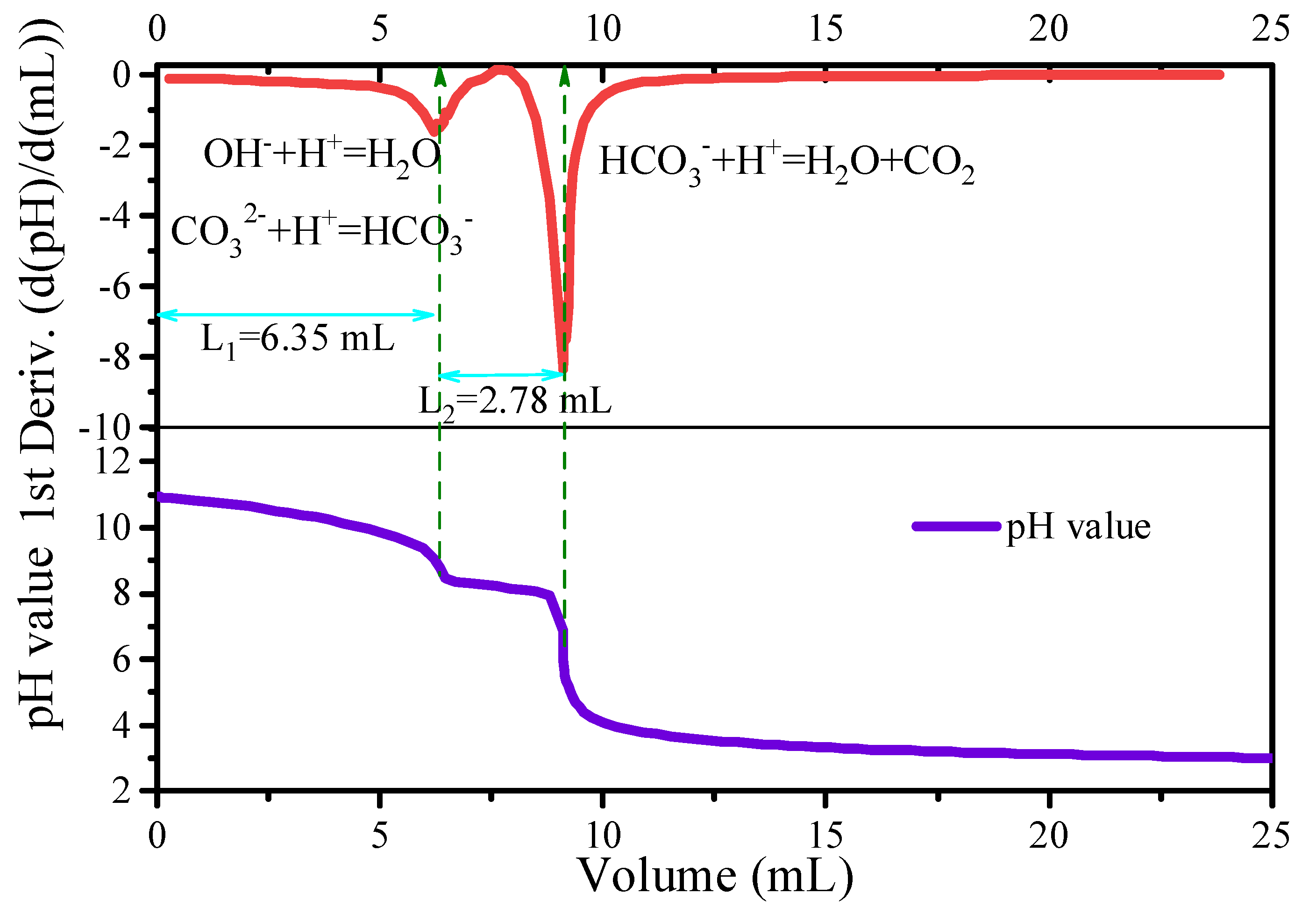
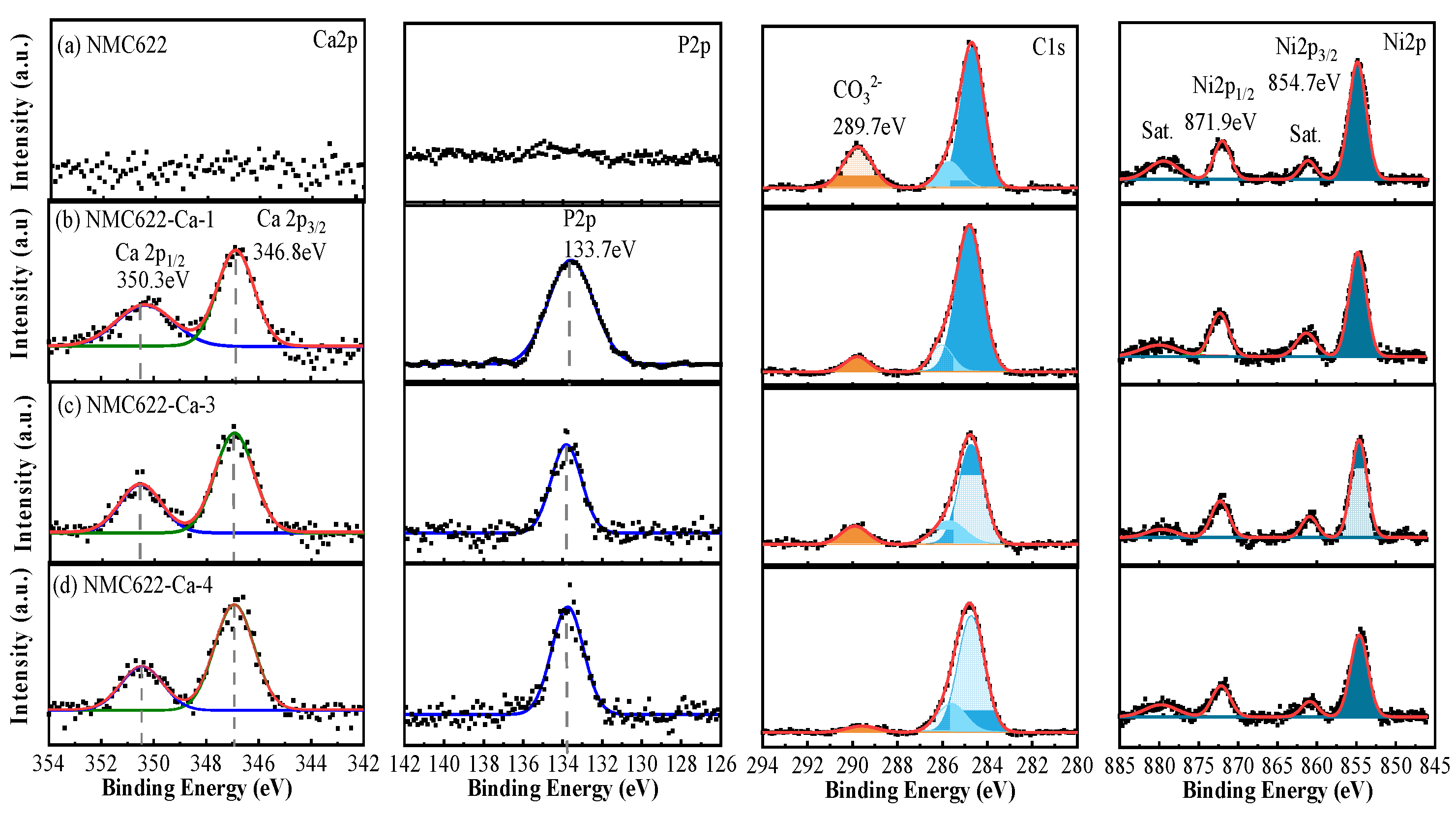
3.2. Surface Chemistry of NMC622
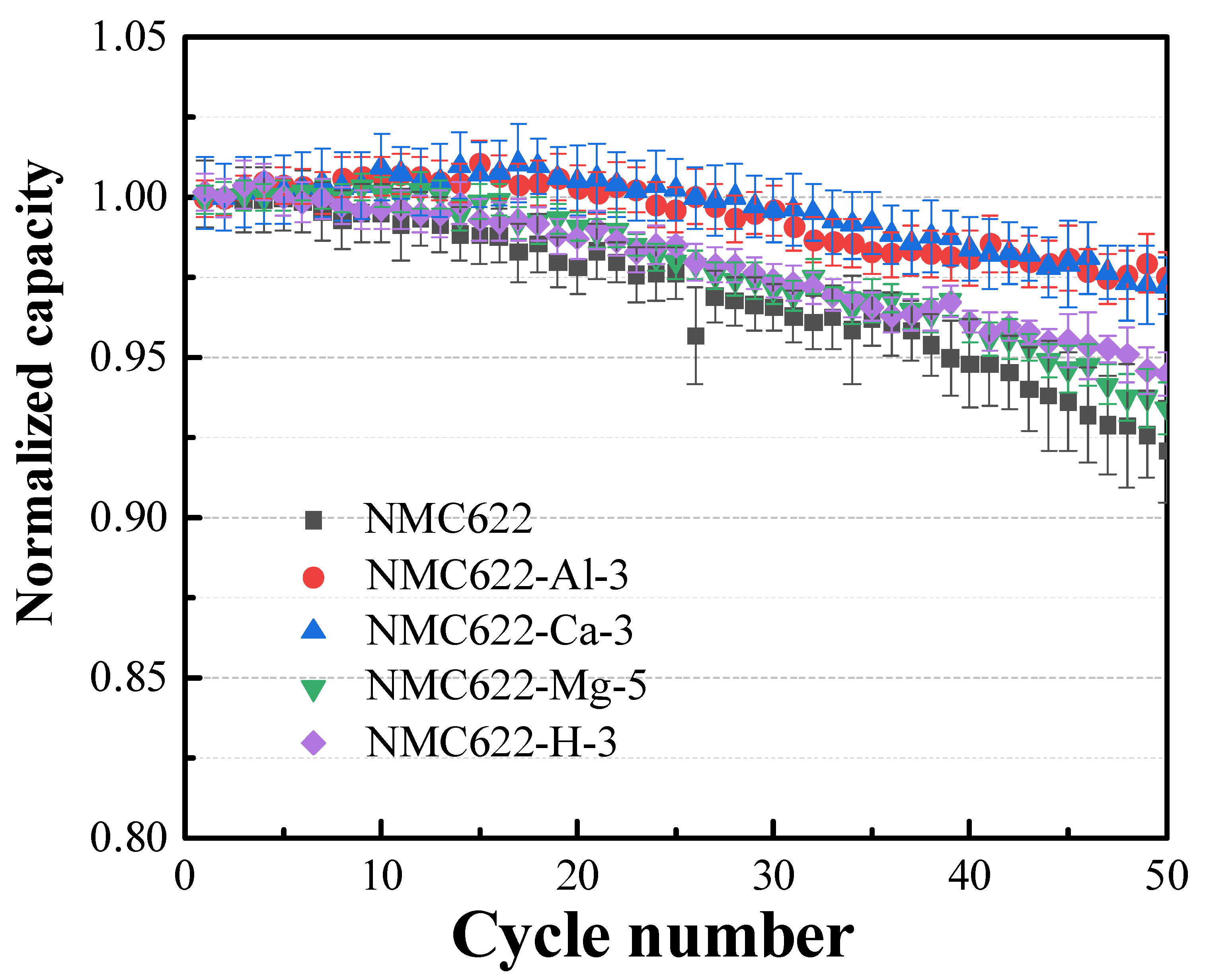
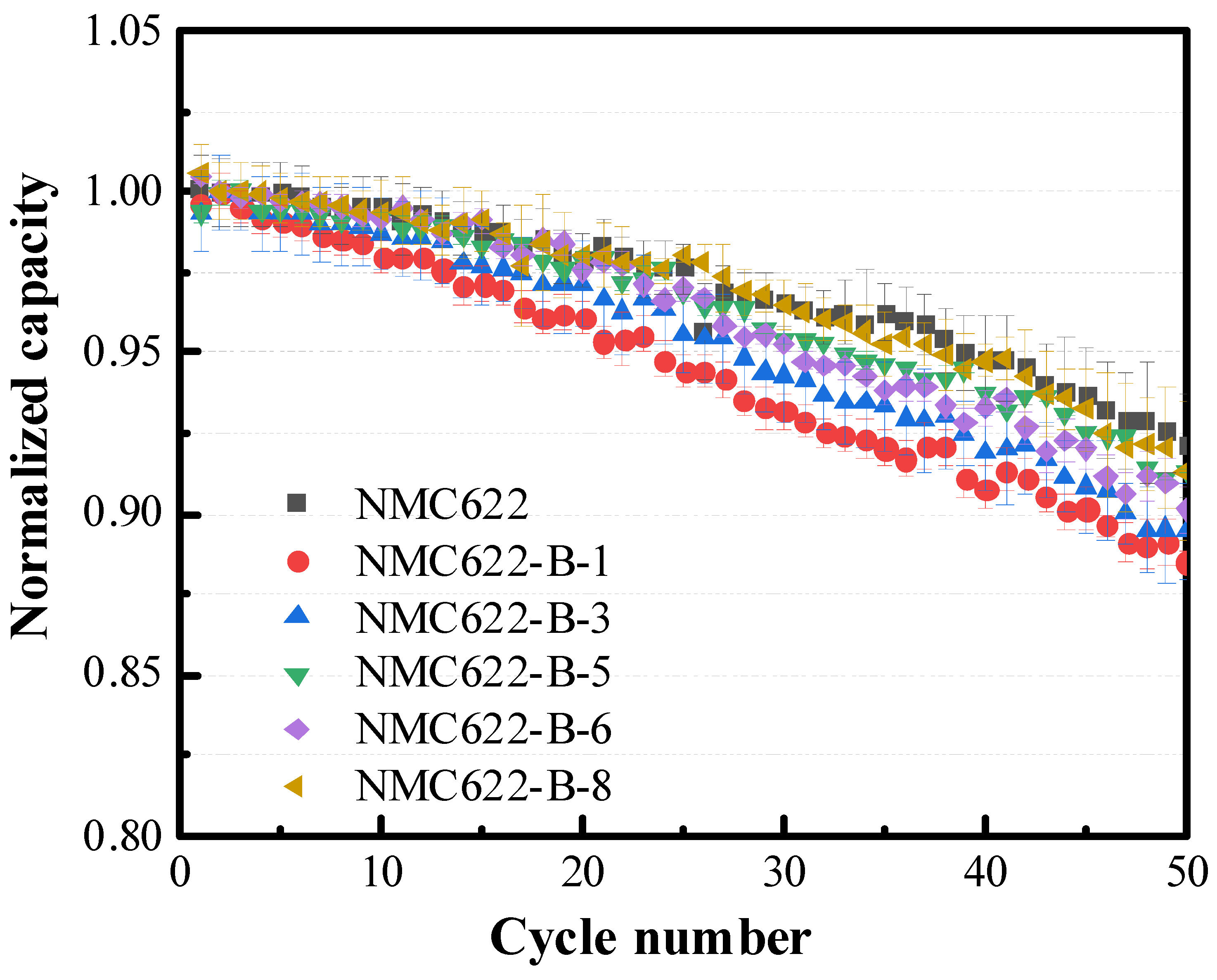
3.3. Electrochemical Performance of NMC622
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.-K.; Lee, D.-J.; Lee, Y.J.; Chen, Z.; Myung, S.-T. Cobalt-Free Nickel Rich Layered Oxide Cathodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 11434–11440. [Google Scholar] [CrossRef]
- Dose, W.M.; Temprano, I.; Allen, J.P.; Björklund, E.; O’Keefe, C.A.; Li, W.; Mehdi, B.L.; Weatherup, R.S.; De Volder, M.F.L.; Grey, C.P. Electrolyte Reactivity at the Charged Ni-Rich Cathode Interface and Degradation in Li-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 13206–13222. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Feng, Y.; Zhu, J.; Mo, C.; Cai, Q.; Liao, Y.; Li, W. Achieving a Highly Stable Electrode/Electrolyte Interface for a Nickel-Rich Cathode via an Additive-Containing Gel Polymer Electrolyte. ACS Appl. Mater. Interfaces 2022, 14, 36656–36667. [Google Scholar] [CrossRef]
- Wang, S.; Dai, A.; Cao, Y.; Yang, H.; Khalil, A.; Lu, J.; Li, H.; Ai, X. Enabling stable and high-rate cycling of a Ni-rich layered oxide cathode for lithium-ion batteries by modification with an artificial Li+-conducting cathode-electrolyte interphase. J. Mater. Chem. A 2021, 9, 11623–11631. [Google Scholar] [CrossRef]
- Liu, T.; Yu, L.; Lu, J.; Zhou, T.; Huang, X.; Cai, Z.; Dai, A.; Gim, J.; Ren, Y.; Xiao, X.; et al. Rational design of mechanically robust Ni-rich cathode materials via concentration gradient strategy. Nat. Commun. 2021, 12, 6024. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, Z.; Dahn, J.R. Staging Phase Transitions in Lix CoO2. J. Electrochem. Soc. 2002, 149, A1604. [Google Scholar] [CrossRef]
- Tan, S.; Shadike, Z.; Li, J.; Wang, X.; Yang, Y.; Lin, R.; Cresce, A.; Hu, J.; Hunt, A.; Waluyo, I.; et al. Additive engineering for robust interphases to stabilize high-Ni layered structures at ultra-high voltage of 4.8 V. Nat. Energy 2022, 7, 484–494. [Google Scholar] [CrossRef]
- Zhan, C.; Lu, J.; Jeremy Kropf, A.; Wu, T.; Jansen, A.N.; Sun, Y.-K.; Qiu, X.; Amine, K. Mn(II) deposition on anodes and its effects on capacity fade in spinel lithium manganate–carbon systems. Nat. Commun. 2013, 4, 2437. [Google Scholar] [CrossRef]
- Jung, R.; Linsenmann, F.; Thomas, R.; Wandt, J.; Solchenbach, S.; Maglia, F.; Stinner, C.; Tromp, M.; Gasteiger, H.A. Nickel, Manganese, and Cobalt Dissolution from Ni-Rich NMC and Their Effects on NMC622-Graphite Cells. J. Electrochem. Soc. 2019, 166, A378. [Google Scholar] [CrossRef]
- Wachs, S.J.; Behling, C.; Ranninger, J.; Möller, J.; Mayrhofer, K.J.J.; Berkes, B.B. Online Monitoring of Transition-Metal Dissolution from a High-Ni-Content Cathode Material. ACS Appl. Mater. Interfaces 2021, 13, 33075–33082. [Google Scholar] [CrossRef]
- Xu, C.; Märker, K.; Lee, J.; Mahadevegowda, A.; Reeves, P.J.; Day, S.J.; Groh, M.F.; Emge, S.P.; Ducati, C.; Layla Mehdi, B.; et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 2021, 20, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, J.; Zhang, M.; Zheng, B.; Zhao, D.; Cheng, Y.; He, Z.; Su, M.; Xie, C.; Luo, M.; et al. Mitigating the Surface Reconstruction of Ni-Rich Cathode via P2-Type Mn-Rich Oxide Coating for Durable Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 30398–30409. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, A.; Tang, Z.; Meng, F.; Shang, T.; Guo, S.; Ding, J.; Luo, Y.; Xiao, D.; Wang, X.; et al. Robust Surface Reconstruction Induced by Subsurface Ni/Li Antisites in Ni-Rich Cathodes. Adv. Funct. Mater. 2021, 31, 2010291. [Google Scholar] [CrossRef]
- Xu, G.-L.; Liu, Q.; Lau, K.K.S.; Liu, Y.; Liu, X.; Gao, H.; Zhou, X.; Zhuang, M.; Ren, Y.; Li, J.; et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes. Nat. Energy 2019, 4, 484–494. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Zhang, J.-G.; Wang, C.-M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 2017, 8, 14101. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, L.A.; Huang, T.-Y.; Lee, D.; McCloskey, B.D. Particle Surface Cracking Is Correlated with Gas Evolution in High-Ni Li-Ion Cathode Materials. ACS Appl. Mater. Interfaces 2022, 14, 39959–39964. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Liu, J.; Wang, B.; Cheng, X.; Zhang, Y.; Sun, X.; Wang, C.; Zhang, J.-G. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 2018, 3, 600–605. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.-T.; Zhao, C.; Weng, Q.-S.; Deng, J.; Hwang, I.; Jiang, Y.; Sun, C.; Li, T.; Xu, W.; et al. Conformal PEDOT Coating Enables Ultra-High-Voltage and High-Temperature Operation for Single-Crystal Ni-Rich Cathodes. ACS Nano 2022, 16, 14527–14538. [Google Scholar] [CrossRef]
- Bi, Y.; Tao, J.; Wu, Y.; Li, L.; Xu, Y.; Hu, E.; Wu, B.; Hu, J.; Wang, C.; Zhang, J.-G.; et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 2020, 370, 1313–1317. [Google Scholar] [CrossRef]
- Song, B.; Li, W.; Oh, S.-M.; Manthiram, A. Long-Life Nickel-Rich Layered Oxide Cathodes with a Uniform Li2ZrO3 Surface Coating for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 9718–9725. [Google Scholar] [CrossRef]
- Hou, D.; Han, J.; Geng, C.; Xu, Z.; AlMarzooqi, M.M.; Zhang, J.; Yang, Z.; Min, J.; Xiao, X.; Borkiewicz, O.; et al. Surface coating by mechanofusion modulates bulk charging pathways and battery performance of Ni-rich layered cathodes. Proc. Natl. Acad. Sci. USA 2022, 119, e2212802119. [Google Scholar] [CrossRef]
- Sungjemmenla, S.K.V.; Soni, C.B.; Kumar, V.; Seh, Z.W. Understanding the Cathode–Electrolyte Interphase in Lithium-Ion Batteries. Energy Technol. 2022, 10, 2200421. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Nicolau, B.G.; Esbenshade, J.L.; Gewirth, A.A. Characterization of the Cathode Electrolyte Interface in Lithium Ion Batteries by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 7171–7177. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; He, Y.; Wang, C.; Zou, P.; Hu, E.; Yang, X.-Q.; Xu, K.; Xin, H.L. Characterization of the structure and chemistry of the solid–electrolyte interface by cryo-EM leads to high-performance solid-state Li-metal batteries. Nat. Nanotechnol. 2022, 17, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Wheatcroft, L.; Klingner, N.; Heller, R.; Hlawacek, G.; Özkaya, D.; Cookson, J.; Inkson, B.J. Visualization and Chemical Characterization of the Cathode Electrolyte Interphase Using He-Ion Microscopy and In Situ Time-of-Flight Secondary Ion Mass Spectrometry. ACS Appl. Energy Mater. 2020, 3, 8822–8832. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.M.; Burns, J.C.; Stevens, D.A.; Dahn, H.M.; Dahn, J.R. Improving Precision and Accuracy in Coulombic Efficiency Measurements of Li-Ion Batteries. J. Electrochem. Soc. 2013, 160, A521. [Google Scholar] [CrossRef]
- Hall, D.S.; Gauthier, R.; Eldesoky, A.; Murray, V.S.; Dahn, J.R. New chemical insights into the beneficial role of Al2O3 cathode coatings in lithium-ion cells. ACS Appl. Mater. Interfaces 2019, 11, 14095–14100. [Google Scholar] [CrossRef]
- Ma, T.; Xu, G.-L.; Li, Y.; Wang, L.; He, X.; Zheng, J.; Liu, J.; Engelhard, M.H.; Zapol, P.; Curtiss, L.A.; et al. Revisiting the Corrosion of the Aluminum Current Collector in Lithium-Ion Batteries. J. Phys. Chem. Lett. 2017, 8, 1072–1077. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, H.; Gim, J.; Ngo, A.T.; Ma, Z.-F.; Chen, Z. Identifying Active Sites for Parasitic Reactions at the Cathode–Electrolyte Interface. J. Phys. Chem. Lett. 2019, 10, 589–594. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, G.-L.; Li, Y.; Luo, X.; Maglia, F.; Bauer, C.; Lux, S.F.; Paschos, O.; Kim, S.-J.; Lamp, P.; et al. Kinetic Study of Parasitic Reactions in Lithium-Ion Batteries: A Case Study on LiNi0.6Mn0.2Co0.2O2. ACS Appl. Mater. Interfaces 2016, 8, 3446–3451. [Google Scholar] [CrossRef]
- Chen, Z. Chasing protons in lithium-ion batteries. Chem. Commun. 2022, 58, 10127–10135. [Google Scholar] [CrossRef]
- Cai, J.; Yang, Z.; Zhou, X.; Wang, B.; Suzana, A.; Bai, J.; Liao, C.; Liu, Y.; Chen, Y.; Song, S.; et al. Unveiling the parasitic-reaction-driven surface reconstruction in Ni-rich cathode and the electrochemical role of Li2CO3. J. Energy Chem. 2023, 85, 126–136. [Google Scholar] [CrossRef]
- Renfrew, S.E.; McCloskey, B.D. Residual Lithium Carbonate Predominantly Accounts for First Cycle CO2 and CO Outgassing of Li-Stoichiometric and Li-Rich Layered Transition-Metal Oxides. J. Am. Chem. Soc. 2017, 139, 17853–17860. [Google Scholar] [CrossRef]
- Renfrew, S.E.; McCloskey, B.D. Quantification of surface oxygen depletion and solid carbonate evolution on the first cycle of LiNi0.6Mn0.2Co0.2O2 electrodes. ACS Appl. Energy Mater. 2019, 2, 3762–3772. [Google Scholar] [CrossRef]
- Sattar, T.; Sim, S.-J.; Jin, B.-S.; Kim, H.-S. Dual function Li-reactive coating from residual lithium on Ni-rich NCM cathode material for Lithium-ion batteries. Sci. Rep. 2021, 11, 18590. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Thomas, T.; Gong, H.; Li, F.; Li, Q.; Song, L.; Azhagan, T.; Jiang, H.; Yang, M. A mechanism of alkali metal carbonates catalysing the synthesis of β-hydroxyethyl sulfide with mercaptan and ethylene carbonate. Org. Biomol. Chem. 2019, 17, 9367–9374. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, S.; Li, J.; Zhao, N.; Wei, W.; Sun, Y. Effective synthesis of propylene carbonate from propylene glycol and carbon dioxide by alkali carbonates. Catal. Lett. 2006, 112, 187–191. [Google Scholar] [CrossRef]



| Surface Modification Agent | Targeted Concentration |
|---|---|
| Ca(H2PO4)2·H2O | 6.5 wt% |
| Al(H2PO4)3 | 5.0 wt% |
| MgHPO4·3H2O | 17.5 wt% |
| H3PO4 | 3.3 wt% |
| Sample ID | Processing Solution | 1st Charge Capacity (mAh g−1) | 1st Discharge Capacity (mAh g−1) | 1st Cycle Columbic Efficiency, % | Capacity Retention @ 50th Cycle, % |
|---|---|---|---|---|---|
| NMC622 | 205.0 ± 1.84 | 182.2 ± 2.40 | 89.04 ± 0.35 | 92.08 ± 1.59 | |
| NMC622-Ca-1 | 1 wt% Ca(H2PO4)2 | 207.9 ± 2.25 | 181.1 ± 1.94 | 87.10 ±0.08 | 96.21 ± 0.28 |
| NMC622-Ca-3 | 3 wt% Ca(H2PO4)2 | 209.5 ± 2.04 | 180.7 ± 0.64 | 86.23 ± 0.53 | 97.25 ± 0.87 |
| NMC622-Ca-4 | 4 wt% Ca(H2PO4)2 | 202.8 ± 0.85 | 175.7 ± 1.13 | 86.65 ± 0.21 | 95.48 ± 0.25 |
| NMC622-Al-p5 | 0.5 wt% Al(H2PO4)3 | 207.6 ± 1.91 | 184.2 ± 1.34 | 88.73 ± 0.16 | 94.09 ± 1.17 |
| NMC622-Al-1 | 1 wt% Al(H2PO4)3 | 211.3 ± 1.97 | 187.4 ± 1.33 | 88.73 ± 0.05 | 94.49 ± 0.45 |
| NMC622-Al-2 | 2 wt% Al(H2PO4)3 | 209.4 ± 1.94 | 180.2 ± 0.40 | 86.06 ± 0.01 | 96.53 ± 0.03 |
| NMC622-Al-3 | 3 wt% Al(H2PO4)3 | 208.8 ± 0.78 | 180.2 ± 0.42 | 86.32 ± 0.12 | 97.56 ± 0.73 |
| NMC622-Mg-5 | 5 wt% MgHPO4 | 207.9 ± 1.01 | 183.7 ± 0.95 | 88.33 ± 0.05 | 93.42 ± 0.81 |
| NMC622-Mg-10 | 10 wt% MgHPO4 | 203.4 ± 0.92 | 179.3 ± 0.45 | 88.33 ± 0.06 | 89.01 ± 3.26 |
| NMC622-Mg-15 | 15 wt% MgHPO4 | 196.1 ± 2.82 | 173.2 ± 1.70 | 88.32 ± 0.03 | 89.77 ± 0.95 |
| NMC622-H-1 | 1 wt% H3PO4 | 199.9±1.22 | 177.0± 1.23 | 88.53± 0.21 | 94.50± 0.67 |
| NMC622-H-3 | 3 wt% H3PO4 | 205.9±0.90 | 184.5± 0.85 | 89.96± 0.07 | 94.50± 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Li, M.; Li, J.; Huang, X.; Cai, J.; Yang, Z.; Nguyen, H.; Shaik sulaiman, B.a.; Karami, N.; Chernova, N.A.; et al. Functional Surface Coating to Enhance the Stability of LiNi0.6Mn0.2Co0.2O2. Batteries 2023, 9, 485. https://doi.org/10.3390/batteries9100485
Xie Y, Li M, Li J, Huang X, Cai J, Yang Z, Nguyen H, Shaik sulaiman Ba, Karami N, Chernova NA, et al. Functional Surface Coating to Enhance the Stability of LiNi0.6Mn0.2Co0.2O2. Batteries. 2023; 9(10):485. https://doi.org/10.3390/batteries9100485
Chicago/Turabian StyleXie, Yingying, Matthew Li, Jiantao Li, Xiaozhou Huang, Jiyu Cai, Zhenzhen Yang, Hoai Nguyen, Baasit ali Shaik sulaiman, Niloofar Karami, Natalya A. Chernova, and et al. 2023. "Functional Surface Coating to Enhance the Stability of LiNi0.6Mn0.2Co0.2O2" Batteries 9, no. 10: 485. https://doi.org/10.3390/batteries9100485
APA StyleXie, Y., Li, M., Li, J., Huang, X., Cai, J., Yang, Z., Nguyen, H., Shaik sulaiman, B. a., Karami, N., Chernova, N. A., Upreti, S., Prevel, B., Wang, F., & Chen, Z. (2023). Functional Surface Coating to Enhance the Stability of LiNi0.6Mn0.2Co0.2O2. Batteries, 9(10), 485. https://doi.org/10.3390/batteries9100485