Silicon/Carbon Nanoparticles Assembled with Multifunction Carbon Nanotubes/Sheets as High-Performance Anode of Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of SiO2@C
2.3. Synthesis of Si@C
2.4. Synthesis of Si@C-CNTs/CS
2.5. Material Characterizations
2.6. Electrochemical Characterizations
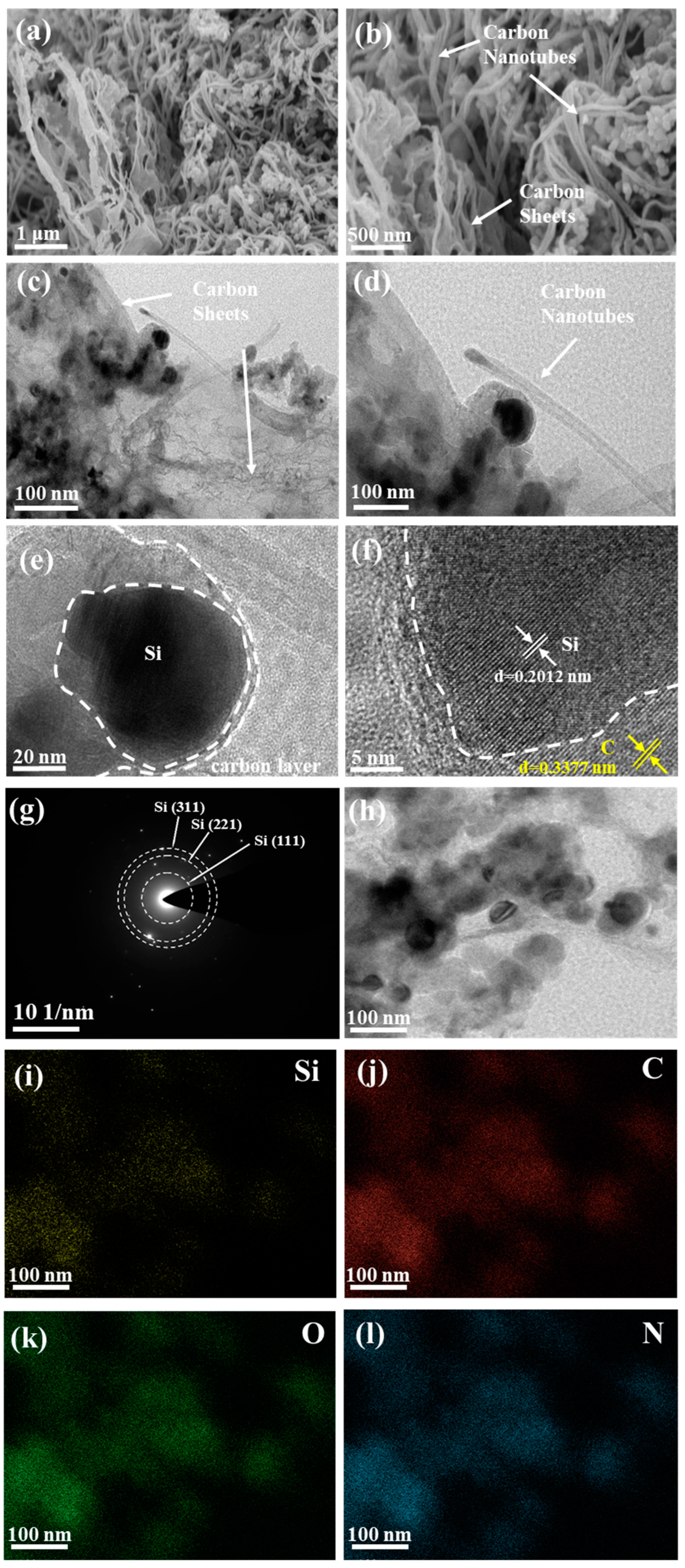
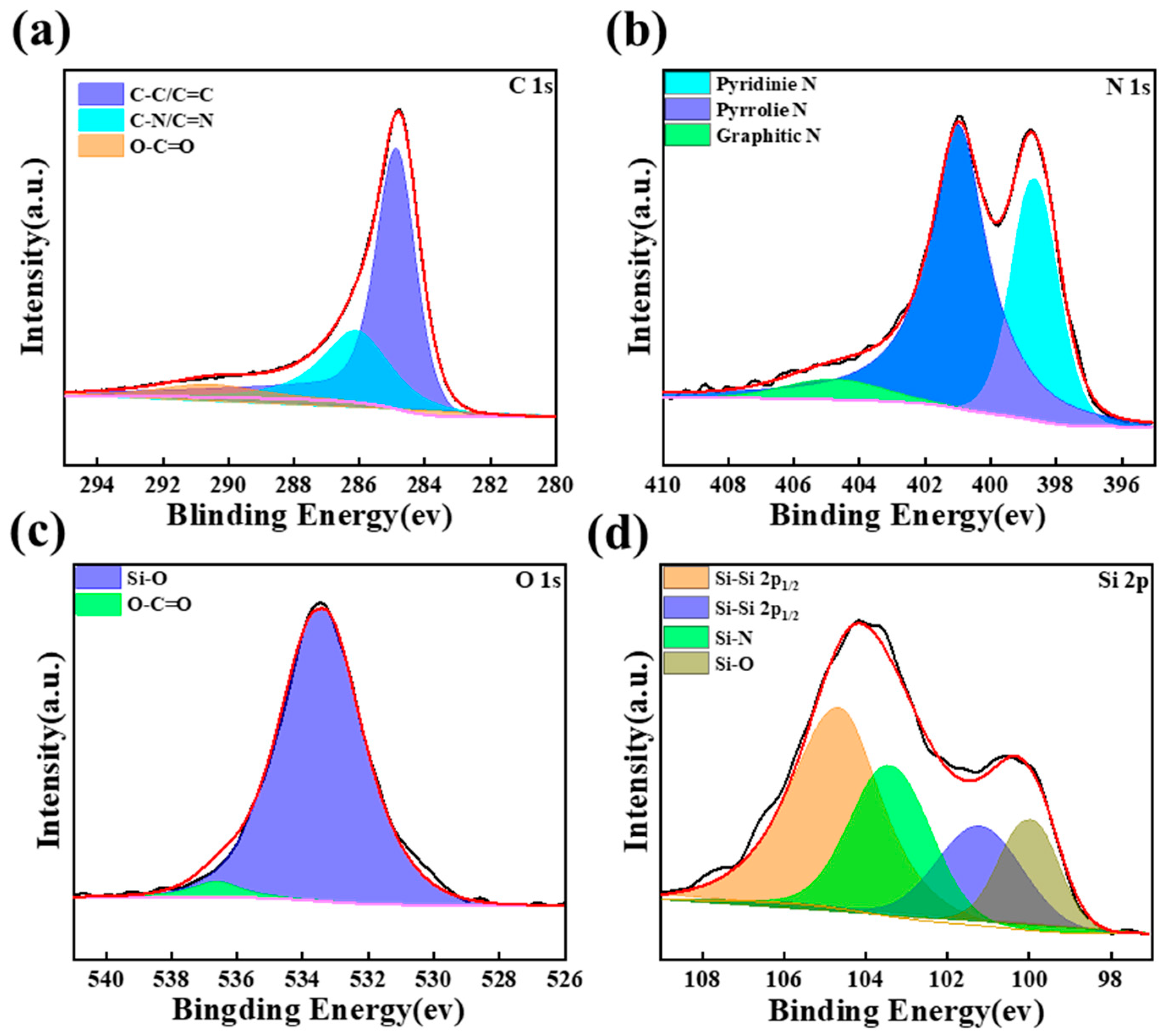
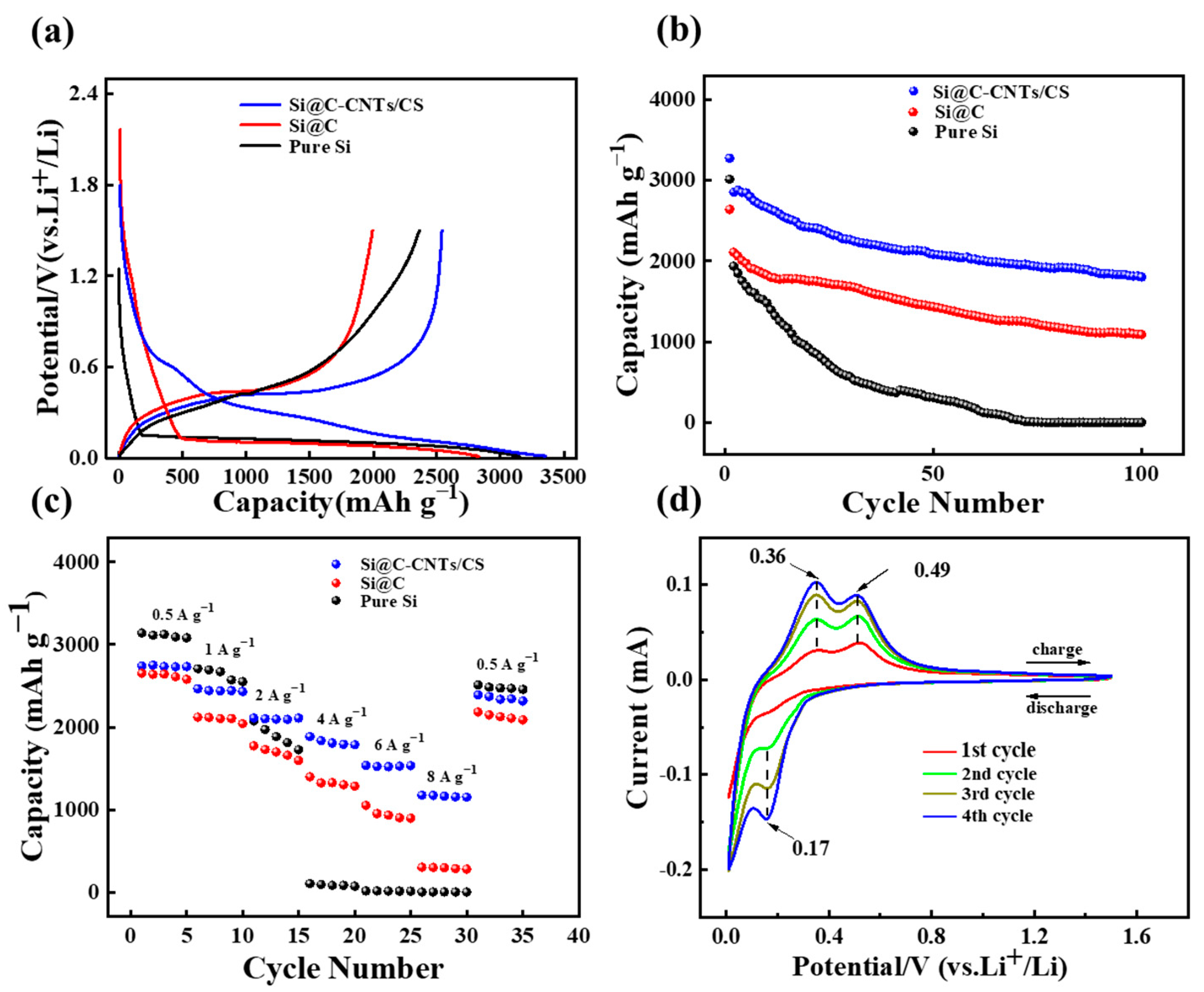
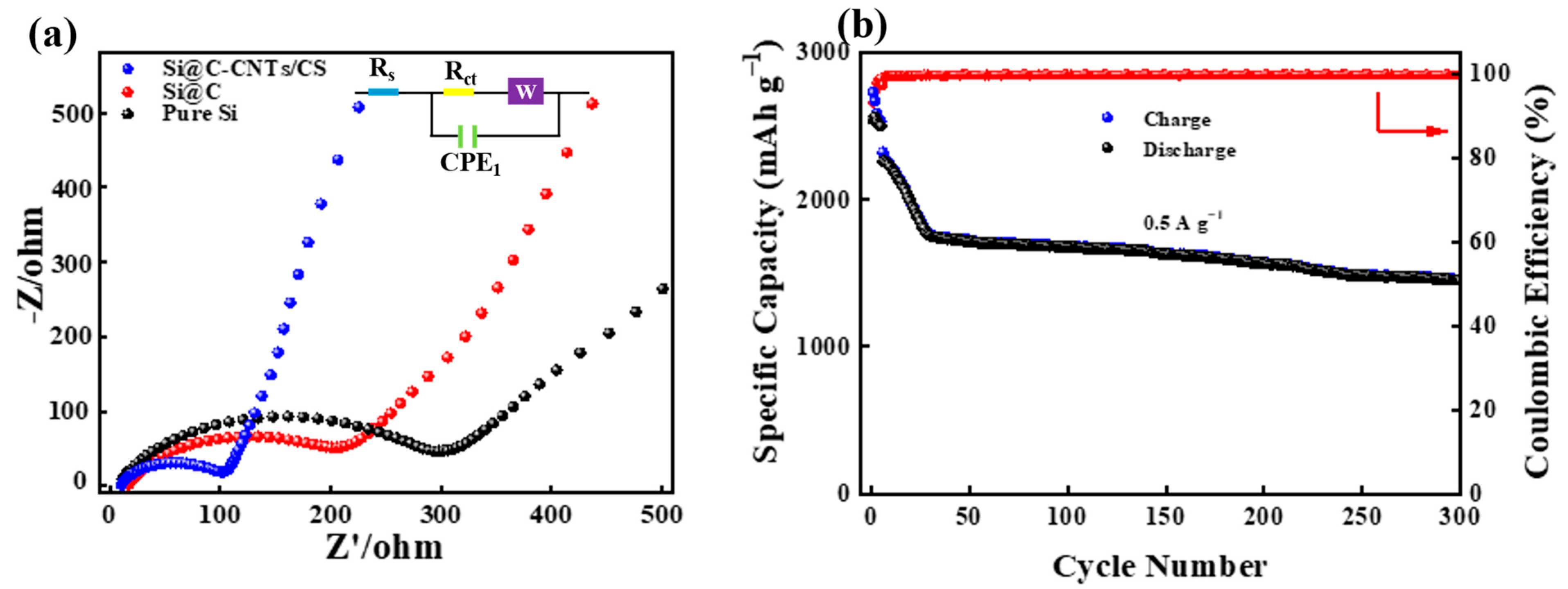
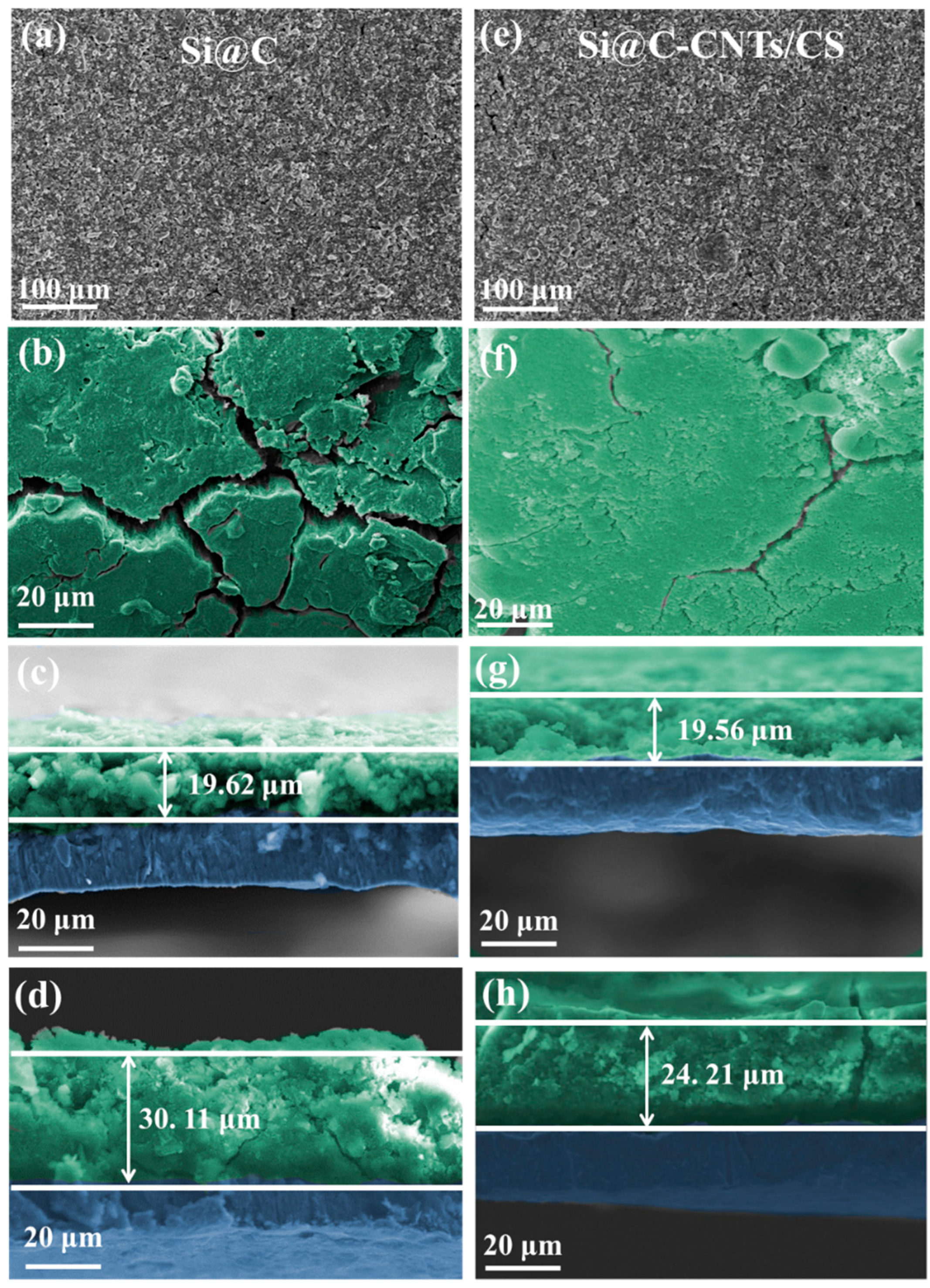
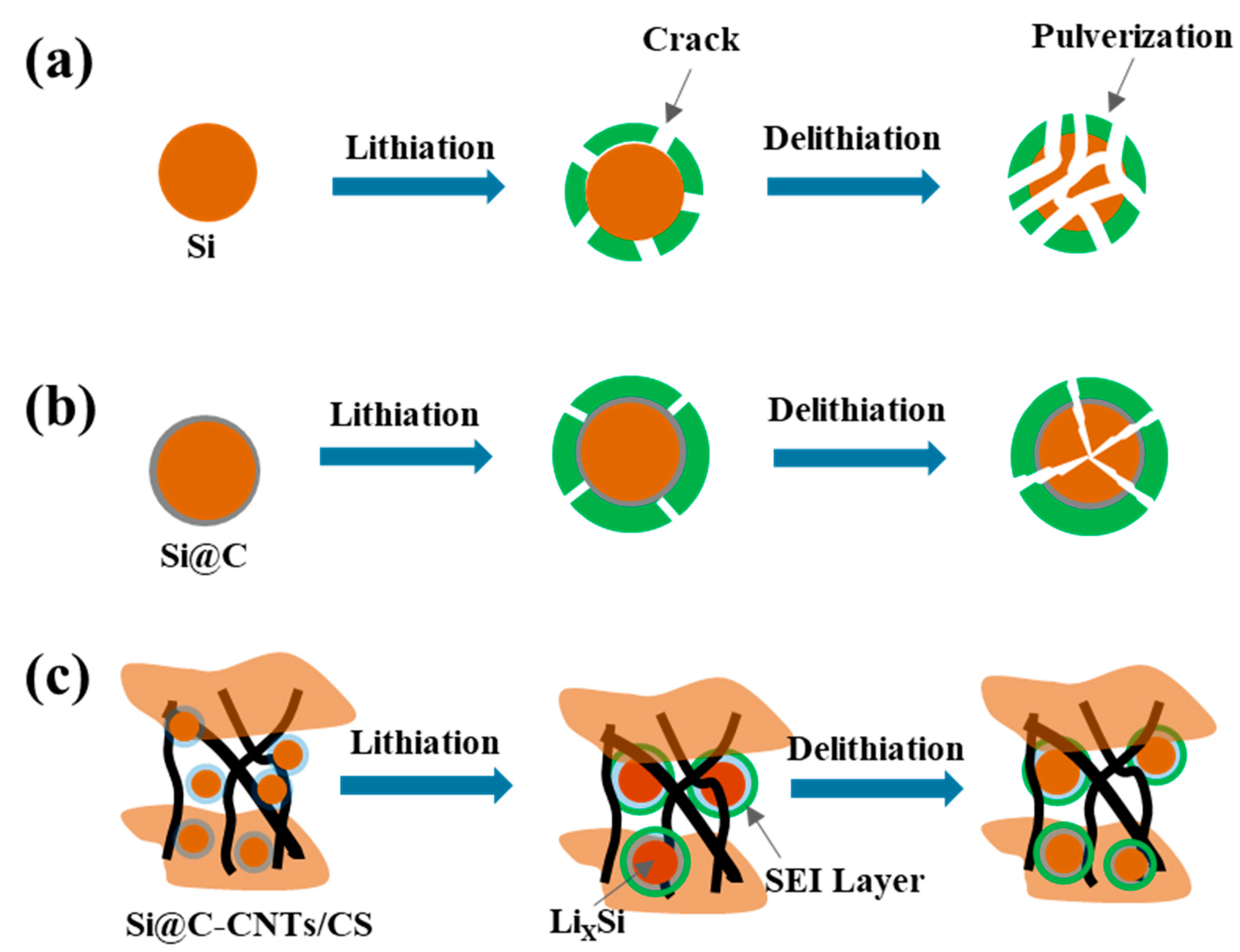
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rangarajan, S.S.; Sunddararaj, S.P.; Sudhakar, A.V.V.; Shiva, C.K.; Subramaniam, U.; Collins, E.R.; Senjyu, T. Lithium-Ion Batteries—The Crux of Electric Vehicles with Opportunities and Challenges. Clean Technol. 2022, 4, 908–930. [Google Scholar] [CrossRef]
- Camargos, P.H.; dos Santos, P.H.J.; dos Santos, I.R.; Ribeiro, G.S.; Caetano, R.E. Perspectives on Li-ion battery categories for electric vehicle applications: A review of state of the art. Int. J. Energy Res. 2022, 46, 19258–19268. [Google Scholar] [CrossRef]
- Chen, Z.L.; Zhang, Q.; Liang, Q.J. Carbon-Coatings Improve Performance of Li-Ion Battery. Nanomaterials 2022, 12, 1936. [Google Scholar] [CrossRef]
- Xu, X.L.; Cai, S.; Song, X.D.; Zhao, Y.M.; Zhou, G.W.; Liu, Y. A Mini Review on the Excellent Nanostructures in Electrochemical Energy Storage and Conversion. Nano 2022, 17, 2230002. [Google Scholar] [CrossRef]
- Xia, F.; Kwon, S.; Lee, W.W.; Liu, Z.; Kim, S.; Song, T.; Choi, K.J.; Paik, U.; Park, W.I. Graphene as an Interfacial Layer for Improving Cycling Performance of Si Nanowires in Lithium-Ion Batteries. Nano Lett. 2015, 15, 6658–6664. [Google Scholar] [CrossRef]
- Palomares, V.; Nieto, N.; Rojo, T. Negative electrode materials for high-energy density Li- and Na-ion batteries. Curr. Opin. Electrochem. 2022, 31, 100840. [Google Scholar] [CrossRef]
- Wu, Z.S.; Kong, D.F. Comparative life cycle assessment of lithium-ion batteries with lithium metal, silicon nanowire, and graphite negative electrodes. Clean Technol. Environ. Policy 2018, 20, 1233–1244. [Google Scholar] [CrossRef]
- Bitew, Z.; Tesemma, M.; Beyene, Y.; Amare, M. Nano-structured silicon and silicon based composites as negative electrode materials for lithium ion batteries: Recent progress and perspectives. Sustain. Energy Fuels 2022, 6, 1014–1050. [Google Scholar] [CrossRef]
- Du, A.M.; Li, H.; Chen, X.W.; Han, Y.Y.; Zhu, Z.P.; Chu, C.C. Recent Research Progress of Silicon-Based Negative electrode Materials for Lithium-Ion Batteries. Chemistryselect 2022, 7, e202201269. [Google Scholar] [CrossRef]
- Chadha, U.; Hafiz, M.; Bhardwaj, P.; Padmanaban, S.; Sinha, S.; Hariharan, S.; Kabra, D.; Venkatarangan, V.; Khanna, M.; Selvaraj, S.K.; et al. Theoretical progresses in silicon negative electrode substitutes for Lithium-ion batteries. J. Energy Storage 2022, 55, 105352. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Q.; Chen, Z.; Rong, C.; Chao, D. Application and Development of Silicon Negative electrode Binders for Lithium-Ion Batteries. Material 2023, 16, 4266. [Google Scholar] [CrossRef]
- Kamali, A.R.; Zhao, H.Y. Electrochemical conversion of natural graphite minerals into carbon nanostructures incorporated with Fe3Si for Li-ion storage application. J. Alloys Compd. 2023, 949, 169819. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.Y.; Wu, H.L.; Ding, H.L.; Zhang, C.T.; Huang, L.Q.; Luo, X.T. Constructing triple-protected Si/SiOx@ZnO@C negative electrode derived from volatile silicon waste for enhanced lithium storage capacity. Appl. Surf. Sci. 2023, 634, 157651. [Google Scholar] [CrossRef]
- Su, Y.J.; Yu, S.M.; Lang, X.S.; Wang, T.; Qu, T.T.; Wang, Q.S.; Li, L.; Yao, C.G.; Bai, Z.X.; Zhao, Y.Y.; et al. Cross-linked Si@SiC nanowires prepared by vacuum DC arc method embedded in phenolic resin as high electrochemical performance negative electrode active materials for lithium-ion batteries. Electrochim. Acta 2023, 463, 142808. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhu, X.J.; Pan, D. Solutions for the problems of silicon-carbon negative electrode materials for lithium-ion batteries. R. Soc. Open Sci. 2018, 5, 172370. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Wang, G.L.; Wang, S.; Zhang, J.; Wang, J.; Zhong, W.; Tang, F.; Yang, Z.L.; Zheng, J.; Li, X. In situ catalytic growth 3D multi-layers graphene sheets coated nano-silicon negative electrode for high performance lithium-ion batteries. Chem. Eng. J. 2019, 356, 895–903. [Google Scholar] [CrossRef]
- Zhang, X.; Weng, J.Z.; Ye, C.X.; Liu, M.R.; Wang, C.Y.; Wu, S.R.; Tong, Q.S.; Zhu, M.Q.; Gao, F. Strategies for Controlling or Releasing the Influence Due to the Volume Expansion of Silicon inside Si-C Composite Negative electrode for High-Performance Lithium-Ion Batteries. Materials 2022, 15, 4264. [Google Scholar] [CrossRef]
- Jiang, M.M.; Chen, J.L.; Zhang, Y.B.; Song, N.; Jiang, W.; Yang, J.P. Assembly: A Key Enabler for the Construction of Superior Silicon-Based Negative electrodes. Adv. Sci. 2022, 9, 2203162. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.L.; Lv, G.X.; Mu, Y.; Zhao, Y.L.; Wang, Y.X.; Li, X.Q.; Yao, P.C.; Deng, Y.; Cui, Y.; et al. Minimized lithium trapping by isovalent isomorphism for high initial Coulombic efficiency of silicon negative electrodes. Sci. Adv. 2019, 5, eaax0651. [Google Scholar] [CrossRef]
- Wu, S.; Di, F.; Zheng, J.G.; Zhao, H.W.; Zhang, H.; Li, L.X.; Geng, X.; Sun, C.G.; Yang, H.M.; Zhou, W.M.; et al. Self-healing polymer binders for the Si and Si/carbon negative electrodes of lithium-ion batteries. New Carbon Mater. 2022, 37, 802–826. [Google Scholar] [CrossRef]
- Min, J.H.; Bae, Y.S.; Kim, J.Y.; Kim, S.S.; Song, S.W. Self-organized Artificial SEI for Improving the Cycling Ability of Silicon-based Battery Negative electrode Materials. Bull. Korean Chem. Soc. 2013, 34, 1296–1299. [Google Scholar] [CrossRef]
- Xu, C.H.Y.; Jing, P.; Luo, H.; Cai, W.L.; Wu, H.; Wang, Q.; Zhang, Y. Oriented tuning yolk-shell structure for well-confinement and constructing durability of silicon/carbon composite. J. Alloys Compd. 2023, 942, 168955. [Google Scholar] [CrossRef]
- Patel, Y.; Vanpariya, A.; Mukhopadhyay, I. Si-decorated CNT network as negative electrode for lithium-ion battery. J. Solid State Electrochem. 2023, 27, 501–510. [Google Scholar] [CrossRef]
- Fu, K.; Wang, Y.; Mao, L.C.; Yang, X.X.; Jin, J.H.; Yang, S.L.; Li, G. Strongly coupled Co, N co-doped carbon nanotubes/graphene-like carbon nanosheets as efficient oxygen reduction electrocatalysts for primary Zinc-air battery. Chem. Eng. J. 2018, 351, 94–102. [Google Scholar] [CrossRef]
- Ke, C.Z.; Liu, F.; Zheng, Z.M.; Zhang, H.H.; Cai, M.T.; Li, M.; Yan, Q.Z.; Chen, H.X.; Zhang, Q.B. Boosting lithium storage performance of Si nanoparticles via thin carbon and nitrogen/phosphorus co-doped two-dimensional carbon sheet dual encapsulation. Rare Met. 2021, 40, 1347–1356. [Google Scholar] [CrossRef]
- Wang, F.F.; Lin, S.; Lu, X.S.; Hong, R.Y.; Liu, H.Y. Poly-dopamine carbon-coated stable silicon/graphene/CNT composite as negative electrode for lithium ion batteries. Electrochim. Acta 2022, 404, 139708. [Google Scholar] [CrossRef]
- Zeng, Y.F.; Huang, Y.D.; Liu, N.T.; Wang, X.C.; Zhang, Y.; Guo, Y.; Wu, H.H.; Chen, H.X.; Tang, X.C.; Zhang, Q.B. N-doped porous carbon nanofibers sheathed pumpkin-like Si/C composites as free-standing negative electrodes for lithium-ion batteries. J. Energy Chem. 2021, 54, 727–735. [Google Scholar] [CrossRef]
- Yao, R.R.; Xie, L.; Wu, Y.Q.; Meng, W.J.; He, Y.J.; Zhao, D.L. Controllable self-assembled mesoporous silicon nanocrystals framework as negative electrode material for Li-ion battery. Electrochim. Acta 2021, 390, 138850. [Google Scholar] [CrossRef]
- Shen, L.Y.; Guo, X.W.; Fang, X.P.; Wang, Z.X. Magnesiothermically reduced diatomaceous earth as a porous silicon negative electrode material for lithium ion batteries. J. Power Sources 2012, 213, 229–232. [Google Scholar] [CrossRef]
- Ryu, J.; Hong, D.; Choi, S.; Park, S. Synthesis of ultrathin Si nanosheets from natural clays for lithium-ion battery negative electrodes. ACS NANO 2016, 10, 2843–2851. [Google Scholar] [CrossRef]
- Park, A.R.; Nam, M.G.; Kim, A.Y.; Kim, K.S.; Shah, M.; Lee, J.Y.; Kim, W.J.; Lee, J.K.; Yoo, P.J. Si/Co-CoSi2/reduced graphene oxide ternary nanocomposite negative electrodes for Li-Ion batteries with enhanced capacity and cycling stability. J. Alloys Compd. 2017, 724, 1134–1142. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, R.X.; Xie, H.M.; Zhang, D.D.; Liu, X.Y.; Si, Y.J.; Li, N. Electronic structure tuning of FeCo nanoparticles embedded in multi-dimensional carbon matrix for enhanced bifunctional oxygen electrocatalysis. J. Alloys Compd. 2021, 853, 157070. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical Properties of Protoporphyrin IX, Pyropheophorbide-a, and Photofrin (R) in Different Conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Wrogemann, J.M.; Fromm, O.; Deckwirth, F.; Beltrop, K.; Heckmann, A.; Winter, M.; Placke, T. Impact of Degree of Graphitization, Surface Properties and Particle Size Distribution on Electrochemical Performance of Carbon Negative electrodes for Potassium-Ion Batteries. Batter. Supercaps 2022, 5, e202200045. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, P.; Chen, M.; Jin, H.; Bai, Y.; Li, S.W.; Ma, F.; Xu, H.; Lian, K. In Situ Synthesis of Multilayer Carbon Matrix Decorated with Copper Particles: Enhancing the Performance of Si as Negative electrode for Li-Ion Batteries. Acs Nano 2019, 13, 3054–3062. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.R.; Xiang, Y.X.; Xu, L.F.; Luo, H.; Wang, B.L.; Liu, Y.; Han, X.; Zhao, W.M.; Chen, S.J.; Chen, H.L.; et al. Controlling Surface Oxides in Si/C Nanocomposite Negative electrodes for High-Performance Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1801718. [Google Scholar] [CrossRef]
- He, H.Z.; Zhang, Y.; Wang, P.; Hu, D.M. Preparation of sponge-cake-like N-doped porous carbon materials derived from silk fibroin by chemical activation. Microporous Mesoporous Mater. 2021, 317, 110998. [Google Scholar] [CrossRef]
- Dai, X.Q.; Liu, H.T.; Liu, X.; Liu, Z.L.; Liu, Y.S.; Cao, Y.H.; Tao, J.Y.; Shan, Z.Q. Silicon nanoparticles encapsulated in multifunctional crosslinked nano-silica/carbon hybrid matrix as a high-performance negative electrode for Li-ion batteries. Chem. Eng. J. 2021, 418, 129468. [Google Scholar] [CrossRef]
- Wang, S.H.; Teng, J.; Xie, Y.Y.; Wei, Z.W.; Fan, Y.A.; Jiang, J.J.; Wang, H.P.; Liu, H.G.; Wang, D.W.; Su, C.Y. Embedding CoO nanoparticles in a yolk-shell N-doped porous carbon support for ultrahigh and stable lithium storage. J. Mater. Chem. A 2019, 7, 4036–4046. [Google Scholar] [CrossRef]
- Hu, L.; Luo, B.; Wu, C.H.; Hu, P.F.; Wang, L.Z.; Zhang, H.J. Yolk-shell Si/C composites with multiple Si nanoparticles encapsulated into double carbon shells as lithium-ion battery anodes. J. Energy Chem. 2019, 32, 124–130. [Google Scholar] [CrossRef]
- Cai, H.Y.; Han, K.; Jiang, H.; Wang, J.W.; Liu, H. Self-standing silicon-carbon nanotube/graphene by a scalable in situ approach from low-cost Al-Si alloy powder for lithium ion batteries. J. Phys. Chem. Solid 2017, 109, 9–17. [Google Scholar] [CrossRef]
- Zhu, X.; Choi, S.H.; Tao, R.; Jia, X.L.; Lu, Y.F. Building high-rate silicon anodes based on hierarchical Si@C@CNT nanocomposite. J. Alloys Compd. 2019, 791, 1105–1113. [Google Scholar] [CrossRef]
- Jin, Y.M.; Ai, Z.Q.; Song, Y.; Zhang, X.W.; Shi, J.L.; Ma, C. Enhanced lithium storage performance of Si/C composite nanofiber membrane with carbon coating as binder-free and self-supporting anode for lithium-ion battery. Mater. Res. Bull. 2023, 167, 112429. [Google Scholar] [CrossRef]
- Zhang, M.X.; Zhao, L.; Sun, D.; Sun, Y.K.; Xu, C.M.; Lu, S.C.; Li, T.; Li, Y.F.; Xiao, Z.H. S doped CNTs scaffolded Si@C spheres anode toward splendid High-Temperature performance in Lithium-ion Battery. Appl. Surf. Sci. 2023, 626, 157254. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Li, C.; Li, X.; Zhang, G.; Liu, Y.; Deng, J. Silicon/Carbon Nanoparticles Assembled with Multifunction Carbon Nanotubes/Sheets as High-Performance Anode of Lithium-Ion Batteries. Batteries 2023, 9, 460. https://doi.org/10.3390/batteries9090460
Yang R, Li C, Li X, Zhang G, Liu Y, Deng J. Silicon/Carbon Nanoparticles Assembled with Multifunction Carbon Nanotubes/Sheets as High-Performance Anode of Lithium-Ion Batteries. Batteries. 2023; 9(9):460. https://doi.org/10.3390/batteries9090460
Chicago/Turabian StyleYang, Ruifeng, Canbing Li, Xinxi Li, Guoqing Zhang, Yanlin Liu, and Jian Deng. 2023. "Silicon/Carbon Nanoparticles Assembled with Multifunction Carbon Nanotubes/Sheets as High-Performance Anode of Lithium-Ion Batteries" Batteries 9, no. 9: 460. https://doi.org/10.3390/batteries9090460
APA StyleYang, R., Li, C., Li, X., Zhang, G., Liu, Y., & Deng, J. (2023). Silicon/Carbon Nanoparticles Assembled with Multifunction Carbon Nanotubes/Sheets as High-Performance Anode of Lithium-Ion Batteries. Batteries, 9(9), 460. https://doi.org/10.3390/batteries9090460







