Facile Constructing Hierarchical Fe3O4@C Nanocomposites as Anode for Superior Lithium-Ion Storage
Abstract
1. Introduction
2. Experiments
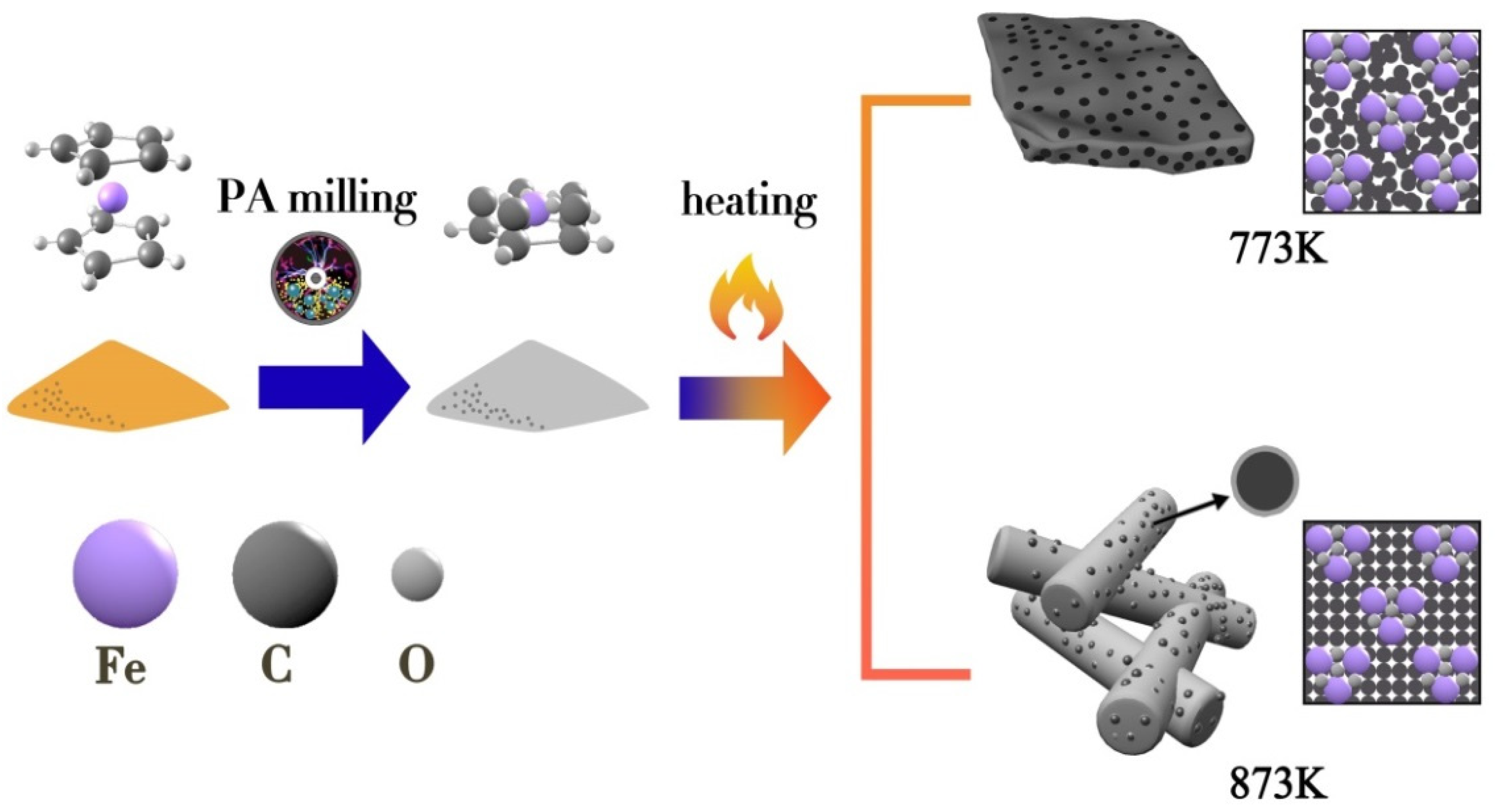
2.1. Material Preparation and Characterization
2.2. Electrochemical Measurements
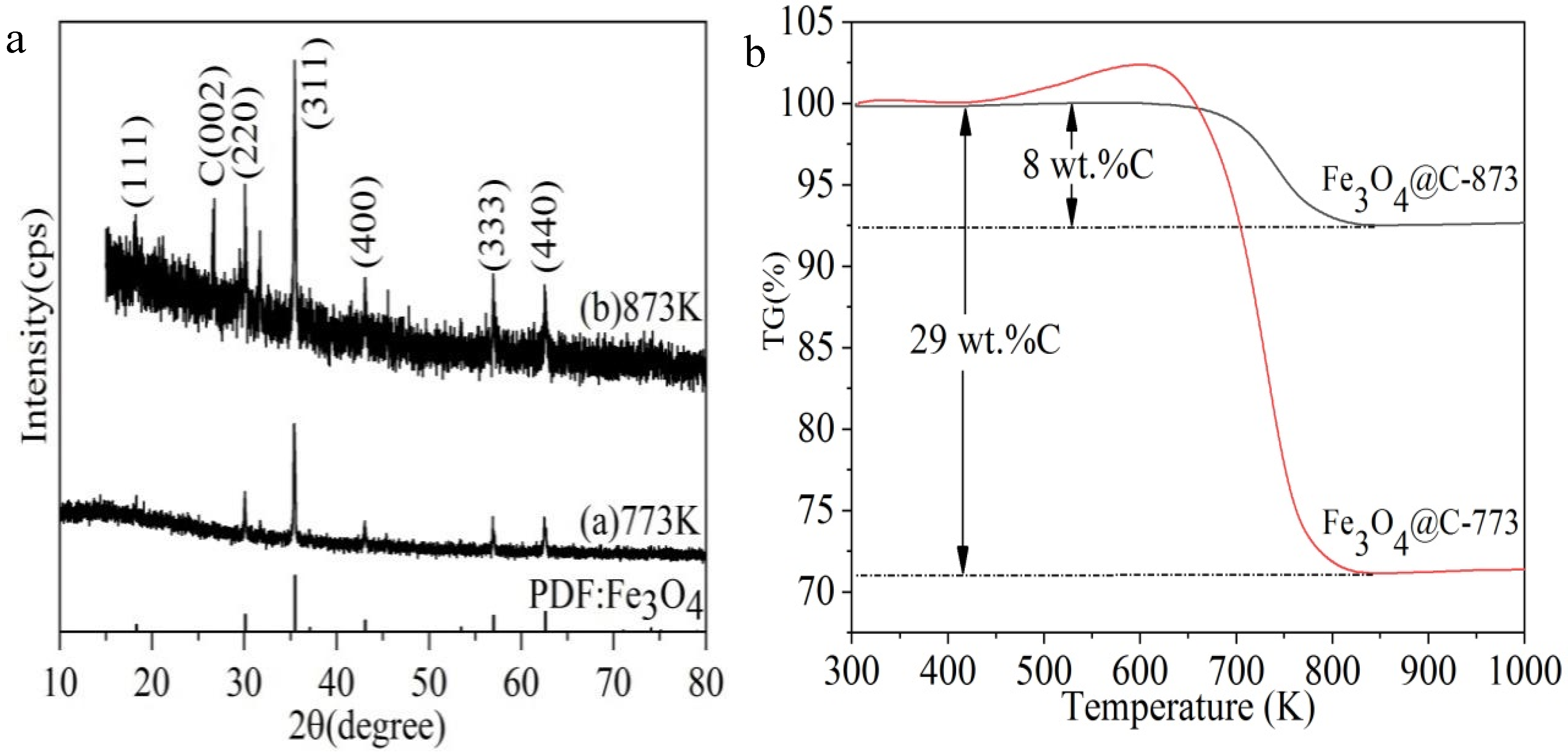
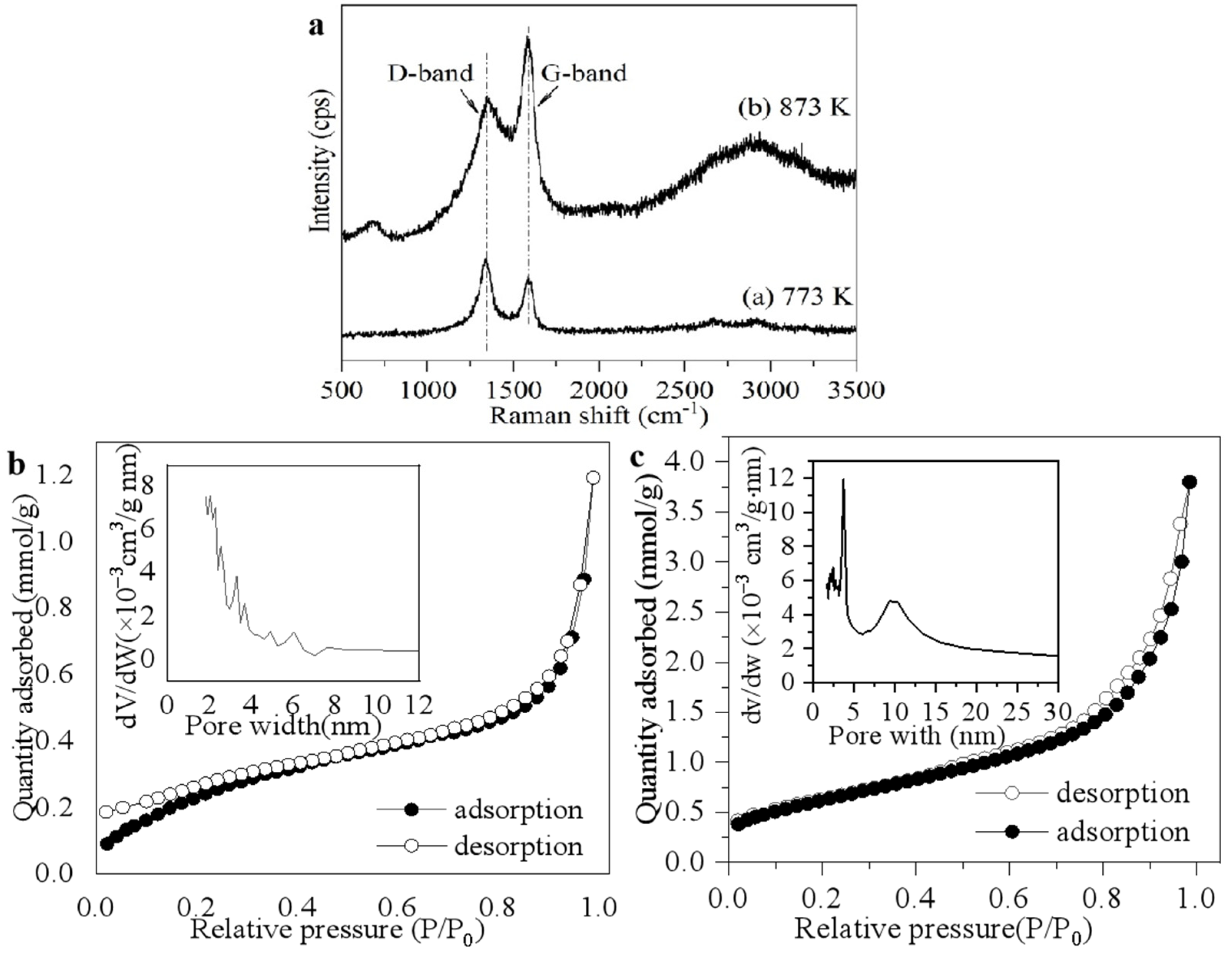
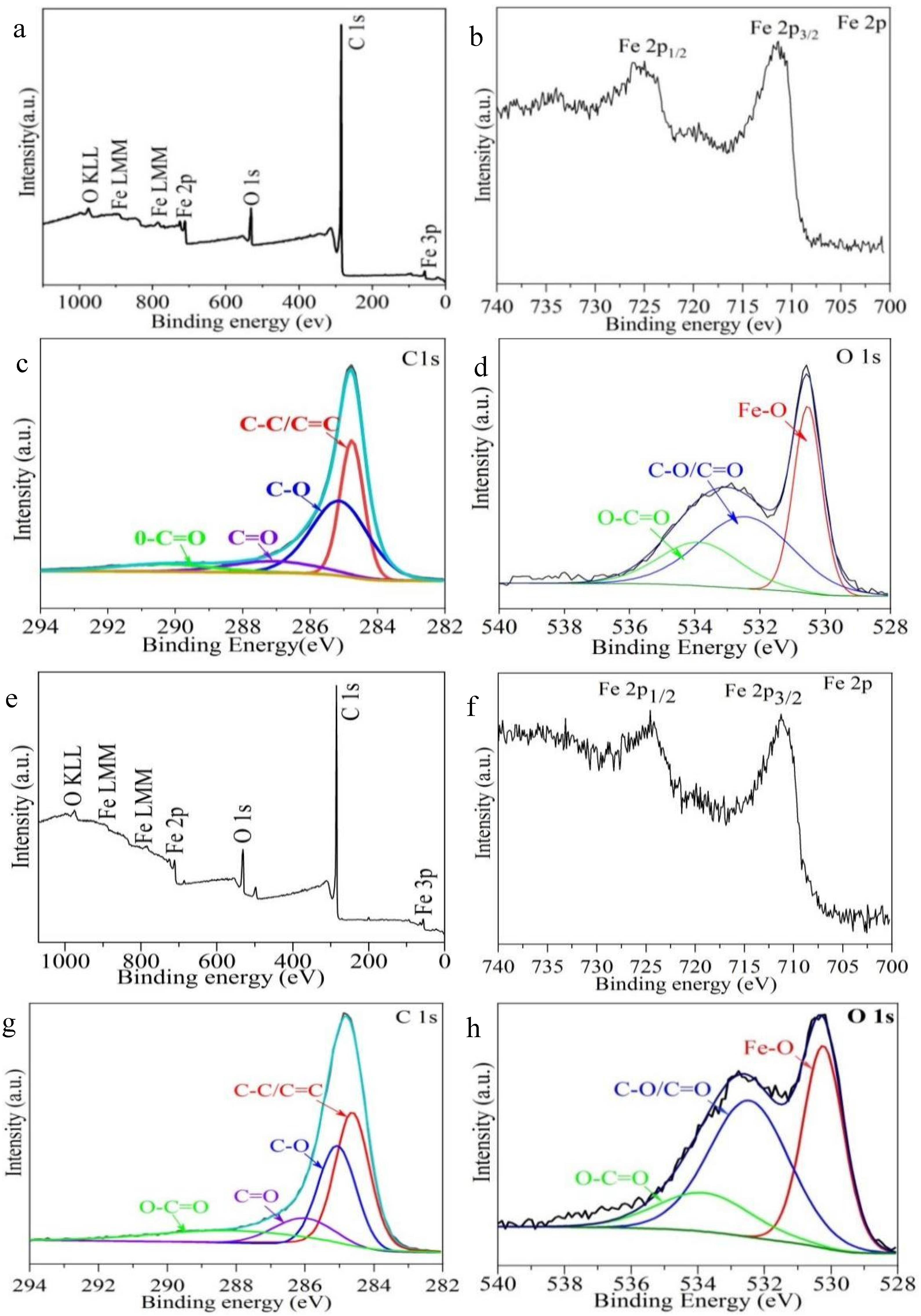
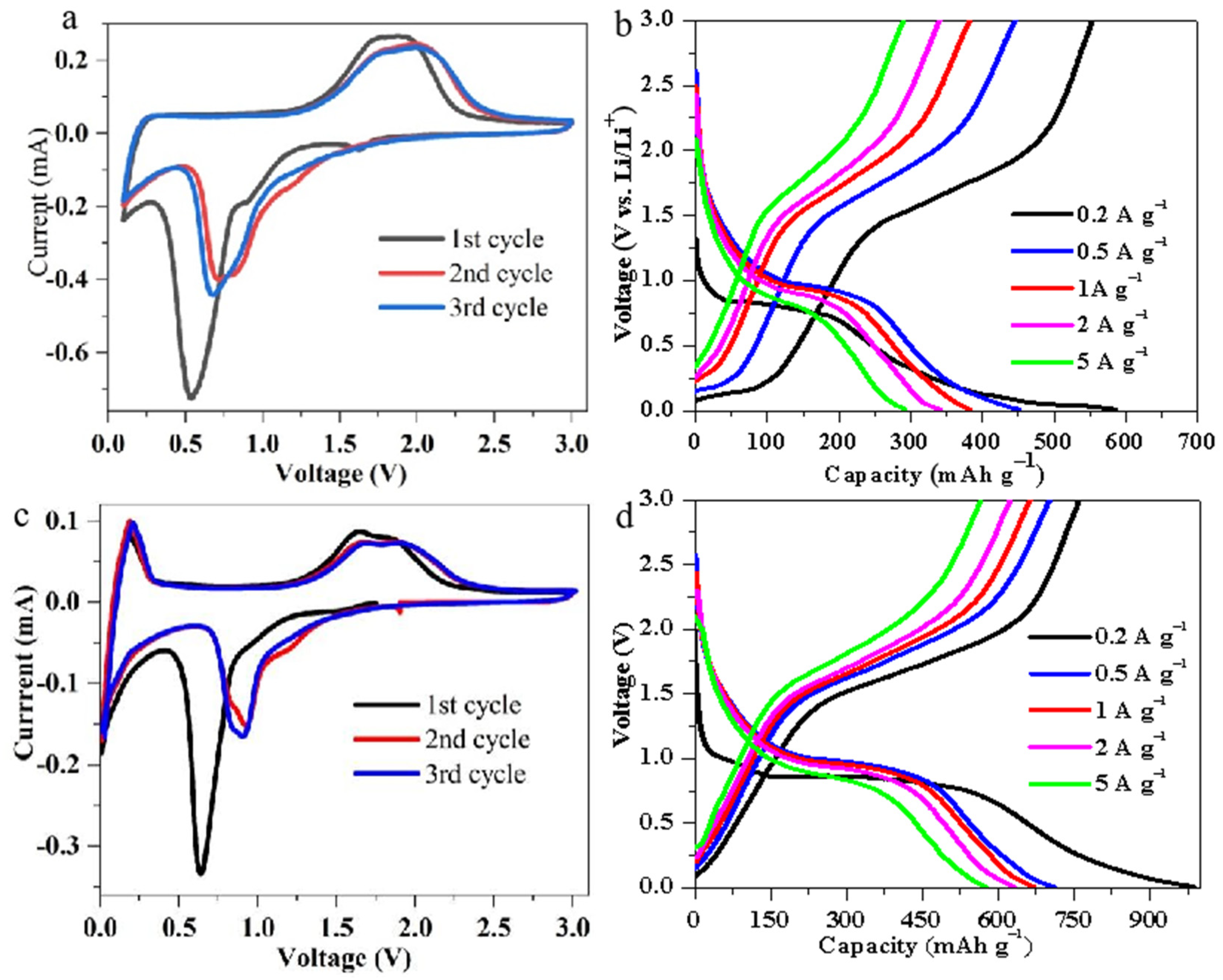
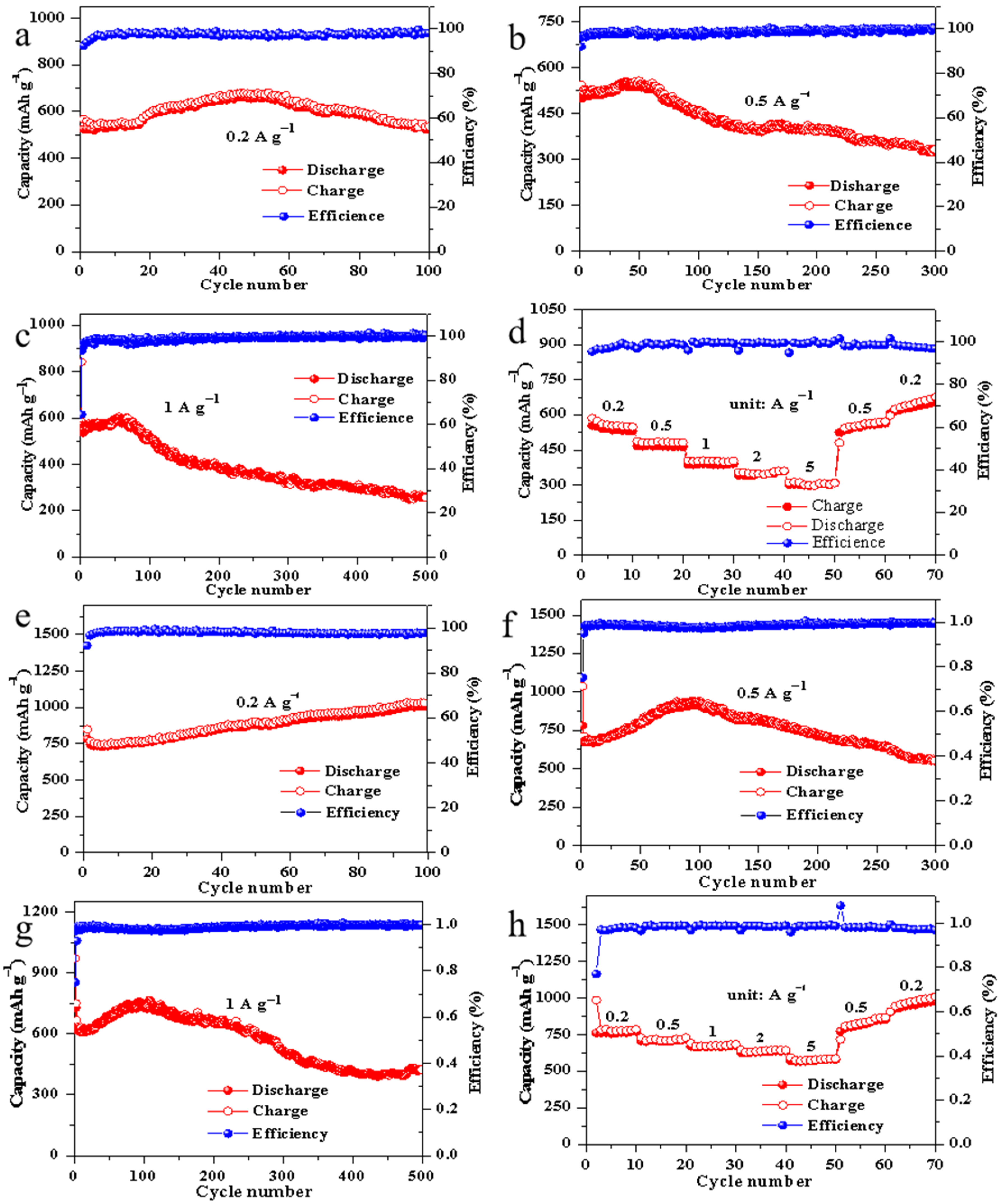
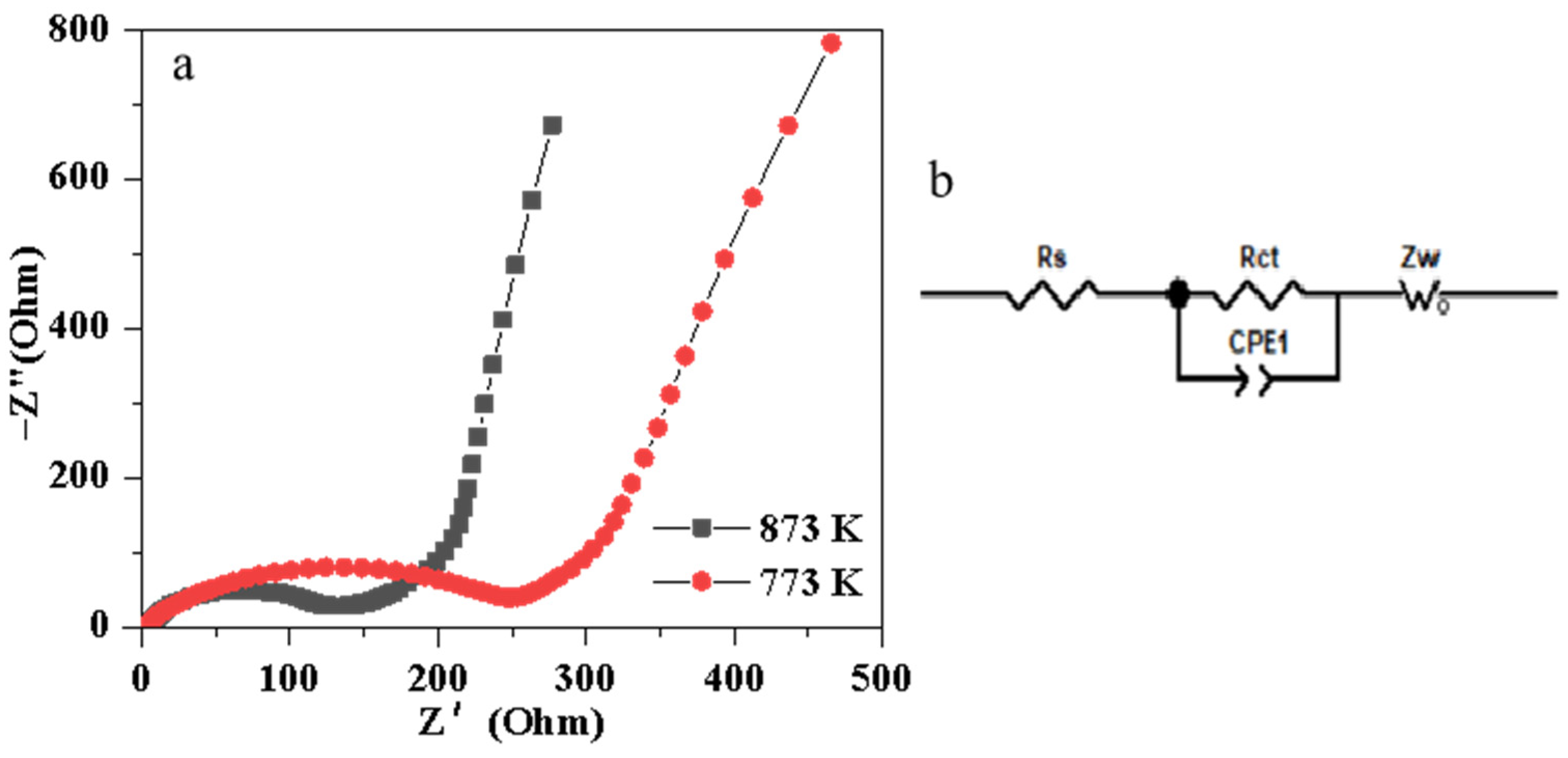
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and Opportunities towards Fast-Charging Battery Materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and Trends for Next-Generation Rechargeable Lithium and Lithium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, J.; Bruck, A.M.; Zhang, Y.; Li, J.; Stach, E.A.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Yu, G. A Tunable 3D Nanostructured Conductive Gel Framework Electrode for High-Performance Lithium Ion Batteries. Adv. Mater. 2017, 29, 1603922. [Google Scholar] [CrossRef]
- Goodenough, J.B. Evolution of Strategies for Modern Rechargeable Batteries. Acc. Chem. Res. 2013, 46, 1053–1061. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, H.-S.; Hu, J.; Liu, H.; Wei, L.; Wu, S.; Huang, Y.; Li, J.; Tang, K. Construction of Hierarchical MnSe@SnSe2@N–C Nanorods for High-Performance Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 6586–6596. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.; Wu, C.; Wen, Y.; Yin, K.; Chiang, F.-K.; Hu, R.; Liu, J.; Sun, L.; Gu, L.; et al. New Nanoconfined Galvanic Replacement Synthesis of Hollow Sb@C Yolk–Shell Spheres Constituting a Stable Anode for High-Rate Li/Na-Ion Batteries. Nano Lett. 2017, 17, 2034–2042. [Google Scholar] [CrossRef]
- Li, X.; Dhanabalan, A.; Gu, L.; Wang, C. Three-Dimensional Porous Core-Shell Sn@Carbon Composite Anodes for High-Performance Lithium-Ion Battery Applications. Adv. Energy Mater. 2012, 2, 238–244. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Xu, X.; Zhang, L.; Hu, R.; Liu, J.; Yang, L.; Zhu, M. Metal–Organic Framework-Derived NiSb Alloy Embedded in Carbon Hollow Spheres as Superior Lithium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2017, 9, 2516–2525. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, R.; Liu, H.; Sun, W.; Lu, Z.; Liu, J.; Yang, L.; Zhang, Y.; Zhu, M. A Spherical Sn–Fe3O4@graphite Composite as a Long-Life and High-Rate-Capability Anode for Lithium Ion Batteries. J. Mater. Chem. A 2016, 4, 10321–10328. [Google Scholar] [CrossRef]
- Hao, S.; Zhang, B.; Wang, Y.; Li, C.; Feng, J.; Ball, S.; Srinivasan, M.; Wu, J.; Huang, Y. Hierarchical Three-Dimensional Fe3O4@porous Carbon Matrix/Graphene Anodes for High Performance Lithium Ion Batteries. Electrochim. Acta 2018, 260, 965–973. [Google Scholar] [CrossRef]
- Marzuki, N.S.; Taib, N.U.; Hassan, M.F.; Idris, N.H. Enhanced Lithium Storage in Co3O4/Carbon Anode for Li-Ion Batteries. Electrochim. Acta 2015, 182, 452–457. [Google Scholar] [CrossRef]
- Kim, W.-S.; Hwa, Y.; Kim, H.-C.; Choi, J.-H.; Sohn, H.-J.; Hong, S.-H. SnO2@Co3O4 Hollow Nano-Spheres for a Li-Ion Battery Anode with Extraordinary Performance. Nano Res. 2014, 7, 1128–1136. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Yi, R.; Li, Z.; Chen, Y.; Li, Y.; Mitrovic, I.; Taylor, S.; Chalker, P.; Yang, L.; et al. Facile Preparation of Co3O4 Nanoparticles Incorporating with Highly Conductive MXene Nanosheets as High-Performance Anodes for Lithium-Ion Batteries. Electrochim. Acta 2020, 345, 136203. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Torelli, M.; Ren, C.E.; Ghidiu, M.; Ling, Z.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. 2D Titanium Carbide and Transition Metal Oxides Hybrid Electrodes for Li-Ion Storage. Nano Energy 2016, 30, 603–613. [Google Scholar] [CrossRef]
- Li, L.; Kovalchuk, A.; Fei, H.; Peng, Z.; Li, Y.; Kim, N.D.; Xiang, C.; Yang, Y.; Ruan, G.; Tour, J.M. Enhanced Cycling Stability of Lithium-Ion Batteries Using Graphene-Wrapped Fe3O4-Graphene Nanoribbons as Anode Materials. Adv. Energy Mater. 2015, 5, 1500171. [Google Scholar] [CrossRef]
- Ma, F.-X.; Hu, H.; Wu, H.B.; Xu, C.-Y.; Xu, Z.; Zhen, L.; David Lou, X.W. Formation of Uniform Fe3O4 Hollow Spheres Organized by Ultrathin Nanosheets and Their Excellent Lithium Storage Properties. Adv. Mater. 2015, 27, 4097–4101. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Li, B.; Wang, X.; Shi, Z.; Lu, J.; Wang, Z. MOF-Derived Fe3O4 Hierarchical Nanocomposites Encapsulated by Carbon Shells as High-Performance Anodes for Li-Storage Systems. J. Alloys Compd. 2021, 875, 159906. [Google Scholar] [CrossRef]
- Hu, X.; Ma, M.; Zeng, M.; Sun, Y.; Chen, L.; Xue, Y.; Zhang, T.; Ai, X.; Mendes, R.G.; Rümmeli, M.H.; et al. Supercritical Carbon Dioxide Anchored Fe3O4 Nanoparticles on Graphene Foam and Lithium Battery Performance. ACS Appl. Mater. Interfaces 2014, 6, 22527–22533. [Google Scholar] [CrossRef]
- Bulut Kopuklu, B.; Tasdemir, A.; Alkan Gursel, S.; Yurum, A. High Stability Graphene Oxide Aerogel Supported Ultrafine Fe3O4 Particles with Superior Performance as a Li-Ion Battery Anode. Carbon 2021, 174, 158–172. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Xie, Z.; An, C.; Jiang, G.; Wang, Y. 3D Graphene-Encapsulated Nearly Monodisperse Fe3O4 Nanoparticles as High-Performance Lithium-Ion Battery Anodes. J. Alloys Compd. 2020, 815, 152337. [Google Scholar] [CrossRef]
- Mao, J.; Niu, D.; Zheng, N.; Jiang, G.; Zhao, W.; Shi, J.; Li, Y. Fe3O4 -Embedded and N-Doped Hierarchically Porous Carbon Nanospheres as High-Performance Lithium Ion Battery Anodes. ACS Sustain. Chem. Eng. 2019, 7, 3424–3433. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, K.; Wang, W.; Zuo, X.; Yang, Q.; Tang, H.; Wu, M.; Li, G. Remarkable Enhancement in the Photoelectric Performance of Uniform Flower-like Mesoporous Fe3O4 Wrapped in Nitrogen-Doped Graphene Networks. ACS Appl. Mater. Interfaces 2018, 10, 19564–19572. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, D.; Yang, Y.; Wang, Y.; Bai, Z.; Chu, P.K.; Luo, Y. MXene-Encapsulated Hollow Fe3O4 Nanochains Embedded in N-Doped Carbon Nanofibers with Dual Electronic Pathways as Flexible Anodes for High-Performance Li-Ion Batteries. Nanoscale 2021, 13, 4624–4633. [Google Scholar] [CrossRef]
- Liu, J.; Xu, X.; Hu, R.; Yang, L.; Zhu, M. Uniform Hierarchical Fe3O4 @Polypyrrole Nanocages for Superior Lithium Ion Battery Anodes. Adv. Energy Mater. 2016, 6, 1600256. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Li, Y.; Zhao, Y.; Tian, Y.; Kurmanbayeva, I.; Bakenov, Z. Hierarchical Sandwiched Fe3O4@C/Graphene Composite as Anode Material for Lithium-Ion Batteries. J. Electroanal. Chem. 2019, 847, 113240. [Google Scholar] [CrossRef]
- Yan, Z.; Jiang, X.; Dai, Y.; Xiao, W.; Li, X.; Du, N.; He, G. Pulverization Control by Confining Fe3O4 Nanoparticles Individually into Macropores of Hollow Carbon Spheres for High-Performance Li-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 2581–2590. [Google Scholar] [CrossRef]
- Li, M.; Du, H.; Kuai, L.; Huang, K.; Xia, Y.; Geng, B. Scalable Dry Production Process of a Superior 3D Net-Like Carbon-Based Iron Oxide Anode Material for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2017, 56, 12649–12653. [Google Scholar] [CrossRef]
- Park, G.D.; Hong, J.H.; Jung, D.S.; Lee, J.-H.; Kang, Y.C. Unique Structured Microspheres with Multishells Comprising Graphitic Carbon-Coated Fe3O4 Hollow Nanopowders as Anode Materials for High-Performance Li-Ion Batteries. J. Mater. Chem. A 2019, 7, 15766–15773. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Pal, A.; Ning, Y.; Wang, Z.; Zhao, B.; Bradley, R.; Wu, W. Ultra-Small Fe3O4 Nanoparticles Encapsulated in Hollow Porous Carbon Nanocapsules for High Performance Supercapacitors. Carbon 2021, 179, 327–336. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, J.; Qi, J.; Li, Z.; Wang, C.; Chen, M. N-Doped Dual Carbon-Confined 3D Architecture RGO/Fe3O4/AC Nanocomposite for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 13470–13478. [Google Scholar] [CrossRef]
- Wei, W.; Yang, S.; Zhou, H.; Lieberwirth, I.; Feng, X.; Müllen, K. 3D Graphene Foams Cross-Linked with Pre-Encapsulated Fe3O4 Nanospheres for Enhanced Lithium Storage. Adv. Mater. 2013, 25, 2909–2914. [Google Scholar] [CrossRef]
- Liu, H.; Jia, M.; Zhu, Q.; Cao, B.; Chen, R.; Wang, Y.; Wu, F.; Xu, B. 3D-0D Graphene-Fe3O4 Quantum Dot Hybrids as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 26878–26885. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, H.; Wu, J.; Chu, X.; He, Y.-B.; Han, C.; Miao, C.; Wang, S.; Li, B.; Kang, F. Fe3O4 Nanoparticles Encapsulated in Electrospun Porous Carbon Fibers with a Compact Shell as High-Performance Anode for Lithium Ion Batteries. Carbon 2015, 87, 347–356. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, B.; Zheng, J.; Hu, J.; Wen, J.; Miller, D.J.; Yan, P.; Liu, T.; Guo, H.; Li, W.; et al. Excess Li-Ion Storage on Reconstructed Surfaces of Nanocrystals To Boost Battery Performance. Nano Lett. 2017, 17, 6018–6026. [Google Scholar] [CrossRef]
- Jiao, J.; Qiu, W.; Tang, J.; Chen, L.; Jing, L. Synthesis of Well-Defined Fe3O4 Nanorods/N-Doped Graphene for Lithium-Ion Batteries. Nano Res. 2016, 9, 1256–1266. [Google Scholar] [CrossRef]
- Xiao, Z.; Xia, Y.; Ren, Z.; Liu, Z.; Xu, G.; Chao, C.; Li, X.; Shen, G.; Han, G. Facile Synthesis of Single-Crystalline Mesoporous α-Fe2O3 and Fe3O4 Nanorods as Anode Materials for Lithium-Ion Batteries. J. Mater. Chem. 2012, 22, 20566. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Park, J.J.; Housel, L.M.; Minnici, K.; Zhang, G.; Lee, S.R.; Lee, S.W.; Chen, Z.; Noda, S.; Takeuchi, E.S.; et al. Carbon Nanotube Web with Carboxylated Polythiophene “Assist” for High-Performance Battery Electrodes. ACS Nano 2018, 12, 3126–3139. [Google Scholar] [CrossRef]
- Gao, T.; Xu, C.; Li, R.; Zhang, R.; Wang, B.; Jiang, X.; Hu, M.; Bando, Y.; Kong, D.; Dai, P.; et al. Biomass-Derived Carbon Paper to Sandwich Magnetite Anode for Long-Life Li-Ion Battery. ACS Nano 2019, 13, 11901–11911. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Liu, H.; Xiong, Z.; Zhao, L.; Liu, S.; Huang, C.; Zhao, Y. Cornlike Ordered N-Doped Carbon Coated Hollow Fe3O4 by Magnetic Self-Assembly for the Application of Li-Ion Battery. Chem. Eng. J. 2019, 356, 746–755. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, X.; Liu, X.; Wang, G.; Wang, H.; Bai, J. Rational Design of Fe3O4@C Yolk-Shell Nanorods Constituting a Stable Anode for High-Performance Li/Na-Ion Batteries. J. Colloid Interface Sci. 2018, 528, 225–236. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Liu, Z.; Shen, J.; Hu, R.; Liu, J.; Ouyang, L.; Zhang, L.; Zhu, M. Robust Pitaya-Structured Pyrite as High Energy Density Cathode for High-Rate Lithium Batteries. ACS Nano 2017, 11, 9033–9040. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.-Y.; Paik, U. Etching-in-a-Box: A Novel Strategy to Synthesize Unique Yolk-Shelled Fe3O4 @Carbon with an Ultralong Cycling Life for Lithium Storage. Adv. Energy Mater. 2016, 6, 1502318. [Google Scholar] [CrossRef]
- Guo, W.; Sun, W.; Lv, L.-P.; Kong, S.; Wang, Y. Microwave-Assisted Morphology Evolution of Fe-Based Metal–Organic Frameworks and Their Derived Fe2O3 Nanostructures for Li-Ion Storage. ACS Nano 2017, 11, 4198–4205. [Google Scholar] [CrossRef]
- Du, J.; Tang, Y.; Wang, Y.; Shi, P.; Fan, J.; Xu, Q.; Min, Y. A MOF-Derived Method to Construct Well-Arranged Porous Nanosheets for Lithium Ion Batteries. Dalton Trans. 2018, 47, 7571–7577. [Google Scholar] [CrossRef]
- Dai, R.; Sun, W.; Wang, Y. Ultrasmall Tin Nanodots Embedded in Nitrogen-Doped Mesoporous Carbon: Metal-Organic-Framework Derivation and Electrochemical Application as Highly Stable Anode for Lithium Ion Batteries. Electrochim. Acta 2016, 217, 123–131. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Zhao, H.; Wang, Z.; Zhu, B.; Zhang, X.; He, P.; Liu, Y.; Yang, T. MOF-Derived Long Spindle-like Carbon-Coated Ternary Transition-Metal-Oxide Composite for Lithium Storage. ACS Omega 2022, 7, 16837–16846. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy in carbons: From nanotubes to diamond-Preface. Philos. Trans. R. Soc. Lond. Ser. Math. Phys. Eng. Sci. 2004, 362, 2269–2270. [Google Scholar] [CrossRef]
- Lv, L.-P.; Du, P.; Liu, P.; Li, X.; Wang, Y. Integrating Mixed Metallic Selenides/Nitrogen-Doped Carbon Heterostructures in One-Dimensional Carbon Fibers for Efficient Oxygen Reduction Electrocatalysis. ACS Sustain. Chem. Eng. 2020, 8, 8391–8401. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, S.; Chen, Z.; Xu, A.; Zhan, S.; Wu, S. Self-Formed Channel Boosts Ultrafast Lithium Ion Storage in Fe3O4@Nitrogen-Doped Carbon Nanocapsule. ACS Appl. Mater. Interfaces 2020, 12, 527–537. [Google Scholar] [CrossRef]
- Xie, Y.; Qiu, Y.; Tian, L.; Liu, T.; Su, X. Ultrafine Hollow Fe3O4 Anode Material Modified with Reduced Graphene Oxides for High-Power Lithium-Ion Batteries. J. Alloys Compd. 2022, 894, 162384. [Google Scholar] [CrossRef]
- Pan, Q.; Ding, Y.; Yan, Z.; Cai, Y.; Zheng, F.; Huang, Y.; Wang, H.; Li, Q. Designed Synthesis of Fe3O4@NC Yolk-Shell Hollow Spheres as High Performance Anode Material for Lithium-Ion Batteries. J. Alloys Compd. 2020, 821, 153569. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, Y.; Sun, Z.; Bakenov, Z. Rational Design of MOFs-Derived Fe3O4@C Interwoven with Carbon Nanotubes as Sulfur Host for Advanced Lithium-sulfur Batteries. J. Electroanal. Chem. 2020, 877, 114608. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Vaz, A.R.; Savu, R.; Moshkalev, S.A. Self-Assembled and One-Step Synthesis of Interconnected 3D Network of Fe3O4/Reduced Graphene Oxide Nanosheets Hybrid for High-Performance Supercapacitor Electrode. ACS Appl. Mater. Interfaces 2017, 9, 8880–8890. [Google Scholar] [CrossRef]
- Yi, Q.; Du, M.; Shen, B.; Ji, J.; Dong, C.; Xing, M.; Zhang, J. Hollow Fe3O4/Carbon with Surface Mesopores Derived from MOFs for Enhanced Lithium Storage Performance. Sci. Bull. 2020, 65, 233–242. [Google Scholar] [CrossRef]
- Ma, T.; Liu, X.; Sun, L.; Xu, Y.; Zheng, L.; Zhang, J. Strongly Coupled N-Doped Carbon/Fe3O4/N-Doped Carbon Hierarchical Micro/Nanostructures for Enhanced Lithium Storage Performance. J. Energy Chem. 2019, 34, 43–51. [Google Scholar] [CrossRef]
- Cong, B.; Hu, Y.; Sun, S.; Wang, Y.; Wang, B.; Kong, H.; Chen, G. Metal–Organic Framework Derived Amorphous VOx Coated Fe3O4/C Hierarchical Nanospindle as Anode Material for Superior Lithium-Ion Batteries. Nanoscale 2020, 12, 16901–16909. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Q.; Cui, C.; Ren, H.; Lu, X.; Hong, Y.; Woo Joo, S. Cake-like Porous Fe3O4@C Nanocomposite as High-Performance Anode for Li-Ion Battery. J. Electroanal. Chem. 2022, 918, 116508. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. EIS Study on the Formation of Solid Electrolyte Interface in Li-Ion Battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Moss, P.L.; Au, G.; Plichta, E.J.; Zheng, J.P. Investigation of Solid Electrolyte Interfacial Layer Development during Continuous Cycling Using Ac Impedance Spectra and Micro-Structural Analysis. J. Power Sources 2009, 189, 66–71. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, M.; Zhang, J.; Liu, S.; Zhang, N.; Yao, W.; Ye, Y.; Luo, C.; Gong, Z.; Wang, C. Dual-defect surface engineering of bimetallic sulfide nanotubes towards flexible asymmetric solid-state supercapacitors. J. Mater. Chem. A 2020, 8, 24053–24064. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jung, E.; Lee, S.; Jun, S.C. Structural engineering and surface modification of MOF-derived cobalt-based hybrid nanosheets for flexible solid-state supercapacitors. Energy Storage Mater. 2020, 32, 167–177. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Huang, W.; Wei, Y.; Yang, X.; Jiang, C.; Liu, H.; Zhang, W.; Liang, C.; Dai, L.; Xu, X. Facile Constructing Hierarchical Fe3O4@C Nanocomposites as Anode for Superior Lithium-Ion Storage. Batteries 2023, 9, 403. https://doi.org/10.3390/batteries9080403
Zhong H, Huang W, Wei Y, Yang X, Jiang C, Liu H, Zhang W, Liang C, Dai L, Xu X. Facile Constructing Hierarchical Fe3O4@C Nanocomposites as Anode for Superior Lithium-Ion Storage. Batteries. 2023; 9(8):403. https://doi.org/10.3390/batteries9080403
Chicago/Turabian StyleZhong, Haichang, Wenlong Huang, Yukun Wei, Xin Yang, Chunhai Jiang, Hui Liu, Wenxian Zhang, Chu Liang, Leyang Dai, and Xijun Xu. 2023. "Facile Constructing Hierarchical Fe3O4@C Nanocomposites as Anode for Superior Lithium-Ion Storage" Batteries 9, no. 8: 403. https://doi.org/10.3390/batteries9080403
APA StyleZhong, H., Huang, W., Wei, Y., Yang, X., Jiang, C., Liu, H., Zhang, W., Liang, C., Dai, L., & Xu, X. (2023). Facile Constructing Hierarchical Fe3O4@C Nanocomposites as Anode for Superior Lithium-Ion Storage. Batteries, 9(8), 403. https://doi.org/10.3390/batteries9080403








