Rational Design of Bismuth Metal Anodes for Sodium-/Potassium-Ion Batteries: Recent Advances and Perspectives
Abstract
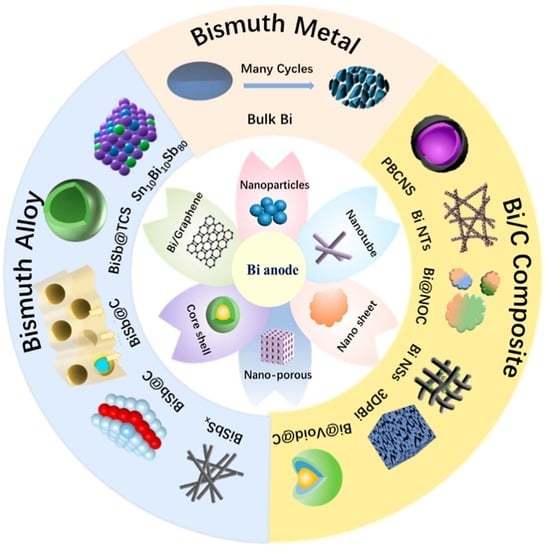
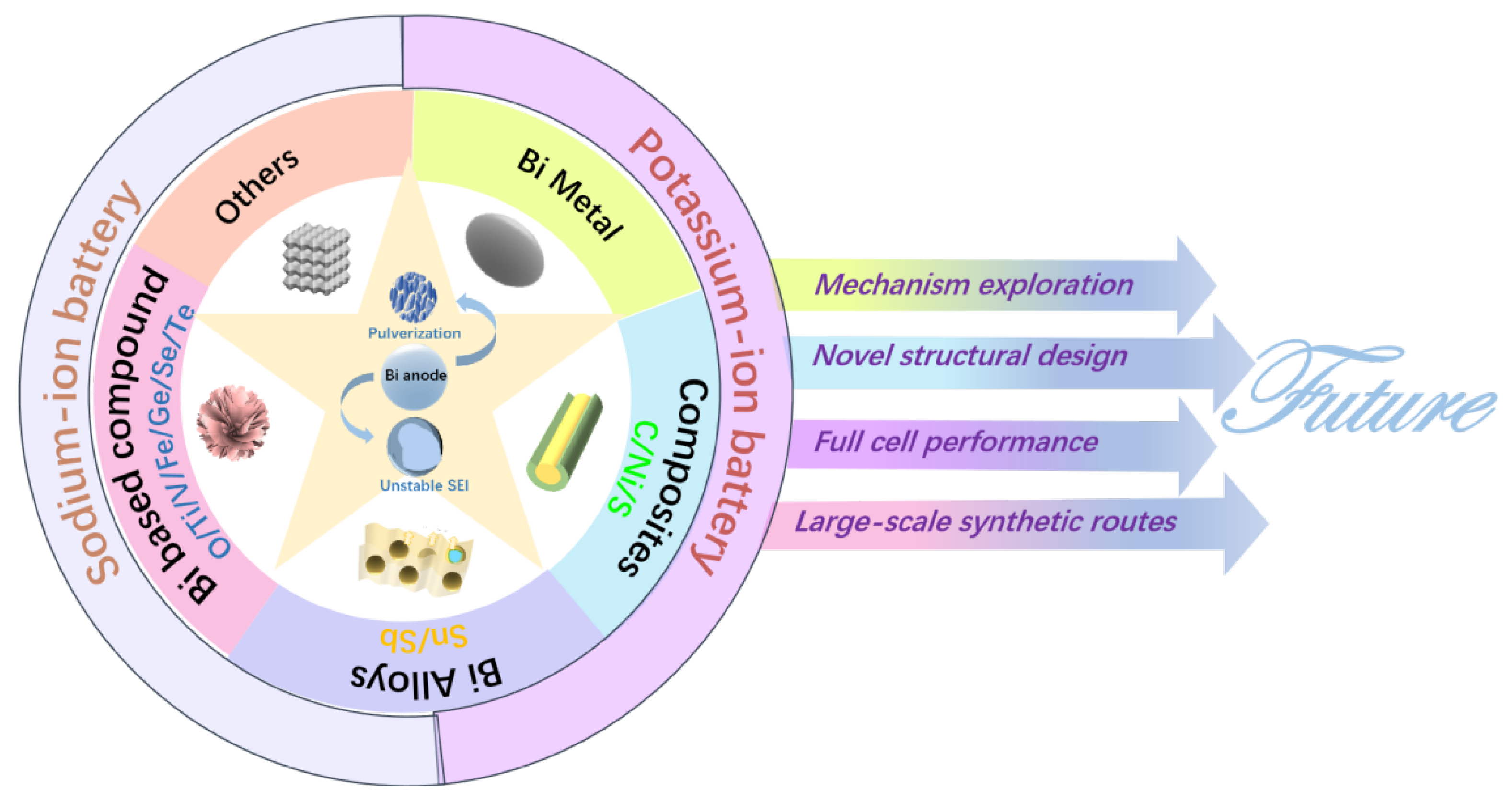
:1. Introduction
2. Bismuth Metal
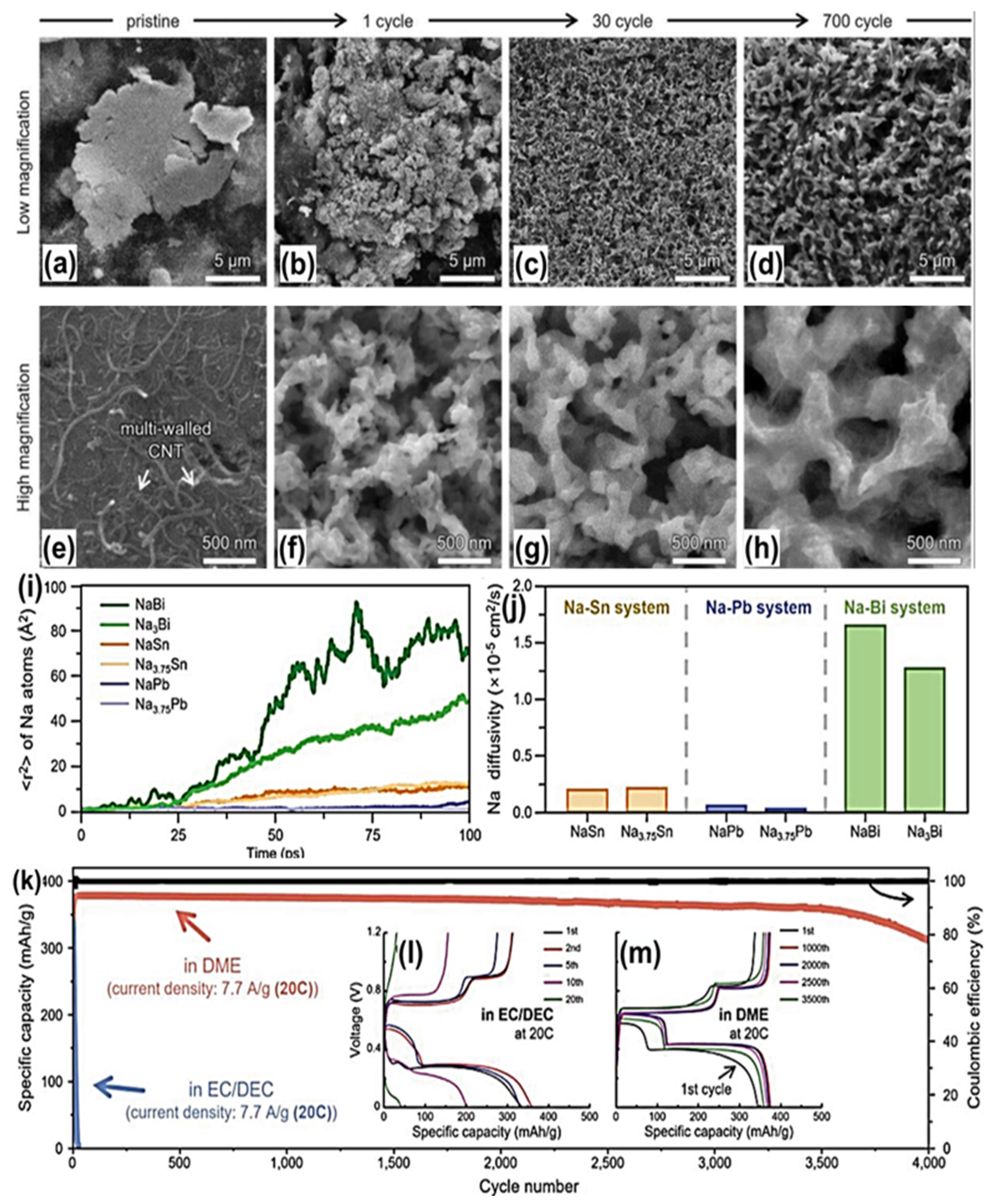
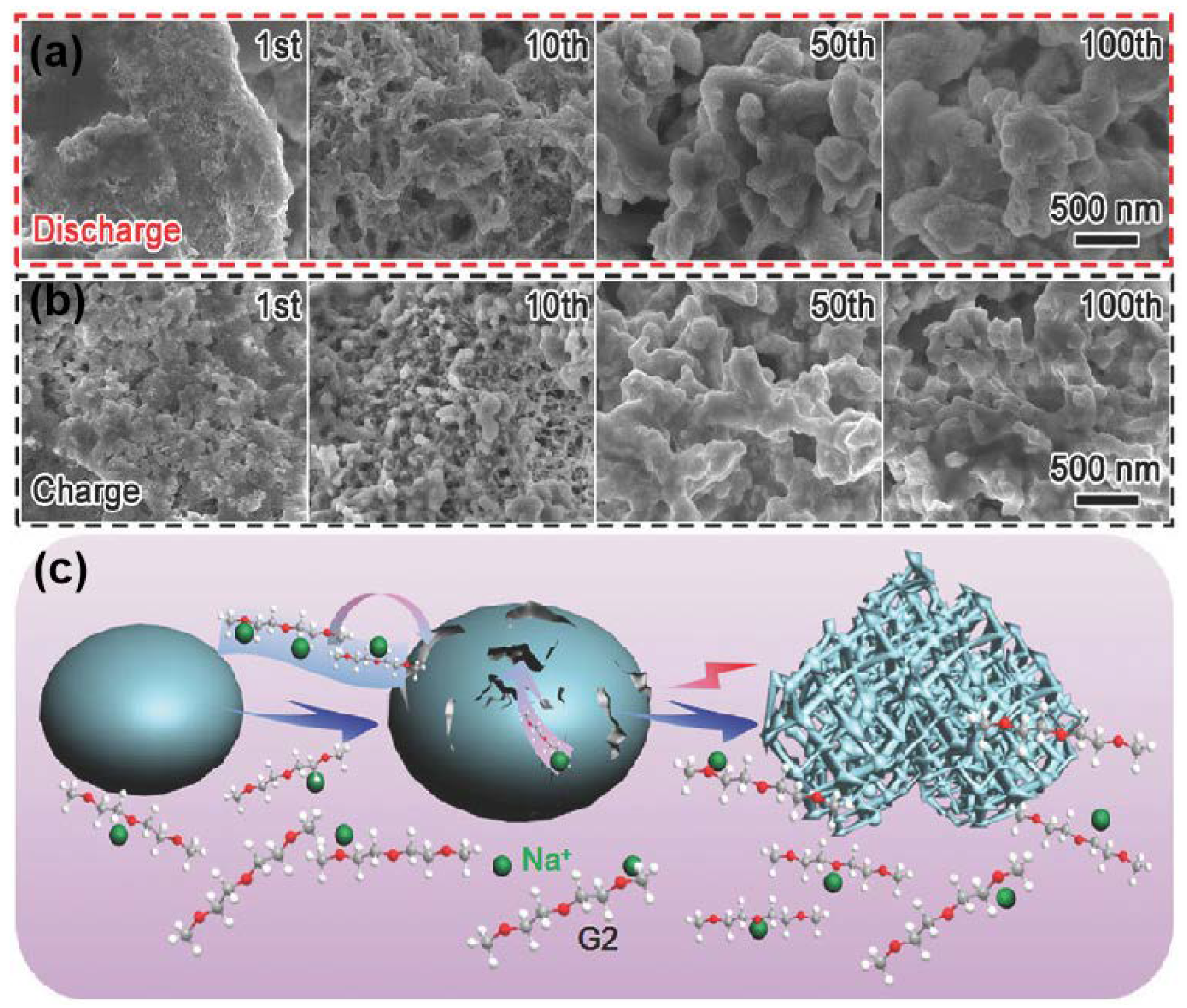
2.1. Bulk Bismuth
2.2. Nanostructured Bismuth
3. Bi/C Composite
3.1. Zero-Dimensional Bi/C Composite
3.1.1. Hollow Structure
3.1.2. Yolk–Shell/Core–Shell Structure
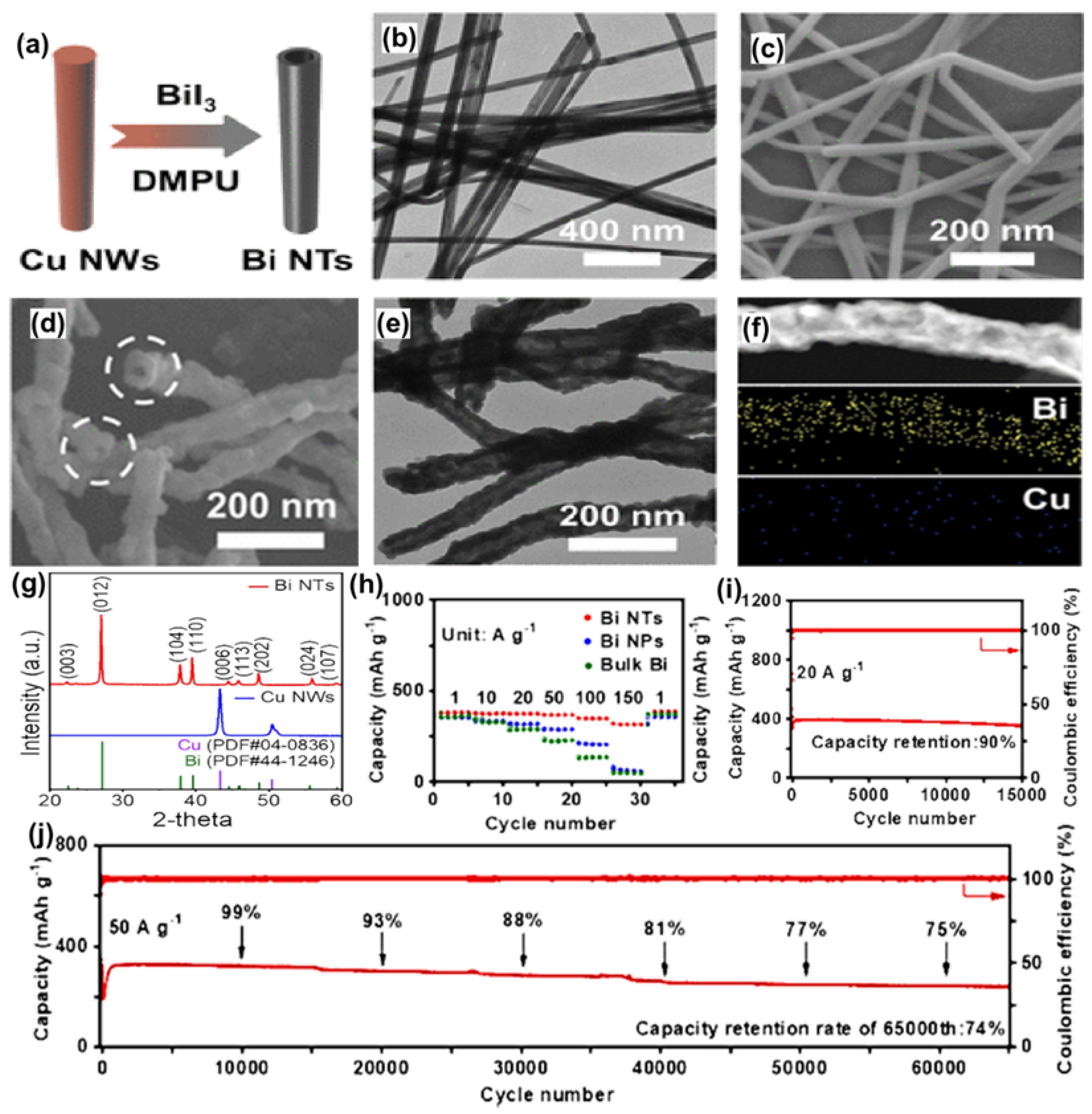
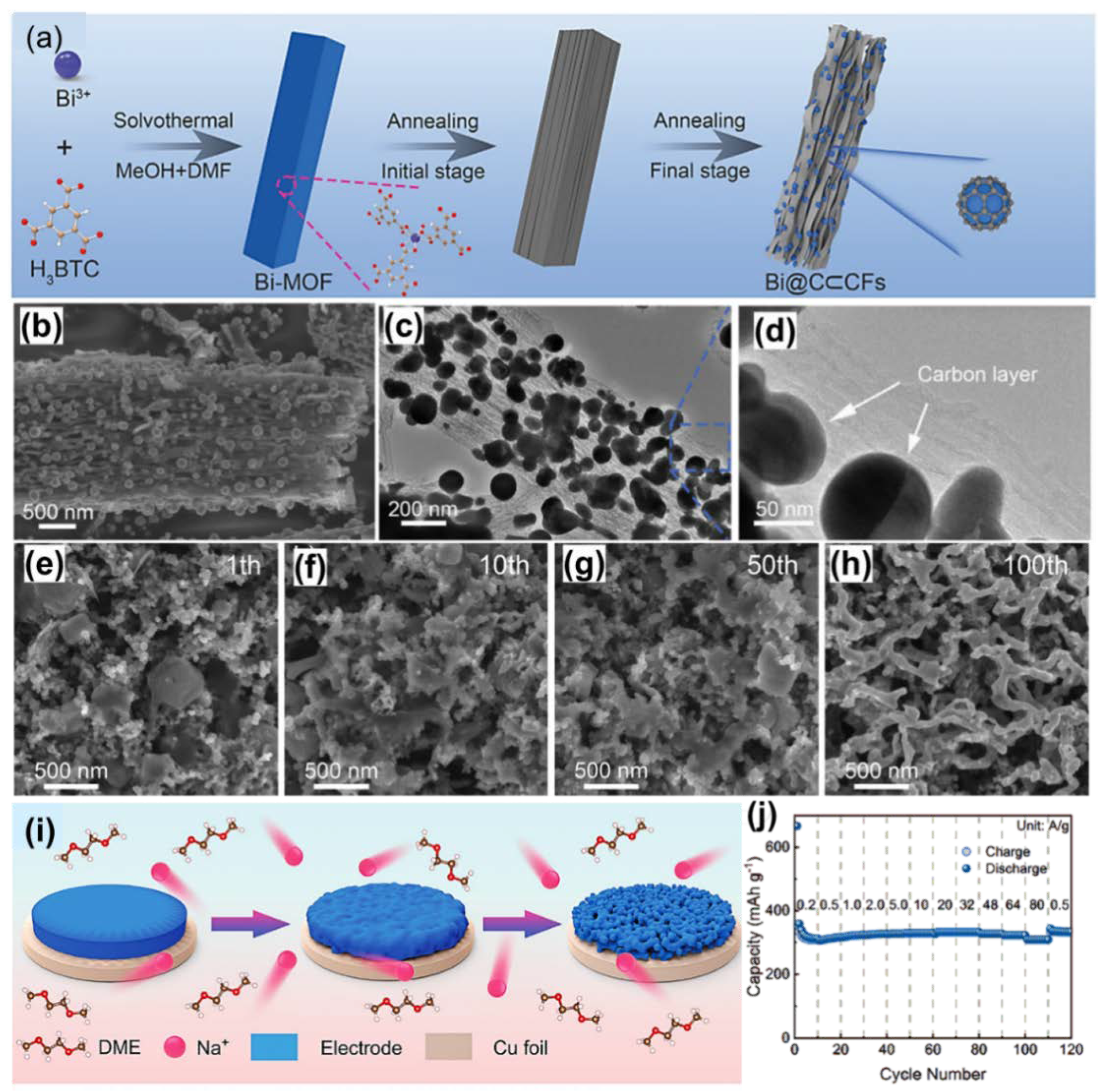
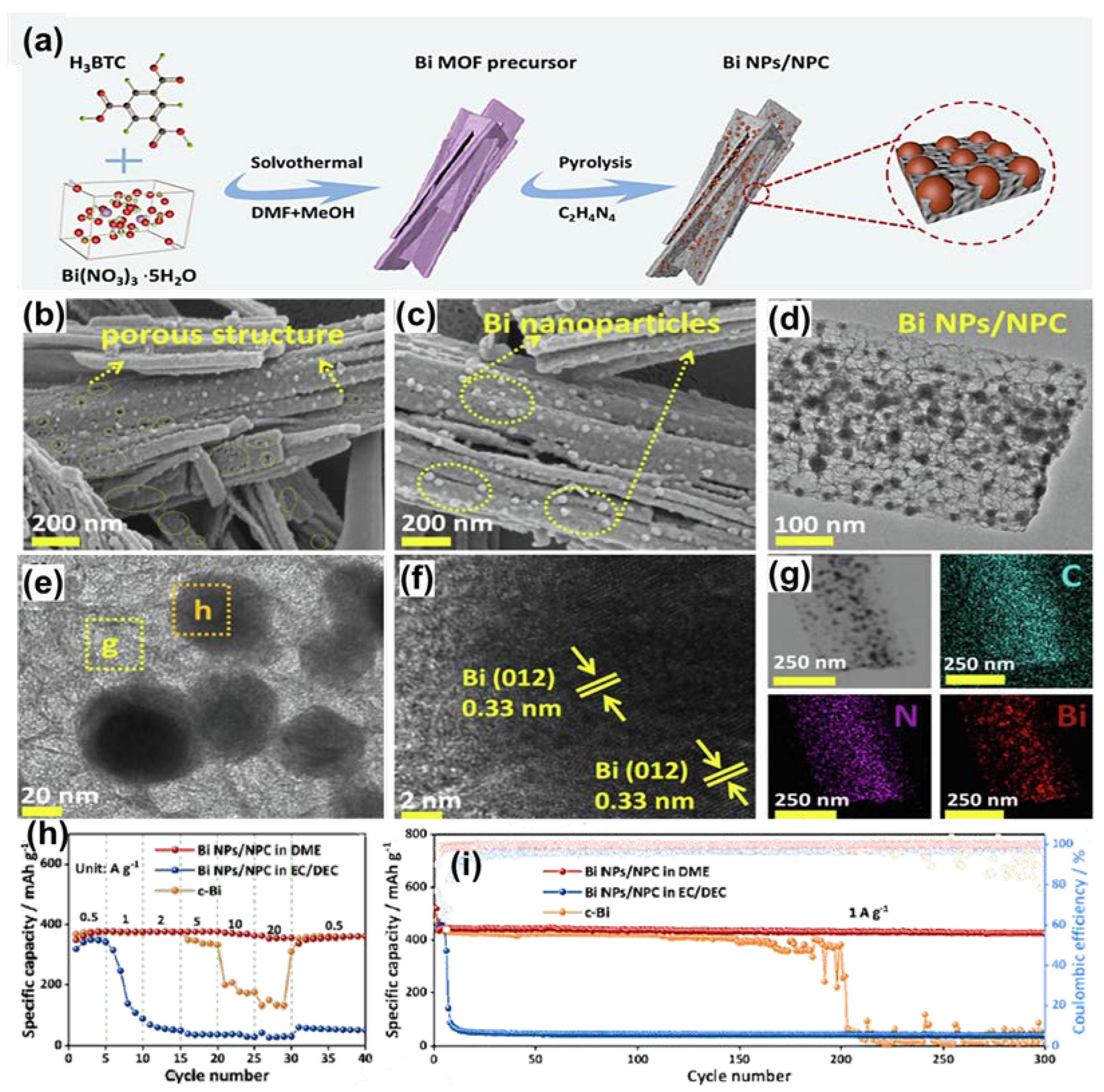
3.2. One-Dimensional Bi/C Composite
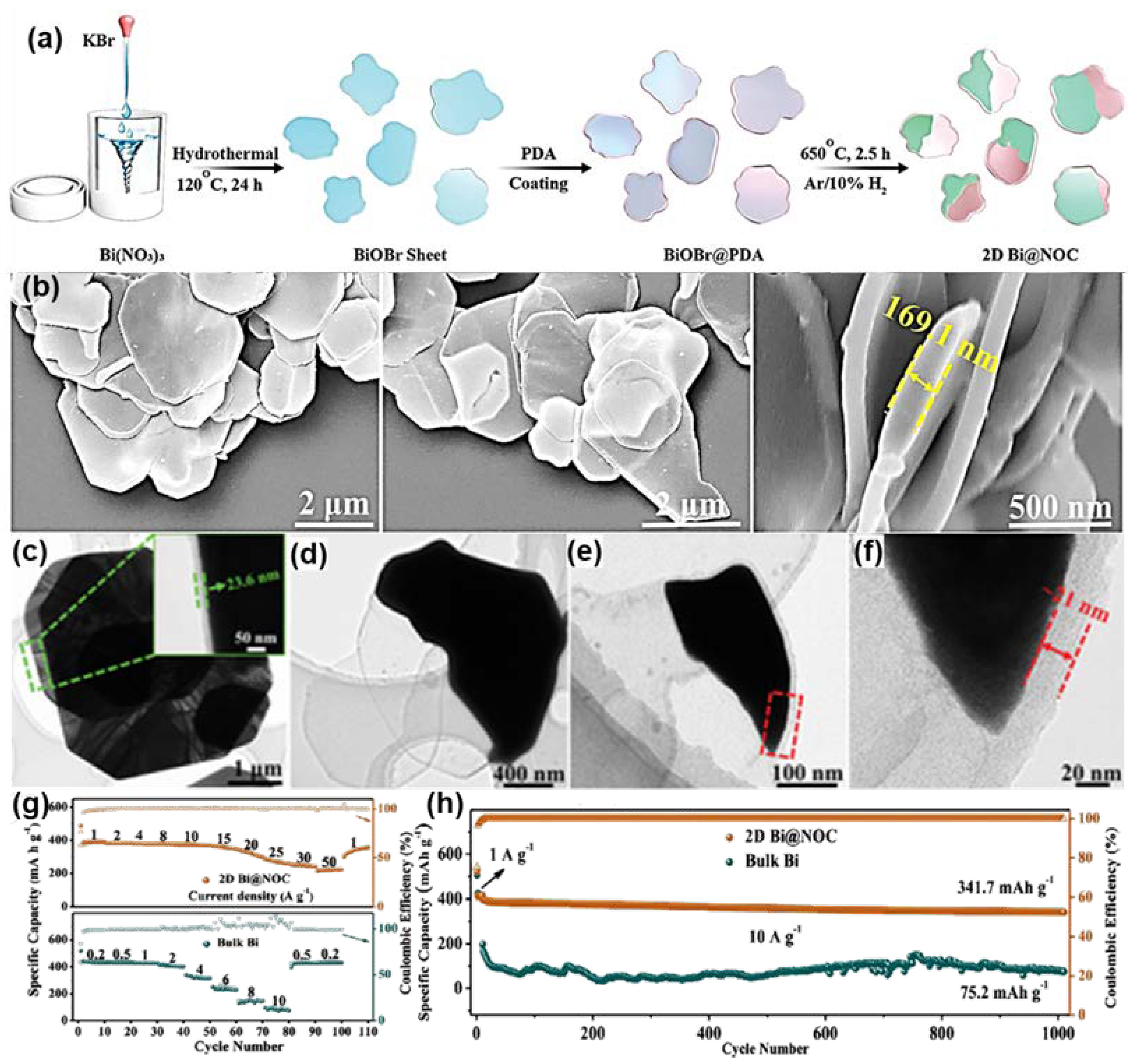
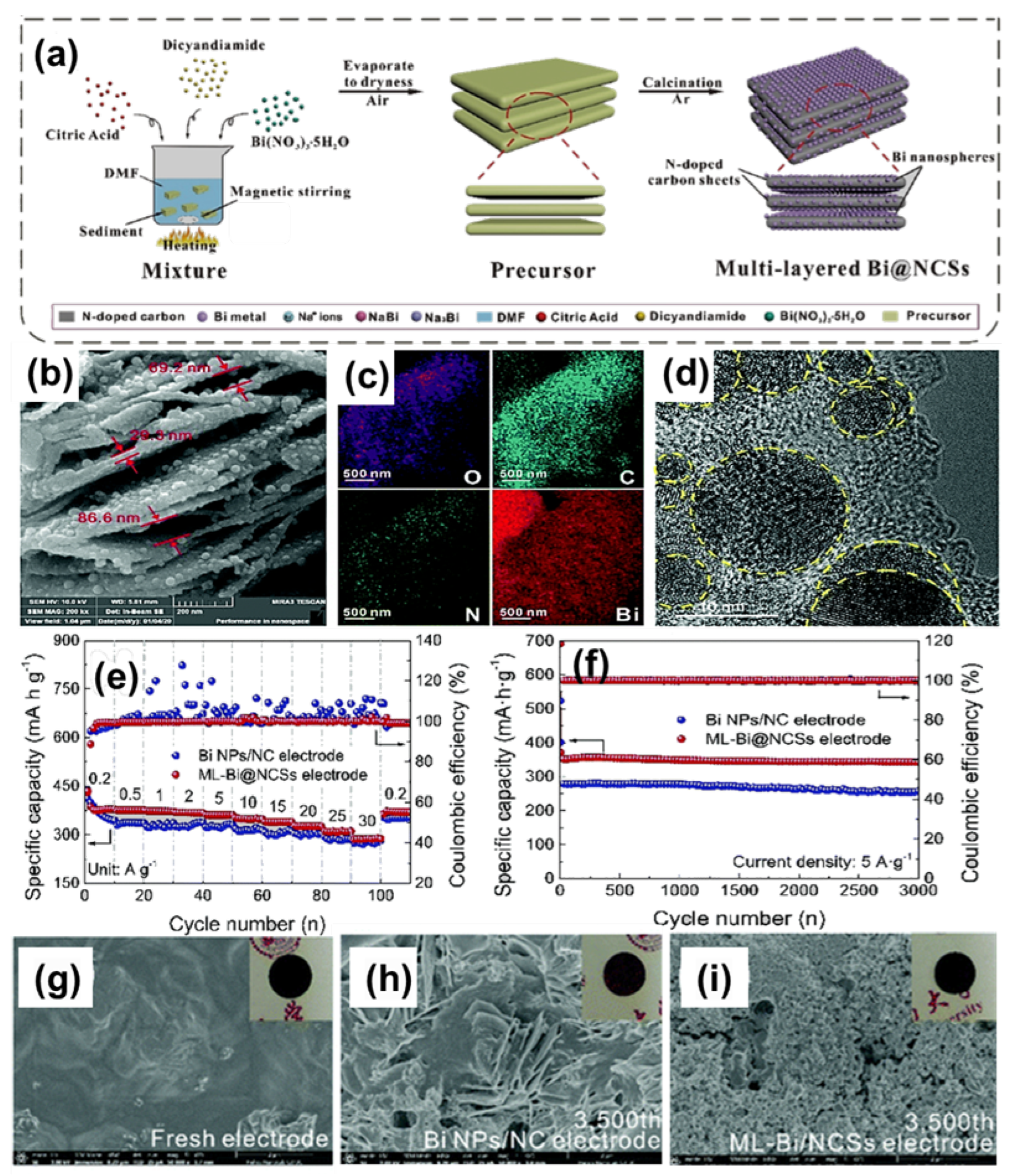
3.3. Two-Dimensional Bi/C Composite
3.4. Three-Dimensional Bi/C Composite
4. Bismuth Alloys
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhou, X.; Ou, X.; Lee, C.; Zhou, J.; Tang, Y. Ultrahigh Nitrogen Doping of Carbon Nanosheets for High Capacity and Long Cycling Potassium Ion Storage. Adv. Energy Mater. 2019, 9, 1902672. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Wang, Z.; Zhao, L.; Li, P.; Yang, Y.; Yang, C.; Huang, H.; Guo, S. Short-Range Order in Mesoporous Carbon Boosts Potassium-Ion Battery Performance. Adv. Energy Mater. 2018, 8, 1701648. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Guo, Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, 7412. [Google Scholar] [CrossRef]
- Ji, B.; Yao, W.; Zheng, Y.; Kidkhunthod, P.; Zhou, X.; Tunmee, S.; Sattayaporn, S.; Cheng, H.; He, H.; Tang, Y. A fluoroxalate cathode material for potassium-ion batteries with ultra-long cyclability. Nat. Commun. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Wang, D.; Gao, X.; Chen, Y.; Jin, L.; Kuss, C.; Bruce, P. Plating and stripping calcium in an organic electrolyte. Nat. Mater. 2018, 17, 16–20. [Google Scholar] [CrossRef]
- Nayak, P.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Eftekhari, A.; Jian, Z.; Ji, X. Potassium Secondary Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4404–4419. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602. [Google Scholar] [CrossRef]
- Lim, C.; Selvaraj, B.; Song, Y.; Wang, C.; Jin, J.; Huang, S.; Chuang, C.; Sheu, H.; Liao, Y.; Wu, N. Insight into microstructural and phase transformations in electrochemical sodiation–desodiation of a bismuth particulate anode. J. Mater. Chem. A 2017, 5, 21536. [Google Scholar] [CrossRef]
- Ge, X.; Liu, S.; Qiao, M.; Du, Y.; Li, Y.; Bao, J.; Zhou, X. Enabling Superior Electrochemical Properties for Highly Efficient Potassium Storage by Impregnating Ultrafine Sb Nanocrystals within Nanochannel-Containing Carbon Nanofibers. Angew. Chem. Int. Ed. 2019, 58, 14578–14583. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Lv, W.; Li, J.; Zhou, G.; Zhao, Y.; Fan, S.; Liu, B.; Li, B.; Kang, F.; Yang, Q. Twinborn TiO2–TiN heterostructures enabling smooth trapping–diffusion–conversion of polysulfides towards ultralong life lithium–sulfur batteries. Energy Environ. Sci. 2017, 10, 1694–1703. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, Y.; Li, D.; Yang, H.; Chen, F.; Huang, F.; Jiang, Y.; Wu, Y.; An, X.; Yu, Y. From 0D to 3D: Dimensional Control of Bismuth for Potassium Storage with Superb Kinetics and Cycling Stability. Adv. Energy Mater. 2021, 11, 2102263. [Google Scholar] [CrossRef]
- Huang, J.; Lin, X.; Tan, H.; Zhang, B. Bismuth Microparticles as Advanced Anodes for Potassium-Ion Battery. Adv. Energy Mater. 2018, 8, 1703496. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Tang, Y.; Ji, X.; Jia, C.; Wang, H. Advancements and Challenges in Potassium Ion Batteries: A Comprehensive Review. Adv. Funct. Mater. 2020, 30, 1909486. [Google Scholar] [CrossRef]
- Xue, P.; Wang, N.; Fang, Z.; Lu, Z.; Xu, X.; Wang, L.; Du, Y.; Ren, X.; Bai, Z.; Dou, S.; et al. Rayleigh-Instability-Induced Bismuth Nanorod@Nitrogen-Doped Carbon Nanotubes as A Long Cycling and High Rate Anode for Sodium-Ion Batteries. Nano Lett. 2019, 19, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xian, J.; Liu, Q.; Sun, Y.; Li, G. Bi nanoparticles in situ encapsulated by carbon film as high-performance anode materials for Li-ion batteries. J. Energy Chem. 2022, 69, 524–530. [Google Scholar] [CrossRef]
- Gao, H.; Niu, J.; Zhang, C.; Peng, Z.; Zhang, Z. A Dealloying Synthetic Strategy for Nanoporous Bismuth–Antimony Anodes for Sodium Ion Batteries. ACS Nano 2018, 12, 3568–3577. [Google Scholar] [CrossRef]
- Zhang, J.; Kim, G.; Park, M.; Zhang, J.; Lee, S.; Cui, Y.; Zhang, K.; Zou, F.; Kang, Y. Nanostructuring-Promoted Non-Equilibrium Phase Transformation of Bi Anodes toward Diffusion-Controlled Reaction for K-Ion Batteries. Adv. Energy Mater. 2022, 12, 2202446. [Google Scholar] [CrossRef]
- Kim, Y.; An, J.; Kim, S.; Li, X.; Song, E.; Park, J.; Chung, K.; Choi, Y.; Scanlon, D.O.; Ahn, H.; et al. Enabling 100C Fast-Charging Bulk Bi Anodes for Na-Ion Batteries. Adv. Mater. 2022, 34, 2201446. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Li, F.; Cheng, F.; Chen, J. Bulk Bismuth as a High-Capacity and Ultralong Cycle-Life Anode for Sodium-Ion Batteries by Coupling with Glyme-Based Electrolytes. Adv. Mater. 2017, 29, 1702212. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Wang, C.; Liu, L.; Luo, Y.; Mu, C.; Li, F.; Chen, J. A Porous Network of Bismuth Used as the Anode Material for High-Energy-Density Potassium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 4687–4691. [Google Scholar] [CrossRef] [PubMed]
- Pu, B.; Liu, Y.; Bai, J.; Chu, X.; Zhou, X.; Qing, Y.; Wang, Y.; Zhang, M.; Ma, Q.; Xu, Z.; et al. Iodine-Ion-Assisted Galvanic Replacement Synthesis of Bismuth Nanotubes for Ultrafast and Ultrastable Sodium Storage. ACS Nano 2022, 16, 18746–18756. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shao, R.; Li, D.; Yang, H.; Wu, Y.; Wang, B.; Sun, C.; Jiang, Y.; Zhang, Q.; Yu, Y. A Self-Healing Volume Variation Three-Dimensional Continuous Bulk Porous Bismuth for Ultrafast Sodium Storage. Adv. Funct. Mater. 2021, 31, 2011264. [Google Scholar] [CrossRef]
- Xiong, P.; Bai, P.; Li, A.; Li, B.; Cheng, M.; Chen, Y.; Huang, S.; Jiang, Q.; Bu, X.; Xu, Y. Bismuth Nanoparticle@Carbon Composite Anodes for Ultralong Cycle Life and High-Rate Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1904771. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; Lee, Y.; Byun, D.; Kim, H.; Choid, W. Synthesis of Bi2S3/C yolk-shell composite based on sulfur impregnation for efficient sodium storage. Chem. Eng. J. 2020, 383, 123094. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Li, G.; Huang, L.; Cao, M.; Wu, Y. Heterogeneous structured pomegranate-like Bi@C nanospheres for high-performance sodium storage. J. Mater. Chem. A 2020, 8, 25746–25755. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, W.; Cheng, D.; Zhang, H. One-step large-scale fabrication of Bi@N-doped carbon for ultrahigh-rate and long-life sodium-ion battery anodes. J. Mater. Sci. 2021, 56, 11000. [Google Scholar] [CrossRef]
- Nam, K.; Park, C. Bismuth and its nanocomposite: Reaction mechanism and rational nanocomposite fabrication process for superior sodium-ion battery anodes. Int. J. Energy Res. 2022, 46, 9486–9497. [Google Scholar] [CrossRef]
- Yang, F.; Yu, F.; Zhang, Z.; Zhang, K.; Lai, Y.; Li, J. Bismuth Nanoparticles Embedded in Carbon Spheres as Anode Materials for Sodium/Lithium-Ion Batteries. Chem. Eur. J. 2016, 22, 2333–2338. [Google Scholar] [CrossRef]
- Ma, H.; Wang, T.; Li, J.; Yang, J.; Liu, Z.; Wang, N.; Su, D.; Wang, C. Nitrogen Doped Carbon Coated Bi Microspheres as High-performance Anode for Half and Full Sodium Ion Batteries. Chem. Asian J. 2021, 16, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, X.; Wang, B.; Wang, G.; Wang, H. Bi@C Nanospheres with the Unique Petaloid Core–Shell Structure Anchored on Porous Graphene Nanosheets as an Anode for Stable Sodium- and Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 59867–59881. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, Z.; Zhang, J.; Shang, C.; Yang, M.; He, L.; Lu, Z. Hierarchical ball-in-ball structured nitrogen-doped carbon microspheres as high-performance anode for sodium-ion batteries. Energy Storage Mater. 2017, 7, 229–235. [Google Scholar] [CrossRef]
- Sun, X.; Zeng, S.; Man, R.; Wang, L.; Zhang, B.; Tian, F.; Qian, Y.; Xu, L. Yolk–shell structured CoSe2/C nanospheres as multifunctional anode materials for both full/half sodium-ion and full/half potassium-ion batteries. Nanoscale 2021, 13, 10385–10392. [Google Scholar] [CrossRef]
- Gao, H.; Lee, J.; Lu, Q.; Kim, Y.; Shin, K.H.; Park, H.S.; Zhang, Z.; Lee, L.Y.S. Highly Stable Sb/C Anode for K+ and Na+ Energy Storage Enabled by Pulsed Laser Ablation and Polydopamine Coating. Small 2023, 19, 220568. [Google Scholar] [CrossRef]
- Yang, S.; Park, S.; Park, G.; Kim, J.; Kang, Y. Rational synthesis of uniform yolk–shell Ni–Fe bimetallic sulfide nanoflakes@porous carbon nanospheres as advanced anodes for high-performance potassium-/sodium-ion batteries. Chem. Eng. J. 2021, 417, 127963. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, C.; Yang, G.; Sha, M.; Dong, Y.; Fu, Q.; Wu, Y.; Zhao, H.; Wu, M.; Lei, Y. Bismuth Nanoparticles Confined in Carbonaceous Nanospheres as Anodes for High-Performance Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 31766–31774. [Google Scholar] [CrossRef]
- Yang, H.; Xu, R.; Yao, Y.; Ye, S.; Zhou, X.; Yu, Y. Multicore–Shell Bi@N-doped Carbon Nanospheres for High Power Density and Long Cycle Life Sodium- and Potassium-Ion Anodes. Adv. Funct. Mater. 2019, 29, 1809195. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Tong, Y.; Li, H. Unique Spindle-Like Bismuth-Based Composite toward Ultrafast Potassium Storage. Small 2022, 18, 2204045. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, H.; Kim, S.; Kim, T.; Choi, J.; Cho, J.; Kang, Y.; Park, G. Controllable Synthesis of Carbon Yolk-Shell Microsphere and Application of Metal Compound–Carbon Yolk-Shell as Effective Anode Material for Alkali-Ion Batteries. Small Methods 2023, 7, 2201370. [Google Scholar] [CrossRef]
- Wan, S.; Cheng, M.; Chen, H.; Zhu, H.; Liu, Q. Nanoconfined bimetallic sulfides (CoSn)S heterostructure in carbon microsphere as a high-performance anode for half/full sodium-ion batteries. J. Colloid Inter. Sci. 2022, 609, 403–413. [Google Scholar] [CrossRef]
- Gao, Z.; Han, L.; Gao, H.; Chen, J.; Sun, Z.; Zhu, C.; Zhang, Y.; Shi, J.; Chen, S.; Wang, H. Coupling core–shell Bi@Void@TiO2 heterostructures into carbon nanofibers for achieving fast potassium storage and long cycling stability. J. Mater. Chem. A 2022, 10, 12908–12920. [Google Scholar] [CrossRef]
- Yang, H.; Chen, L.; He, F.; Zhang, J.; Feng, Y.; Zhao, L.; Wang, B.; He, L.; Zhang, Q.; Yu, Y. Optimizing the Void Size of Yolk–Shell Bi@Void@C Nanospheres for High-Power-Density Sodium-Ion Batteries. Nano Lett. 2020, 20, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jiu, H.; Zhang, L.; Song, W.; Gao, T.; Guo, F.; Li, X.; Wei, H.; Wang, C.; Liu, Y.; et al. Rational Design of 1D Porous Carbon Microtubes Supporting Multi-size Bi2O3 Nanoparticles for Ultra-long Cycle Life Lithium-Ion Battery Anodes. ChemElectroChem 2022, 9, e202101321. [Google Scholar] [CrossRef]
- Kumar, P.; Wahyudi, W.; Sharma, A.; Yuan, Y.; Harrison, G.T.; Gedda, M.; Wei, X.; El-Labban, A.; Ahmad, S.; Kumar, V.; et al. Bismuth-based mixed-anion compounds for anode materials in rechargeable batteries. Chem. Commun. 2022, 58, 3354–3357. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Qin, Y.; Zhao, Z.; Xu, Y.; Yi, Y.; Guan, H.; Fu, Y.; Liu, P.; Li, D. Sb2S3-Bi2S3 microrods with the combined action of carbon encapsulation and rGO confinement for improving high cycle stability in sodium/potassium storage. Chem. Eng. J. 2021, 414, 128787. [Google Scholar] [CrossRef]
- Xu, C.; Tian, Y.; Sun, J.; Li, M.; Song, W.; You, J.; Feng, M.; Wang, X.; Wang, P.; Li, H.; et al. Novel Preoxidation-Assisted Mechanism to Preciously Form and Disperse Bi2O3 Nanodots in Carbon Nanofibers for Ultralong-Life and High-Rate Sodium Storage. ACS Appl. Mater. Interfaces 2023, 15, 1891–1902. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, B.; Chen, M.; Wang, L.; Wang, D.; Man, R.; Iqbal, S.; Tian, F.; Qian, Y.; Xu, L. Space-confined growth of Bi2Se3 nanosheets encapsulated in N-doped carbon shell lollipop-like composite for full/half potassium-ion and lithium-ion batteries. Nano Today 2022, 43, 101408. [Google Scholar] [CrossRef]
- Tong, H.; Chen, S.; Tu, J.; Zeng, X.; Wang, C.; Wang, P.; Chen, Q. Bi2O3 particles embedded in carbon matrix as high-performance anode materials for potassium ion batteries. J. Power Sources 2022, 549, 232140. [Google Scholar] [CrossRef]
- Hu, Z.; Li, X.; Qu, J.; Zhao, Z.; Xie, H.; Yin, H. Electrolytic bismuth/carbon nanotubes composites for high-performance sodium-ion battery anodes. J. Power Sources 2021, 496, 229830. [Google Scholar] [CrossRef]
- Li, W.; Zhou, M.; Li, H.; Wang, K.; Cheng, S.; Jiang, K. A high performance sulfur-doped disordered carbon anode for sodium ion batteries. Energy Environ. Sci. 2015, 8, 2916–2921. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Lu, Y.; Ge, Y.; Jiang, H.; Fu, K.; Zhang, X. Nitrogen-doped carbon nanofibers derived from polyacrylonitrile for use as anode material in sodium-ion batteries. Carbon 2015, 94, 189–195. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Wu, D.; Zhao, X.; Zhou, Z. S-Doped N-Rich Carbon Nanosheets with Expanded Interlayer Distance as Anode Materials for Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1604108. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Yin, Y.; Zou, Y.; Xia, Y.; An, Q.; Jian, Z.; Chen, W. N-Doped carbon coated bismuth nanorods with a hollow structure as an anode for superior-performance potassium-ion batteries. Nanoscale 2020, 12, 4309–4313. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Liu, D.; Zhua, X.; Fang, K.; Zhou, K.; Tanga, H.; Xie, Z.; Li, J.; Zhenga, H.; Qu, D. Evaporation-induced formation of hollow Bismuth@N-doped Carbon Nanorods for Enhanced Electrochemical Potassium Storage. Appl. Surf. Sci. 2020, 514, 145947. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Pan, Q.; Li, Y.; Wang, G.; Ou, X.; Zheng, F.; Xiong, X.; Liu, M.; Zhang, Q. Nitrogen-doped bamboo-like carbon nanotubes as anode material for high performance potassium ion batteries. J. Mater. Chem. A 2018, 6, 15162–15169. [Google Scholar] [CrossRef]
- Liang, Y.; Song, N.; Zhang, Z.; Chen, W.; Feng, J.; Xi, B.; Xiong, S. Integrating Bi@C Nanospheres in Porous Hard Carbon Frameworks for Ultrafast Sodium Storage. Adv. Mater. 2022, 34, 2202673. [Google Scholar] [CrossRef]
- Liang, H.; Su, Q.; Xu, J.; Yang, Z.; Li, S.; Wang, J. Preparation of anode by MOF pyrolysis enabled long-life rechargeable zinc nickel batteries. J. Alloys Compd. 2023, 949, 169870. [Google Scholar] [CrossRef]
- Su, S.; Liu, Q.; Wang, J.; Fan, L.; Ma, R.; Chen, S.; Han, X.; Lu, B. Control of SEI Formation for Stable Potassium-Ion Battery Anodes by Bi-MOF-Derived Nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 22474. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Ganesan, S.; Nguyen, M.T.; Yonezawa, T.; Praserthdam, S.; Pornprasertsuk, R.; Kheawhom, S. Critical roles of metal–organic frameworks in improving the Zn anode in aqueous zinc-ion batteries. Chem. Eng. J. 2023, 457, 141334. [Google Scholar] [CrossRef]
- Wang, A.; Hong, W.; Li, L.; Guo, R.; Xiang, Y.; Ye, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Bi3Se4 nanodots in porous carbon: A new anode candidate for fast lithium/sodium storage. Energy Storage Mater. 2022, 53, 1–12. [Google Scholar] [CrossRef]
- Xie, L.; Yang, Z.; Sun, J.; Zhou, H.; Chi, X.; Chen, H.; Li, A.X.; Yao, Y.; Chen, S. Bi2Se3/C Nanocomposite as a New Sodium-Ion Battery Anode Material. Nano-Micro Lett. 2018, 10, 50. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Guo, S.; Cai, B.; Yang, S.A.; Shan, F.; Pumer, M.; Zeng, H. Advances of 2D bismuth in energy sciences. Chem. Soc. Rev. 2020, 49, 263–285. [Google Scholar] [CrossRef]
- Shen, C.; Cheng, T.; Liu, C.; Huang, L.; Cao, M.; Song, G.; Wang, D.; Lu, B.; Wang, J.; Qin, C.; et al. Bismuthene from sonoelectrochemistry as a superior anode for potassium-ion batteries. J. Mater. Chem. A 2020, 8, 453–460. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, Z.; Song, T. Hierarchical iron sulfide-graphene nanocubes consisting of multiple nanoparticles with superior sodium ion storage properties. Electrochim. Acta 2018, 283, 683–690. [Google Scholar] [CrossRef]
- Xu, A.; Zhu, Q.; Li, G.; Gong, C.; Li, X.; Chen, H.; Cui, J.; Wu, S.; Xu, Z.; Yan, Y. 2D Bismuth@N-Doped Carbon Sheets for Ultrahigh Rate and Stable Potassium Storage. Small 2022, 18, 2203976. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Huang, P.; Chen, P.; Wang, Z.; Xu, X.; Xie, J.; Yan, J.; Li, S.; Tu, J.; et al. A multi-layered composite assembly of Bi nanospheres anchored on nitrogen-doped carbon nanosheets for ultrastable sodium storage. Nanoscale 2020, 12, 23682–23693. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, Y.; Ma, G.; Tian, F.; Zhou, Y.; Yang, J.; Qian, Y. Sandwich-structured dual carbon modified bismuth nanosphere composites as long-cycle and high-rate anode materials for sodium-ion batteries. Electrochim. Acta 2021, 365, 137379. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, X.; Xing, Z.; Zhu, D.; Tian, W.; Guan, C.; Yang, Y.; Qin, W.; Wang, J.; Zhang, L.; et al. In Situ Formation of Hierarchical Bismuth Nanodots/Graphene Nanoarchitectures for Ultrahigh-Rate and Durable Potassium-Ion Storage. Small 2020, 16, 1905789. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, F.; Peng, L.; Qin, T.; Kang, F.; Yang, C. Inlaying Bismuth Nanoparticles on Graphene Nanosheets by Chemical Bond for Ultralong-Lifespan Aqueous Sodium Storage. Angew. Chem. Int. Ed. 2023, 62, e202212439. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Xu, H.; Liu, Y.; Zhao, X.; Zhang, Z.; Li, L. Three-Dimensional Hierarchical Ternary Nanostructures Bismuth/Polypyrrole/CNTs for High Performance Potassium-Ion Battery Anodes. Chin. J. Chem. 2022, 40, 1585–1591. [Google Scholar] [CrossRef]
- Hsieh, Y.; Tuan, H. Architectural van der Waals Bi2S3/Bi2Se3 topological heterostructure as a superior potassium-ion storage material. Energy Storage Mater. 2022, 51, 789–805. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Li, C.; Wang, D.; Sikandar, I.; Man, R.; Tian, F.; Qian, Y.; Xu, L. Dandelion-Like Bi2S3/rGO hierarchical microspheres as high-performance anodes for potassium-ion and half/full sodium-ion batteries. Nano Res. 2021, 14, 4696–4703. [Google Scholar] [CrossRef]
- Han, M.; Zhou, Z.; Li, Y.; Chen, Q.; Chen, M. Constructing Bi2Se3/Bi2O3 heterostructure as promising anode for efficient sodium-ion storage. J. Alloys Compd. 2021, 892, 162143. [Google Scholar] [CrossRef]
- Qiu, J.; Li, S.; Su, X.; Wang, Y.; Xu, L.; Yuan, S.; Li, H.; Zhang, S. Bismuth nano-spheres encapsulated in porous carbon network for robust and fast sodium storage. Chem. Eng. J. 2017, 320, 300–307. [Google Scholar] [CrossRef]
- Cheng, X.; Li, D.; Wu, Y.; Xua, R.; Yu, Y. Bismuth nanospheres embedded in three-dimensional (3D) porous graphene frameworks as high performance anodes for sodium- and potassium-ion batteries. J. Mater. Chem. A 2019, 7, 4913–4921. [Google Scholar] [CrossRef]
- Wang, L.; Voskanyan, A.; Chan, K.Y.; Qin, B.; Li, F. Combustion Synthesized Porous Bismuth/N-Doped Carbon Nanocomposite for Reversible Sodiation in a Sodium-Ion Battery. ACS Appl. Energy Mater. 2020, 3, 565–572. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Y.; Ye, W.; Zhang, J.; Wang, Y.; Lin, Y.; Hou, L.; Wang, M.; Yua, C. Unveiling Intrinsic Potassium Storage Behaviors of Hierarchical Nano Bi@N-Doped Carbon Nanocages Framework via In Situ Characterizations. Angew. Chem. Int. Ed. 2021, 60, 7180–7187. [Google Scholar] [CrossRef]
- Cui, R.; Zhou, H.; Li, J.; Yang, C.; Jiang, Q. Ball-Cactus-Like Bi Embedded in N-Riched Carbon Nanonetworks Enables the Best Potassium Storage Performance. Adv. Funct. Mater. 2021, 31, 2103067. [Google Scholar] [CrossRef]
- Zhang, R.; Bao, J.; Wang, Y.; Sun, C. Concentrated electrolytes stabilize bismuth–potassium batteries. Chem. Sci. 2018, 9, 6193. [Google Scholar] [CrossRef]
- Abel, P.R.; Fields, M.G.; Heller, A.; Mullins, C.B. Tin–Germanium Alloys as Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 15860. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.D.; Green, S.; Prieto, A.L. Electrochemical Performance of Electrodeposited Zn4Sb3 Films for Sodium-Ion Secondary Battery Anodes. ACS Appl. Mater. Interfaces 2015, 7, 7447–7450. [Google Scholar] [CrossRef]
- Farbod, B.; Cui, K.; Kalisvaart, W.P.; Kupsta, M.; Zahiri, B.; Kohandehghan, A.; Lotfabad, E.M.; Li, Z.; Luber, E.J.; Mitlin, D. Anodes for Sodium Ion Batteries Based on Tin–Germanium–Antimony Alloys. ACS Nano 2014, 8, 4415–4429. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Han, Q.; Geng, D.; Wu, Y.; Cheng, Y.; Wang, L. One-pot chemical route for morphology-controllable fabrication of Sn-Sb micro/nano-structures: Advanced anode materials for lithium and sodium storage. J. Power Sources 2017, 342, 861–871. [Google Scholar] [CrossRef]
- Ji, L.; Gu, M.; Shao, Y.; Li, X.; Engelhard, M.H.; Arey, B.W.; Wang, W.; Nie, Z.; Xiao, J.; Wang, C.; et al. Controlling SEI Formation on SnSb-Porous Carbon Nanofibers for Improved Na Ion Storage. Adv. Mater. 2014, 26, 2901–2908. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Cao, Y.; Xiao, J.; Wang, W.; Kovarik, L.; Nie, Z.; Liu, J. High capacity, reversible alloying reactions in SnSb/C nanocomposites for Na-ion battery applications. Chem. Commun. 2012, 48, 3321–3323. [Google Scholar] [CrossRef]
- Gu, Y.; Pei, Y.; Zhao, M.; Yang, C.; Jiang, Q. Sn−, Sb− and Bi− Based Anodes for Potassium Ion Battery. Chem. Rec. 2022, 22, e202200098. [Google Scholar] [CrossRef]
- Xiong, P.; Wu, J.; Zhou, M.; Xu, Y. Bismuth–Antimony Alloy Nanoparticle@Porous Carbon Nanosheet Composite Anode for High-Performance Potassium-Ion Batteries. ACS Nano 2020, 14, 1018–1026. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Lin, C.; Wang, Y.; Lei, Z.; Xiong, P.; Luo, Y.; Chen, Q.; Wei, M.L.; Qian, Q. Structure Engineering of BiSbSx Nanocrystals Embedded within Sulfurized Polyacrylonitrile Fibers for High Performance of Potassium-Ion Batteries. Chem. Eur. J. 2022, 28, e202200028. [Google Scholar] [CrossRef]
- Huang, C.; Xu, A.; Li, G.; Sun, H.; Wu, S.; Xu, Z.; Yan, Y. Alloyed BiSb Nanoparticles Confined in Tremella-Like Carbon Microspheres for Ultralong-Life Potassium Ion Batteries. Small 2021, 17, 2100685. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, B.; Xie, H.; Bai, X.; Liang, M.; Wu, Z.; Jin, X.; He, C.; Zhao, N. Bismuth-antimony alloy nanoparticles encapsulated in 3D carbon framework: Synergistic effect for enhancing interfacial potassium storage. Chem. Eng. J. 2022, 430, 132906. [Google Scholar] [CrossRef]
- Xie, H.; Kalisvaart, W.P.; Olsen, B.C.; Luber, E.J.; David, M.; Buriak, J.M. Sn–Bi–Sb alloys as anode materials for sodium ion batteries. J. Mater. Chem. A 2017, 5, 9661–9670. [Google Scholar] [CrossRef]
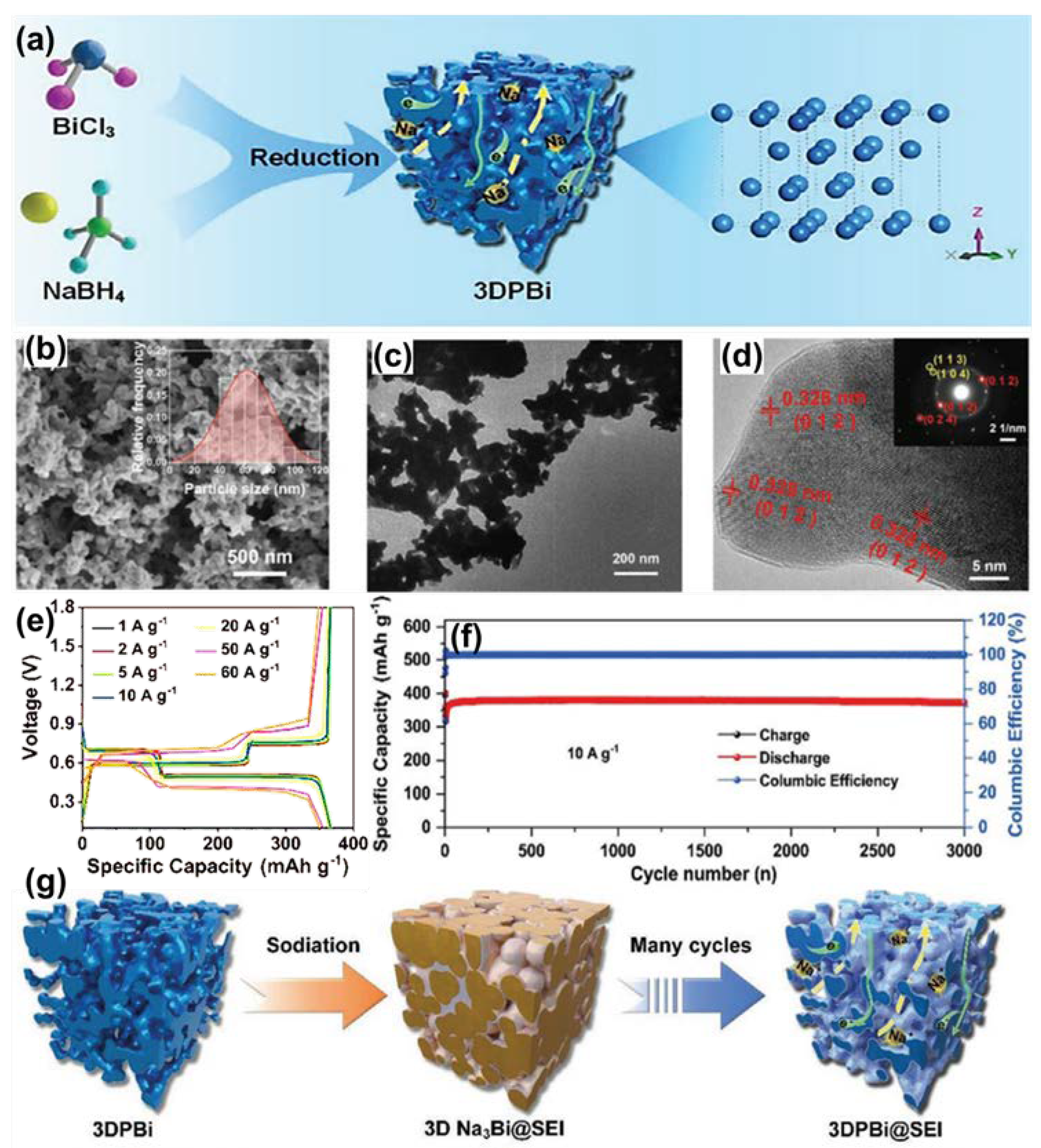
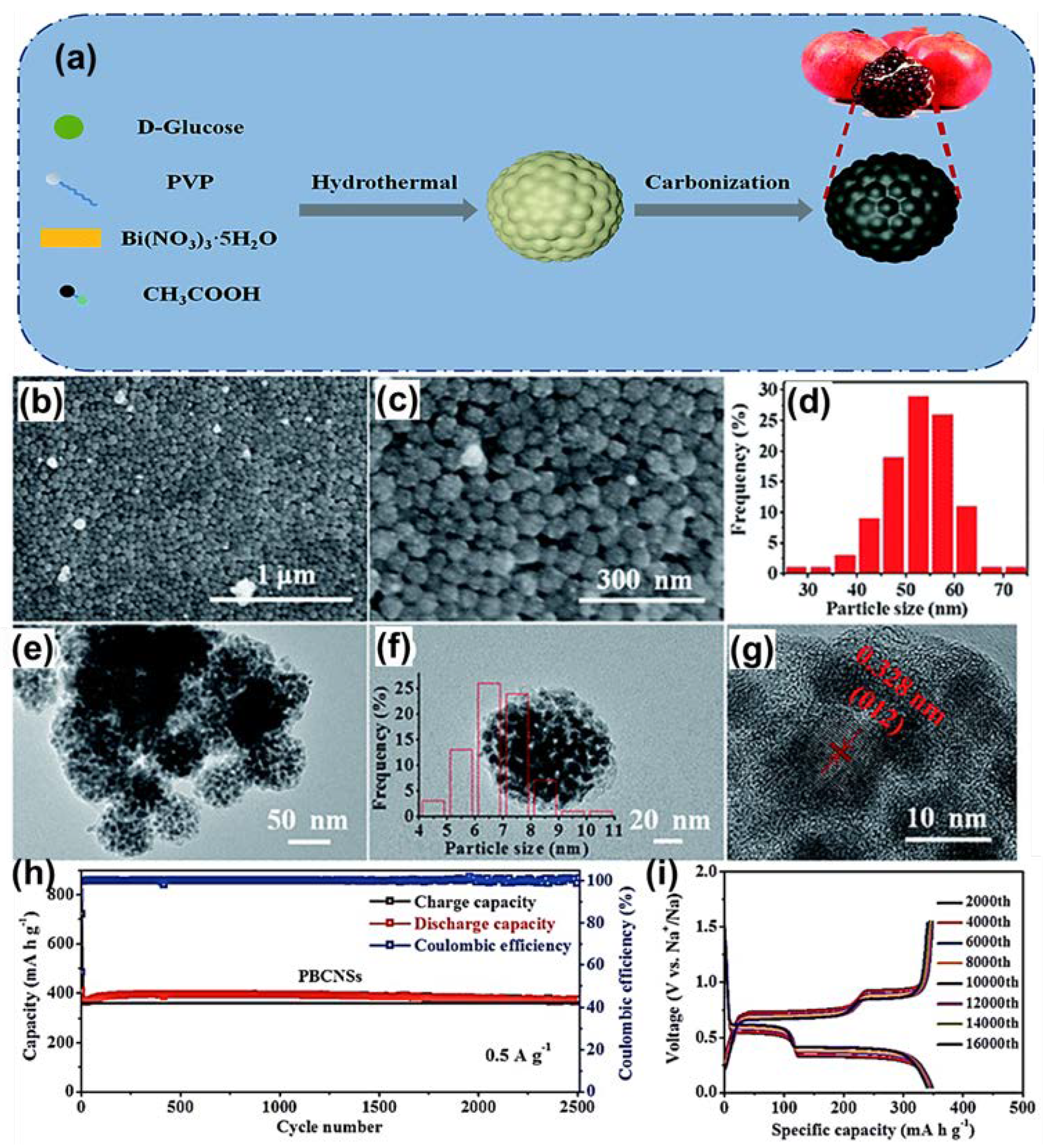
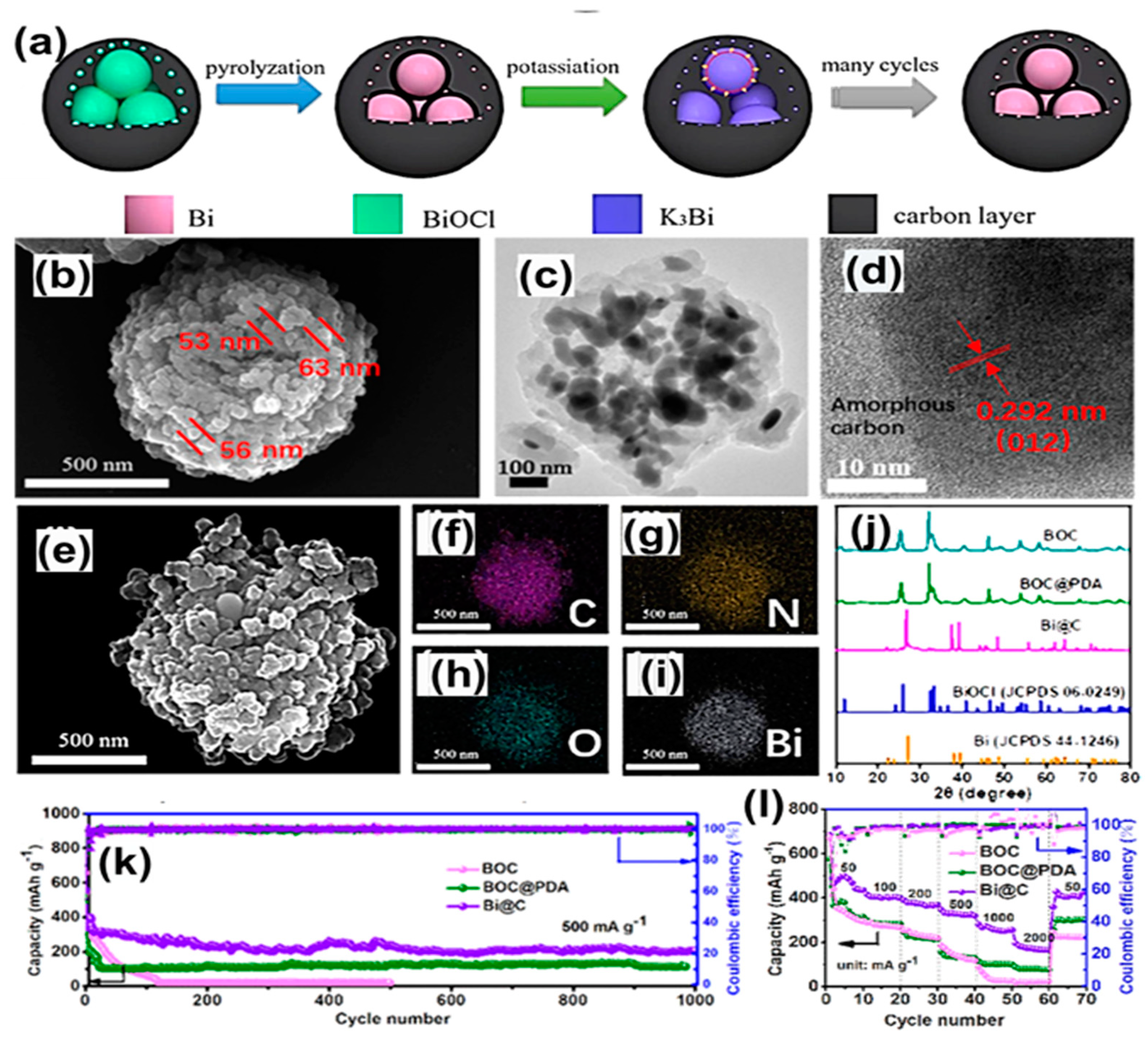
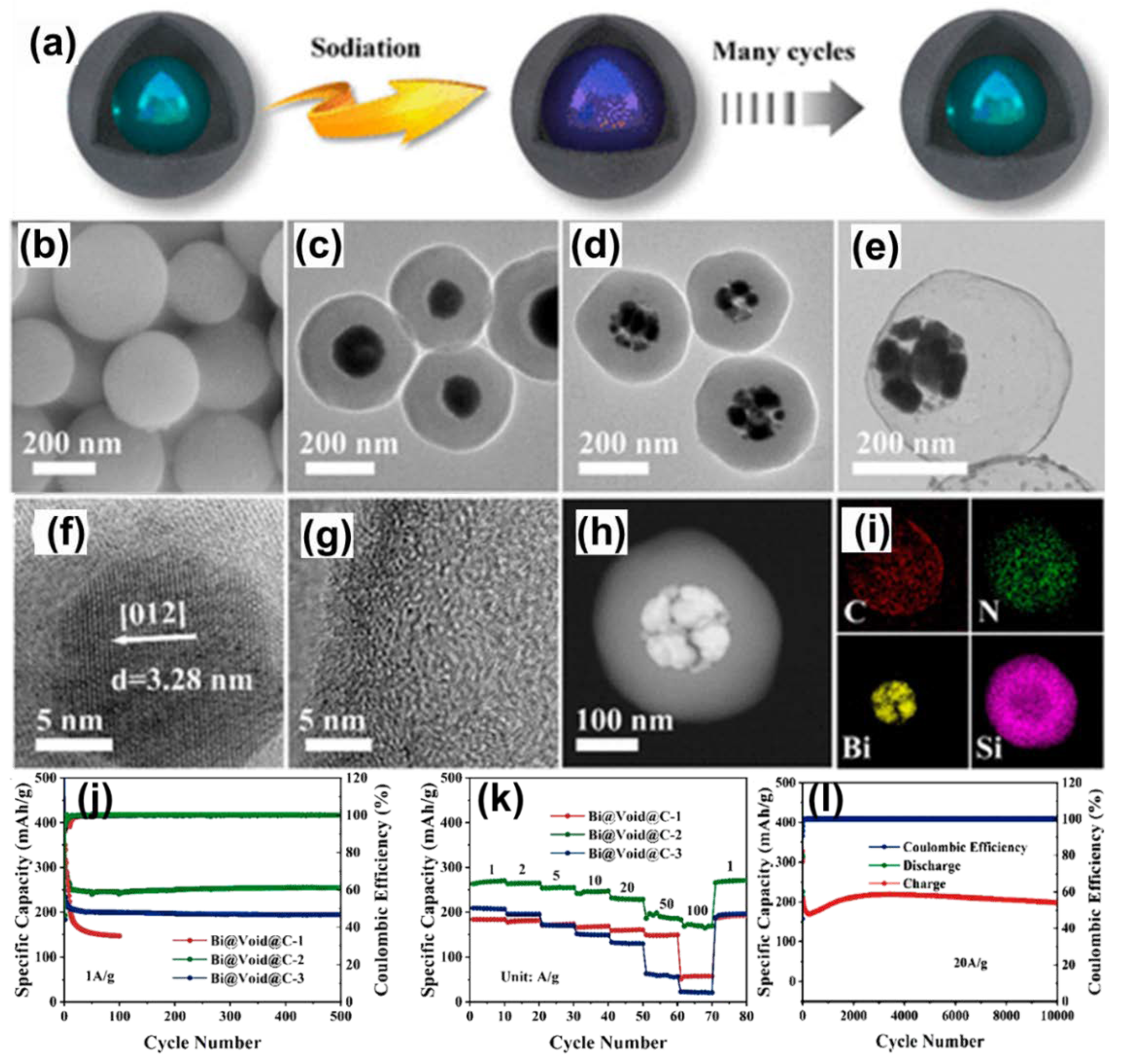
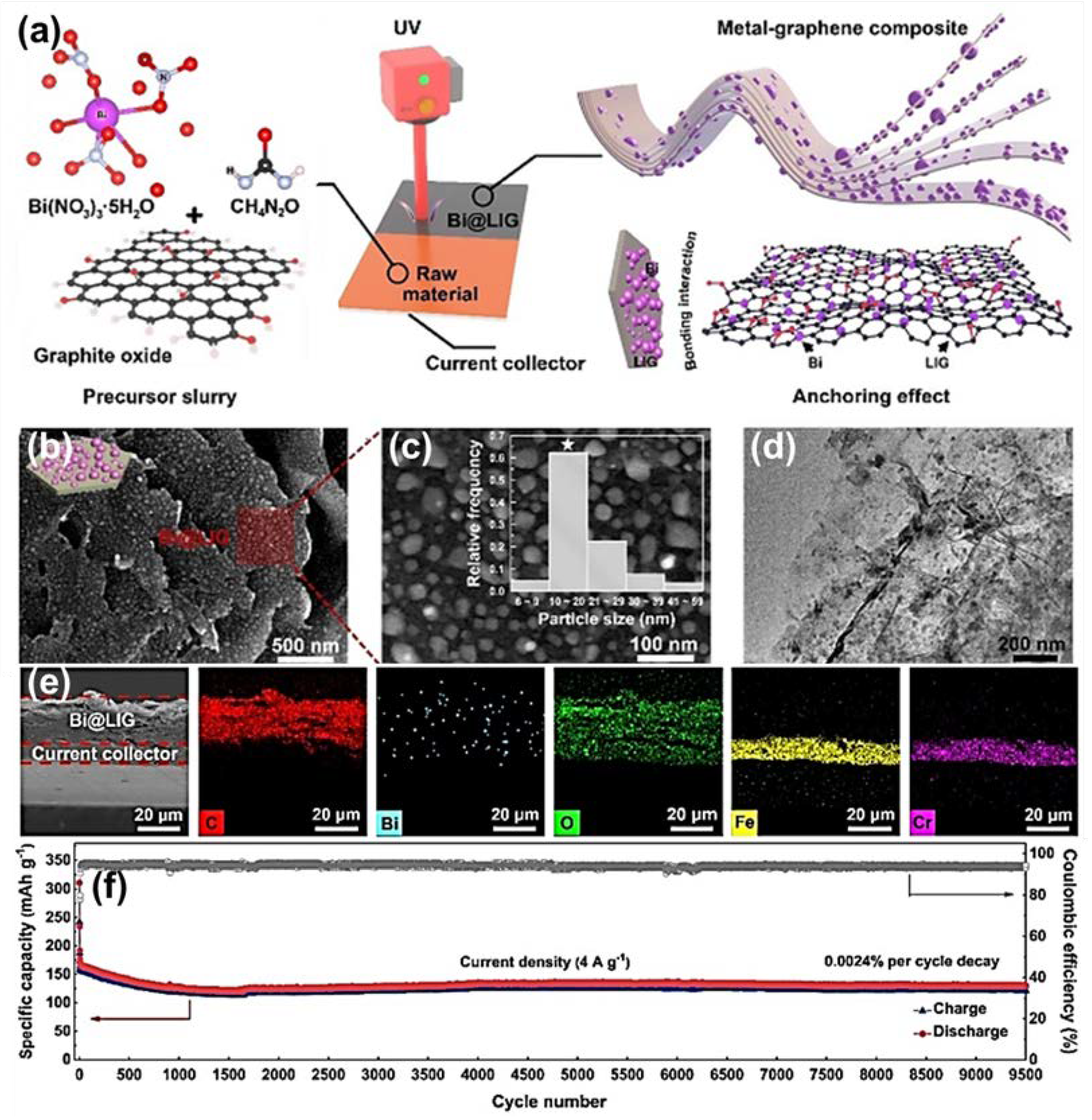
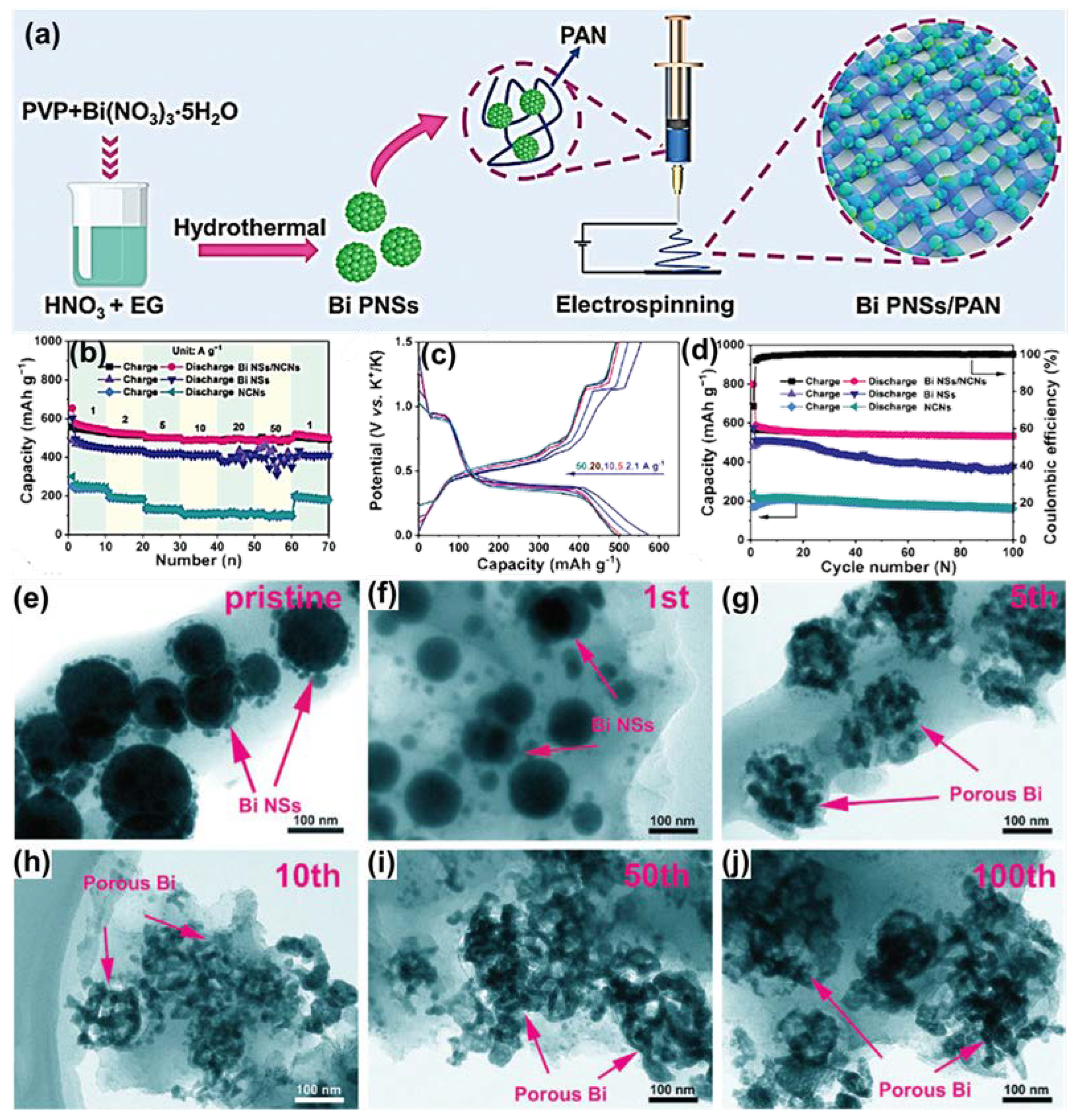
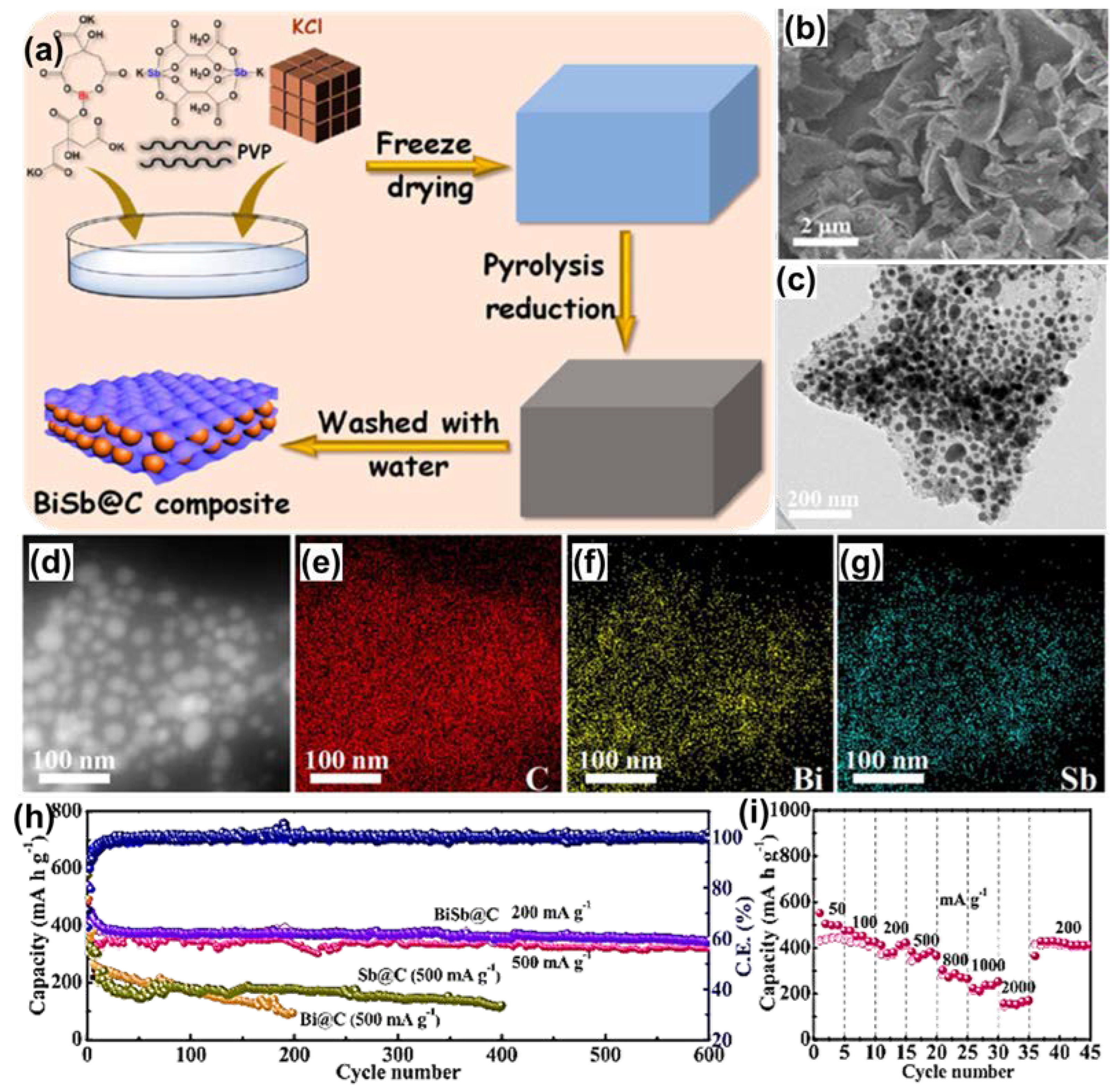
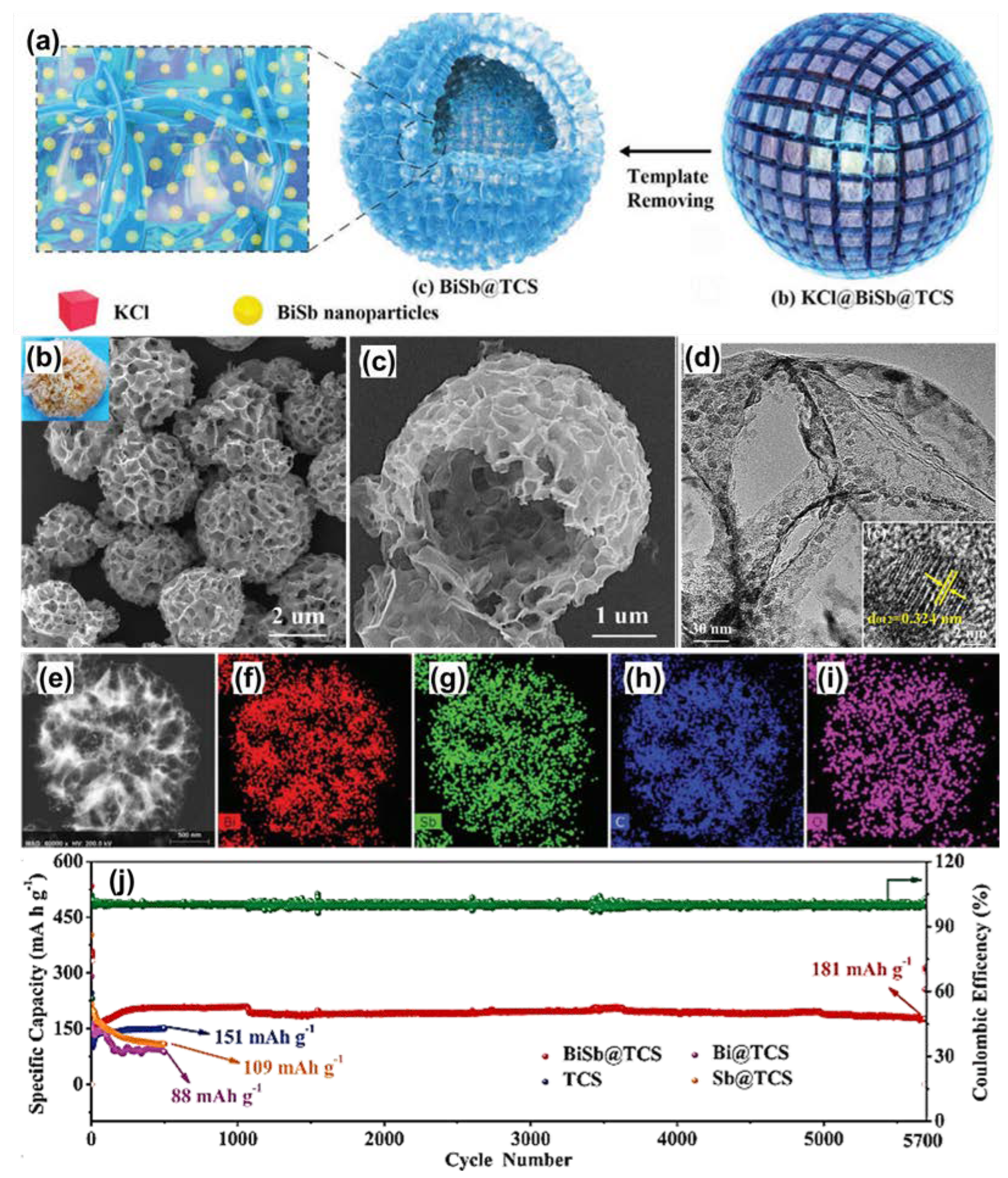
| Anode | Morphology | Current Density | Cycles | Capacity | Ref | |
|---|---|---|---|---|---|---|
| mA g−1 | mA h g−1 | |||||
| Bismuth metal | Bismuth (Na+) | Bulk | 7700 | 3500 | 379 | [20] |
| Bismuth (Na+) | Bulk | 400 | 2000 | 400 | [21] | |
| Bismuth (K+) | Bulk | 371 | 300 | 800 | [22] | |
| Bi NTs (Na+) | Nanotubes | 1000 | 5000 | 351.7 | [23] | |
| Bi/C composite material | 3DPBi (Na+) | Nano-porous | 1000 | 200 | 380 | [24] |
| PBCNS (Na+) | Nanospheres | 500 | 2500 | 373.4 | [27] | |
| Bi@C (Na+) | Nanospheres | 1000 | 1500 | 344 | [28] | |
| Bi@a-C (Na+) | Nanospheres | 50 | 100 | 202.1 | [29] | |
| p-Bi@C (Na+) | Bi-MOF | 50 | 100 | 301.9 | [29] | |
| Bi@C (Na+) | Microsphere | 100 | 100 | 123 | [30] | |
| Bi@NC (Na+) | Nanospheres | 200 | 100 | 371 | [31] | |
| Bi@C@GR-2 (Na+) | Nanospheres | 100 | 80 | 300 | [32] | |
| Bi@C@GR-2 (K+) | Nanospheres | 100 | 70 | 200 | [32] | |
| Bi@C (K+) | Nanospheres | 100 | 100 | 389 | [37] | |
| Bi@N-C (Na+) | Core–shell | 1000 | 400 | 300 | [38] | |
| Bi@N-C (K+) | Core–shell | 1000 | 100 | 268 | [38] | |
| Bi@Void@TiO2 (K+) | Core–shell | 2000 | 3000 | 171 | [42] | |
| Bi@Void@C (Na+) | Core–shell | 1000 | 500 | 253 | [43] | |
| Bi/CNTs (Na+) | Nanotubes | 1000 | 200 | 350 | [50] | |
| Bi@N-CT (K+) | Nanorods | 3850 | 1000 | 266 | [54] | |
| Bi@C (K+) | Nanorods | 50 | 300 | 353 | [55] | |
| NBCNTs (K+) | Nanotubes | 500 | 1000 | 204 | [56] | |
| Bi@C⊂CFs (Na+) | Nanorods | 1000 | 1000 | 325 | [57] | |
| UCF@CNs@BiN (K+) | Nanorods | 100 | 600 | 425 | [58] | |
| Bi/CFC (Na+) | Nanosheet | 50 | 300 | 350 | [65] | |
| FBN (K+) | Nanosheet | 7500 | 100 | 318 | [66] | |
| 2D Bi@NOC (K+) | Nanosheet | 1000 | 1000 | 341.7 | [67] | |
| ML-Bi@NCSs (Na+) | Nanosheet | 5000 | 3000 | 343 | [68] | |
| C PVP + C2H2/Bi/rGO (Na+) | Nanosheet | 5000 | 1200 | 327.6 | [69] | |
| BiND/G (K+) | Nanosheet | 5000 | 500 | 213 | [70] | |
| Bi@LIG (Na+) | Nanosheet | 250 | 2500 | 481.4 | [71] | |
| Bi/PPy/CNT (K+) | 3D porous | 100 | 200 | 302 | [72] | |
| Bi-NS@C (Na+) | Nano-porous | 200 | 1000 | 106 | [76] | |
| Bi@3DGF (Na+) | Nano-porous | 100 | 95 | 208 | [77] | |
| Bi/N–C (Na+) | 3D porous | 5000 | 1600 | 203 | [78] | |
| Bi@N-CNCs (K+) | Nanocages | 1000 | 300 | 327.5 | [79] | |
| Bi NSs/NCNs (K+) | Nano network | 1000 | 100 | 525.1 | [80] | |
| Bismuth Alloy | BiSb@C (K+) | Nano sheet | 500 | 600 | 320 | [88] |
| BiSbSx@SPAN (K+) | Nanocrystals | 100 | 50 | 790 | [89] | |
| BiSb@TCS (K+) | Nanospheres | 500 | 100 | 265 | [90] | |
| Sn10Bi10Sb80 (Na+) | Alloy film | 200 | 100 | 621 | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, X.; Li, F.; Ji, S.; Zhao, J.; Liu, J.; Huo, Y. Rational Design of Bismuth Metal Anodes for Sodium-/Potassium-Ion Batteries: Recent Advances and Perspectives. Batteries 2023, 9, 440. https://doi.org/10.3390/batteries9090440
Wang Y, Xu X, Li F, Ji S, Zhao J, Liu J, Huo Y. Rational Design of Bismuth Metal Anodes for Sodium-/Potassium-Ion Batteries: Recent Advances and Perspectives. Batteries. 2023; 9(9):440. https://doi.org/10.3390/batteries9090440
Chicago/Turabian StyleWang, Yan, Xijun Xu, Fangkun Li, Shaomin Ji, Jingwei Zhao, Jun Liu, and Yanping Huo. 2023. "Rational Design of Bismuth Metal Anodes for Sodium-/Potassium-Ion Batteries: Recent Advances and Perspectives" Batteries 9, no. 9: 440. https://doi.org/10.3390/batteries9090440
APA StyleWang, Y., Xu, X., Li, F., Ji, S., Zhao, J., Liu, J., & Huo, Y. (2023). Rational Design of Bismuth Metal Anodes for Sodium-/Potassium-Ion Batteries: Recent Advances and Perspectives. Batteries, 9(9), 440. https://doi.org/10.3390/batteries9090440