Low-Temperature-Aged Synthesis of CeO2-Coated Li-Rich Oxide as Cathode for Low-Cost High-Energy Density Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Physical Characterization
2.3. Electrochemical Measurements
3. Results and Discussions
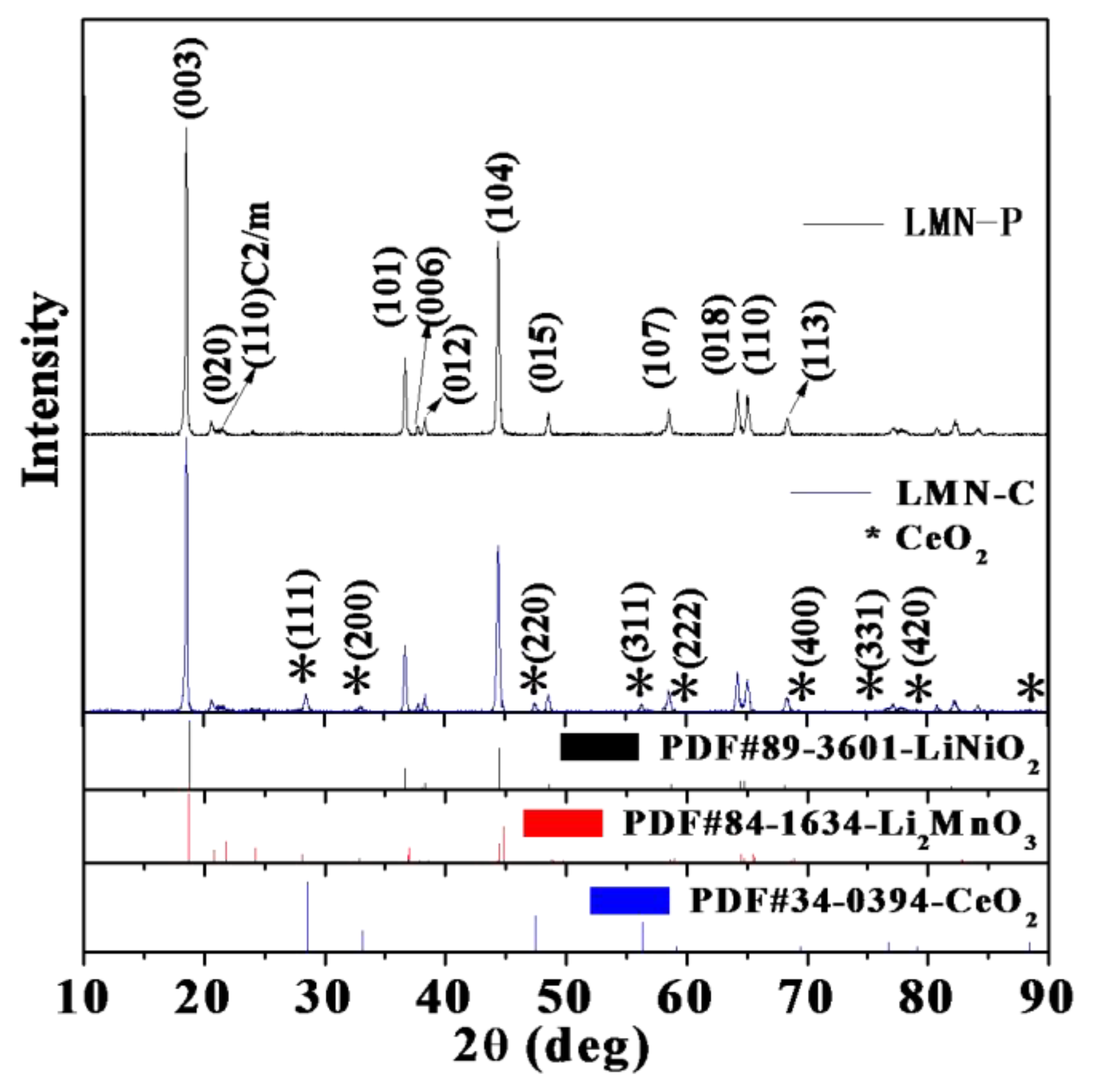
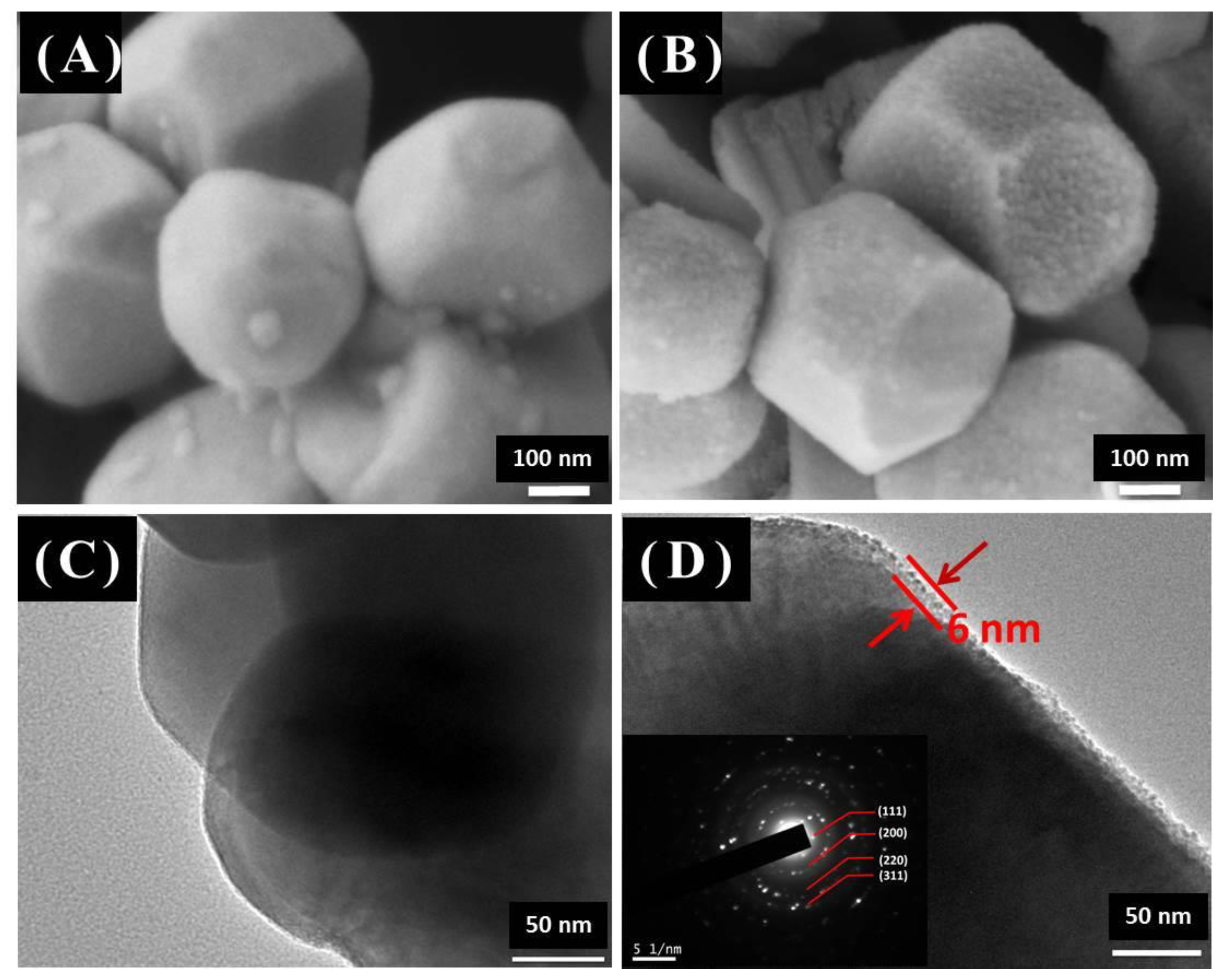
3.1. Structure and Morphology
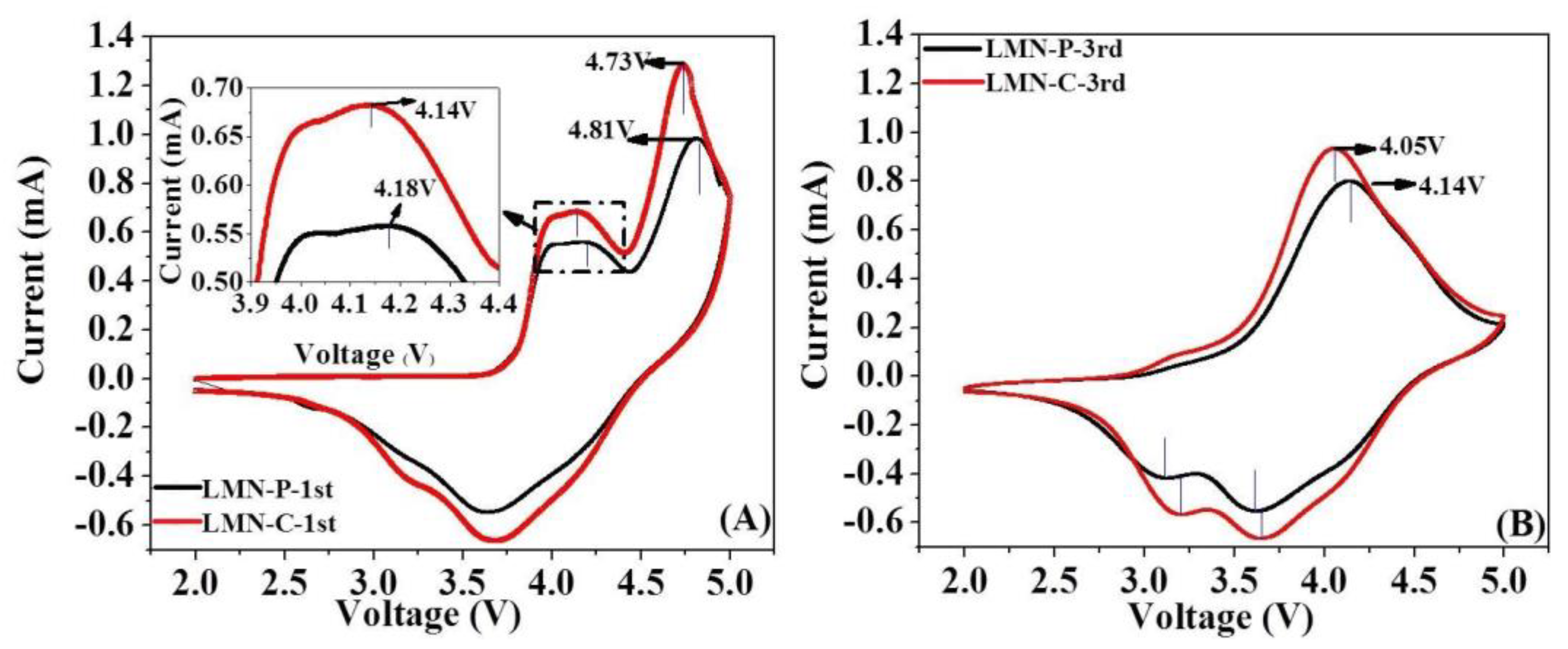
3.2. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; We, X.; Yang, Z. Pulsed laser 3D-micro/nanostructuring of materials for electrochemical energy storage and conversion. Prog. Mater. Sci. 2023, 133, 101052. [Google Scholar] [CrossRef]
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond—A 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef]
- Wu, K.; Cui, J.; Yi, J.; Liu, X.; Ning, F.; Liu, Y.; Zhang, J. Biodegradable Gel Electrolyte Suppressing Water-Induced Issues for Long-Life Zinc Metal Anodes. ACS Appl. Mater. Interfaces 2022, 14, 34612–34619. [Google Scholar] [CrossRef] [PubMed]
- Molaiyan, P.; Reis, G.S.D.; Karuppiah, D.; Subramaniyam, C.M.; Flaviano, G.A.; Lassi, U. Recent Progress in Biomass-Derived Carbon Materials for Li-Ion and Na-Ion Batteries—A Review. Batteries 2023, 9, 116. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Tu, W.; Wang, Z.; Xu, M.; Xing, L.; Li, W. Improving cyclic stability of lithium nickel manganese oxide cathode for high voltage lithium ion battery by modifying electrode/electrolyte interface with electrolyte additive. Electrochim. Acta 2014, 147, 636–642. [Google Scholar] [CrossRef]
- Rozier, P.; Tarascon, J.M. Review-Li-Rich Layered Oxide Cathodes for Next-Generation Li-Ion Batteries: Chances and Challenges. J. Electrochem. Soc. 2015, 162, A2490. [Google Scholar] [CrossRef]
- Fang, L.; Chen, M.; Nam, K.W.; Kang, Y.M. Redox Evolution of Li-Rich Layered Cathode Materials. Batteries 2022, 8, 132. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Johnson, C.S.; Vaughey, J.T.; Li, N.; Hackney, S.A. Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J. Mater. Chem. 2005, 15, 2257–2267. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Q.; Xiang, X.; Chen, M.; Chen, Z.; Song, S.; Xiong, L.; Liao, Y.; Xing, L.; Li, W. Porous layered lithium-rich oxide nanorods: Synthesis and performances as cathode of lithium ion battery. Electrochim. Acta 2015, 154, 83–93. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Holzapfel, M.; Novák, P.; Johnson, C.S.; Kang, S.; Thackeray, M.M.; Bruce, P.G. Demonstrating Oxygen Loss and Associated Structural Reorganization in the Lithium Battery Cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 2006, 128, 8694–8698. [Google Scholar] [CrossRef]
- Xiang, X.; Li, X.; Li, W. Preparation and characterization of size-uniform Li[Li0.131Ni0.304Mn0.565]O2 particles as cathode materials for high energy lithium ion battery. J. Power Sources 2013, 230, 89–95. [Google Scholar] [CrossRef]
- Ji, X.; Xia, Q.; Xu, Y.; Feng, H.; Wang, P.; Tan, Q. A review on progress of lithium-rich manganese-based cathodes for lithium ion batteries. J. Power Sources 2021, 487, 229362. [Google Scholar] [CrossRef]
- Zuo, W.; Luo, M.; Liu, X.; Wu, J.; Liu, H.; Li, J.; Winter, M.; Fu, R.; Yang, W.; Yang, Y. Li-rich cathodes for rechargeable Li-based batteries: Reaction mechanisms and advanced characterization techniques. Energy Environ. Sci. 2020, 13, 4450–4497. [Google Scholar] [CrossRef]
- Xiang, X.; Li, W. Significant influence of insufficient lithium on electrochemical performance of lithium-rich layered oxide cathodes for lithium ion batteries. Electrochim. Acta 2014, 133, 422–427. [Google Scholar] [CrossRef]
- Lee, S.H.; Moon, J.S.; Lee, M.S.; Yu, T.H.; Kim, H.; Park, B.M. Enhancing phase stability and kinetics of lithium-rich layered oxide for an ultra-high performing cathode in Li-ion batteries. J. Power Sources 2015, 281, 77–84. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Park, B.; Wu, J.; Yang, W.; McCloskey, B.D. Extended Interfacial Stability through Simple Acid Rinsing in a Li-Rich Oxide Cathode Material. J. Am. Chem. Soc. 2020, 142, 8522–8531. [Google Scholar] [CrossRef]
- Fan, J.; Li, G.; Luo, D.; Fu, C.; Li, Q.; Zheng, J.; Li, L. Hydrothermal-Assisted Synthesis of Li-Rich Layered Oxide Microspheres with High Capacity and Superior Rate-capability as a Cathode for Lithium-ion Batteries. Electrochim. Acta 2015, 173, 7–16. [Google Scholar] [CrossRef]
- Ji, X.; Xu, Y.; Xia, Q.; Zhou, Y.; Song, J.; Feng, H.; Wang, P.; Yang, J.; Tan, Q. Li-Deficient Materials-Decoration Restrains Oxygen Evolution Achieving Excellent Cycling Stability of Li-Rich Mn-Based Cathode. ACS Appl. Mater. Interfaces 2022, 14, 30133–30143. [Google Scholar] [CrossRef]
- Cao, X.; Sun, J.; Chang, Z.; Wang, P.; Yue, X.; Okagaki, J.; He, P.; Yoo, E.; Zhou, H. Enabling Long-Term Cycling Stability within Layered Li-Rich Cathode Materials by O2/O3-Type Biphasic Design Strategy. Adv. Funct. Mater. 2022, 32, 2205199. [Google Scholar] [CrossRef]
- Ates, M.N.; Jia, Q.; Shah, A.; Busnaina, A.; Mukerjee, S.; Abraham, K.M. Mitigation of Layered to Spinel Conversion of a Li-Rich LayeredMetal Oxide Cathode Material for Li-Ion Batteries. J. Electrochem. Soc. 2014, 161, A290–A301. [Google Scholar] [CrossRef]
- Chen, G.; An, J.; Meng, Y.; Yuan, C.; Matthews, B.; Dou, F.; Shi, L.; Zhou, Y.; Song, P.; Wu, G.; et al. Cation and anion Co-doping synergy to improve structural stability of Li- and Mn-rich layered cathode materials for lithium-ion batteries. Nano Energy 2019, 57, 157–165. [Google Scholar] [CrossRef]
- Li, L.; Song, B.H.; Chang, Y.L.; Xia, H.; Yang, J.R.; Lee, K.S.; Lu, L. Retarded phase transition by fluorine doping in Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material. J. Power Sources 2015, 283, 162–170. [Google Scholar] [CrossRef]
- Qiu, Y.; Peng, X.; Zhou, L.; Song, Y.; Bi, L.; Long, X.; He, L.; Xie, Q.; Wang, S.; Liao, J. Building Ultrathin Li4Mn5O12 Shell for Enhancing the Stability of Cobalt-Free Lithium-Rich Manganese Cathode Materials. Batteries 2023, 9, 123. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Wang, Z.; Zheng, F.; Zhong, R.; Hong, R. AlF3 coating improves cycle and voltage decay of Li-rich manganese oxides. J. Mater. Sci. 2023, 58, 4525–4540. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, Y.; Hai, C.; Zeng, J.; Sun, Y.; Ma, Y.; Shen, Y.; Li, X.; Ren, X.; Sun, C.; et al. Enhanced Cathode Performance: Mixed Al2O3 and LiAlO2 Coating of Li1.2Ni0.13Co0.13Mn0.54O2. ACS Appl. Mater. Interfaces 2020, 12, 38153–38162. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Yue, Y.; Hu, X.; Xia, M. Carbon Modified Li-rich Cathode Materials Li1.26Fe0.22Mn0.52O2 Synthesized via Molten Salt Method with Excellent Rate Ability for Li-ion Batteries. Electrochim. Acta 2014, 130, 66–75. [Google Scholar] [CrossRef]
- Hou, M.; Liu, J.; Guo, S.; Yang, J.; Wang, C.; Xia, Y. Enhanced electrochemical performance of Li-rich layered cathode materials by surface modification with P2O5. Electrochem. Commun. 2014, 49, 83–87. [Google Scholar] [CrossRef]
- Shobana, M.K. Metal oxide coated cathode materials for Li ion batteries—A review. J. Alloys Compd. 2019, 802, 477–487. [Google Scholar] [CrossRef]
- Seu, C.S.; Davis, V.K.; Pasalic, J.; Bugga, R.V. Aluminum Borate Coating on High-Voltage Cathodes for Li-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2259–A2265. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Xiao, J.; Polzin, B.J.; Yan, P.; Chen, X.; Wang, C.; Zhang, J. Functioning Mechanism of AlF3 Coating on the Li- and Mn-Rich Cathode Materials. Chem. Mater. 2014, 26, 6320–6327. [Google Scholar] [CrossRef]
- Moghadam, Y.S.; Kharbachi, A.E.; Cambaz, M.A.; Dinda, S.; Diemant, T.; Hu, Y.; Melinte, G.; Fichtner, M. Borate-Based Surface Coating of Li-Rich Mn-Based Disordered Rocksalt Cathode Materials. Adv. Mater. Interfaces 2022, 9, 2201200. [Google Scholar] [CrossRef]
- Kehoe, A.B.; Scanlon, D.O.; Watson, G.W. Role of lattice distortions in the oxygen storage capacity of divalently doped CeO2. Chem. Mater. 2011, 23, 4464–4468. [Google Scholar] [CrossRef]
- Jaksic, J.M.; Nan, F.; Papakonstantinou, G.D.; Botton, G.A.; Jaksic, M.M. Theory Substantiation and Properties of Novel Reversible Electrocatalysts for Oxygen Electrode Reactions. J. Phys. Chem. C 2015, 119, 11267–11285. [Google Scholar] [CrossRef]
- Suzuki, T.; Kosacki, I.; Anderson, H.U. Electrical Conductivity and Lattice Defects in Nanocrystalline Cerium Oxide Thin Films. J. Am. Ceram. Soc. 2001, 84, 2007–2014. [Google Scholar] [CrossRef]
- Tuller, H.L. Ionic conduction in nanocrystalline materials. Solid State Ion. 2000, 131, 143–157. [Google Scholar] [CrossRef]
- Uberuaga, B.P.; Sickafus, K.E. Interpreting oxygen vacancy migration mechanisms in oxides using the layered structure motif. Comput. Mater. Sci. 2015, 103, 216–223. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Lavik, E.B.; Kosacki, I.; Tuller, H.L.; Chiang, Y.M.; Ying, J.Y. Nonstoichiomerty and electrical conductivity of nanocrystalline CeO2−x. J. Electroceram. 1997, 1, 7–14. [Google Scholar] [CrossRef]
- Ko, H.; Yang, G.; Wang, M.; Zhao, X. Isothermal crystallization kinetics and effect of crystallinity on the optical properties of nanosized CeO2 powder. Ceram. Int. 2014, 40, 6663–6671. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Dillip, G.R.; Lee, Y.R. Picrasma quassioides mediated cerium oxide nanostructures and their post-annealing treatment on the microstructural, morphological and enhanced catalytic performance. Ceram. Int. 2016, 42, 6610–6618. [Google Scholar] [CrossRef]
- Ha, H.; Yun, N.J.; Kim, M.H.; Woo, M.H.; Kim, K. Enhanced electrochemical and thermal stability of surface-modified LiCoO2 cathode by CeO2 coating. Electrochim. Acta 2006, 51, 3297–3302. [Google Scholar] [CrossRef]
- Yao, J.; Wu, F.; Qiu, X.; Li, N.; Su, Y. Effect of CeO2-coating on the electrochemical performances of LiFePO4/C cathode material. Electrochim. Acta 2011, 56, 5587–5592. [Google Scholar] [CrossRef]
- Ha, H.; Yun, N.J.; Kim, K. Improvement of electrochemical stability of LiMn2O4 by CeO2 coating for lithium-ion batteries. Electrochim. Acta 2007, 52, 3236–3241. [Google Scholar] [CrossRef]
- Ha, H.; Jeong, K.H.; Yun, N.J.; Hong, M.Z.; Kim, K. Effects of surface modification on the cycling stability of LiNi0.8Co0.2O2 electrodes by CeO2 coating. Electrochim. Acta 2005, 50, 3764–3769. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, H.Z.; Liu, Q.; Li, G.R.; Gao, X.P. Surface modification of Li(Li0.17Ni0.2Co0.05Mn0.58)O2 with CeO2 as cathode material for Li-ion batteries. Electrochim. Acta 2014, 135, 199–207. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, J.; Qiu, S.; Tian, F.; Potapenko, O.; Zhong, S.; Potapenko, H.; Liang, Z. Structural and Electrochemical Properties of Li1.2Ni0.16Mn0.54Co0.08O2-Al2O3 Composite Prepared by Atomic Layer Deposition as the Cathode Material for LIBs. Int. J. Electrochem. Sci. 2020, 15, 10759–10771. [Google Scholar] [CrossRef]
- Han, W.Q.; Wu, L.; Zhu, Y. Formation and Oxidation State of CeO2-x Nanotubes. J. Am. Chem. Soc. 2005, 127, 12814–12815. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.B.; Yu, F.D.; Que, L.F.; Wang, M.J.; Xia, Y.F.; Xue, Y.; Wu, J. Studies on stability and capacity for long-life cycle performance of Li(Ni0.5Co0.2Mn0.3)O2 by Mo modification for lithium-ion battery. J. Power Sources 2017, 358, 1–12. [Google Scholar] [CrossRef]
- Johnson, C.S.; Li, N.; Lefief, C.; Thackeray, M.M. Anomalous capacity and cycling stability of xLi2MnO3 · (1 − x)LiMO2 electrodes (M = Mn, Ni, Co) in lithium batteries at 50 °C. Electrochem. Commun. 2007, 9, 787–795. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Xie, K. Effect of annealing on the first-cycle perfomance and reversible capabilities of lithium-rich layered oxide cathodes. J. Phys. Chem. C 2014, 118, 11505–11511. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Z.; Yu, M.; Hu, H.; Zhang, Y.; Zhang, K.; Du, Z.; Cheng, F.; Chen, J. Building Homogenous Li2TiO3 Coating Layer on Primary Particles to Stabilize Li-Rich Mn-Based Cathode Materials. Small 2022, 18, 2106337. [Google Scholar] [CrossRef]
- Kang, S.H.; Kempgens, P.; Greenbaum, S.; Kropf, A.J.; Aminea, K.; Thackeraya, M.M. Interpreting the structural and electrochemical complexity of 0.5Li2MnO3·0.5LiMO2 electrodes for lithium batteries (M = Mn0.5−xNi0.5−xCo2x, 0 ≤ x ≤ 0.5). J. Mater. Chem. 2007, 17, 2069–2077. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Genc, A.; Xiao, J.; Xu, P.; Chen, X.; Zhu, Z.; Zhao, W.; Pullan, L.; Wang, C.; et al. Mitigating voltage fade in cathode materials by improving the atomic level uniformity of elemental distribution. Nano Lett. 2014, 14, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, P.; Chitra, S.; Mohan, T.; Gopukumar, S. Lithium metal rechargeable cells using Li2MnO3 as the positive electrode. J. Power Sources 1999, 80, 103–106. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, X.; Liao, Y.; Huang, Q.; Xing, L.; Xu, M.; Li, W. Maintaining structural integrity of 4.5 V lithium cobalt oxide cathode with fumaronitrile as a novel electrolyte additive. J. Power Sources 2017, 338, 108–116. [Google Scholar] [CrossRef]
- Li, J.; Xing, L.; Wang, Z.; Tu, W.; Yang, X.; Lin, Y.; Liao, Y.; Xu, M.; Li, W. Insight into the capacity fading of layered lithium-rich oxides and its suppression via a film-forming electrolyte additive. RSC Adv. 2018, 8, 25794–25801. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.; Li, C.; Zhang, H.; Liu, J.; Zhang, Q. Interface modification in high voltage spinel lithium-ion battery by using N-methylpyrrole as an electrolyte additive. Electrochim. Acta 2015, 178, 127–133. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Yu, L.; Fan, W.; Wang, Z.; Yang, X.; Lin, Y.; Xing, L.; Xu, M.; Li, W. Understanding Interfacial Properties between Li-Rich Layered Oxide and Electrolyte Containing Triethyl Borate. J. Phys. Chem. C 2016, 120, 26899–26907. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Day, S.J.; Sui, T.; Lu, L.; Tang, C.C.; Korsunsky, A.M. Mitigated phase transition during first cycle of a Li-rich layered cathode studied by in operando synchrotron X-ray powder diffraction. Phys. Chem. Chem. Phys. 2016, 18, 4745–4752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Shi, J.L.; Liang, J.Y.; Yin, Y.Y.; Zhang, J.Z.; Yu, X.Q.; Guo, Y.G. Suppressing Surface Lattice Oxygen Release of Li-Rich Cathode Materials via Heterostructured Spinel Li4Mn5O12 Coating. Adv. Mater. 2018, 30, 1801751. [Google Scholar] [CrossRef]
- Choi, S.M.; Kang, I.S.; Sun, Y.K.; Song, J.H.; Chung, S.M.; Kim, D.W. Cycling characteristics of lithium metal batteries assembled with a surface modified lithium electrode. J. Power Sources 2013, 244, 363–368. [Google Scholar] [CrossRef]
- Ruff, Z.; Xu, C.; Grey, C.P. Transition Metal Dissolution and Degradation in NMC811-Graphite Electrochemical Cells. J. Electrochem. Soc. 2021, 168, 060518. [Google Scholar] [CrossRef]
- Li, W. An Unpredictable Hazard in Lithium-ion Batteries from Transition Metal Ions: Dissolution from Cathodes, Deposition on Anode and Elimination Strategies. J. Electrochem. Soc. 2020, 167, 090514. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Rong, H.; Wang, Y.; Liu, J.; Xing, L.; Xu, M.; Li, W. A novel electrolyte with the ability to form a solid electrolyte interface on the anode and cathode of a LiMn2O4/graphite battery. J. Mater. Chem. A 2013, 1, 12954–12961. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novak, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Dekoven, B.M.; Hagans, P.L. XPS studies of metal/polymer interfaces—Thin films of Al on polyacrylic acid and polyethylene. Appl. Surf. Sci. 1986, 27, 199–213. [Google Scholar] [CrossRef]
- Lazarraga, M.G.; Pascual, L.; Gadjov, H.; Kovacheva, D.; Petrov, K.; Amarilla, J.M.; Rojas, R.M.; Luengo-Martin, M.A.; Rojo, J.M. Nanosize LiNiyMn2−yO4 (0 < y ≤ 0.5) spinels synthesized by a sucrose-aided combustion method: Characterization and electrochemical performance. J. Mater. Chem. 2004, 14, 1640–1647. [Google Scholar]
- Guo, K.; Qi, S.; Wang, H.; Huang, J.; Wu, M.; Yang, Y.; Li, X.; Ren, Y.; Ma, J. High-Voltage Electrolyte Chemistry for Lithium Batteries. Small Sci. 2022, 2, 2100107. [Google Scholar] [CrossRef]
- Gao, H.; Maglia, F.; Lamp, P.; Amine, K.; Chen, Z. Mechanistic Study of Electrolyte Additives to Stabilize High-Voltage Cathode–Electrolyte Interface in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 44542–44549. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Lin, H.; Liu, J.; Xing, L.; Xu, M.; Li, W. Improving high voltage stability of lithium cobalt oxide/graphite battery via forming protective films simultaneously on anode and cathode by using electrolyte additive. Electrochim. Acta 2014, 141, 263–270. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Lin, Y.; Zhu, Y.; Tu, W.; Xu, M.; Liu, X.; Li, B.; Li, W. Tetrafluoroterephthalonitrile: A Novel Electrolyte Additive for High-Voltage Lithium Cobalt Oxide/Graphite Battery. Electrochim. Acta 2017, 256, 307–315. [Google Scholar] [CrossRef]


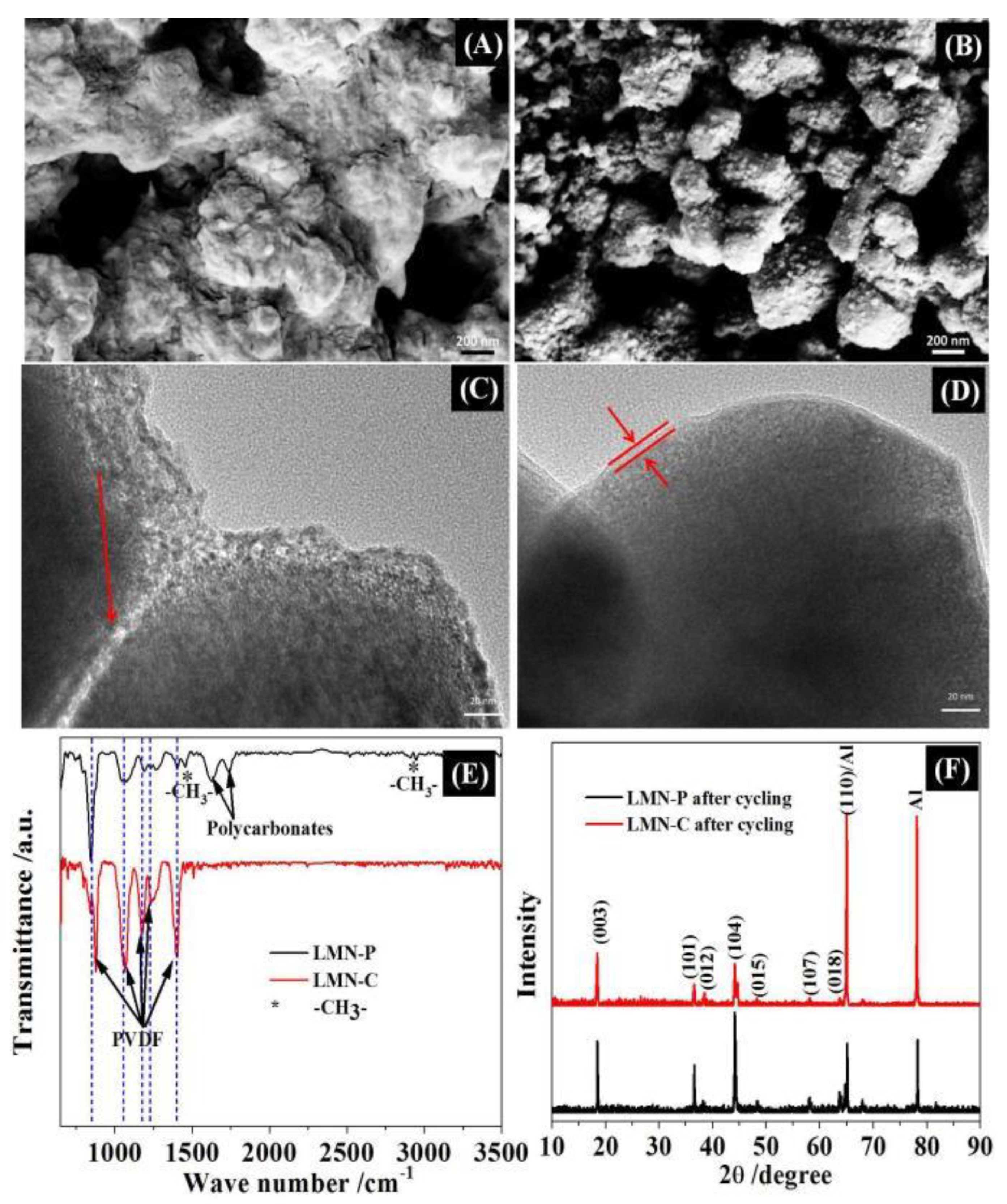

| Resistance/Ω | Charge Transfer (Rct) | Film (Rf) |
|---|---|---|
| LMN-P | 181 | 106 |
| LMN-C | 172 | 93 |
| Sample | R-3m | C2/m | ||||
|---|---|---|---|---|---|---|
| a (Å) | b (Å) | a (Å) | b (Å) | c (Å) | ||
| Before cycling | LMN-P | 2.8611 | 14.25785 | 4.9586 | 8.57046 | 5.03293 |
| lMN-C | 2.8635 | 14.25752 | 4.9559 | 8.54779 | 5.03707 | |
| After cycling | LMN-P | 2.87467 | 14.43984 | |||
| lMN-C | 2.87601 | 14.26065 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, B.; Chen, M.; Li, W. Low-Temperature-Aged Synthesis of CeO2-Coated Li-Rich Oxide as Cathode for Low-Cost High-Energy Density Li-Ion Batteries. Batteries 2023, 9, 330. https://doi.org/10.3390/batteries9060330
Liu Y, Li B, Chen M, Li W. Low-Temperature-Aged Synthesis of CeO2-Coated Li-Rich Oxide as Cathode for Low-Cost High-Energy Density Li-Ion Batteries. Batteries. 2023; 9(6):330. https://doi.org/10.3390/batteries9060330
Chicago/Turabian StyleLiu, Yanlin, Bin Li, Min Chen, and Weishan Li. 2023. "Low-Temperature-Aged Synthesis of CeO2-Coated Li-Rich Oxide as Cathode for Low-Cost High-Energy Density Li-Ion Batteries" Batteries 9, no. 6: 330. https://doi.org/10.3390/batteries9060330
APA StyleLiu, Y., Li, B., Chen, M., & Li, W. (2023). Low-Temperature-Aged Synthesis of CeO2-Coated Li-Rich Oxide as Cathode for Low-Cost High-Energy Density Li-Ion Batteries. Batteries, 9(6), 330. https://doi.org/10.3390/batteries9060330









