Facile Synthesis of Nickel Phosphide @ N-Doped Carbon Nanorods with Exceptional Cycling Stability as Li-Ion and Na-Ion Battery Anode Material
Abstract
1. Introduction
2. Materials and Methods
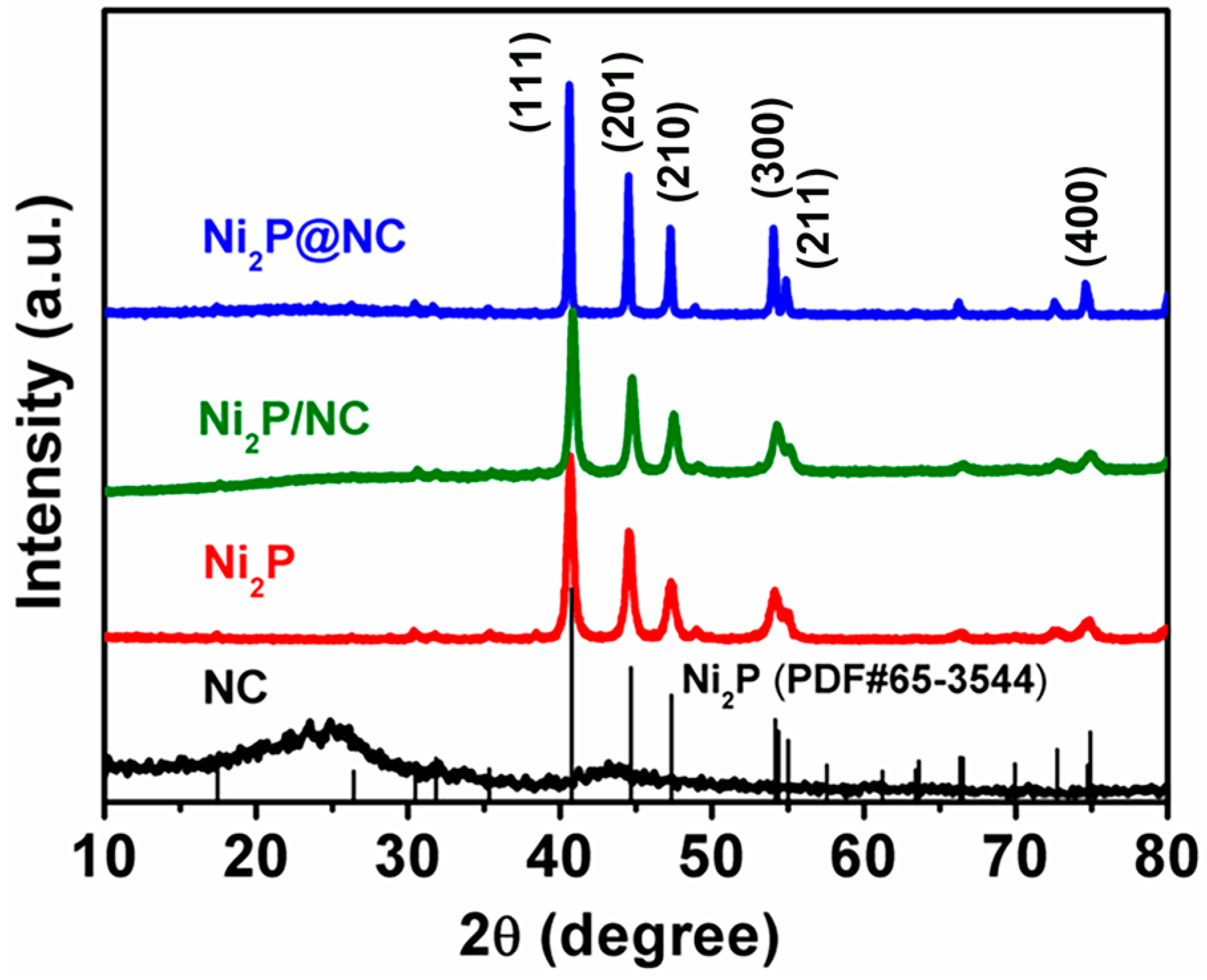
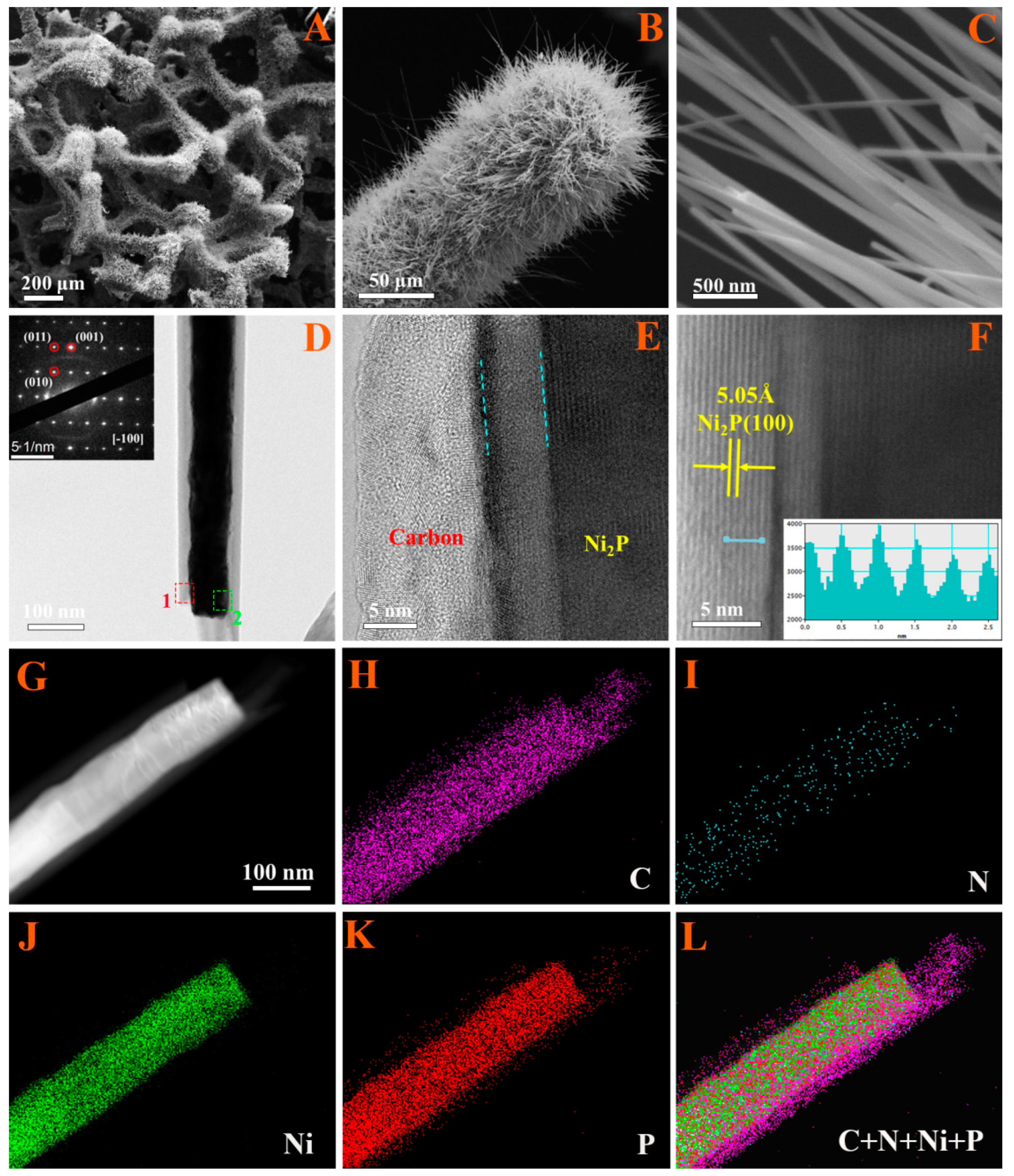
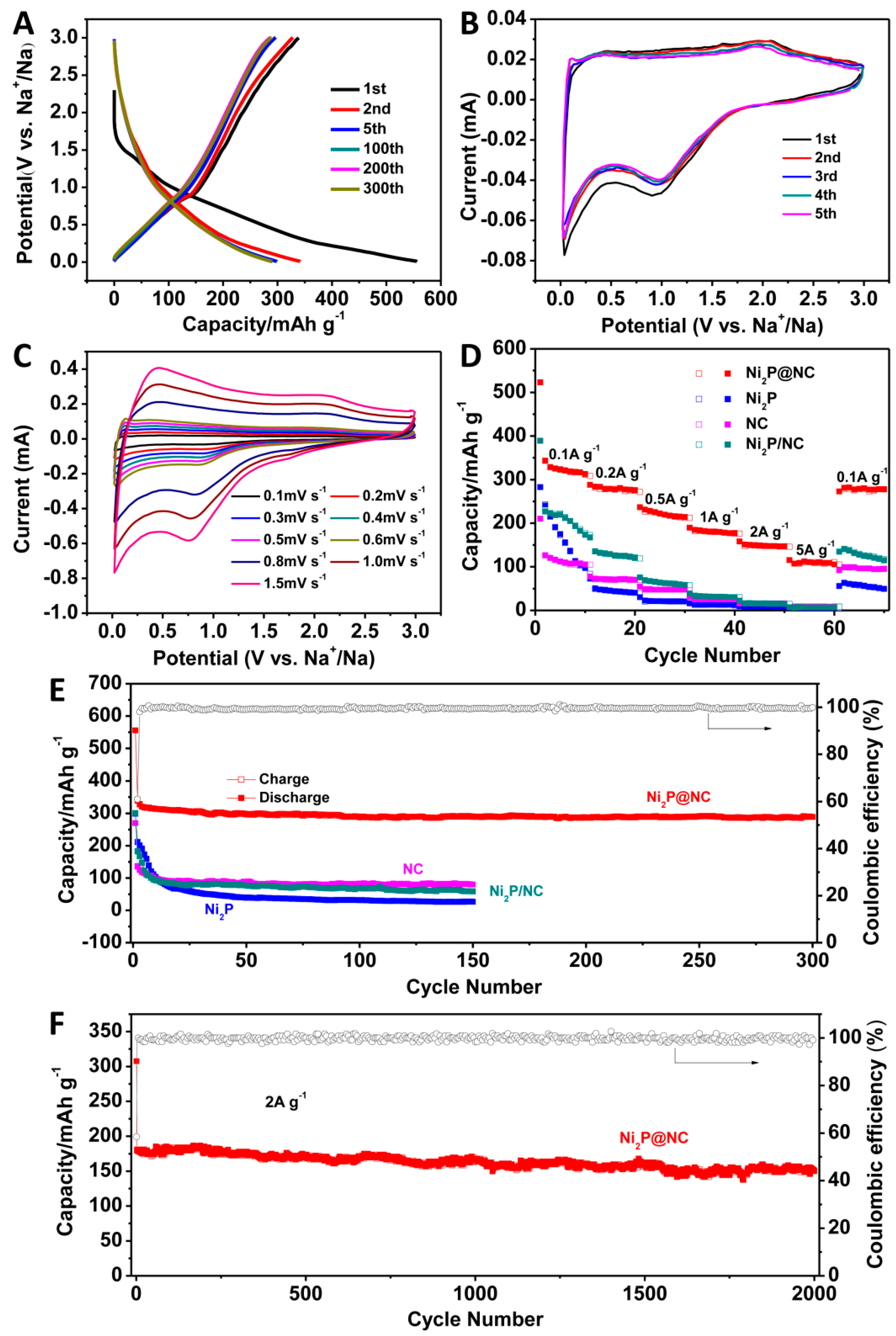
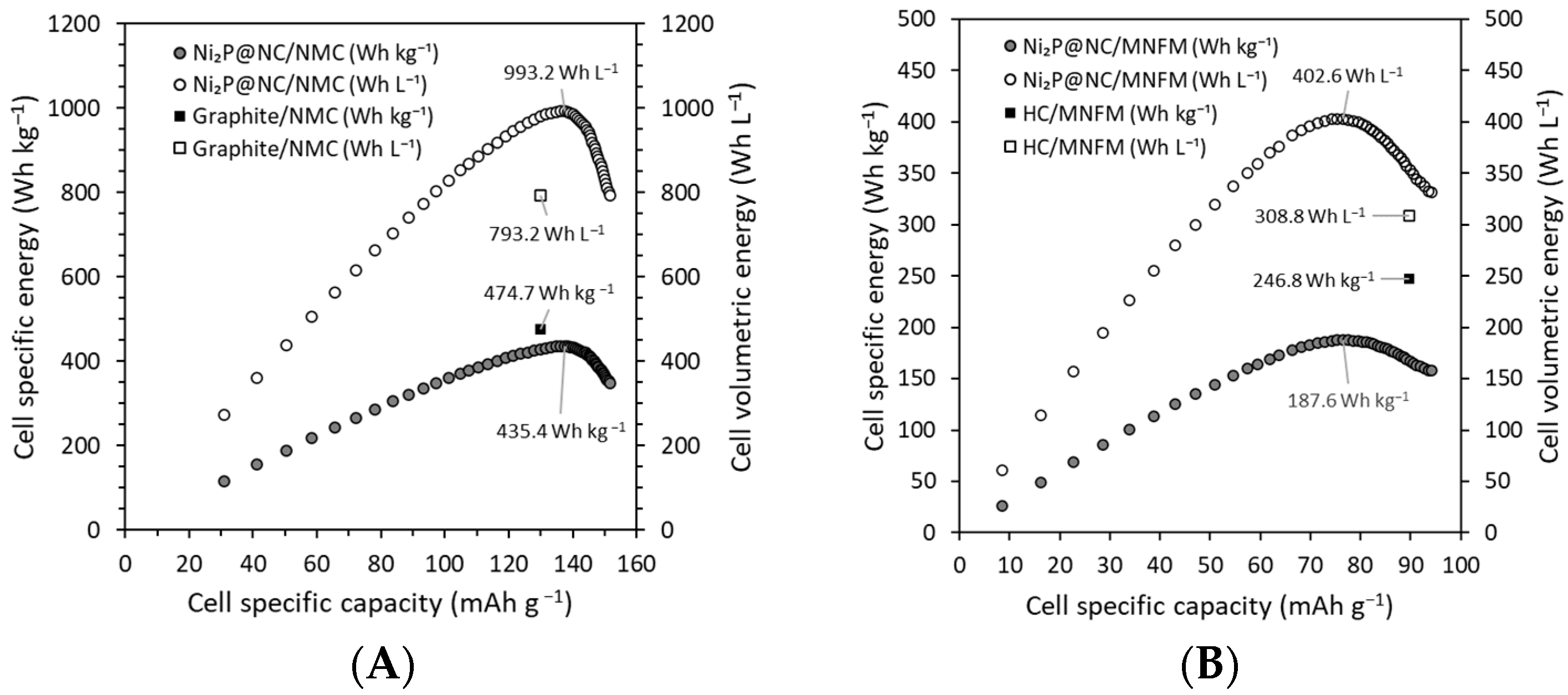
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Mao, C.; Wood, M.; David, L.; Jin An, S.; Yangping Sheng, Y.; Du, Z.; Meyer, H.; Rose, E.; Ruther, R.E.; Wood, D.L. Selecting the Best Graphite for Long-Life, High-Energy Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A1837. [Google Scholar] [CrossRef]
- Moon, H.; Innocenti, A.; Liu, H.; Zhang, H.; Weil, M.; Zarrabeitia, M.; Passerini, S. Hard carbon cycling Bio-Waste-Derived Hard Carbon Anodes Through a Sustainable and Cost-Effective Synthesis Process for Sodium-Ion Batteries. ChemSusChem 2023, 16, e202201713. [Google Scholar]
- Liu, L.; Tian, Y.; Abdussalam, A.; Gilani, M.R.H.S.; Zhang, W.; Xu, G. Hard Carbons as Anodes in Sodium-Ion Batteries: Sodium Storage Mechanism and Optimi-zation Strategies. Molecules 2022, 27, 6516. [Google Scholar] [CrossRef] [PubMed]
- Burchell, T.D.; McEnaney, B. Carbon Materials for Advanced Technologies (Structure and Bonding in Carbon Materials); Elsevier: Amsterdam, The Netherlands, 1999; Volume 1, p. 2. [Google Scholar]
- Pistoia, G. Lithium-Ion Batteries. Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, p. 25. [Google Scholar]
- Bates, A.M.; Preger, Y.; Torres-Castro, L.; Harrison, K.L.; Harris, S.J.; Hewson, J. Are solid-state batteries safer than lithium-ion batteries? Joule 2022, 6, 742–755. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, C.; Huang, Y.; Zhuang, Y.; Fan, M.; Lin, J.; Wang, L.; Xie, Q.; Peng, D.-L. Challenge and Strategies in Room Temperature Sodium–Sulfur Batteries: A Comparison with Lithium–Sulfur Batteries. Small 2022, 18, 2107368. [Google Scholar] [CrossRef]
- Xu, X.; Feng, J.; Liu, J.; Lv, F.; Hu, R.; Fang, F.; Yang, L.; Ouyang, L.; Zhu, M. Robust spindle-structured FeP@C for high-performance alkali-ion batteries anode. Electrochimi. Acta 2019, 312, 224–233. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, W.; Yuan, S.; Zhang, L.; Yang, Y.; Ge, P.; Ji, X. Designing Rational Interfacial Bonds for Hierarchical Mineral-Type Trogtalite with Double Carbon towards Ultra-Fast Sodium-Ions Storage Properties. Adv. Funct. Mater. 2021, 31, 2100156. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Yan, Z.; Chen, X.; Cheng, F.; Weiss, P.S.; Chen, J. Porous Multishelled Ni2P Hollow Microspheres as an Active Electrocatalyst for Hydrogen and Oxygen Evolution. Chem. Mater. 2017, 29, 8539–8547. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, G.; Zhang, Y.; Liang, H.; Huang, W. Conversion of hydroxide into carbon-coated phosphide using plasma for sodium ion batteries. Nano Res. 2022, 15, 2023–2029. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Li, D.; Ding, J.; Liu, Y.; Cai, Q. Fabrication of Core-Shell Ni2P@N, P−Co-Doped Carbon/Reduced Graphene Oxide Composite as Anode Material for Lithium- and Sodium-Ion Batteries. ChemElectroChem 2019, 6, 5492–5498. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Chen, H.; Lv, X.; Jiang, Y.; Feng, Y.; Rui, X.; Yu, Y. Advances in metal phosphides for sodium-ion batteries. Sustain. Mat. 2021, 1, 359–392. [Google Scholar]
- Jain, A.; Ong, S.P.; Hautier, G.; Wei Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation; APL Materials 1; AIP: Long Island, NY, USA, 2013; p. 011002. [Google Scholar]
- Zhou, S.; Huang, P.; Xiong, T.; Yang, F.; Yang, H.; Huang, Y.; Li, D.; Deng, J.; Balogun, M.S. Sub-Thick Electrodes with Enhanced Transport Kinetics via In Situ Epitaxial Heterogeneous Interfaces for High Areal-Capacity Lithium Ion Batteries. Small 2021, 17, 2100778. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, C.; Zhao, C.; Guo, W.; Yu, J.; Qiu, J. Interface Inversion: A Promising Strategy to Configure Ultrafine Nanoparticles over Graphene for Fast Sodium Storage. Small 2021, 17, 2005119. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, K.; Zhu, K.; Liu, X.; Ye, K.; Yan, J.; Wang, G.; Cao, D. Cable-like polyimide@carbon nanotubes composite as a capable anode for lithium ion batteries. Chem. Eng. J. 2022, 446, 137208. [Google Scholar] [CrossRef]
- Liu, S.; Niu, K.; Chen, S.; Sun, X.; Liu, L.; Jiang, B.; Chu, L.; Lv, X.; Li, M. TiO2 bunchy hierarchical structure with effective enhancement in sodium storage behaviors. Carbon Energy 2022, 4, 645–653. [Google Scholar] [CrossRef]
- Kim, C.; Kim, H.; Choi, Y.; Lee, H.A.; Jung, Y.S.; Park, J. Facile Method to Prepare for the Ni2P Nanostructures with Controlled Crystallinity and Morphology as Anode Materials of Lithium-Ion Batteries. ACS Omega 2018, 3, 7655–7662. [Google Scholar] [CrossRef]
- Miao, X.; Yin, R.; Ge, X.; Li, Z.; Yin, L. Ni2P@Carbon Core–Shell Nanoparticle-Arched 3D Interconnected Graphene Aerogel Architectures as Anodes for High-Performance Sodium-Ion Batteries. Small 2017, 13, 1702138. [Google Scholar] [CrossRef]
- Xiong, X.; You, C.; Cao, X.; Pang, L.; Kong, R.; Sun, X. Ni2P nanosheets array as a novel electrochemical catalyst electrode for non-enzymatic H2O2 sensing. Electrochim. Acta 2017, 253, 517–521. [Google Scholar] [CrossRef]
- Stern, L.-A.; Feng, L.; Song, F.; Hu, X. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Shi, F.; Xie, D.; Zhong, Y.; Wang, D.H.; Xia, X.H.; Gu, C.D.; Wang, X.L.; Tu, J.P. Facile synthesis of self-supported Ni2P nanosheet@Ni sponge composite for high-rate battery. J. Power Sources 2016, 328, 405–412. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, C.; Wang, L.; Jiang, Q.; Ji, C.; He, X. Preparation of mesoporous Ni2P nanobelts with high performance for electrocatalytic hydrogen evolution and supercapacitor. Int. J. Hydrogen Energy 2018, 43, 3697–3704. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Gu, J.; Li, Y.; Tian, Z.; Pang, H. Porous rod-like Ni2P/Ni assemblies for enhanced urea electrooxidation. Nano Res. 2021, 14, 1405–1412. [Google Scholar] [CrossRef]
- Chen, T.; Liu, D.; Lu, W.; Wang, K.; Du, G.; Asiri, A.M.; Sun, X. Three-Dimensional Ni2P Nanoarray: An Efficient Catalyst Electrode for Sensitive and Selective Nonenzymatic Glucose Sensing with High Specificity. Anal. Chem. 2016, 88, 7885–7889. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, X.; Pan, X.; Teng, C.; Wang, N. Yolk-shelled Ni2P@carbon nanocomposite as high-performance anode material for lithium and sodium ion batteries. Appl. Surf. Sci. 2019, 473, 699–705. [Google Scholar] [CrossRef]
- Duan, C.W.; Hu, L.X.; Ma, J.L. Ionic liquids as an efficient medium for the mechanochemical synthesis of α-AlH3 nano-composites. J. Mater. Chem A 2018, 6, 6309–6318. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Lin, X.-M.; Chen, J.-H.; Fan, J.-J.; Ma, Y.; Radjenovic, P.; Xu, Q.-C.; Huang, L.; Passerini, S.; Tian, Z.-Q.; Li, J.-F. Synthesis and Operando Sodiation Mechanistic Study of Nitrogen-Doped Porous Carbon Coated Bimetallic Sulfide Hollow Nanocubes as Advanced Sodium Ion Battery Anode. Adv. Energy Mater. 2019, 9, 1902312. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Zhao, X.; Liu, Y.; Nan, C.-W.; Fan, L.-Z. Two Birds with One Stone: Metal–Organic Framework Derived Micro-/Nanostructured Ni2P/Ni Hybrids Embedded in Porous Carbon for Electrocatalysis and Energy Storage. Adv. Funct. Mater. 2019, 29, 1901510. [Google Scholar] [CrossRef]
- Yuan, H.; Wu, M.; Zheng, J.; Chen, Z.-G.; Zhang, W.; Luo, J.; Jin, C.; Sheng, O.; Liang, C.; Gan, Y.; et al. Empowering Metal Phosphides Anode with Catalytic Attribute toward Superior Cyclability for Lithium-Ion Storage. Adv. Funct. Mater. 2019, 29, 1809051. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, T.; Fang, X.; Fan, Y.; Qian, S.; Gao, Y.; Cheng, N.; Xue, H.; Tian, J. Interface engineering of S-doped Co2P@Ni2P core–shell heterostructures for efficient and energy-saving water splitting. Chem. Eng. J. 2022, 439, 135743. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Zou, R.; Shi, G.; Huang, Y.; Yang, W.; Yang, W.; Liu, C.; Peng, X. Coupling overall water splitting and biomass oxidation via Fe-doped Ni2P@C nanosheets at large current density. Appl. Catal. B 2022, 307, 121170. [Google Scholar] [CrossRef]
- Wan, Y.; Song, K.; Chen, W.; Qin, C.; Zhang, X.; Zhang, J.; Dai, H.; Hu, Z.; Yan, P.; Liu, C.; et al. Ultra-High Initial Coulombic Efficiency Induced by Interface Engineering Enables Rapid, Stable Sodium Storage. Angew. Chem. Int. Ed. 2021, 60, 11481–11486. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; He, Q.; Liu, Y.; Key, J.; Nie, S.; Wu, M.; Shen, P.K. Three-dimensional, hetero-structured, Cu3P@C nanosheets with excellent cycling stability as Na-ion battery anode material. J. Mater. Chem A 2019, 7, 16999–17007. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Q.; Key, J.; Wu, M.; Shen, P.K. Self-assembled superstructure of carbon-wrapped, single-crystalline Cu3P porous nanosheets: One-step synthesis and enhanced Li-ion battery anode performance. Energy Storage Mater. 2018, 15, 75–81. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, C.; Zhang, W.; He, Y.; Li, X.; Yuan, X. Controlled design for integration of FeP into 3D carbon frameworks for superior Na storage. Chem. Eng. J. 2022, 429, 132271. [Google Scholar] [CrossRef]
- Wei, P.; Zhu, J.; Qiu, Y.; Wang, G.; Xu, X.; Ma, S.; Shen, P.K.; Wu, X.-L.; Yamauchi, Y. One-dimensional core–shell motif nanowires with chemically-bonded transition metal sulfide-carbon heterostructures for efficient sodium-ion storage. Chem. Sci. 2021, 12, 15054–15060. [Google Scholar] [CrossRef]
- Abdullaeva, Z.; Omurzak, E.; Iwamoto, C.; Ganapathy, H.S.; Sulaimankulova, S.; Liliang, C.; Mashimo, T. Onion-like carbon-encapsulated Co, Ni, and Fe magnetic nanoparticles with low cytotoxicity synthesized by a pulsed plasma in a liquid. Carbon 2012, 50, 1776–1785. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Fang, L.; Liu, L.; Qiu, H.; Wang, Y. Novel peapod array of Ni2P@graphitized carbon fiber composites growing on Ti substrate: A superior material for Li-ion batteries and the hydrogen evolution reaction. J. Mater. Chem A 2015, 3, 5434–5441. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Li, X.; Liu, L.; Xu, H.; Qiu, H.; Wang, Y. Novel peapod-like Ni2P nanoparticles with improved electrochemical properties for hydrogen evolution and lithium storage. Nanoscale 2015, 7, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, Y.; Tian, Z.Q.; Pan, Z.; Key, J.; Shen, P.K. Low temperature synthesis of polyhedral hollow porous carbon with high rate capability and long-term cycling stability as Li-ion and Na-ion battery anode material. J. Power Sources 2018, 398, 149–158. [Google Scholar] [CrossRef]
- Sun, C.; Chen, S.; Li, Z. Controllable synthesis of Fe2O3-carbon fiber composites via a facile sol-gel route as anode materials for lithium ion batteries. Appl. Surf. Sci. 2018, 427, 476–484. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Borstel, G. Towards a practical rechargeable 5 V Li ion battery. Phys. Stat. Sol. A. 2005, 202, R13–R15. [Google Scholar]
- Eglitis, R.I. Theoretical prediction of the 5 V rechargeable Li ion battery using Li2CoMn3O8 as a cathode. Phys. Scr. 2015, 90, 9. [Google Scholar] [CrossRef]
- Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R. Performance of layered Li(Ni1/3Co1/3Mn1/3) O2 as cathode for Li-ion batteries. Electrochim. Acta 2002, 48, 145–151. [Google Scholar] [CrossRef]
- Keller, M.; Buchholz, D.; Passerini, S. Layered Na-Ion Cathodes with Outstanding Performance Resulting from the Synergetic Effect of Mixed P- and O-Type Phases. Adv. Energy Mater. 2016, 6, 1501555. [Google Scholar]
- Chen, Y.; Qin, Z. General Applicability of Nanocrystalline Ni2P as a Noble-Metal-Free Cocatalyst to Boost Photocatalytic Hydrogen Generation. Catal. Sci. Technol. 2016, 6, 8212–8221. [Google Scholar] [CrossRef]
- Mai, Y.-J.; Xia, X.; Jie, X.-H. Cross-Linked Hierarchical Arrays of Ni2P Nanoflakes Prepared Via Directional Phosphorization and Their Applications for Advanced Alkaline Batteries. J. Power Sources 2017, 367, 116–121. [Google Scholar] [CrossRef]
- Liu, G.; He, D.; Yao, R.; Zhao, Y.; Li, J. Enhancing the Water Oxidation Activity of Ni2P Nanocatalysts by Iron-Doping and Electrochemical Activation. Electrochimi. Acta 2017, 253, 498–505. [Google Scholar] [CrossRef]
- Li, Q.; Ma, J.; Wang, H.; Yang, X.; Yuan, R.; Chai, Y. interconnected Ni2P Nanorods Grown on Nickel Foam for Binder Free Lithium Ion Batteries. Electrochim. Acta 2016, 213, 201–206. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, D.; Fan, L.; Wu, X.; Wang, M.; Zhang, N.; Sun, K. Ultra-High Rate Li-S Batteries Based on A Novel Conductive Ni2P Yolk-Shell Material as the Host for the S Cathode. J. Mater. Chem. A 2017, 5, 14519–14524. [Google Scholar] [CrossRef]
- Laursen, A.B.; Wexler, R.B.; Whitaker, M.J.; Izett, E.J.; Calvinho, K.U.D.; Hwang, S.; Rucker, R.; Wang, H.; Ji, J.; Garfunkel, E.; et al. Climbing the Volcano of Electrocatalytic Activity while Avoiding Catalyst Corrosion: Ni3P, a Hydrogen Evolution Electrocatalyst Stable in Both Acid and Alkali. Acs Catal. 2018, 8, 4408–4419. [Google Scholar] [CrossRef]
- Wang, X.D.; Cao, Y.; Teng, Y.; Chen, H.Y.; Xu, Y.F.; Kuang, D.B. Large-Area Synthesis of A Ni2P Honeycomb Electrode for Highly Efficient Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 32812–32819. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, Y.; Zhuo, S.; Zhang, J.; Zhang, B. Ni2P Nanosheets/Ni Foam Composite Electrode for Long-Lived and Ph-Tolerable Electrochemical Hydrogen Generation. ACS Appl. Mater. Interfaces 2015, 7, 2376–2384. [Google Scholar] [CrossRef]
- Liu, S.; Feng, J.; Bian, X.; Liu, J.; Xu, H. Electroless Deposition of Ni3P-Ni Arrays on 3-D Nickel Foam as A High Performance Anode for Lithium-Ion Batteries. RSC Adv. 2015, 5, 60870–60875. [Google Scholar] [CrossRef]
- Chen, G.-F.; Ma, T.Y.; Liu, Z.-Q.; Li, N.; Su, Y.-Z.; Davey, K.; Qiao, S.-Z. Efficient and Stable Bifunctional Electrocatalysts Ni/Nixmy (M = P, S) for Overall Water Splitting. Adv. Funct. Mater. 2016, 26, 3314–3323. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiong, D.; Liu, L. Fast Fabrication of Self-Supported Porous Nickel Phosphide Foam for Efficient, Durable Oxygen Evolution and Overall Water Splitting. J. Mater. Chem. A 2016, 4, 5639–5646. [Google Scholar] [CrossRef]
- Shi, S.; Li, Z.; Sun, Y.; Wang, B.; Liu, Q.; Hou, Y.; Huang, S.; Huang, J.; Zhao, Y. A Covalent Heterostructure of Monodisperse Ni2P Immobilized on N, P-Co-Doped Carbon Nanosheets for High Performance Sodium/Lithium Storage. Nano Energy 2018, 48, 510–517. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, T.; Li, X.; Bai, Y.; Lyu, X.; Liu, G.; Cai, W.; Li, Y. Bifunctional Hybrid Ni/Ni2P Nanoparticles Encapsulated By Graphitic Carbon Supported with N, S Modified 3D Carbon Framework for Highly Efficient Overall Water Splitting. Adv. Mater. Interfaces 2018, 5, 1800473. [Google Scholar] [CrossRef]
- Sun, T.; Dong, J.; Huang, Y.; Ran, W.; Chen, J.; Xu, L. Highly Active and Stable Electrocatalyst of Ni2P Nanoparticles Supported on 3D Ordered Macro-/Mesoporous Co-N-Doped Carbon for Acidic Hydrogen Evolution Reaction. J. Mater. Chem. A 2018, 6, 12751–12758. [Google Scholar] [CrossRef]
- Li, Y.; Cai, P.; Ci, S.; Wen, Z. Strongly Coupled 3D Nanohybrids with Ni2P/Carbon Nanosheets as Ph-Universal Hydrogen Evolution Reaction Electrocatalysts. Chemelectrochem 2017, 4, 340–344. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, H.; Mu, Y.; Li, W.; Sun, J.; Wu, K.; Wang, Y. Monodisperse Sandwich-Like Coupled Quasi-Graphene Sheets Encapsulating Ni2P Nanoparticles for Enhanced Lithium-Ion Batteries. Chemistry 2015, 21, 9229–9235. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Xu, J.; Shen, Y.; Xie, A. Synthesis and Excellent Performance of Porous Ni2P@C/Cnts Nanocomposite Derived From Ni-Mofs as an Anode for Lithium-Ion Batteries. Int. J. Energy Res. 2022, 46, 10875–10884. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Zhu, K.J.; Huang, J.D.; Yang, L.Y.; Li, S.T.; Wang, Z.Y.; Xie, J.R.; Wang, H.; Liu, J. Ultrafine Ni2P Nanoparticles Embedded in one-Dimensional Carbon Skeleton Derived From Metal-Organic Frameworks Template as a High-Performance Anode for Lithium Ion Battery. J. Alloys Compd. 2019, 775, 490–497. [Google Scholar] [CrossRef]
- Dong, C.; Guo, L.; He, Y.; Chen, C.; Qian, Y.; Chen, Y.; Xu, L. Sandwich-Like Ni2P Nanoarray/Nitrogen-Doped Graphene Nanoarchitecture as A High-Performance Anode for Sodium and Lithium Ion Batteries. Energy Storage Mater. 2018, 15, 234–241. [Google Scholar] [CrossRef]
- Zhou, D.; Xue, L.-P.; Wang, N. Robustly Immobilized Ni2P Nanoparticles in Porous Carbon Networks Promotes High-Performance Sodium-Ion Storage. J. Alloys Compd. 2019, 776, 912–918. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhao, Z.; Tian, Z.; Zhang, D.; Wu, Y. Ni2P Nanoflake Array/Three Dimensional Graphene Architecture as Integrated Free-Standing Anode for Boosting the Sodiation Capability and Stability. ChemElectroChem 2019, 6, 404–412. [Google Scholar] [CrossRef]
- Yin, S.; Rho, Y.; Swainson, I.; Nazar, L. X-ray/Neutron Diffraction and Electrochemical Studies of Lithium De/Re-Intercalation in Li1-xCo1/3Ni1/3Mn1/3O2 (x = 0→1). Chem. Mater. 2006, 18, 1901–1910. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, F.; He, Q.; Zhang, X.; Key, J.; Shen, P.; Zhu, J. Facile Synthesis of Nickel Phosphide @ N-Doped Carbon Nanorods with Exceptional Cycling Stability as Li-Ion and Na-Ion Battery Anode Material. Batteries 2023, 9, 267. https://doi.org/10.3390/batteries9050267
Fu F, He Q, Zhang X, Key J, Shen P, Zhu J. Facile Synthesis of Nickel Phosphide @ N-Doped Carbon Nanorods with Exceptional Cycling Stability as Li-Ion and Na-Ion Battery Anode Material. Batteries. 2023; 9(5):267. https://doi.org/10.3390/batteries9050267
Chicago/Turabian StyleFu, Fang, Qiuchen He, Xuan Zhang, Julian Key, Peikang Shen, and Jinliang Zhu. 2023. "Facile Synthesis of Nickel Phosphide @ N-Doped Carbon Nanorods with Exceptional Cycling Stability as Li-Ion and Na-Ion Battery Anode Material" Batteries 9, no. 5: 267. https://doi.org/10.3390/batteries9050267
APA StyleFu, F., He, Q., Zhang, X., Key, J., Shen, P., & Zhu, J. (2023). Facile Synthesis of Nickel Phosphide @ N-Doped Carbon Nanorods with Exceptional Cycling Stability as Li-Ion and Na-Ion Battery Anode Material. Batteries, 9(5), 267. https://doi.org/10.3390/batteries9050267




