A High-Performance Li-O2/Air Battery System with Dual Redox Mediators in the Hydrophobic Ionic Liquid-Based Gel Polymer Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of IL-Based Gel Polymer Electrolyte (IL-GPE)
2.3. Assembling the Li-O2 Batteries and Electrochemical Measurement
2.4. Material Characterization
3. Results and Discussion
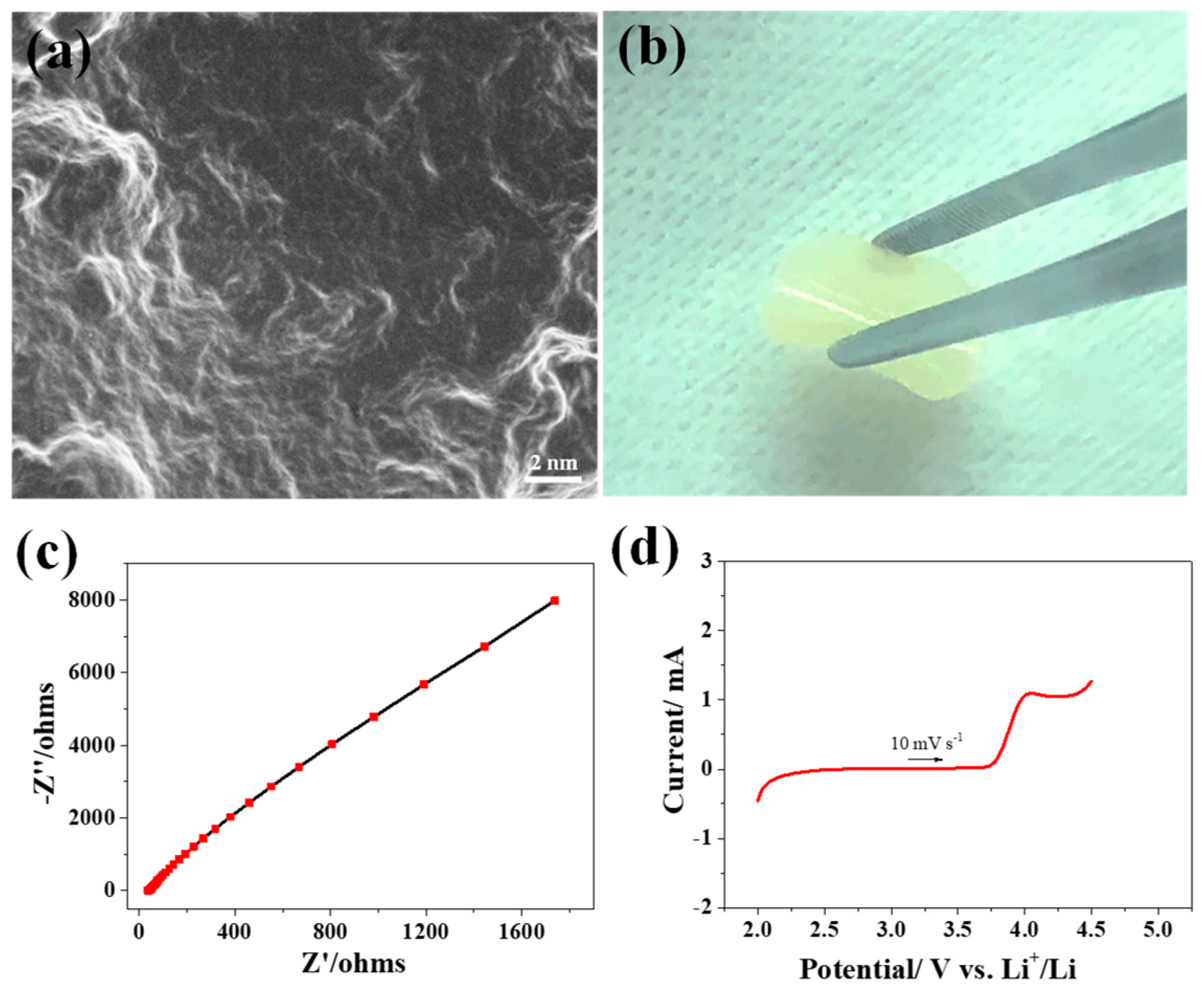
3.1. Characterization of Polymer Electrolyte
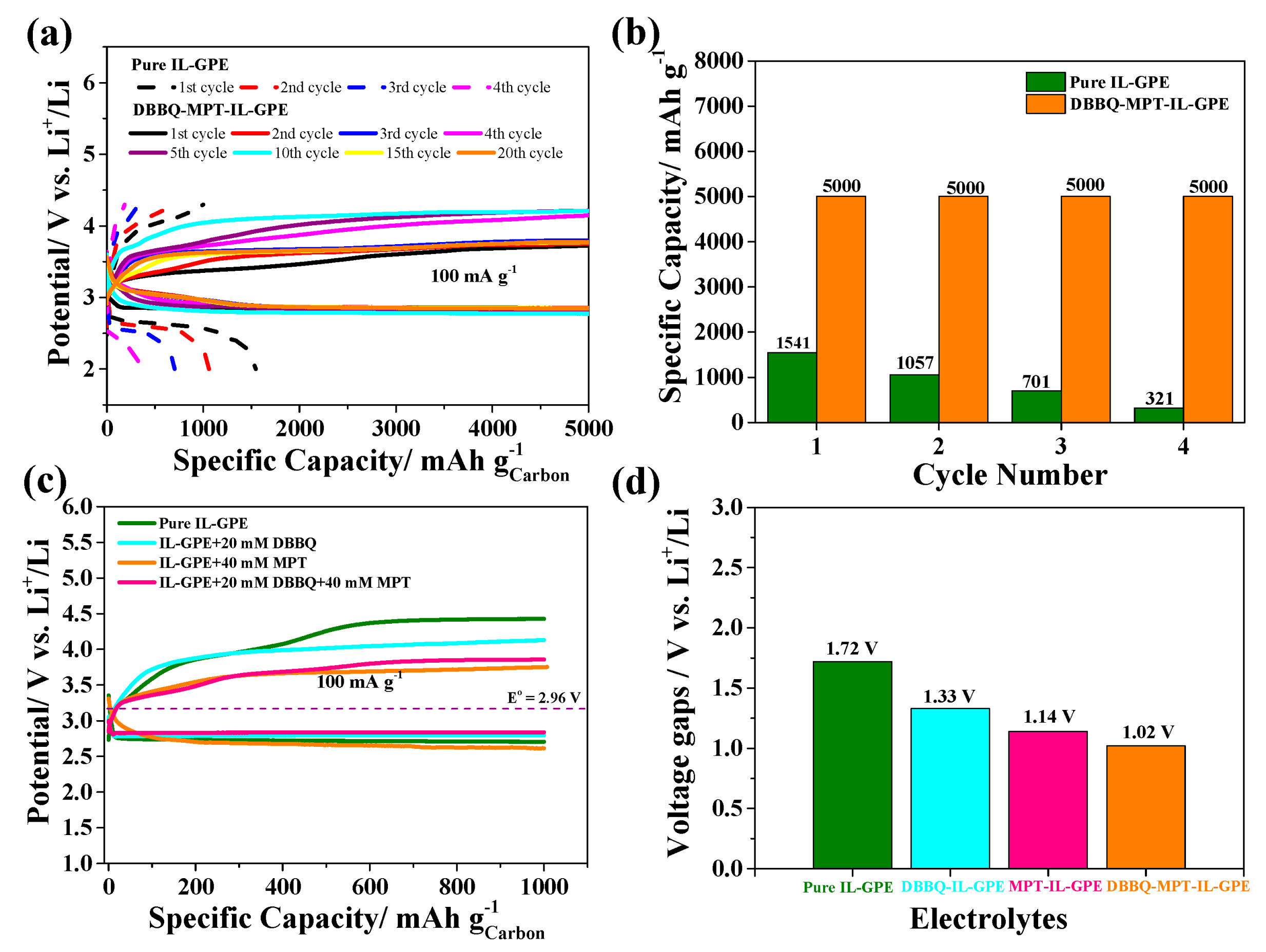
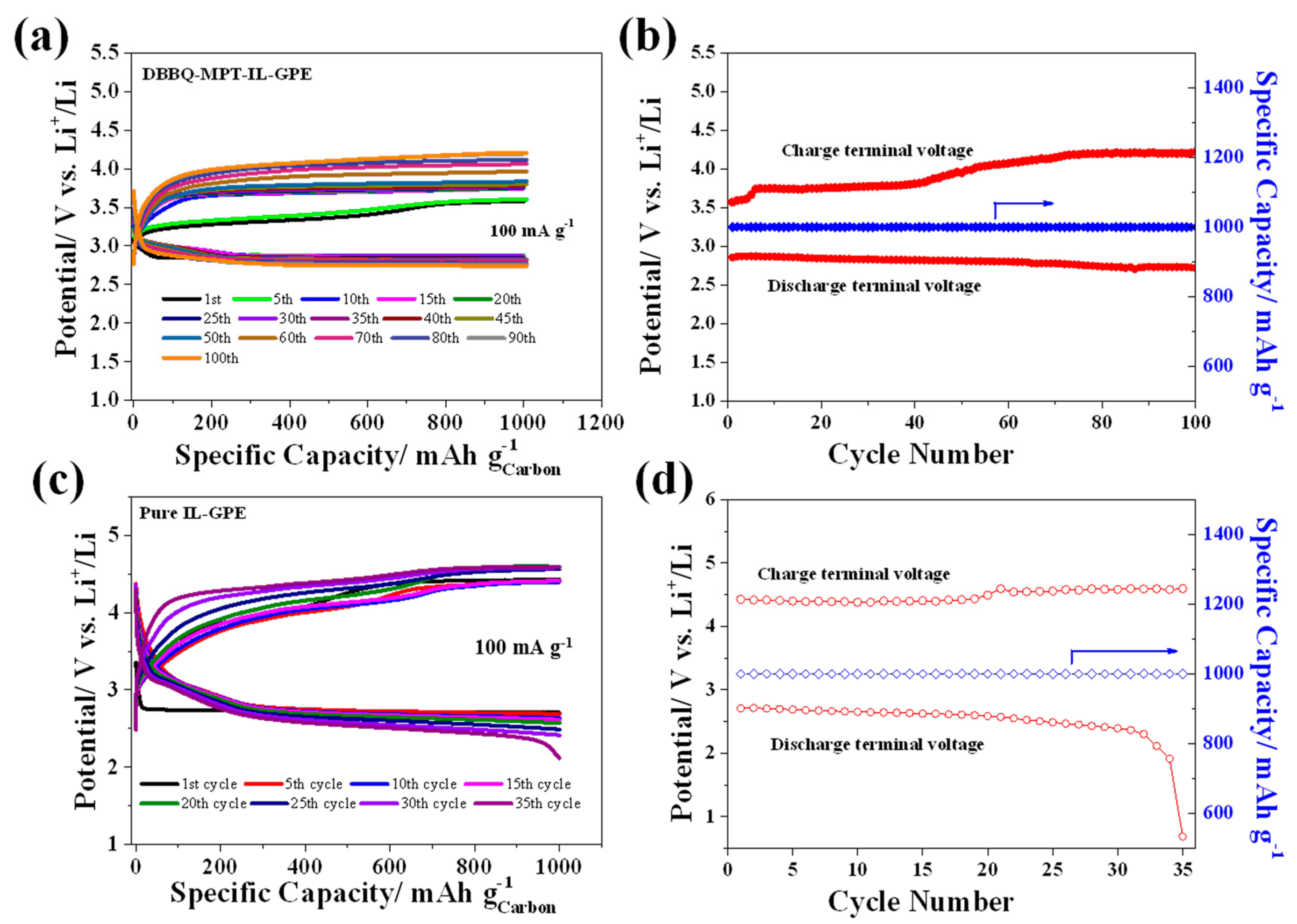
3.2. Electrochemical Performance of the GPE-Based Li-O2 Batteries
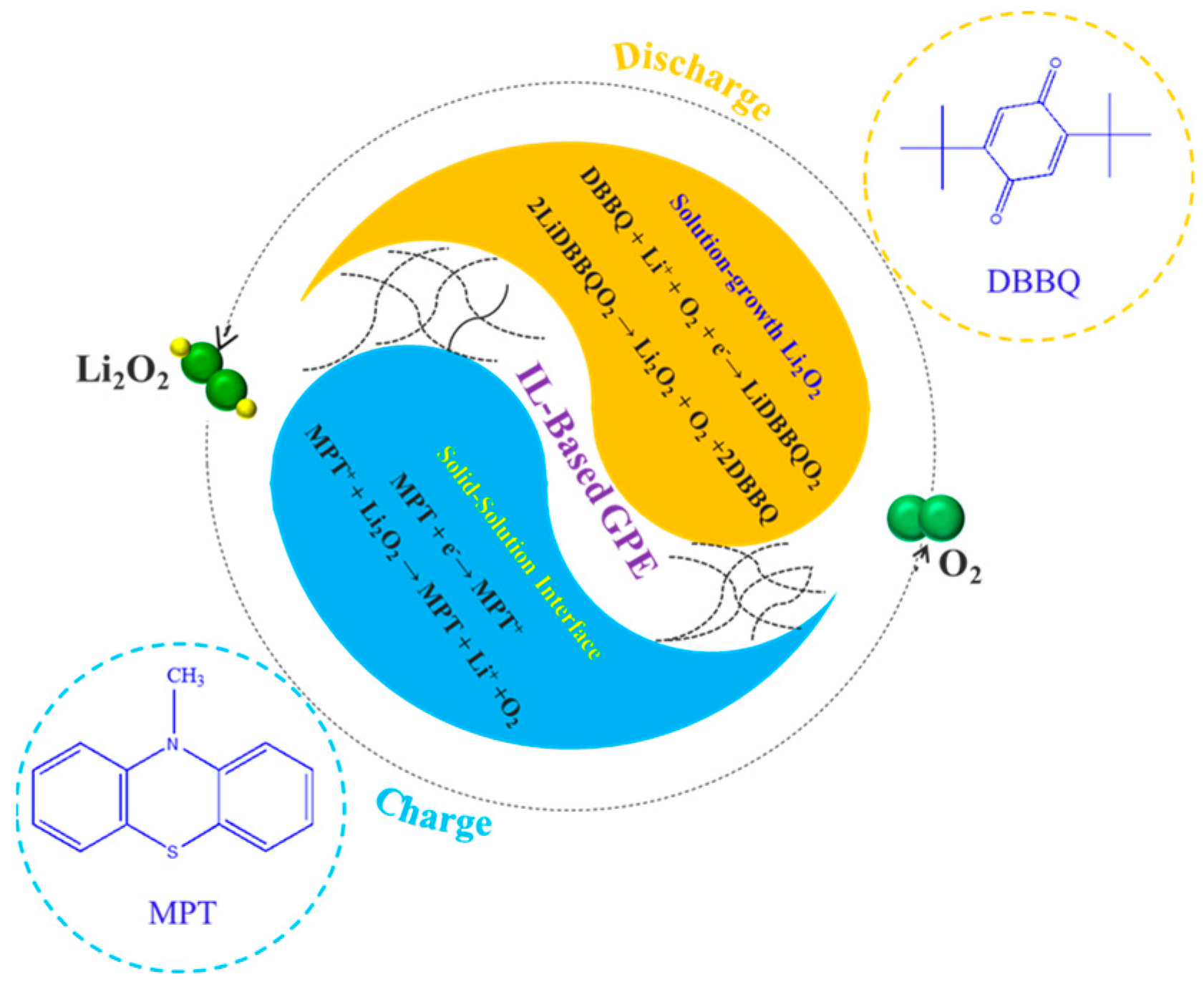
3.3. Products Characterization of Dual RMs-IL-GPE-Based Li-O2 Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kwak, W.J.; Rosy; Sharon, D.; Xia, C.; Kim, H.; Johnson, L.R.; Bruce, P.G.; Nazar, L.F.; Sun, Y.K.; Frimer, A.A.; et al. Lithium-Oxygen Batteries and Related Systems: Potential, Status, and Future. Chem. Rev. 2020, 120, 6626–6683. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Xu, J.J.; He, P.; Qiao, Y.; Guo, S.H.; Yang, H.J.; Zhou, H.S. Metal-air batteries: Progress and perspective. Sci. Bull. 2022, 67, 2449–2486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Dong, P.P.; Song, M.K. Advances in Lithium-Oxygen Batteries Based on Lithium Hydroxide Formation and Decomposition. Front. Chem. 2022, 10, 923936. [Google Scholar] [CrossRef]
- Feng, N.N.; He, P.; Zhou, H.S. Critical Challenges in Rechargeable Aprotic Li-O2 Batteries. Adv. Energy Mater. 2016, 6, 1502303. [Google Scholar] [CrossRef]
- Ma, L.; Yu, T.; Tzoganakis, E.; Amine, K.; Wu, T.; Chen, Z.W.; Lu, J. Fundamental Understanding and Material Challenges in Rechargeable Nonaqueous Li-O2 Batteries: Recent Progress and Perspective. Adv. Energy Mater. 2018, 8, 1800348. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.; Zhang, X. Functional and stability orientation synthesis of materials and structures in aprotic Li-O2 batteries. Chem. Soc. Rev. 2018, 47, 2921–3004. [Google Scholar] [CrossRef]
- Guo, H.; Luo, W.; Chen, J.; Chou, S.L.; Liu, H.K.; Wang, J.Z. Review of Electrolytes in Nonaqueous Lithium-Oxygen Batteries. Adv. Sustain. Syst. 2018, 2, 1700183. [Google Scholar] [CrossRef]
- Amici, J.; Marquez, P.; Mangini, A.; Torchio, C.; Dessantis, D.; Versaci, D.; Francia, C.; Aguirre, M.J.; Bodoardo, S. Sustainable, economic, and simple preparation of an efficient catalyst for Li-O2 batteries. J. Power Sources 2022, 546, 231942. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, S.H.; Jung, H.G.; Aurbach, D.; Sun, Y.K. Redox Mediators for Li-O2 Batteries: Status and Perspectives. Adv. Mater. 2018, 30, 1704162. [Google Scholar] [CrossRef]
- Khan, Z.; Vagin, M.; Crispin, X. Can Hybrid Na–Air Batteries Outperform Nonaqueous Na–O2 Batteries? Adv. Sci. 2020, 7, 1902866. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, S.; Wu, Y.; Chen, Y.H. Promoting Li–O2 Batteries with Redox Mediators. ChemSusChem 2019, 12, 104–114. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Johnson, L.; Bruce, P.G. Promoting solution phase discharge in Li-O2 batteries containing weakly solvating electrolyte solutions. Nat. Mater. 2016, 15, 882–888. [Google Scholar] [CrossRef]
- Chang, Z.W.; Xu, J.J.; Zhang, X.B. Recent Progress in Electrocatalyst for Li-O2 Batteries. Adv. Energy Mater. 2017, 7, 1700875. [Google Scholar] [CrossRef]
- Johnson, L.; Li, C.M.; Liu, Z.; Chen, Y.H.; Freunberger, S.A.; Ashok, P.C.; Praveen, B.B.; Dholakia, K.; Tarascon, J.M.; Bruce, P.G. The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li-O2 batteries. Nat. Chem. 2014, 6, 1091–1099. [Google Scholar] [CrossRef]
- Freunberger, S.A.; Chen, Y.; Drewett, N.E.; Hardwick, L.J.; Bardé, F.; Bruce, P.G. The lithium-oxygen battery with ether-based electrolytes. Angew. Chem. Int. Ed. Engl. 2011, 50, 8609–8613. [Google Scholar] [CrossRef]
- Gu, Z.; Xin, X.; Yang, J.; Guo, D.; Yang, S.; Wu, J.; Sun, Y.; Yao, X. Bilayer NASICON/Polymer Hybrid Electrolyte for Stable Solid-State Li–O2 Batteries. ACS Appl. Energy Mater. 2022, 5, 9149–9157. [Google Scholar] [CrossRef]
- Asadi, M.; Sayahpour, B.; Abbasi, P.; Ngo, A.T.; Karis, K.; Jokisaari, J.R.; Liu, C.; Narayanan, B.; Gerard, M.; Yasaei, P.; et al. A lithium-oxygen battery with a long cycle life in an air-like atmosphere. Nature 2018, 555, 502–506. [Google Scholar] [CrossRef]
- Das, S.; Hojberg, J.; Knudsen, K.B.; Younesi, R.; Johansson, P.; Norby, P.; Vegge, T. Instability of Ionic Liquid-Based Electrolytes in Li-O2 Batteries. J. Phys. Chem. C 2015, 119, 18084–18090. [Google Scholar] [CrossRef]
- Cecchetto, L.; Salomon, M.; Scrosati, B.; Croce, F. Study of a Li-air battery having an electrolyte solution formed by a mixture of an ether-based aprotic solvent and an ionic liquid. J. Power Sources 2012, 213, 233–238. [Google Scholar] [CrossRef]
- Khan, A.; Zhao, C. Enhanced performance in mixture DMSO/ionic liquid electrolytes: Toward rechargeable Li-O2 batteries. Electro. Commun. 2014, 49, 1–4. [Google Scholar] [CrossRef]
- Garcia-Quintana, L.; Ortiz-Vitoriano, N.; Zhu, H.; Nolis, G.M.; Herrero-Martin, J.; Echeverría, M.; López del Amo, J.M.; Forsyth, M.; Bond, A.M.; Howlett, P.C.; et al. Unveiling the Impact of the Cations and Anions in Ionic Liquid/Glyme Hybrid Electrolytes for Na–O2 Batteries. ACS Appl. Mater. Interfaces 2022, 14, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Quartarone, E.; Tomasi, C.; Bini, M.; Galinetto, P.; Fagnoni, M.; Mustarelli, P. Investigation of Ether-Based Ionic Liquid Electrolytes for Lithium-O2 Batteries. J. Electrochem. Soc. 2015, 162, A3001–A3006. [Google Scholar] [CrossRef]
- Jung, K.N.; Lee, J.I.; Jung, J.H.; Shin, K.H.; Lee, J.W. A quasi-solid-state rechargeable lithium-oxygen battery based on a gel polymer electrolyte with an ionic liquid. Chem. Commun. 2014, 50, 5458–5461. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, Y.; Johnson, L.; Jovanov, Z.P.; Bruce, P.G. A rechargeable lithium–oxygen battery with dual mediators stabilizing the carbon cathode. Nat. Energy 2017, 2, 17118. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Lang, S.Y.; Shi, Y.; Ma, J.M.; Wen, R.; Wan, L.J. Revealing the Surface Effect of the Soluble Catalyst on Oxygen Reduction/Evolution in Li-O2 Batteries. J. Am. Chem. Soc. 2019, 141, 6900–6905. [Google Scholar] [CrossRef]
- Lee, D.; Park, H.; Ko, Y.; Park, H.; Hyeon, T.; Kang, K.; Park, J. Direct Observation of Redox Mediator-Assisted Solution-Phase Discharging of Li-O2 Battery by Liquid-Phase Transmission Electron Microscopy. J. Am. Chem. Soc. 2019, 141, 8047–8052. [Google Scholar] [CrossRef]
- Chen, Y.; Freunberger, S.A.; Peng, Z.; Fontaine, O.; Bruce, P.G. Charging a Li-O2 battery using a redox mediator. Nat. Chem. 2013, 5, 489–494. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Q.; Zhang, R.; Trimm, B.D.; Whittingham, M.S. The electrochemical behaviour of TTF in Li-O2 batteries using a TEGDME-based electrolyte. Chem. Commun. 2016, 52, 7580–7583. [Google Scholar] [CrossRef]
- Bergner, B.J.; Schurmann, A.; Peppler, K.; Garsuch, A.; Janek, J. TEMPO: A mobile catalyst for rechargeable Li-O2 batteries. J. Am. Chem. Soc. 2014, 136, 15054–15064. [Google Scholar] [CrossRef]
- Feng, N.; He, P.; Zhou, H. Enabling catalytic oxidation of Li2O2 at the liquid-solid interface: The evolution of an aprotic Li-O2 battery. ChemSusChem 2015, 8, 600–602. [Google Scholar] [CrossRef]
- Feng, N.; Mu, X.; Zhang, X.; He, P.; Zhou, H.S. Intensive Study on the Catalytical Behavior of N-Methylphenothiazine as a Soluble Mediator to Oxidize the Li2O2 Cathode of the Li-O2 Battery. ACS Appl. Mater. Interfaces 2017, 9, 3733–3739. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Qi, H.; Sun, X.; Song, X.; Guo, Z.Y.; Tamirat, A.G.; Liu, J.; Wang, L.; Feng, S. Drawing a Pencil-Trace Cathode for a High-Performance Polymer-Based Li-CO2 Battery with Redox Mediator. Adv. Funct. Mater. 2019, 29, 1806863. [Google Scholar] [CrossRef]
- Dai, A.; Li, Q.; Liu, T.; Amine, K.; Lu, J. Fundamental Understanding of Water-Induced Mechanisms in Li-O2 Batteries: Recent Developments and Perspectives. Adv. Mater. 2019, 31, e1805602. [Google Scholar] [CrossRef]
- Aetukuri, N.B.; McCloskey, B.D.; Garcia, J.M.; Krupp, L.E.; Viswanathan, V.; Luntz, A.C. Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li-O2 batteries. Nat. Chem. 2015, 7, 50–56. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.; Kim, D.W.; Suk, J.; Park, O.O.; Kang, Y. Flexible binder-free graphene paper cathodes for high-performance Li-O2 batteries. Carbon 2015, 93, 625–635. [Google Scholar] [CrossRef]
- Kundu, D.; Black, R.; Berg, E.J.; Nazar, L.F. A highly active nanostructured metallic oxide cathode for aprotic Li-O2 batteries. Energy Environ. Sci. 2015, 8, 1292–1298. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Speidel, A.; Scheffler, R.; Miller, D.C.; Viswanathan, V.; Hummelshøj, J.S.; Nørskov, J.K.; Luntz, A.C. Twin Problems of Interfacial Carbonate Formation in Nonaqueous Li–O2 Batteries. J. Phys. Chem. Lett. 2012, 3, 997–1001. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Scheffler, R.; Speidel, A.; Girishkumar, G.; Luntz, A.C. On the Mechanism of Nonaqueous Li-O2 Electrochemistry on C and Its Kinetic Overpotentials: Some Implications for Li-Air Batteries. J. Phys. Chem. C 2012, 116, 23897–23905. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, N.; Wang, C.; Wang, J.; Lin, Y.; Yang, G. A High-Performance Li-O2/Air Battery System with Dual Redox Mediators in the Hydrophobic Ionic Liquid-Based Gel Polymer Electrolyte. Batteries 2023, 9, 243. https://doi.org/10.3390/batteries9050243
Feng N, Wang C, Wang J, Lin Y, Yang G. A High-Performance Li-O2/Air Battery System with Dual Redox Mediators in the Hydrophobic Ionic Liquid-Based Gel Polymer Electrolyte. Batteries. 2023; 9(5):243. https://doi.org/10.3390/batteries9050243
Chicago/Turabian StyleFeng, Ningning, Chaoqiang Wang, Jing Wang, Yang Lin, and Gang Yang. 2023. "A High-Performance Li-O2/Air Battery System with Dual Redox Mediators in the Hydrophobic Ionic Liquid-Based Gel Polymer Electrolyte" Batteries 9, no. 5: 243. https://doi.org/10.3390/batteries9050243
APA StyleFeng, N., Wang, C., Wang, J., Lin, Y., & Yang, G. (2023). A High-Performance Li-O2/Air Battery System with Dual Redox Mediators in the Hydrophobic Ionic Liquid-Based Gel Polymer Electrolyte. Batteries, 9(5), 243. https://doi.org/10.3390/batteries9050243








