Impedance Investigation of Silicon/Graphite Anode during Cycling
Abstract
1. Introduction
2. Experimental Section
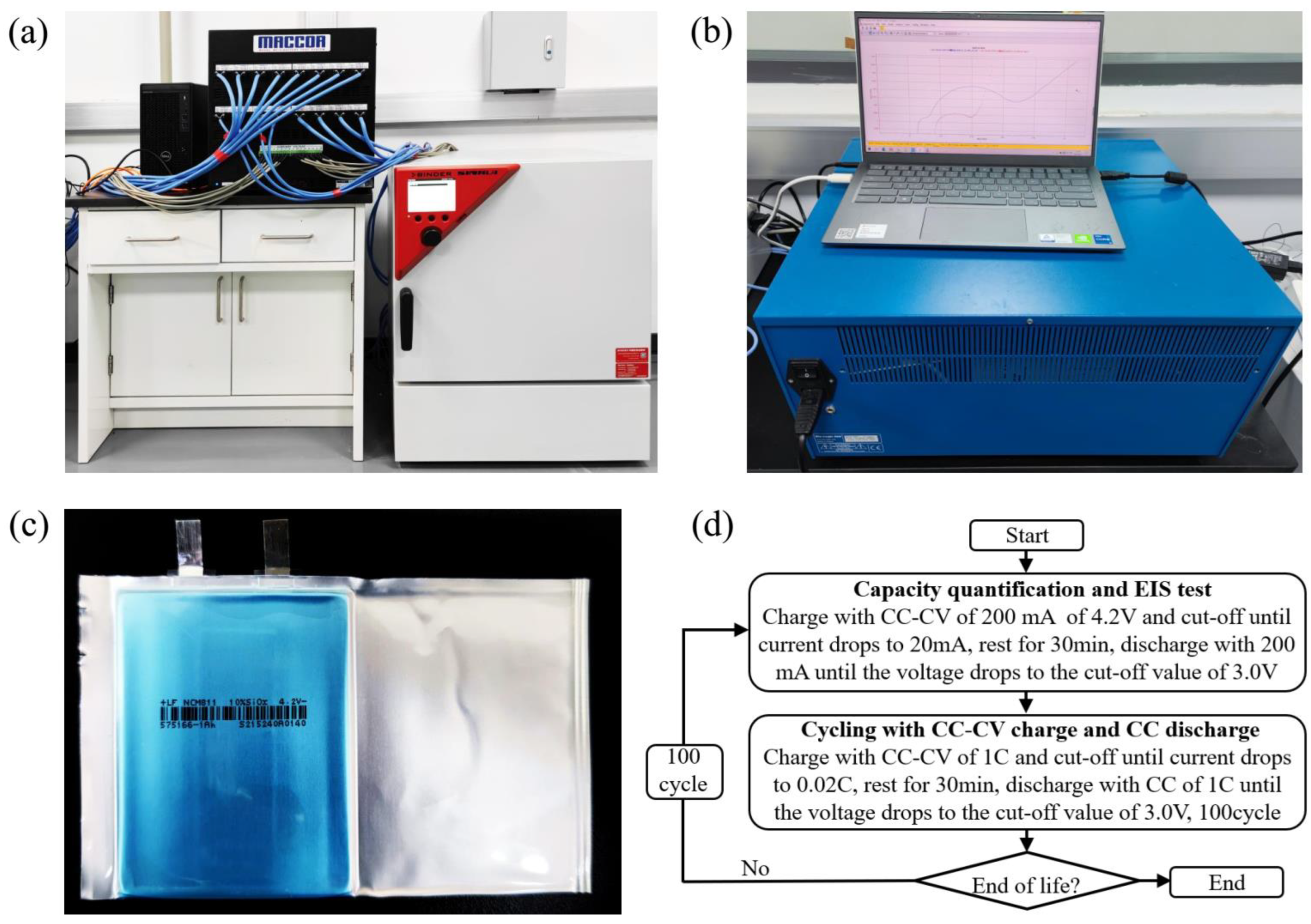
2.1. Cyclic Aging Tests and Electrochemical Impedance Spectroscopy Tests
2.2. Investigating the Effect of SOC on Electromechanical Characterizations
2.3. Post Mortem Characterization Tests
3. Results and Discussion
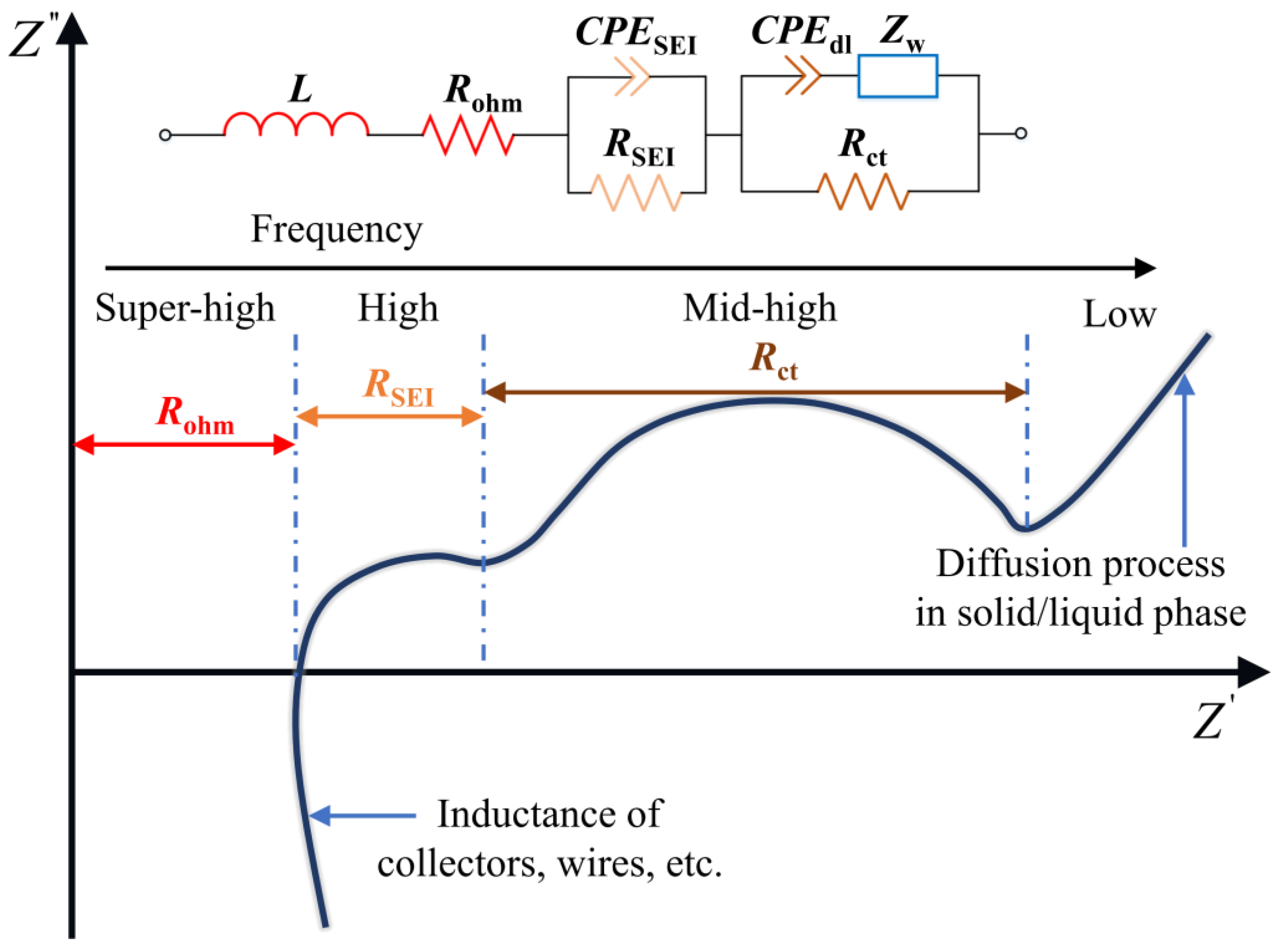
3.1. Battery EIS and Equivalent Circuit Model
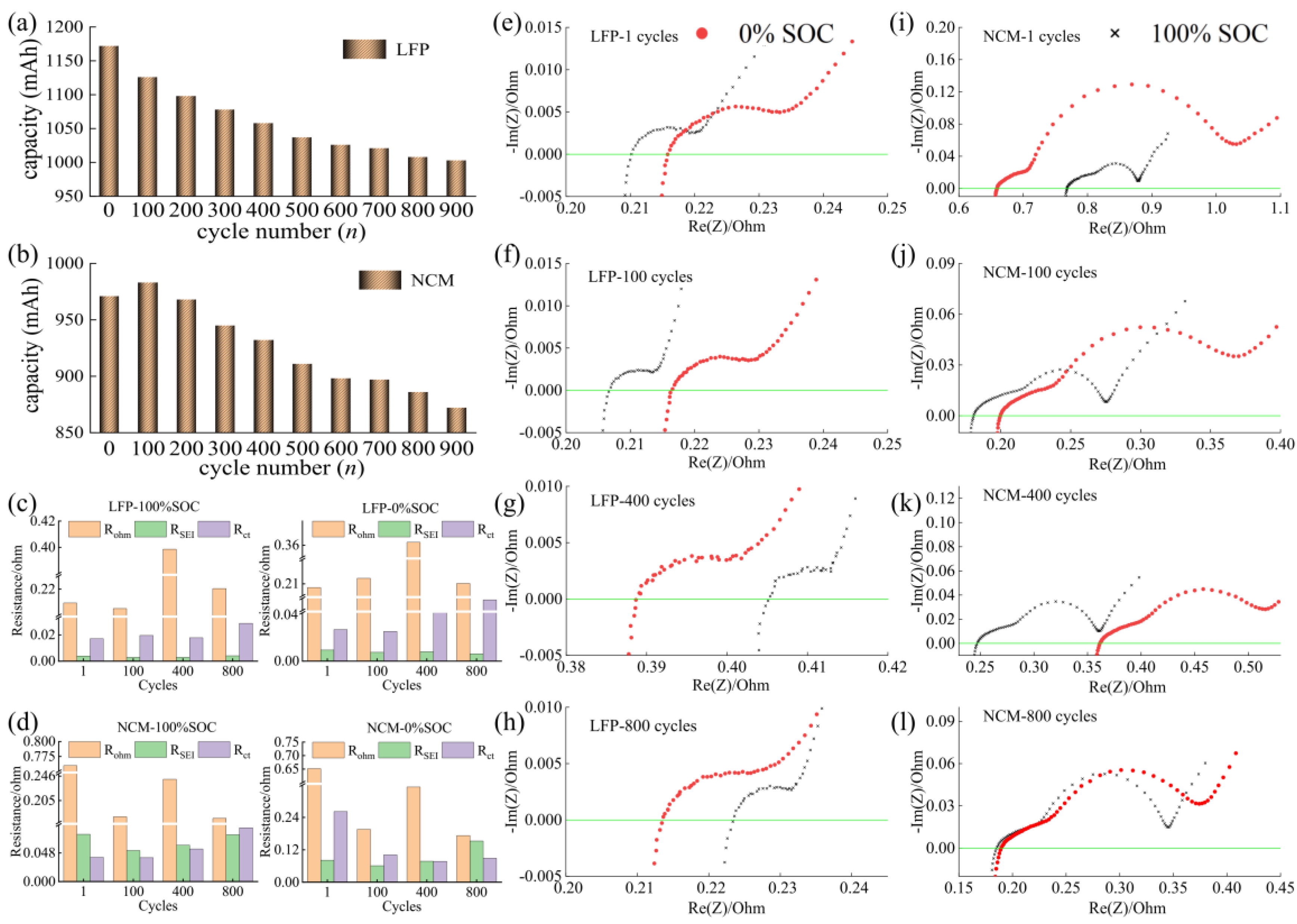
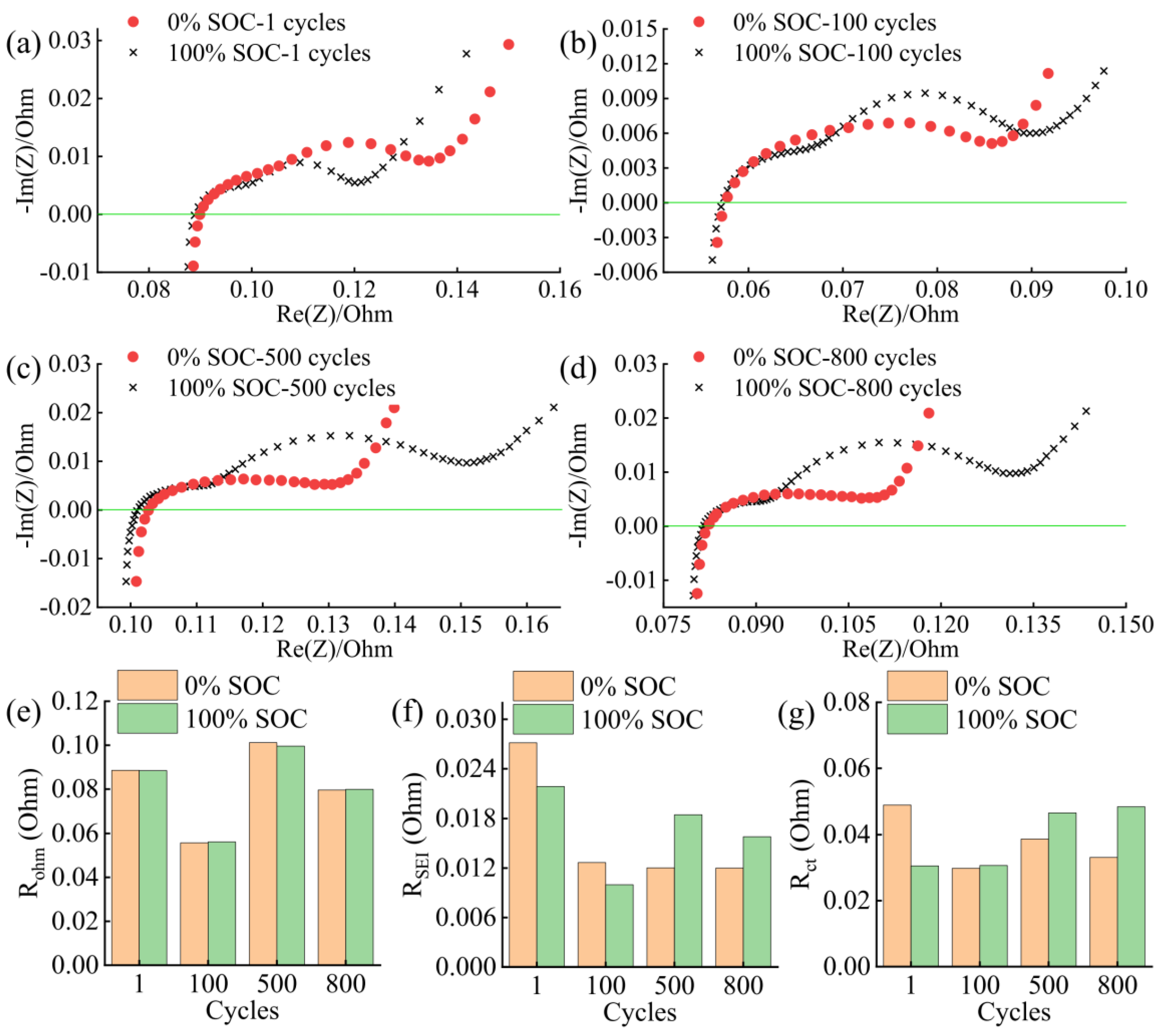
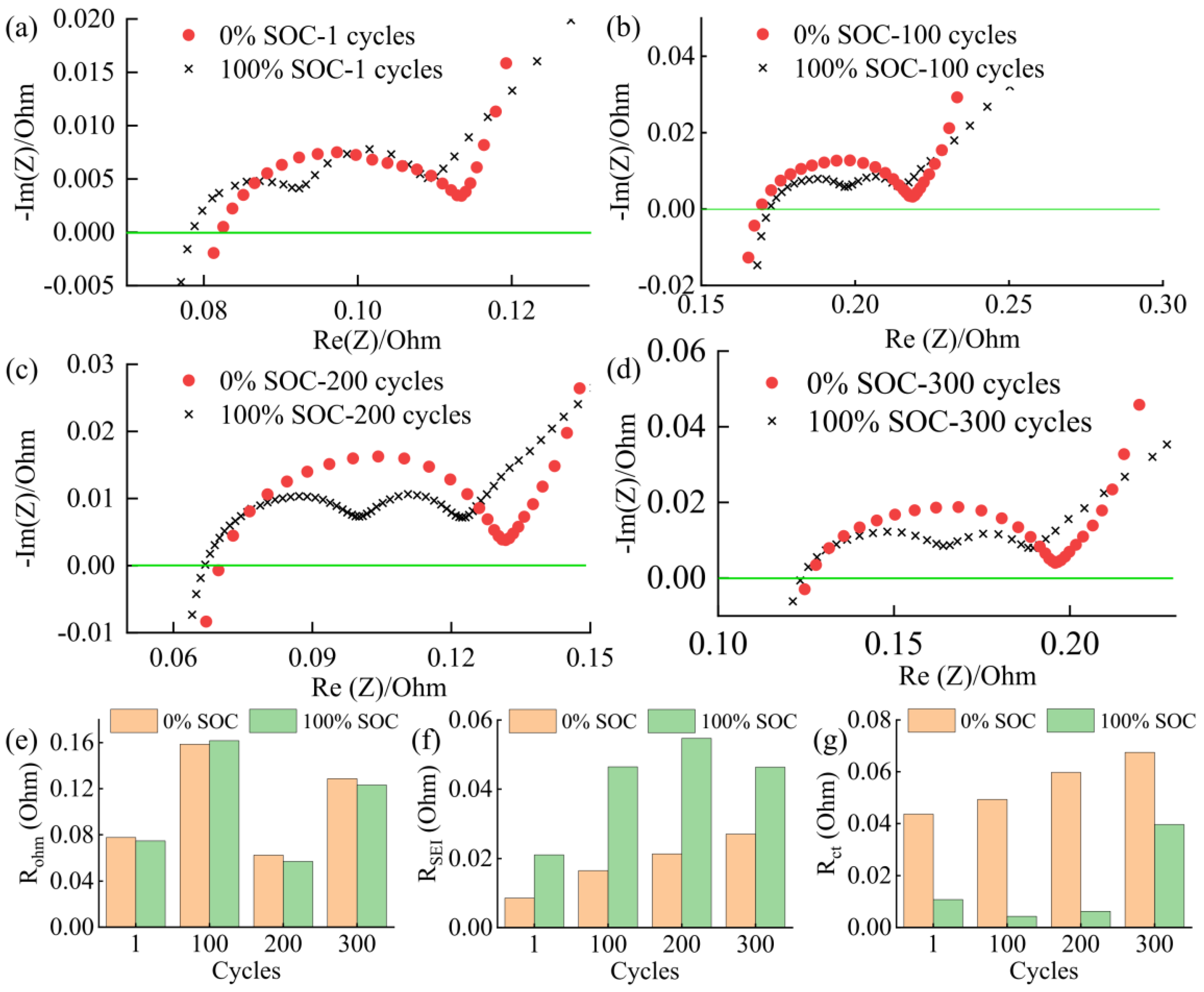
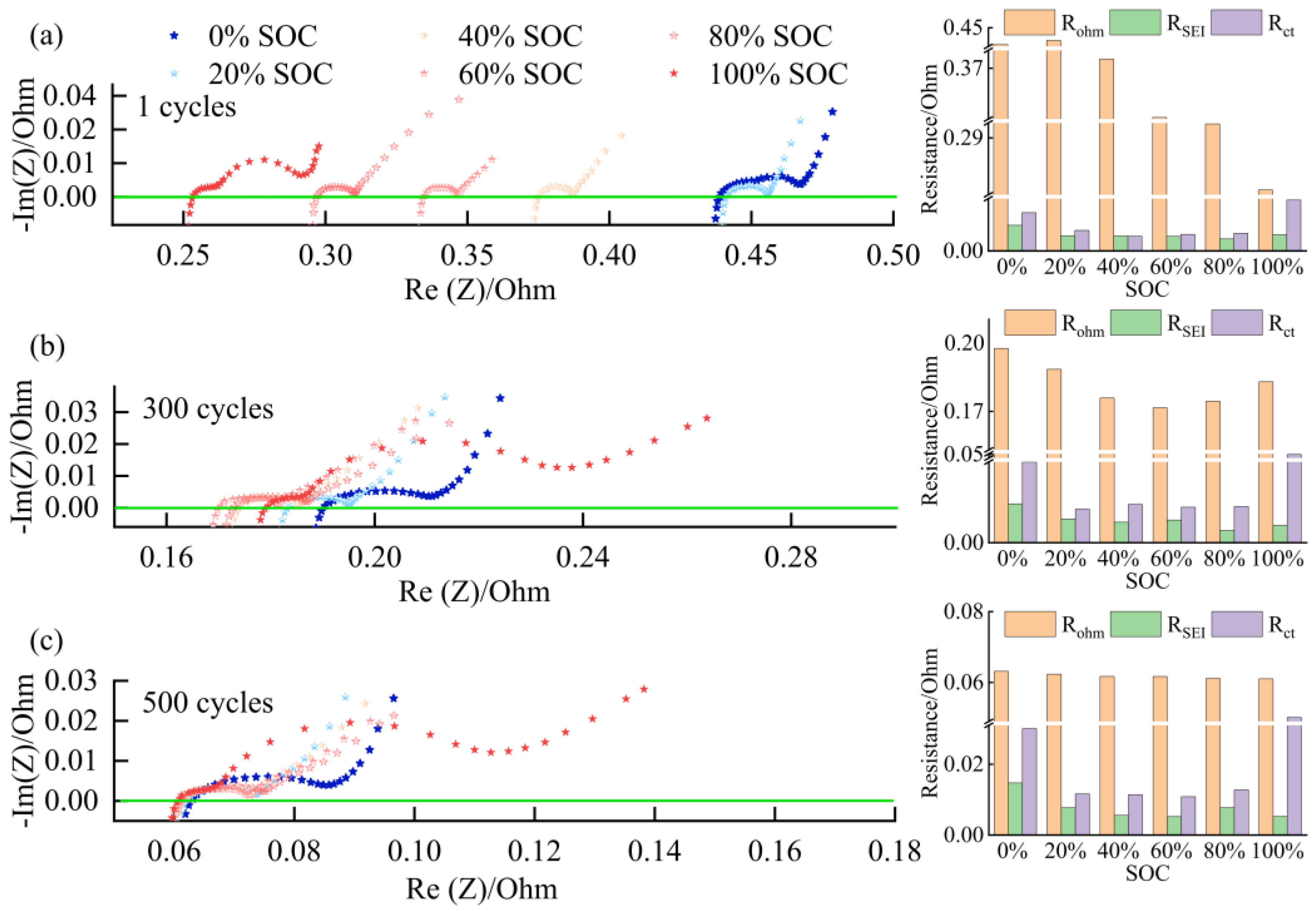
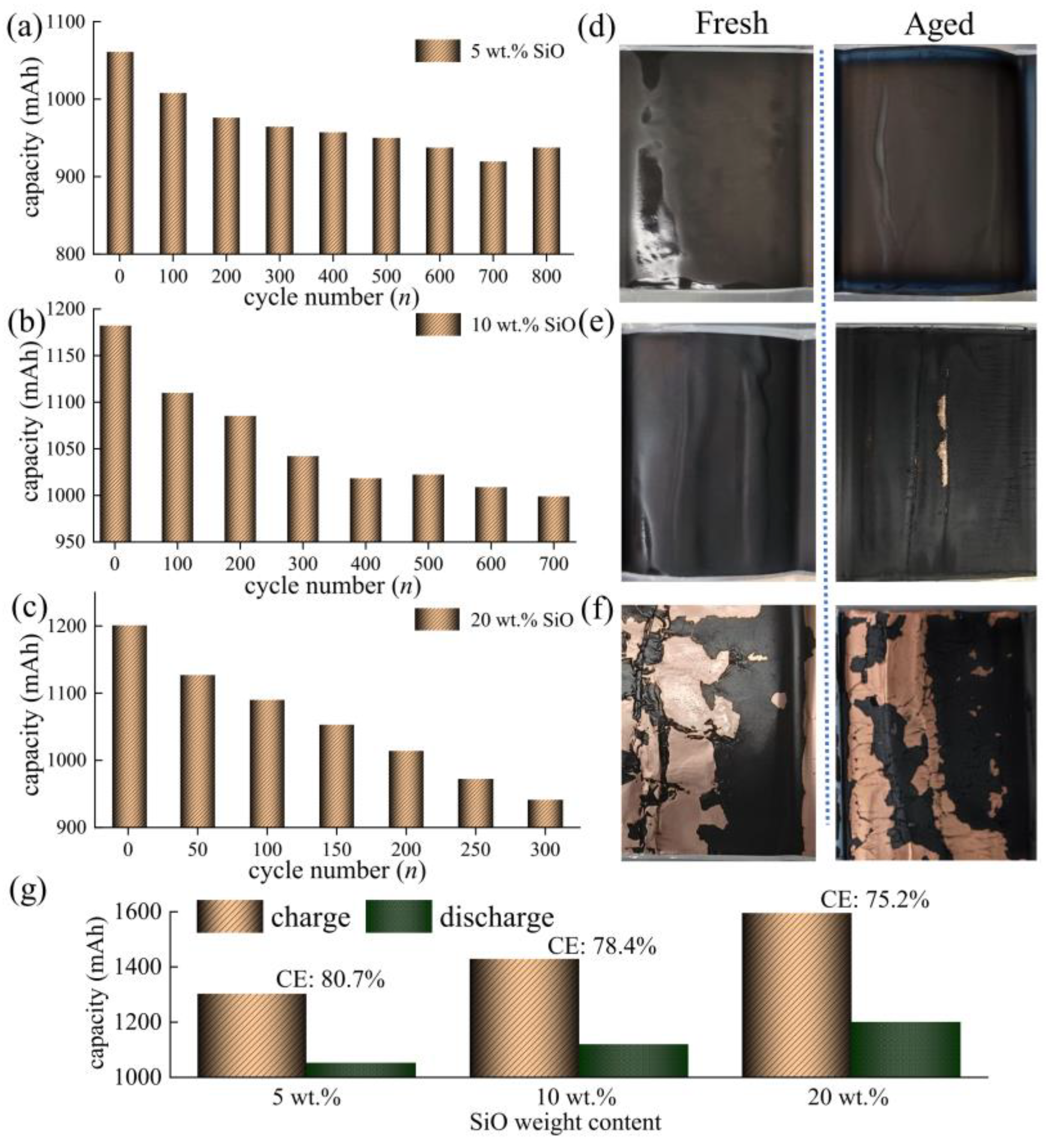
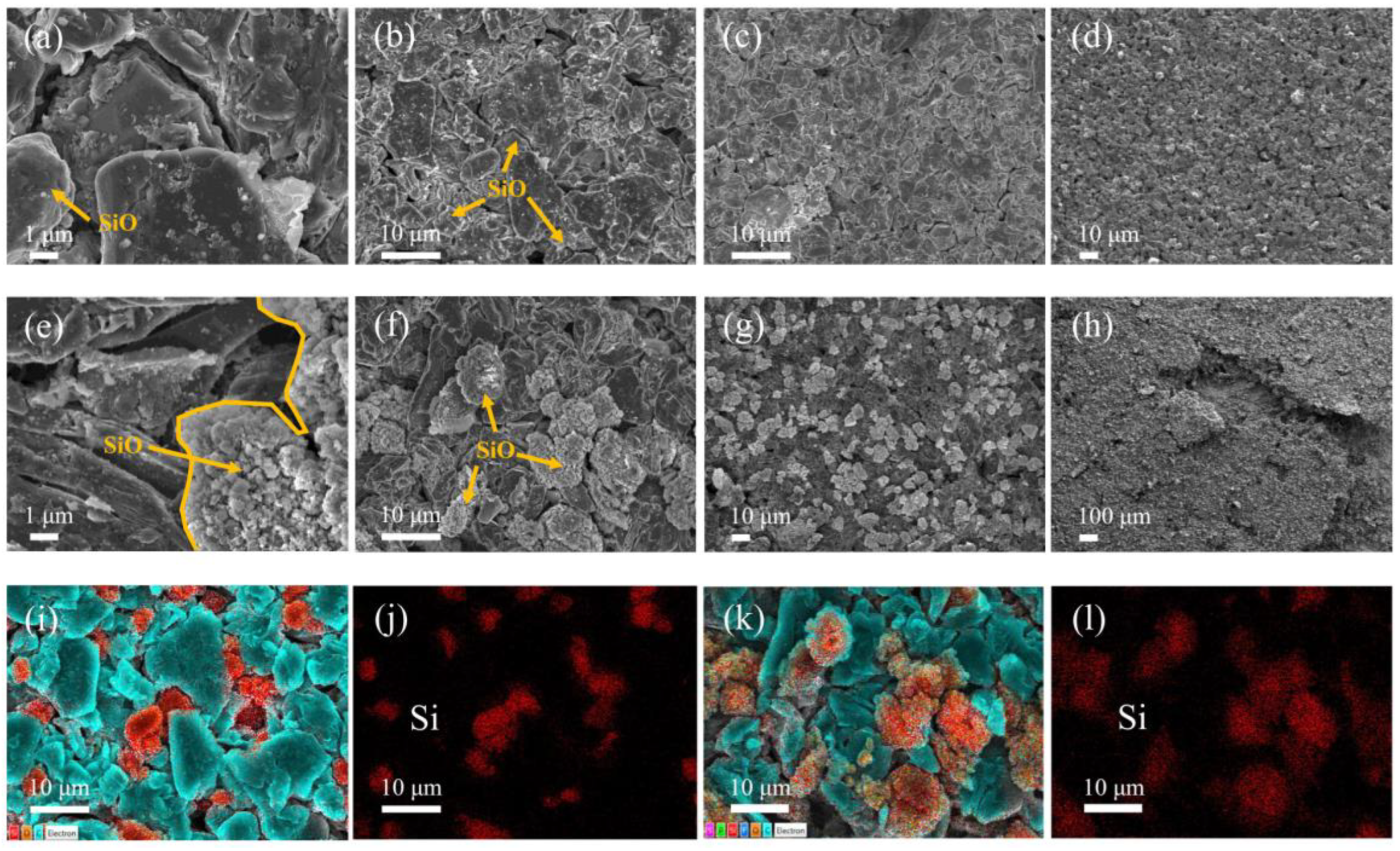
3.2. Battery Degradation and Impedance Analysis
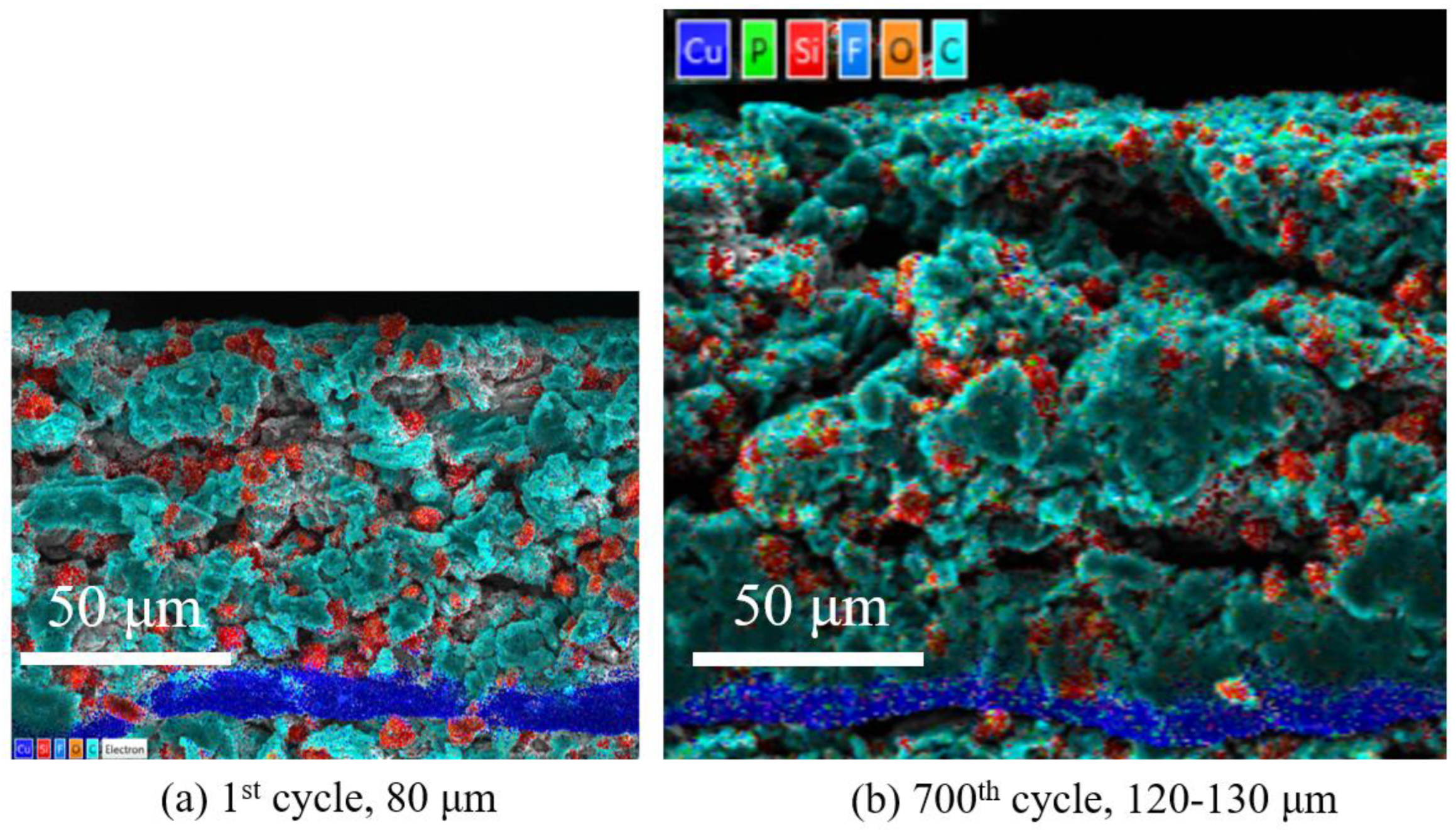
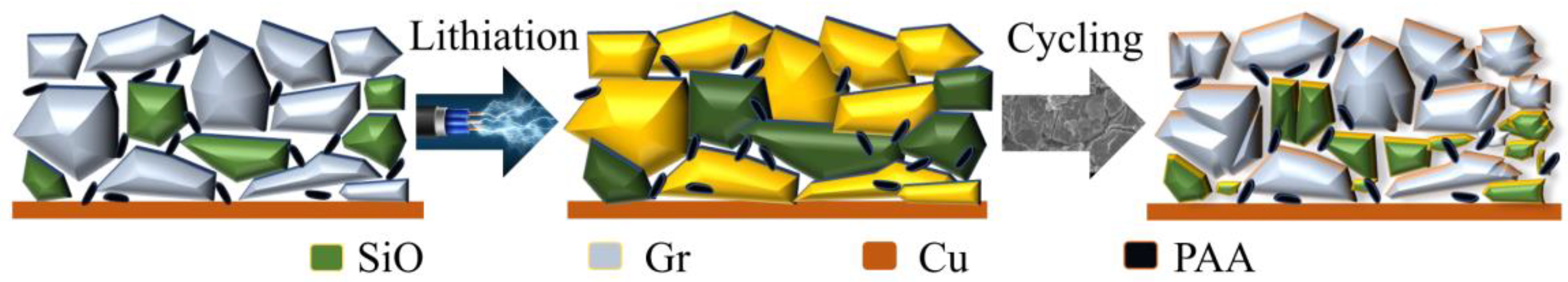
- In this work, each cell is filled with a sufficient quantity of electrolyte, and the effect of electrolyte consumption on ohmic resistance is ignored. In contrast, the active lithium loss and the SiO particles volume expansion of the electrode have a more pronounced and direct effect on the conductivity and diffusivity properties [27].
- For the cell 5 wt.% SiO and the cell 10 wt.% SiO, Rohm decreases during charging due to the moderate swelling of the anode electrodes. However, the volume expansion of the SiO particles and the continued growth of SEI film significantly affect the diffusion of Li+ in the electrode particles and electrolyte, especially when the cell is fully charged. As the mechanical force responds, the effective electrolyte conductivity and diffusivity decrease, causing a gradual increase in charge transfer resistance. For the cell 20 wt.% SiO, the irreversible change of the fracture of SiO particles and the formation of the solid-electrolyte interphase are completed in the early stage during cyclic aging. Therefore, the SOC has no significant effect on Rohm after a few cycles for cell 20 wt.% SiO. On the contrary, incorporating a surplus of SiO particles resulted in significant crack extensions and the flaking of the active material in the anode. The significant swelling of the anode electrode when the cell finished charging will instead precisely fit the electrode to the active material, thus reducing the value of Rct.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef]
- Lin, C.; Burggraf, P.; Liu, L.; Adlon, T.; Mueller, K.; Beyer, M.; Xu, T.; Kammerer, V.; Hu, J.; Liu, S.; et al. Deep-Dive analysis of the latest Lithium-Ion battery safety testing standards and regulations in Germany and China. Renew. Sustain. Energy Rev. 2023, 173, 113077. [Google Scholar] [CrossRef]
- You, H.; Zhu, J.; Wang, X.; Jiang, B.; Sun, H.; Liu, X.; Wei, X.; Han, G.; Ding, S.; Yu, H.; et al. Nonlinear health evaluation for lithium-ion battery within full-lifespan. J. Energy Chem. 2022, 72, 333–341. [Google Scholar] [CrossRef]
- Sun, S.; Yao, N.; Jin, C.; Xie, J.; Li, X.; Zhou, M.; Chen, X.; Li, B.; Zhang, X.; Zhang, Q. The Crucial Role of Electrode Potential of a Working Anode in Dictating the Structural Evolution of Solid Electrolyte Interphase. Angew. Chem. Int. Ed. 2022, 61, 202208743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, J.; Saito, N.; Zhang, Z.; Yang, L.; Hirano, S. Search for Stable Host Materials as Low-voltage Anodes for Lithium-ion Batteries: A Mini-review. Energy Storage Mater. 2023, 55, 364–387. [Google Scholar] [CrossRef]
- Lee, B.; Oh, S.; Choi, Y.; Yi, M.; Kim, S.; Kim, S.; Sung, Y.; Shin, S.; Lee, Y.; Yu, S. SiO-induced thermal instability and interplay between graphite and SiO in graphite/SiO composite anode. Nat. Commun. 2022, 14, 150. [Google Scholar] [CrossRef]
- Xu, S.; Hou, X.; Wang, D.; Zuin, L.; Zhou, J.; Hou, Y.; Mann, M. Insights into the Effect of Heat Treatment and Carbon Coating on the Electrochemical Behaviors of SiO Anodes for Li-Ion Batteries. Adv. Energy Mater. 2022, 12, 2200127. [Google Scholar] [CrossRef]
- Li, D.; Chu, F.; He, Z.; Cheng, Y.; Wu, F. Single-material aluminum foil as anodes enabling high-performance lithium-ion batteries: The roles of prelithiation and working mechanism. Mater. Today 2022, 58, 80–90. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, T.; Coskun, A.; Choi, J. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 2017, 357, 279–283. [Google Scholar] [CrossRef]
- Carthy, K.; Gullapalli, H.; Ryan, K.; Kennedy, T. Review—Use of Impedance Spectroscopy for the Estimation of Li-ion Battery State of Charge, State of Health and Internal Temperature. J. Electrochem. Soc. 2021, 168, 080517. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sust. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Tanim, T.; Yang, Z.; Finegan, D.; Chinnam, P.; Lin, Y.; Weddle, P.; Jansen, A. A Comprehensive Understanding of the Aging Effects of Extreme Fast Charging on High Ni NMC Cathode. Adv. Energy Mater. 2022, 12, 2103712. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Huang, Y.; Gopaluni, R.; Cao, Y.; Heere, M.; Mühlbauer, M.; Mereacre, L.; Dai, H.; Liu, X.; et al. Data-driven capacity estimation of commercial lithium-ion batteries from voltage relaxation. Nat. Commun. 2022, 13, 2261. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, R.; Dai, H.; Zhang, N.; Chen, Q.; Wei, X. A novel dual time scale life prediction method for lithium-ion batteries considering effects of temperature and state of charge. Int. J. Energ. Res. 2021, 45, 14692–14709. [Google Scholar] [CrossRef]
- Zhu, J.; Darma, M.; Knapp, M.; Sørensen, D.R.; Heere, M.; Fang, Q.; Wang, X.; Dai, H.; Mereacre, L.; Senyshyn, A.; et al. Investigation of lithium-ion battery degradation mechanisms by combining differential voltage analysis and alternating current impedance. J. Power Sources 2020, 448, 227575. [Google Scholar] [CrossRef]
- Rodrigues, S.; Munichandraiah, N.; Shukla, A. A review of state-of-charge indication of batteries by means of a.c. impedance measurements. J. Power Sources 2000, 87, 12–20. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, P.; Dai, J.; Gui, W. Estimating the state-of-charge of lithium-ion battery using an h-infinity observer based on electrochemical impedance model. IEEE Access 2020, 8, 26872–26884. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Deng, Z.; Yang, L. Application of electrochemical impedance spectroscopy in battery management system: State of charge estimation for aging batteries. J. Energy Storage 2023, 57, 106275. [Google Scholar] [CrossRef]
- Gao, X.; Li, S.; Xue, J.; Hu, D.; Xu, J. A Mechanistic and Quantitative Understanding of the Interactions between SiO and Graphite Particles. Adv. Energy Mater. 2022, 13, 2202584. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Kim, Y.; Kim, M.; Choi, A.; Jeong, K.; Lee, H. Unveiling the role of electrode-level heterogeneity alleviated in a silicon-graphite electrode under operando microscopy. Energy Storage Mater. 2023, 57, 269–276. [Google Scholar] [CrossRef]
- Kumar, P.; Berhaut, C.; Dominguez, D.; Vito, E.; Tardif, S.; Pouget, S.; Lyonnard, S.; Jouneau, P. Nano-Architectured Composite Anode Enabling Long-Term Cycling Stability for High-Capacity Lithium-Ion Batteries. Small 2020, 16, 1906812. [Google Scholar] [CrossRef]
- Heidrich, B.; Pritzlaff, L.; Börner, M.; Winter, M.; Niehoff, P. Comparative X-ray Photoelectron Spectroscopy Study of the SEI and CEI in Three Different Lithium Ion Cell Formats. J. Electrochem. Soc. 2022, 169, 030533. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Wahl, M.; Spitthoff, L.; Muri, H.; Jinasena, A.; Burheim, O.; Lamb, J. The Importance of Optical Fibres for Internal Temperature Sensing in Lithium-ion Batteries during Operation. Energies 2021, 14, 3617. [Google Scholar] [CrossRef]
- Sabet, P.; Stahl, G.; Sauer, D. Non-invasive investigation of predominant processes in the impedance spectra of high energy lithium-ion batteries with Nickel-Cobalt-Aluminum cathodes. J. Power Sources 2018, 406, 185–193. [Google Scholar] [CrossRef]
- Yuan, C.; Hahn, Y.; Lu, W.; Oancea, V.; Xu, J. Quantification of electrochemical-mechanical coupling in lithium-ion batteries. Cell Rep. Phys. Sci. 2022, 3, 101158. [Google Scholar] [CrossRef]
- Pidaparthy, S.; Luo, M.; Rodrigues, M.; Zuo, J.; Abraham, D. Physicochemical Heterogeneity in Silicon Anodes from Cycled Lithium-Ion Cells. ACS Appl. Mater. Interfaces 2022, 14, 38660–38668. [Google Scholar] [CrossRef]
- Dai, C.; Li, C.; Huang, H.; Wang, Z.; Zhu, X.; Liao, X.; Chen, X.; Pan, Y.; Fang, D. In situ strain measurements and stress analysis of SiO@C composite electrodes during electrochemical cycling by using digital image correlation. Solid State Ionics 2019, 331, 56–65. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C. Stress evolution and capacity fade in constrained lithium-ion pouch cells. J. Power Sources 2014, 245, 745–751. [Google Scholar] [CrossRef]
- Li, R.; Ren, D.; Wang, S.; Xie, Y.; Hou, Z.; Lu, L.; Ouyang, M. Non-destructive local degradation detection in large format lithium-ion battery cells using reversible strain heterogeneity. J. Energy Storage 2021, 40, 102788. [Google Scholar] [CrossRef]

| Cell Type | Voltage Window | Charge (CC-CV) | Discharge (CC) | EIS Test | Test Temperature |
|---|---|---|---|---|---|
| LFP | 2.4–3.65 V | 1 C with upper cut-off voltage until 0.02 C | 1 C | 10 kHz–10 mHz | 25 °C |
| NCM 523 | 3.0–4.2 V | ||||
| 5 wt.% SiO | 3.0–4.2 V | ||||
| 10 wt.% SiO | |||||
| 20 wt.% SiO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhu, J.; Dai, H.; Yu, C.; Wei, X. Impedance Investigation of Silicon/Graphite Anode during Cycling. Batteries 2023, 9, 242. https://doi.org/10.3390/batteries9050242
Wang X, Zhu J, Dai H, Yu C, Wei X. Impedance Investigation of Silicon/Graphite Anode during Cycling. Batteries. 2023; 9(5):242. https://doi.org/10.3390/batteries9050242
Chicago/Turabian StyleWang, Xiuwu, Jiangong Zhu, Haifeng Dai, Chao Yu, and Xuezhe Wei. 2023. "Impedance Investigation of Silicon/Graphite Anode during Cycling" Batteries 9, no. 5: 242. https://doi.org/10.3390/batteries9050242
APA StyleWang, X., Zhu, J., Dai, H., Yu, C., & Wei, X. (2023). Impedance Investigation of Silicon/Graphite Anode during Cycling. Batteries, 9(5), 242. https://doi.org/10.3390/batteries9050242







