Abstract
Layered lithium transition metal (TM) oxides LiTMO2 (TM = Ni, Co, Mn, Al, etc.) are the most promising cathode materials for lithium-ion batteries because of their high energy density, good rate capability and moderate cost. However, the safety issue arising from the intrinsic thermal instability of nickel-based cathode materials is still a critical challenge for further applications in electric vehicles and energy storage power stations. The main reasons include side reactions between the highly reactive Ni3+/4+ and liquid electrolyte, oxygen release accompanied by structural phase transition, and internal microcrack propagation owing to the low strength of spherical secondary particles. Great efforts have been invested to modify nickel-based cathode materials such as stabilization of bulk structure by element doping, surface engineering, nanostructure design, and particle mono-crystallization. In this review, we summarize these advances and try to give an in-depth insight into the origin of the thermal instability of nickel-based cathode materials. More importantly, some effective strategies to improve thermal stability are outlined, expecting to accelerate the future development of layered TM oxides with high safety.
1. Introduction
Lithium-ion batteries have become the market leader in portable electronic devices (such as smartphones and laptops) due to their high energy density, long cycle life, low self-discharge rate, and reduced cost [1,2,3]. This rechargeable battery laid the foundation of a wireless and portable lifestyle as the Nobel Prize in Chemistry 2019 said [4]. From a clean energy perspective, electric cars using lithium-ion batteries are becoming increasingly popular, and large-scale energy storage power stations are being widely constructed jointly with wind or photovoltaic power generations [5].
Basically, a lithium-ion battery (cell) is composed of cathode (positive) material, anode (negative) material, separator, electrolyte, and other auxiliary materials, such as a binder, current collector, lug and shell. In commercial lithium-ion batteries, the practical capacity of cathode material determines the upper limit of the battery energy density given the fact that graphite still dominates the anode electrode today. The commercialized cathode materials include layered structure materials, spinel type materials (LiMn2O4 for example), and olivine type materials (LiFePO4 for example). As shown in Table 1, the layered Ni-based cathode materials have good comprehensive performance, such as high energy density, good cycle performance, and moderate cost [6]. Compared to LiFePO4 (~150 mAh g−1) and LiMn2O4 (~110 mAh g−1), the layered nickel-based materials, such as LiNi0.5Co0.2Mn0.3O2 (NCM523), LiNi0.8Co0.1Mn0.1O2 (NCM811) and LiNi0.8Co0.15Al0.05O2 (NCA), are taking the market share of power battery because of their higher capacity (>165 mAh g−1) [7].

Table 1.
A comparison of several commercial cathode materials.
However, safety accidents (such as smoke and fire), hundreds for electric cars, and scores for energy storage power stations, have been reported every year worldwide, most of which were caused by the thermal runaway of lithium-ion batteries according to the investigation reports [2,8,9,10]. Therefore, it’s necessary to figure out the main reasons behind the thermal runaway of lithium-ion batteries.
At present, the mechanism and failure sequence of battery thermal runaway have been deeply studied. It is generally believed that under the conditions of mechanical abuse, electrical abuse, and heat abuse, an irreversible temperature rise occurs inside the battery, which then leads to a series of exothermic side reactions such as separator melting, short circuit, and cathode material decomposition, and finally leads to thermal runaway of the battery [11]. Based on the results of hundreds of accelerating rate calorimetry (ARC) tests with various types of batteries (including cylinder, prismatic, and pouch cell), three temperatures, {T1, T2, T3}, were clearly observed and summarized as the common characteristics of battery thermal runaway [12,13]. T1 represents the onset temperature of abnormal heat generation mainly attributed to the decomposition of the solid electrolyte interface (SEI), reflecting the overall thermal stability of a battery cell. T1 is usually observed between 70~150 °C, relating to the composition of electrolytes and aging conditions. Notably, the growth of lithium dendrites during cycling at a low temperature can significantly decrease T1 and may pierce the holes of the separator. T2 is the triggering temperature of battery TR, i.e., the tipping point that separates the gradual temperature increase and the sharp temperature rise. The temperature rise rate, dT·dt−1, can exceed 1 °C·s−1 when reaching T2, with separator melting, short circuit, and decomposition of cathode materials. T3 is the maximum temperature that can be reached after the battery is out of control, which is directly related to the type and capacity of the cell and affects the heat spread rate in the battery pack. In the above three stages, the process of the internal temperature rising from T1 to T2 is the key period for the battery management system (BMS) to be able to conduct safety identification, early warning, and active intervention management, which can provide a certain time for personnel escape and fire-fighting. When the cell temperature reaches T2, it is basically out of control, and can reach the combustion state in a short time. It can only rely on system-level passive protection measures to prevent the further heat spread of the battery pack. Therefore, it’s very important to inhibit or slow down the reaction and heat release in the cell at T1 and T2 stages to prolong safety warning time and reduce personnel and property losses.
In a word, the thermal/chemical stabilities of liquid electrolytes, anode/cathode materials and separators are all related to the safety of lithium-ion batteries, and many ideas have been proposed accordingly to improve the safety of lithium-ion batteries [14]. While during this process, the decomposition of delithiated cathode materials indicates the accelerated stage of thermal runaway with a temperature rise over 1°C min−1 inside the battery and a sharply drop in voltage [13,15]. Clearly, the cathode materials play a critical role in ensuring the batteries’ reliability. Indeed, different cathode materials greatly affect the severity of safety accidents. Batteries using LiFePO4, one of the most commonly used cathode materials in electric cars, might only smoke when thermal runaway occurs, while those involving layered LiTMO2 materials always end with fire, deflagration, and even explosion [16,17,18]. The main reasons for these differences will be discussed in more detail in the following sections.
Herein, we focus on the safety issue of nickel-based cathode materials from the aspects of composition, structure, and chemical stability, and try to give an in-depth insight into the origin of thermal instability in Section 2. Then Section 3 summarizes the advances and the strategies to improve the thermal stability of layered LiTMO2 materials. Finally, some conclusions and perspectives are provided in Section 4.
2. Thermal Stability of Layered LTMO2
The thermal stability of layered LiTMO2 materials is influenced by multiple factors, mainly including composition, structure, and morphology as well as chemical activity. Specifically, the onset temperature of decomposition, exothermic reaction peak temperature, total enthalpy, and decomposition products are important evaluation parameters for the thermal stability of electrode materials [19].
2.1. Composition
Layered lithium transition-metal oxides have a common chemical formula LiTMO2 (TM = Ni, Co, Mn, Al, etc.), in which TM representatives one or more of the transition metals. LiCoO2 is the most widely used cathode material in electronic devices because of its easy synthesis process, stable electrochemical performance, and highest compaction density [20,21]. However, the high cost and toxicity of cobalt make it unsuitable for electric vehicles and other large applications. LiNiO2 is another promising layered cathode material with a similar theoretical specific capacity of 274 mAh g−1 vs. LiCoO2 (275 mAh g−1), while the practical specific capacity of LiNiO2 (>220 mAh g−1) is much higher than that of LiCoO2 (~160 mAh g−1) below 4.3 V [22]. Unfortunately, it’s very difficult to synthesize a pure LiNiO2 since lithium vacancies, cation disordering, and spinel phase are easier to form in the sintering process [23].
As mentioned before, single transition metal oxide (such as LiCoO2 and LiNiO2) cannot meet the increasing requirements of the battery market due to various deficiencies. Ternary cathode materials, especially LiNixCoyMnzO2 (x + y + z = 1), have attracted great attention since 1999 when Liu first proposed a co-substitution of Ni by Co and Mn in LiNiO2 [24]. Nickel, Cobalt, and Manganese are randomly and uniformly distributed in the transition metal layer with performing their own functions, i.e., Ni for capacity, Co for layered characteristics, and Mn for stability. The synergistical effect of the three elements significantly improves the electrochemical performance of NCM and makes it a very successful commercial cathode material. In recent years, nickel fraction in commercial NCM materials has exceeded 60% in order to pursue higher energy density, which is called Ni-rich materials, such as NCA, NCM811 and LiNi0.89Co0.05Mn0.05Al0.01O2 (NCMA) [15,25,26]. Besides, the rapidly rising cost of cobalt also promotes the development of cobalt-free Ni-rich cathode materials (LiNi0.883Mn0.056Al0.061O2 for example).
However, the biggest challenge for Ni-rich materials is how to balance the contradiction between high energy density and reduced thermal stability. It’s well known that the thermal stability of layered LiTMO2 materials strongly depends on their composition, especially the Ni content, i.e., the higher the Ni content, the lower the stability. As shown in Figure 1a, Noh [27] compared the thermal stability of delithiated NCM materials with different nickel contents and found that the exothermic peak temperature drastically decreased from 306 °C to 225 °C for Li0.37Ni1/3Co1/3Mn1/3O2 and Li0.21Ni0.85Co0.075Mn0.075O2 respectively, while the heat generation significantly increased from 512.5 J g−1 to 971.5 J g−1. These differences can be attributed to the varied valence with different Ni content. According to the results of in situ time-resolved X-ray diffraction and mass spectroscopy (TR-XRD/MS), Bak et al. schematically depicted the phase stability map of the charged NMC cathode materials during heating (Figure 1b), which also clearly showed that the thermal stability of the charged NCM samples decreases with increasing Ni content [28]. Further study shows that the average valence of Ni increases from +2 to +3 with the increasing Ni content, while Ni3+-TM bond strengths decrease and Ni3+-O bonds become increasingly covalent, leading to the intrinsic thermodynamical instability of the layered structure [29].
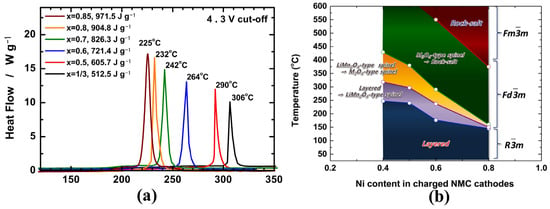
Figure 1.
(a) DSC results of the Li1-δ[NixCoyMnz]O2 materials (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) [27]; (b) Schematic illustration depicting the phase stability map of the charged NMC cathode materials during heating [28].
2.2. Structure and Morphology
All of the layered LiTMO2 compounds correspond to a hexagonal α-NaFeO2 structure with the space group. Specifically, oxygen atoms form a cubic close-packed lattice with rhombohedral distortion along the c-direction, resulting in layers formed by edge-sharing octahedra sites which are occupied by transition metals (3a) and lithium (3b) alternately [30]. But there is a common problem in layered nickel-based cathode materials named Li+/Ni2+ anti-site defects or cation disordering which mainly result from their similar ionic radii (Li+: 0.072 nm, Ni2+: 0.069 nm) [31]. These disordering occupations hinder the Li+ diffusion in the two-dimensional channels and greatly affect the electrochemical performance. Besides that [32], the distribution of TM ions inside the layer is no longer uniform, but the minority of TM ions form clusters at the atomic level when Ni content exceed 80%. The clusters make Mn4+ reduce to Mn3+, leading to aggravating Jahn–Teller distortions. These atomic-level structure deficiencies weaken the TM-TM interactions and TM-O bonds, thus significantly affecting the thermal stability of nickel-based materials.
In the delithiation process, the structure phase converts from hexagonal (H1) over monoclinic (M) to hexagonal (H2 and H3) phases, and the phase transitions reverse in the discharging process [33,34,35]. With the increase of lithium vacancy in the charging process, the distortion of TM-O octahedra gradually increases along the c-axis direction. As shown in Figure 2, when the amount of lithium removal exceeds the threshold (depending on the nickel content and charging cutoff voltage), parts of the region (H3 phase) cannot remain the layered characteristic anymore and convert to spinel Ni3O4 with active oxygen release [35]. The H2 to H3 phase transition usually occurs at a high delithiated state and results in oxygen evolution, which is also one of the main reasons for thermal runaway caused by overcharge [18]. With regard to the thermal decomposition under abusive conditions, the delithiated layered LiTMO2 materials go through phase transitions from layered structure to the disordered spinel structure, and finally to the rock-salt structure, accompanied by a large amount of oxygen gas release and CO2 formation [35,36]. Noted that the oxygen gas release occurs around 200 °C, at which the electrolyte has been decomposed or oxidized into combustible gas. As we all know, oxygen is the most common combustion promoter among the three elements of combustion. Therefore, batteries using layered LiTMO2 materials always catch fire or even explode when thermal runaway happens. Similarly, layered oxides like LiCoO2 and Mn-based Li-rich cathode materials also have the common problem of oxygen release. Samsung’s Galaxy Note 7 explosion strongly indicates that LiCoO2 battery with design defects may cause serious accidents. In contrast, the dilithiated LiFePO4 remains quite stable even above 350 °C and with little oxygen release, thus the weaknesses of the LiFePO4 battery are the separator and electrolyte rather than the cathode material.
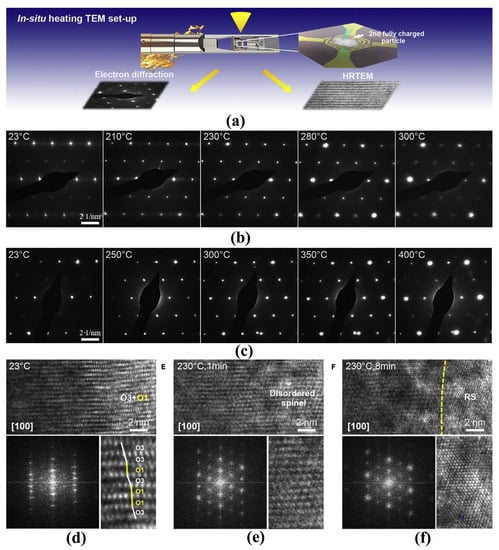
Figure 2.
In situ structural evolution of delithiated LNO and doped LNO induced by oxygen loss. (a) Schematic illustration of the in-situ heating TEM experimental setup. (b,c) Time-resolved EDPs of LNO (b) and doped LNO (c) during in situ heating at different temperatures. (d–f) Time-resolved HRTEM images showing structural transformations of delithiated LNO from layered structure (O3) with O1 phases (d) to disordered spinel (e) and then to rock-salt structure (f) induced by oxygen loss during in situ heating. The lower panels show corresponding fast Fourier transforms and enlarged images of local regions. O3 and O1 phases are indicated by white and yellow lines, respectively [35].
The particle microstructures and morphologies also play an important role in influencing the electrochemical properties and thermal stability of electrode materials. For commercial Ni-based materials, the spherical precursors are firstly agglomerated by nano-sized primary crystals with random orientations and a large number of defects in the coprecipitation process. Then the subsequent high-temperature treatment makes nanocrystals grow up and poly-crystallization, which means many crystal defects, such as grain boundaries, still exist inside the particle [23]. As shown in Figure 3a, the resulting unbalanced mechanical stress inside the particles during the charge-discharge process can cause microcracks that weaken the structural stability of particles and aggravate the undesired cathode-electrolyte side reactions [37,38,39,40]. In particular, the particles in at highly delithiated state may smash at high temperatures and accelerate side reactions and oxygen release (Figure 3b–d) [36].
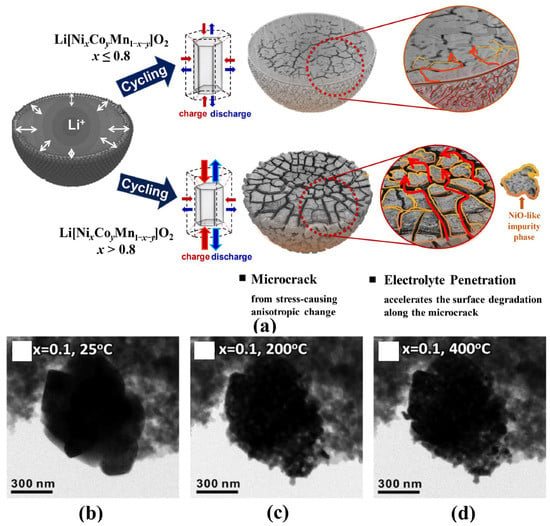
Figure 3.
(a) Scheme of microcrack generation and electrolyte penetration [37]. In situ TEM images (b) before heating (25 °C), after heating at (c) 200 °C, and (d) 400 °C for a Li0.1Ni0.8Co0.15Al0.05O2 particle [36].
2.3. Chemical Activity
The chemical activity of electrode materials is directly related to the gas production inside the battery. Because of the different out shell electron configurations for Ni and Co, the Ni2+/Ni3+/Ni4+ redox contributes to most of the capacity of Ni-based materials below 4.3 V (vs. Li/Li+) while Co3+/Co4+ redox occurs at around 4.3 V in the charging process [41]. However, the highly active Ni4+ on the particle surface can easily be reactive with liquid electrolytes accompanied by the gas generation (mainly including CO2 and CO) [42,43]. Secondly, the lithium residues (such as Li2CO3 and LiOH) introduced in the synthesis process or by-products of the slow reduction of Ni3+ to Ni2+ for Ni-rich materials when exposed to moisture, can also induce gas generation inside the battery [44,45]. Finally, transition metals (especially Mn) can slowly dissolve into the electrolyte, then migrate and incorporate into solid electrolyte interphase (SEI) film of the graphite-based negative electrode, resulting in the damage of SEI and continues alkane gas evolution [46,47]. Actually, the gas evolution related to the chemical activity of electrode materials is a common phenomenon of battery failure, which makes the battery bulge and leakage, and causes safety problems.
In summary, the thermal instability of layered LiTMO2 materials attributes to: (1) compositions, specifically, the higher the Ni content, the worse the thermal stability; (2) oxygen release from delithiated materials due to the phase transitions under overcharge or heated conditions; (3) gas evolution caused by the highly oxidative Ni4+, lithium residues, and transition metals dissolution. Nevertheless, the core reason is the oxygen release due to the astable layered structure of delithiated LiTMO2 materials under abusive conditions. Therefore, the key to improving the safety of LiTMO2 batteries is to reduce or delay oxygen release by improving the structural stability of materials, and we will summarize the advances and strategies in the following sections.
3. Safety Strategies for Ni-Based Cathode Materials
With the deepening understanding of the thermal failure mechanism of layered Ni-based materials, corresponding modification measures have been put forward. Great efforts have been devoted to improving the thermal stability of cathode materials from surface to the inside, such as elements doping, surface coating, microstructure design, morphology control, and so on.
3.1. Elements Doping
A lot of experimental and theoretical studies have shown that element doping can effectively stabilize the material structure. Generally speaking, the main role of elements doping or substitution is to prop up the layered structure after the removal of lithium (named pillar effect) so as to prevent or slow down the phase transitions of LiTMO2. According to the different doping sites, it can be classified into cation doping, anion doping, and co-doping. Specifically, cations usually occupy the TM sites or lithium sites, and anions substitute for the oxygen.
High valence cations, such as Al3+, Ti4+, Zr4+, W5+, V5+, Mo6+ and Nb5+, have been fully studied as dopants in the TM layer [48,49,50,51,52,53,54,55,56]. Levartovsky [49] conducted a comparative study of Al and Ti doping in LiNi0.85Co0.1Mn0.05O2 (NCM85), which indicated that both Al and Ti can improve the thermal stability with a close onset temperature of 214 °C, 216 °C and 218 °C, but with a significantly decreased specific total heat release of 468 J g−1, 376 J g−1 and 352 J g−1 for NCM85 pristine, Ti-doped and Al-doped samples, respectively (Figure 4). It may ascribe to their strong bonds with O and the construction of a protective layer that can reduce oxygen release, while the authors also acknowledge that the relevant mechanism is still unclear. Similarly, the strong W-O bond makes the average charge around O in the NiO6 octahedron more negative, which can effectively suppress the oxygen evolution and thus improve the thermal stability of Ni-based cathode materials [53]. Theoretical studies using first-principles density functional theory (DFT) calculations show that, after high valence cations doping, the more stable Ni2+ state is preferred to Ni3+ or Ni4+ because of the charge compensation, and oxygen vacancies are prevented [28]. In addition, the different doping sites in the TM layer also have different effects, i.e., doping at Mn sites (It may be more accurate to call it substitution) reduces Li/Ni disordering and oxygen release, while doping at Co sites improves the phase stability [57].
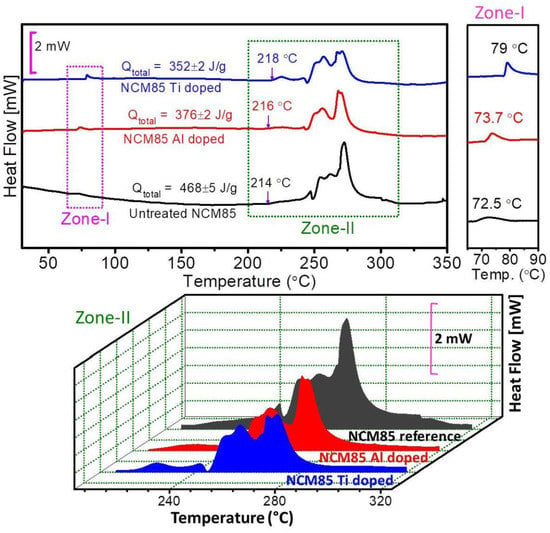
Figure 4.
DSC measurements of NCM85 reference, Al-doped and Ti-doped cathode materials with electrolyte [49].
The low valence cations, mainly including Na+ [58,59,60,61,62] and Mg2+ [63,64], are generally believed that they can enter the lithium layer as inactive pillars. Na+ doping can significantly improve the electrochemical performance of Ni-based cathode materials, but there is little research on the improvement of thermal stability. A few studies show that Mg2+, with a radius (0.072 nm) similar to that of Li (0.076 nm), can stabilize the lithium layer as an effective pillar and thus shift the onset of the exothermic reaction and phase transition toward higher temperature [63]. Interestingly, some investigations indicated that Li+/Ni2+ cation mixing, i.e., Ni2+ doping in the lithium site, can also improve the thermal stability of Ni-based materials to some extent due to the stronger bonds between Ni2+-O than that of Li+-O [50]. Clearly, the lithium sites doping to promote the thermodynamic stability of Ni-based materials prefer +2 ions to +1 ions.
Anions, especially fluoride elements, have also been widely studied as doping elements to substitute oxygen partially in Ni-based cathode materials because of their similar chemical properties to oxygen ions. The reported fluoride sources include lithium fluoride (LiF) [65,66], ammonium fluoride (NH4F) [67,68], polytetrafluoroethylene (PTFE) [69], polyvinylidene fluoride (PVDF) [70], lithium hexafluorophosphate (LiPF6) [71], and so on. Typically, the fluorinated Li1-xNi0.8Co0.1Mn0.1O2-zFz using NH4F as a fluoride source showed greatly improved thermal stability with an exothermic peak temperature of 245 °C and a total generated heat of 1304 J g−1 when z = 0.06, while the pristine Li1-xNi0.8Co0.1Mn0.1O2 had a broadening exothermic peak around 220 °C and a large generated heat of 3285 J g−1 [68]. Similarly, a small amount of fluoride substituted Li1-xNi0.43Co0.22Mn0.35O1.92F0.08 exhibited a higher onset decomposing temperature of 280 °C and a reduced generated heat of 350 J g−1, and those of the pristine sample were 236 °C and 590 J g−1, respectively [66]. The positive effect of fluoride doping on the thermal stability of layered cathode was also observed in manganese-based Li-rich materials [65]. The strong O-F bonds can effectively stabilize the host layered structure and suppress the oxygen evolution at elevated temperatures. Thus, the thermal side reactions between these active oxygen species and the liquid electrolyte can be restarted [72].
Although single ion doping has been widely studied or applied in industrial production with varying degrees of improvement on the structural stability of cathode materials, it is still not enough to significantly improve battery safety. Multi-ion doping using two or more different ions seems to show better properties due to the synergistic effects theoretically. Ti4+ and F− co-doping was conducted in Ni-rich material NCM811, which can induce the formation of an ultra-thin rock-salt phase that acts as a protective layer on the cathode surface and suppress the irreversible phase transition of H2 to H3, alleviating the structural degradation and thus improving the structural stability [73]. Other combinations, such as Ti-B [74], Ti-Mg [75], Na-Mg [76], Ga-Zr [77], Nb-F [78], and K-Cl [79], are also investigated to improve the electrochemical properties, while the promotions in thermal stability are still lacking.
3.2. Surface Coating
The highly oxidative Ni3+/Ni4+ ions on the surface of Ni-based cathode materials, especially at a high delithiated state, can easily react with the liquid electrolyte and induce gas generation, which is one of the main reasons for the safety issues of batteries. The surface coating is like putting a protective layer on the particle surface to reduce the direct contact between the active material and the electrolyte, and thus suppress the side reactions and transition metals dissolution.
Generally speaking, the widely studied coating materials mainly include inactive compounds and conductive materials. The inactive compounds, such as oxides, fluorides, and phosphates, just play the role of a physical protective layer and barely involve chemical reactions. While conductive materials with lithium or electron conductivity, including lithium metal compounds, ionic conductors and electron conductive species, can not only stabilize the cathode interface but also facilitate lithium ions diffusion and electron transfer, improving the electrochemical properties [80]. The electron conductive materials, however, are more suitable for electrochemical properties improvement than thermal stability promotion according to current research. Below we will focus on the improvement of the thermal stability of Ni-based cathode with different coating materials and strategies.
A lot of inactive compounds, such as Al2O3 [81,82,83,84], TiO2 [85,86,87,88], ZrO2 [89], MgO [90,91], AlF3 [92], AlPO4 [93], Gd2O3 [94] and so on, can be used as coating materials for the layered cathodes. Among them, Al2O3 is one of the most successful coating materials and has been used in industrialization. NCM523 coated with a uniform Al2O3 layer of a thickness of about 9 nm by a simple wet chemistry method using Al (NO3)3 as an aluminum source showed a delayed exothermic peak at 444.9 °C and a lower generated heat of 257.5 J g−1, while the bare sample had an exothermic peak with heat generation of 314.2 J g−1 at 430.4 °C [81]. In another study, NCM523 was coated with an ultrathin (~1.65 nm) Al2O3 layer by atomic layer deposition (ALD) method, delaying the exothermic peak from 355 °C to 367 °C after 20 charge-discharge cycles (Figure 5) [83]. Similarly, the TiO2 coating layer can isolate the active oxygen species from electrolytes and postpone the phase transformation, thus slowing down parasitic oxidation and significantly improving battery safety. AlF3 [92] and MnPO4 [95] coating can also obviously improve the thermal stability of Ni-based cathode materials.
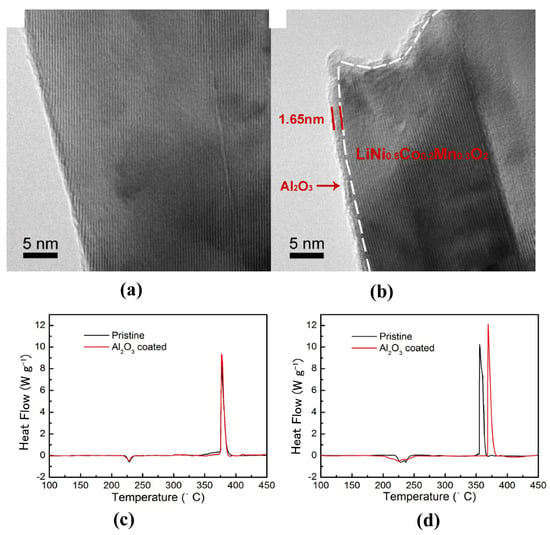
Figure 5.
HRTEM images of NCM523 particles before (a) and after (b) Al2O3 ALD coatings, and DSC curves of the sealed pan with pristine or Al2O3 coated electrode material and electrolyte (c) 1st charged to 4.8 V and (d) Charged to 4.8 V after 20 cycles [83].
From the aspect of thermal improvement mechanism, the inactive coating layer mainly has the following functions: (a) acting as an isolation layer that reduces the harmful side reactions between the oxidative ions and electrolyte; (b) suppressing the phase transition near the surface and slowing down oxygen release; (c) preventing the cathode from HF or moisture attacking, i.e., HF scavenger [83]. It is worth noting that most inactive material coatings (especially metal oxides and fluoride) need to undergo high-temperature treatment, and element diffusion cannot be avoided. Therefore, the inactive materials coating is not only a single surface protective layer, but also has different levels of surface region doping [88]. In addition, the inert coating layer will hinder the rapid removal of lithium ions to a certain extent, and especially affect the rate performance of the battery. Therefore, the thickness, continuity, and uniformity of the coating layer need optimization to further improve the thermal stability of layered cathode materials.
Lithium-ion conductors, including lithium metal oxides (such as Li2TiO3, LiAlO2, and Li2ZrO3) [96,97,98,99], solid electrolytes (such as Li3PO4, Li1.3Al0.3Ti1.7PO4 (LATP) and Li-La-TiO3 (LLTO)) [100,101,102,103,104] and some stable cathode materials (such as LiFePO4 and LiMnPO4) [105,106,107,108] are more promising coating candidates to promote the thermal stability of layered Ni-based materials without sacrificing too much electrochemical performance. For example, Gan et.al. coated NCM811 with a 4~5 nm Li3PO4 layer through a solvent-free in-situ strategy, and identified, using atomic-scale microscopy, ex-situ SXAS, and in-situ Raman, that the Li3PO4 layer can effectively suppress NCM811 phase transition from a layered structure to a rock-salt-like structure and inhibit surface oxygen release [100]. Unfortunately, the sintering temperatures of most solid electrolytes (about 1000 °C or higher) differ significantly from that of layered Ni-based cathode materials (700~900 °C), which makes it difficult to prepare the expected phase of a target solid electrolyte when coating the host materials through a co-sintering method because of the inter diffusion of elements. Another interesting finding is that nano-sized LiFePO4 can be prone to industrialization because of its excellent thermal stability and easy coating method. Typically, the nano-sized LFP particles and NCM spheres are fully mixed through mechanical fusion, forming a continuous LFP coating layer. The thermodynamic stable LFP can effectively protect the active Ni ions from the liquid electrolyte and reduce heat generation. Especially, as tested in 18,650 full batteries by ARC, the time between trigger temperature T0 and the onset temperature Tc of thermal runaway is extended from 796 min to 989 min [105]. The extended time is quite important for early warning when battery safety issues are about to happen.
Evolved from the simple surface coating, Sun and coworkers first proposed and designed the concentration gradient materials, including core-shell structure with concentration gradient (CSG) [109], full concentration gradient (FCG) [110], and two-sloped full concentration gradient (TSFCG) [111]. There is also a lot of research to follow up on the technology [112,113,114,115]. Typically, as shown in Figure 6, the active Ni concentration decreases while the inactive Mn concentration increases linearly from the inside to the outside of the particles. The thermally stable Mn-rich layer can effectively protect the electrochemically active Ni-rich region from the harmful side reactions and phase transitions related to oxygen release, significantly shifting the exothermic peak from 221 °C to 257 °C for FCG LiNi0.75Co0.10Mn0.15O2 and LiNi0.86Co0.1Mn0.04O2 respectively [110].
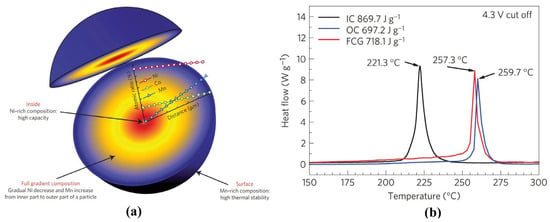
Figure 6.
(a) Schematic diagram of the FCG lithium transition-metal oxide particle; and (b) DSC profiles of the delithiated FCG material, the delithiated inner composition (IC, Li1-xNi0.86Co0.10Mn0.04O2) and the delithiated outer composition (Li1−xNi0.70Co0.10Mn0.2O2) with a scanning rate of 1 °C min−1 after charging to 4.3 V [110].
In short, the surface coating has been proven to be an effective improvement technology in both research and industry. The continuity and thickness of the coating layer, interface interaction between the coating material and host material, and properties of coating materials deserve more attention to in order to more effectively inhibit the phase transition of the layered structure and slow down the release of oxygen. Similar to co-doping, the combination of multiple coating materials is also a promising research area [82,116,117,118].
3.3. Particle Microstructure Design
At present, the coprecipitation method has become the mainstream synthesis method of layered Ni-based cathode materials due to its advantages of easy control of element proportion, uniform element distribution, regular particle morphology, and suitability for large-scale production. However, a large number of crystal defects, such as grain boundaries and holes, inherited from the precursors which are composed of nano-sized primary particles with random orientations largely weaken the structural stability of the secondary spheres [23]. Thereby, the resulted in intergranular cracks expose the newly generated surface to electrolytes and lead to severe side reactions and oxygen release, especially under thermal failure conditions [119]. Therefore, it is very important to design the inner structure to improve the thermal stability of layered Ni-based cathode materials.
The ordering of primary particles is a new strategy to improve thermal stability in recent years. Interestingly, the introduction of W [52], B [120,121], Ta [122] and Sn [123] into NCM materials can selectively lower the (003) surface energy and result in preferential (003) faceting, thereby leading to the formation of elongated primary particles arranging radially from surface to interior. The radial structure can absorb the anisotropic lattice strain and preserve the mechanical integrity of the cathode particles during cycling. As shown in Figure 7, microcrack suppression protects the particle interior from detrimental electrolyte attack and greatly enhances the thermal stability of Ni-based cathode material. In addition to the induction by high-valence ion doping, the radially microstructure can also be designed by regulating ammonia concentration to linear change during the co-precipitation process [124].
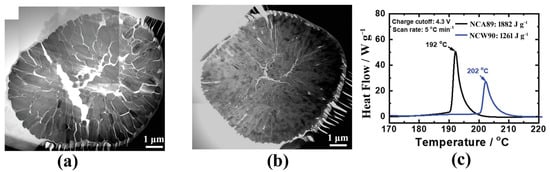
Figure 7.
Bright-field STEM mosaic image of a cross-section of (a) NCA89 and (b) NCW90 at charged to 4.3 V, and (c) DSC profiles for NCA89 and NCW90 in the delithiated state at 4.3 V [52].
Single crystallization is another promising approach to strengthen the particle and mitigate the intergranular cracks. Dahn’s group has done a lot of work in the single crystal (SC) NCM materials and investigated their synthesis conditions and properties in detail [125,126,127,128,129]. Theoretically, single crystal particles have no grain boundaries, which means the side reactions are limited to the particle surface, but little liquid electrolyte can penetrate in. The single crystal NCM523 was charged and held at 4.4, 4.5, and 4.6 V for 100 h under 40 °C, surprisingly, with no oxygen or other gas generation being detected within detection limits, showing greatly enhanced structural stability [126]. Particularly, crystal gaps inside the polycrystalline (PC) cathode materials will increase the inhomogeneity of PC particles during charging and discharging. The structural damage becomes more serious along with the rise of the delithiation state. The damaged structure can reduce the binding of oxygen and become the initiating factor of oxygen release in advance. As shown in Figure 8, the single crystal NCM622 and NCM811 materials clearly have better thermal stabilities [130]. Other studies also indicate that single-crystal materials have a higher exothermic peak or a reduced heat generation [131,132,133,134]. Nevertheless, the large particle size (~4 μm or larger) for single crystals limits the diffusion of lithium ions and scarifies the rate performance especially [135]. In addition, reversible planar gliding and microcracking along the (003) plane in a single-crystalline Ni-rich cathode were also observed during charge-discharge cycles because of the localized stresses induced by a concentration gradient of Li atoms in the lattice [136]. Therefore, it is necessary to further modify the single-crystal Ni-based materials with ion doping and surface engineering to balance the thermal improvement and electrochemical properties.
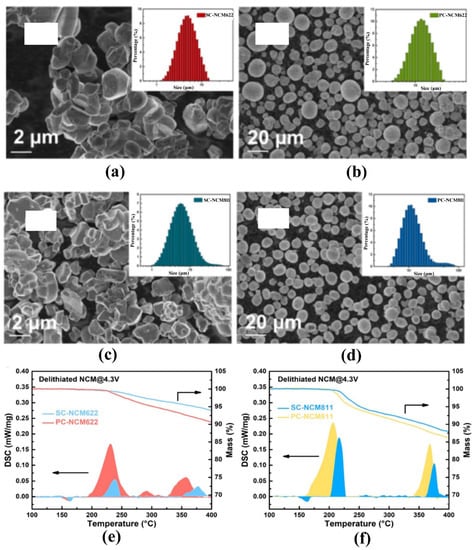
Figure 8.
SEM and particle size analyzer test results of (a) SC-NCM622, (b) PC-NCM622, (c) SC-NCM811, (d) PC-NCM811, and TG-DSC test results of the above materials (e) NCM622 and (f) NCM811 [130].
3.4. Synergistic Effect of Multiple Modifications
According to the current research progress, a single modification technology has improved the thermal stability of the ternary material, but most of the thermal decomposition peak temperatures are only delayed by 10~20 °C, which is not enough to solve the thermal runaway of the battery. Combinations of multiple modifications have attracted more and more attention due to the synergistic effect to improve the performance of cathode materials. Kim et al., synthesized Al-doped NCM622 full gradient material, whose cyclic stability can support more than 10 years of cycle life theoretically [137]. The research of Feng et al. shows that the electrochemical performance of ternary materials can be significantly improved by using doping and coating together [138]. Other studies also show that the synergistic use of single crystallization and doping/coating has better improvement on ternary materials than the single one [139,140,141,142,143]. Therefore, composite modification technology shows excellent synergistic improvement effects and is an important development direction in the field of ternary materials.
3.5. Other Strategies for LiTMO2 Battery Safety
A lithium-ion battery is an integration of many materials, and any one of them may affect the safety of the battery. Generally, the liquid electrolyte is the weakest part of a battery because of its flammability, easy decomposability, and chemical instability. Film-forming additives [144,145,146] and flame retardant additives [147,148,149] can enhance the thermal stability of liquid electrolytes, thus improving the LiTMO2 battery safety. Besides, all-solid-state batteries based on solid electrolytes may be expected to solve the thermal runaway of batteries once and for all since there is no combustible liquid electrolyte at all if the challenge of ionic conductivity, and, in particular, interface problems between electrolyte/electrolyte and electrolyte/electrode would be solved [150,151,152]. Other strategies, such as Al2O3-coated separator [153,154], non-flammable fluorinated polyimide binder [155,156], and positive temperature coefficient (PTC) protecting layer [157,158], have also been investigated to improve battery safety from different aspects.
4. Outlook and Conclusions
Despite the high energy density of layered LiTMO2 materials, the safety issue is still the greatest challenge for large-scale applications although there are already millions of electric vehicles running around the world. The battery safety is strongly affected by the thermal stability of layered Ni-based cathode materials, which attributes to the compositions, oxygen release due to phase transition, and gas evolution. The oxygen release from delithiated layered cathode materials (such as Ni-based materials and Li-rich materials) is the greatest obstacle to improving battery safety, and the mechanism of oxygen evolution needs more detailed research. The corresponding strategies, mainly including elements doping, surface engineering, and microstructure design have been extensively studied and some of the technologies have been successfully applied in industrial productions. The combined use of various modifications can bring synergistic effects to achieve further improvement on the thermal stability of layered Ni-based cathode materials and deserves more explorations. Nevertheless, it makes us feel disappointed and pessimistic that no single technology, at least we have not found it for now, can completely solve the oxygen release problem of layered oxides cathodes.
According to the fire tetrahedron, essentially all four elements, including fuel, heat, oxygen, and a chemical chain reaction, must be present for fire to occur. Removal of any one of these essential elements will result in the fire being extinguished. As illustrated in Figure 9, it is expected to improve the safety of a lithium-ion battery using layered Ni-based materials from the aspects of heat (exothermic), fuel (electrolyte), and chemical reaction chain. Given the fact that most of the combustibles are organic electrolyte, all solid-state batteries based on solid electrolyte may be expected to solve the thermal runaway of batteries once and for all. The PTC inside a cell and combustion blocking layer (asbestos, for example) in a battery pack can effectively cut off the reaction chain. While the heat generation inside a battery is more complicated. We found that almost all cathode materials have a sharp exothermic peak, which corresponds to the sharp temperature rise of T2 inside the battery. Thus, it is possible to split the sharp peak into two or more small peaks by the above modifications in order to slow down the heat accumulation inside a battery. From a macroscopic perspective, it is a systematic engineering to solve the problem of thermal runaway of the battery from the aspects of materials, cell, battery-package structure design and system integration.
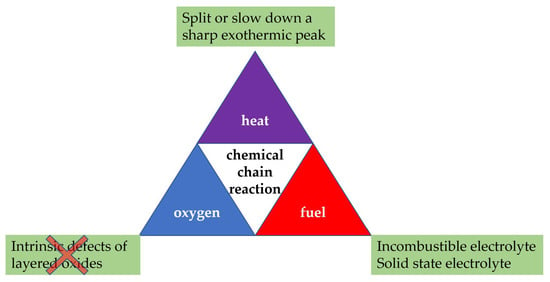
Figure 9.
Scheme of the fire tetrahedron and possible corresponding strategies to improve battery safety.
Author Contributions
Conceptualization, Z.T., D.F., Y.X., L.C., X.Z. and Q.M.; methodology, D.F. and Y.X.; validation, Y.X., L.C. and Q.M.; investigation, Z.T., D.F., L.C., X.Z. and Q.M.; resources, Y.X. and X.Z.; data curation, Z.T., D.F. and X.Z.; writing—original draft preparation, Z.T. and D.F.; writing—review and editing, Z.T., D.F., Q.M. and L.C.; supervision, Z.T. and L.C.; funding acquisition, D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Scientific Research Projects of Universities in Henan province (20B150027); and the Project of Fundamental Researches and Applied Fundamental Researches of Zhengzhou Science and Technology Bureau (zkz202107).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Viswanathan, V.; Epstein, A.H.; Chiang, Y.-M.; Takeuchi, E.; Bradley, M.; Langford, J.; Winter, M. The Challenges and Opportunities of Battery-Powered Flight. Nature 2022, 601, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Yao, J.; Jin, C.Y.; Feng, X.N.; Wang, H.B.; Xu, C.S.; Zheng, Y.J. A Review of Lithium-Ion Battery Failure Hazards: Test Standards, Accident Analysis, and Safety Suggestions. Batteries 2022, 8, 248–275. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.C. A Retrospective on Lithium-Ion Batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef]
- Jung, W.W.; Jeong, J.; Kim, J.; Chang, D. Optimization of Hybrid Off-Grid System Consisting of Renewables and Li-Ion Batteries. J. Power Sources 2020, 451, 227754. [Google Scholar] [CrossRef]
- Wentker, M.; Greenwood, M.; Leker, J. A Bottom-Up Approach to Lithium-Ion Battery Cost Modeling with a Focus on Cathode Active Materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. NCA, NCM811, and the Route to Ni-richer Lithium-Ion Batteries. Energies 2020, 13, 6363. [Google Scholar] [CrossRef]
- Hu, G.; Huang, P.; Bai, Z.; Wang, Q.; Qi, K. Comprehensively Analysis the Failure Evolution and Safety Evaluation of Automotive Lithium Ion Battery. ETransportation 2021, 10, 100140. [Google Scholar] [CrossRef]
- Sun, P.; Bisschop, R.; Niu, H.; Huang, X. A Review of Battery Fires in Electric Vehicles. Fire Technol. 2020, 56, 1361–1410. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, T.; Zhou, Q.; Sun, Y.; Qu, M.; Zeng, Z.; Ju, Y.; Li, L.; Wang, K.; Chi, F. A Review of Technologies and Applications on Versatile Energy Storage Systems. Renew. Sustain. Energy Rev. 2021, 148, 111263. [Google Scholar] [CrossRef]
- Chombo, P.V.; Laoonual, Y. A Review of Safety Strategies of a Li-Ion Battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Feng, X.N.; Ren, D.; He, X.; Ouyang, M.G. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Feng, X.N.; Zheng, S.Q.; He, X.M.; Wang, L.; Wang, Y.; Ren, D.S.; Ouyang, M.G. Time Sequence Map for Interpreting the Thermal Runaway Mechanism of Lithium-Ion Batteries with LiNixCoyMnzO2 Cathode. Front. Energy Res. 2018, 6, 126. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Kang, Y.Q.; Zhao, Y.; Wang, L.; Liu, J.L.; Li, Y.X.; Liang, Z.; He, X.M.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Liang, C.; Jiang, L.H.; Wei, Z.S.; Zhang, W.H.; Wang, Q.S.; Sun, J.H. Insight into the Structural Evolution and Thermal Behavior of LiNi0.8Co0.1Mn0.1O2 Cathode Under Deep Charge. J. Energy Chem. 2022, 65, 424–432. [Google Scholar] [CrossRef]
- Yang, M.J.; Ye, Y.J.; Yang, A.J.; Jiang, Z.Y.; Wang, X.H.; Yuan, H.; Rong, M.Z. Comparative Study on Aging and Thermal Runaway of Commercial LiFePO4/Graphite Battery undergoing Slight Overcharge Cycling. J. Energy Chem. 2022, 50, 104691. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Zhou, X.D.; Cao, B.; Yang, L.Z.; Liew, K.M. Investigating the Relationship between Heating Temperature and Thermal Runaway of Prismatic Lithium-Ion Battery with LiFePO4 as Cathode. Energy 2022, 256, 124714. [Google Scholar] [CrossRef]
- Wang, Z.P.; Yuan, J.; Zhu, X.Q.; Wang, H.; Huang, L.; Wang, Y.T.; Xu, S.Q. Overcharge-to-Thermal-Runaway Behavior and Safety Assessment of Commercial Lithium-Ion Cells with Different Cathode Materials: A Comparison Study. J. Energy Chem. 2021, 55, 484–498. [Google Scholar] [CrossRef]
- Feng, X.N.; Zheng, S.Q.; Ren, D.S.; He, X.M.; Wang, L.; Liu, X.; Li, M.G.; Ouyang, M.G. Key Characteristics for Thermal Runaway of Li-Ion Batteries. Energy Procedia 2019, 158, 4684–4689. [Google Scholar] [CrossRef]
- Lyu, Y.C.; Wu, X.; Wang, K.; Feng, Z.J.; Cheng, T.; Liu, Y.; Wang, M.; Chen, R.M.; Xu, L.M.; Zhou, J.J.; et al. An Overview on the Advances of LiCoO2 Cathodes for Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2000982. [Google Scholar] [CrossRef]
- Zhang, J.N.; Li, Q.H.; Ouyang, C.; Yu, X.; Ge, M.; Huang, X.J.; Hu, E.; Ma, C.; Li, S.F.; Xiao, R.; et al. Trace Doping of Multiple Elements Enables Stable Battery Cycling of LiCoO2 at 4.6 V. Nat. Energy 2019, 4, 594–603. [Google Scholar] [CrossRef]
- Bianchini, M.; Roca-Ayats, M.; Hartmann, P.; Brezesinski, T.; Janek, J. There and Back Again-the Journey of LiNiO2 as a Cathode Active Material. Angew. Chem. Int. Ed. 2019, 58, 10434–10458. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.F.; Bao, J.J.; Du, Q.X.; Shao, Y.; Gao, M.H.; Zou, B.K.; Chen, C.H. Surface Surgery of the Nickel-Rich Cathode Material LiNi0.815Co0.15Al0.035O2: Toward a Complete and Ordered Surface Layered Structure and Better Electrochemical Properties. ACS Appl. Mater. Interfaces 2016, 8, 34879–34887. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Yu, A.S.; Lee, J.Y. Synthesis and Characterization of LiNi1−x−yCoxMnyO2 as the Cathode Materials of Secondary Lithium Batteries. J. Power Sources 1999, 81, 416–419. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, F.L.; Zhou, X.A.; Wang, P.; Wang, J.; Ding, H.; Dong, H.; Liang, W.B.; Zhang, N.S.; Li, S.Y. Which of the Nickel-Rich NCM and NCA is Structurally Superior as a Cathode Material for Lithium-Ion Batteries? J. Mater. Chem. A 2021, 9, 13540–13551. [Google Scholar] [CrossRef]
- Kim, U.H.; Kuo, L.Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.K. Quaternary Layered Ni-Rich NCMA Cathode for Lithium-Ion Batteries. ACS Energy Lett. 2019, 4, 576–582. [Google Scholar] [CrossRef]
- Noh, H.J.; Youn, S.; Yoon, C.S.; Sun, Y.K. Comparison of the Structural and Electrochemical Properties of Layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) Cathode Material for Lithium-Ion Batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Bak, S.M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.J.; Kim, K.B.; Chung, K.Y.; Yang, X.Q.; Nam, K.W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Dixit, M.; Markovsky, B.; Schipper, F.; Aurbach, D.; Major, D.T. Origin of Structural Degradation during Cycling and Low Thermal Stability of Ni-Rich Layered Transition Metal-Based Electrode Materials. J. Phys. Chem. C 2017, 121, 22628–22636. [Google Scholar] [CrossRef]
- Tuccillo, M.; Palumbo, O.; Pavone, M.; Muñoz-García, A.B.; Paolone, A.; Brutti, S. Analysis of the Phase Stability of LiMO2 Layered Oxides (M = Co, Mn, Ni). Crystals 2020, 10, 526. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, L.; Zhang, M.J.; Chen, Z.; Bai, J.; Amine, K.; Pan, F.; Wang, F. Insights into Li/Ni Ordering and Surface Reconstruction During Synthesis of Ni-Rich Layered Oxides. J. Mater. Chem. A 2019, 7, 513–519. [Google Scholar] [CrossRef]
- Liang, C.; Kong, F.; Longo, R.C.; Kc, S.; Kim, J.S.; Jeon, S.; Choi, S.; Cho, K. Unraveling the Origin of Instability in Ni-Rich LiNi1–2xCoxMnxO2 (NCM) Cathode Materials. J. Phys. Chem. C 2016, 120, 6383–6393. [Google Scholar] [CrossRef]
- Jamil, S.; Wang, G.; Yang, L.; Xie, X.; Cao, S.; Liu, H.; Chang, B.B.; Wang, X. Suppressing H2–H3 Phase Transition in High Ni–Low Co Layered Oxide Cathode Material by Dual Modification. J. Mater. Chem. A 2020, 8, 21306–21316. [Google Scholar] [CrossRef]
- Kuo, L.Y.; Guillon, O.; Kaghazchi, P. Origin of Structural Phase Transitions in Ni-Rich LixNi0.8Co0.1Mn0.1O2 with Lithiation/Delithiation: A First-Principles Study. ACS Sustain. Chem. Eng. 2021, 9, 7437–7446. [Google Scholar] [CrossRef]
- Wang, C.; Han, L.; Zhang, R.; Cheng, H.; Mu, L.; Kisslinger, K.; Zou, P.; Ren, Y.; Cao, P.; Lin, F.; et al. Resolving Atomic-Scale Phase Transformation and Oxygen Loss Mechanism in Ultrahigh-Nickel Layered Cathodes for Cobalt-Free Lithium-Ion Batteries. Matter 2021, 4, 2013–2026. [Google Scholar] [CrossRef]
- Bak, S.M.; Nam, K.W.; Chang, W.; Yu, X.; Hu, E.; Hwang, S.; Stach, E.A.; Kim, K.B.; Yang, X.Q. Correlating Structural Changes and Gas Evolution during the Thermal Decomposition of Charged LixNi0.8Co0.15Al0.05O2 Cathode Materials. Chem. Mater. 2013, 25, 337–351. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity Fading of Ni-Rich Li[NixCoyMn1–x–y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Yin, S.; Deng, W.; Chen, J.; Gao, X.; Zou, G.; Hou, H.; Ji, X. Fundamental and Solutions of Microcrack in Ni-Rich Layered Oxide Cathode Materials of Lithium-Ion Batteries. Nano Energy 2021, 83, 105854. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Zhang, J.G.; Wang, C.M. Intragranular Cracking as a Critical Barrier for High-Voltage Usage of Layer-Structured Cathode for Lithium-Ion Batteries. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Besli, M.M.; Xia, S.; Kuppan, S.; Huang, Y.; Metzger, M.; Shukla, A.K.; Schneider, G.; Hellstrom, S.; Christensen, J.; Doeff, M.M.; et al. Mesoscale Chemomechanical Interplay of the LiNi0.8Co0.15Al0.05O2 Cathode in Solid-State Polymer Batteries. Chem. Mater. 2019, 31, 491–501. [Google Scholar] [CrossRef]
- Liu, T.; Yu, L.; Liu, J.; Lu, J.; Bi, X.; Dai, A.; Li, M.; Li, M.F.; Hu, Z.; Ma, L.; et al. Understanding Co Roles towards Developing Co-Free Ni-Rich Cathodes for Rechargeable Batteries. Nat. Energy 2021, 6, 277–286. [Google Scholar] [CrossRef]
- Kim, Y. Encapsulation of LiNi0.5Co0.2Mn0.3O2 with a Thin Inorganic Electrolyte Film to Reduce Gas Evolution in the Application of Lithium Ion Batteries. Phys. Chem. Chem. Phys. 2013, 15, 6400–6405. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhang, X.; Wei, P.; Sun, S.; Xu, Y.; Li, Q.; Fang, C.; Han, J.; Huang, Y. Tailoring Electrolyte Enables High-Voltage Ni-rich NCM Cathode Against Aggressive Cathode Chemistries for Li-Ion Batteries. Sci. Bull. 2022, 67, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, S.E.; McCloskey, B.D. Residual Lithium Carbonate Predominantly Accounts for First Cycle CO2 and CO Outgassing of Li-Stoichiometric and Li-Rich Layered Transition-Metal Oxides. J. Am. Chem. Soc. 2017, 139, 17853–17860. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H.; Warner, J.H.; Manthiram, A. Unraveling the Intricacies of Residual Lithium in High-Ni Cathodes for Lithium-Ion Batteries. ACS Energy Lett. 2021, 6, 941–948. [Google Scholar] [CrossRef]
- Wachs, S.J.; Behling, C.; Ranninger, J.; Möller, J.; Mayrhofer, K.J.; Berkes, B.B. Online Monitoring of Transition-Metal Dissolution from a High-Ni-Content Cathode Material. ACS Appl. Mater. Interfaces 2021, 13, 33075–33082. [Google Scholar] [CrossRef]
- Li, W. An Unpredictable Hazard in Lithium-Ion Batteries from Transition Metal Ions: Dissolution from Cathodes, Deposition on Anodes and Elimination Strategies. J. Electrochem. Soc. 2020, 167, 90514. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, H.; Lee, W.; Ahn, S.J.; Lee, E.; Yoon, W.S. Stabilizing Effects of Al-Doping on Ni-Rich LiNi0.80Co0.15Mn0.05O2 Cathode for Li Rechargeable Batteries. J. Power Sources 2020, 474, 228592. [Google Scholar] [CrossRef]
- Levartovsky, Y.; Wu, X.; Erk, C.; Maiti, S.; Grinblat, J.; Talianker, M.; Aurbach, D. Enhancement of Structural, Electrochemical, and Thermal Properties of Ni-Rich LiNi0.85Co0.1Mn0.05O2 Cathode Materials for Li-Ion Batteries by Al and Ti Doping. Batter. Supercaps 2021, 4, 221–231. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, K.; Li, N.; Deng, X.; Jiao, J.; Zhao, E.; Yin, W.; Wang, B.; Zhao, J.; Xiao, X. Enhancing Thermal and High-Voltage Cycling Stability of Ni-Rich Layered Cathodes through a Ti-Doping-Induced Surface-Disordered Structure. ACS Appl. Energy Mater. 2022, 5, 12673–12681. [Google Scholar] [CrossRef]
- Lipson, A.L.; Durham, J.L.; LeResche, M.; Abu-Baker, I.; Murphy, M.J.; Fister, T.T.; Wang, L.; Zhou, F.; Liu, L.; Kim, K.; et al. Improving the Thermal Stability of NMC 622 Li-Ion Battery Cathodes through Doping during Coprecipitation. ACS Appl. Mater. Interfaces 2020, 12, 18512–18518. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.H.; Park, K.J.; Yoon, D.R.; Aishova, A.; Yoon, C.S.; Sun, Y.K. Li[Ni0.9Co0.09W0.01]O2: A New Type of Layered Oxide Cathode with High Cycling Stability. Adv. Energy Mater. 2019, 9, 1902698. [Google Scholar] [CrossRef]
- Kim, U.H.; Jun, D.W.; Park, K.J.; Zhang, Q.; Kaghazchi, P.; Aurbach, D.; Major, D.T.; Goobes, G.; Dixit, M.; Leifer, N.; et al. Pushing the Limit of Layered Transition Metal Oxide Cathodes for High-Energy Density Rechargeable Li Ion Batteries. Energy Environ. Sci. 2018, 11, 1271–1279. [Google Scholar] [CrossRef]
- Sim, S.J.; Lee, S.H.; Jin, B.S.; Kim, H.S. Improving the Electrochemical Performances using a V-Doped Ni-Rich NCM Cathode. Sci. Rep. 2019, 9, 8952. [Google Scholar] [CrossRef] [PubMed]
- Susai, F.A.; Kovacheva, D.; Chakraborty, A.; Kravchuk, T.; Ravikumar, R.; Talianker, M.; Grinblat, J.; Burstein, L.; Kauffmann, Y.; Major, D.T.; et al. Improving Performance of LiNi0.8Co0.1Mn0.1O2 Cathode Materials for Lithium-Ion Batteries by Doping with Molybdenum-Ions: Theoretical and Experimental Studies. ACS Appl. Energy Mater. 2019, 2, 4521–4534. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, J.; Li, Y.; Zhang, Y.F.; Huang, S.P.; Lin, W.; Chen, W.K. Effects of Doping High-Valence Transition Metal (V, Nb and Zr) Ions on the Structure and Electrochemical Performance of LIB Cathode Material LiNi0.8Co0.1Mn0.1O2. Phys. Chem. Chem. Phys. 2021, 23, 11528–11537. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Seo, S.W.; Song, Y.Y.; Lee, H.S.; Cho, E. A First-Principles Study of the Preventive Effects of Al and Mg Doping on the Degradation in LiNi0.8Co0.1Mn0.1O2 Cathode Materials. Phys. Chem. Chem. Phys. 2017, 19, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yao, X.; Zhang, J.; Wang, S.; Zhang, D.; Yin, D.; Wang, L.; Zhang, Y.; Hu, J.; Cheng, Y.; et al. Sodium Doping Derived Electromagnetic Center of Lithium Layered Oxide Cathode Materials with Enhanced Lithium Storage. Nano Energy 2022, 94, 106900. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Y.; Wu, L.; Qin, W.; Yang, Z. Enhancement on Inter-Layer Stability on Na-Doped LiNi0.6Co0.2Mn0.2O2 Cathode Material. Powder Technol. 2021, 388, 166–175. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Sun, Y.Y.; Liu, S.; Li, G.R.; Gao, X.P. Na-doped LiNi0.8Co0.15Al0.05O2 with Excellent Stability of Both Capacity and Potential as Cathode Materials for Li-Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 3881–3889. [Google Scholar] [CrossRef]
- Xie, H.; Du, K.; Hu, G.; Peng, Z.; Cao, Y. The Role of Sodium in LiNi0.8Co0.15Al0.05O2 Cathode Material and its Electrochemical Behaviors. J. Phys. Chem. C 2016, 120, 3235–3241. [Google Scholar] [CrossRef]
- Li, C.F.; Chen, L.D.; Wu, L.; Liu, Y.; Hu, Z.Y.; Cui, W.J.; Dong, W.D.; Liu, X.; Yu, W.B.; Li, Y.; et al. Directly Revealing the Structure-Property Correlation in Na+-Doped Cathode Materials. Appl. Surf. Sci. 2023, 612, 155810. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Reissig, F.; Frankenstein, L.; Heidbüchel, M.; Winter, M.; Placke, T.; Schmuch, R. Magnesium Substitution in Ni-Rich NMC Layered Cathodes for High-Energy Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103045. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wang, L.; Wang, K.; Wu, X.; Zhou, P.; Miao, Z.; Zhou, J.; Zhao, Y.; Zhuo, S. Stabilizing the High-Voltage Cycle Performance of LiNi0.8Co0.1Mn0.1O2 Cathode Material by Mg Doping. J. Power Sources 2019, 438, 227017. [Google Scholar] [CrossRef]
- Song, J.H.; Kapylou, A.; Choi, H.S.; Yu, B.Y.; Matulevich, E.; Kang, S.H. Suppression of Irreversible Capacity Loss in Li-Rich Layered Oxide by Fluorine Doping. J. Power Sources 2016, 313, 65–72. [Google Scholar] [CrossRef]
- Shin, H.S.; Park, S.H.; Yoon, C.S.; Sun, Y.K. Effect of Fluorine on the Electrochemical Properties of Layered Li[Ni0.43Co0.22Mn0.35]O2 Cathode Materials via a Carbonate Process. Electrochem. Solid-State Lett. 2005, 8, A559. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.B.; Park, D.H.; Park, K.W. Fluorine-Doped LiNi0.8Mn0.1Co0.1O2 Cathode for High-Performance Lithium-Ion Batteries. Energies 2020, 13, 4808. [Google Scholar] [CrossRef]
- Woo, S.U.; Park, B.C.; Yoon, C.S.; Myung, S.T.; Prakash, J.; Sun, Y.K. Improvement of Electrochemical Performances of Li[Ni0.8Co0.1Mn0.1]O2 Cathode Materials by Fluorine Substitution. J. Electrochem. Soc. 2007, 154, A649. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, G.; Tang, W.; Xiao, Y.; Yan, K. Enhanced Rate Performance and High Potential as well as Decreased Strain of LiNi0.6Co0.2Mn0.2O2 by Facile Fluorine Modification. Electrochim. Acta 2018, 261, 565–577. [Google Scholar] [CrossRef]
- Binder, J.O.; Culver, S.P.; Pinedo, R.; Weber, D.A.; Friedrich, M.S.; Gries, K.I.; Volz, K.; Zeier, W.G.; Janek, J. Investigation of Fluorine and Nitrogen as Anionic Dopants in Nickel-Rich Cathode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 44452–44462. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Q.; Dai, S.; Li, W.; Liu, X.; Ding, F.; Zhang, J. Synergistic Effect of F–Doping and LiF Coating on Improving the High-Voltage Cycling Stability and Rate Capacity of LiNi0.5Co0.2Mn0.3O2 Cathode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 34153–34162. [Google Scholar] [CrossRef] [PubMed]
- Breddemann, U.; Krossing, I. Review on Synthesis, Characterization, and Electrochemical Properties of Fluorinated Nickel-Cobalt-Manganese Cathode Active Materials for Lithium-Ion Batteries. ChemElectroChem 2020, 7, 1389–1430. [Google Scholar] [CrossRef]
- Si, Z.; Shi, B.; Huang, J.; Yu, Y.; Han, Y.; Zhang, J.; Li, W. Titanium and Fluorine Synergetic Modification Improves the Electrochemical Performance of Li(Ni0.8Co0.1Mn0.1)O2. J. Mater. Chem. A 2021, 9, 9354–9363. [Google Scholar] [CrossRef]
- Zhu, F.; Shi, Y.; Hu, G.; Peng, Z.; Cao, Y.; Sun, Q.; Xue, Z.; Zhang, Y.; Du, K. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 via titanium and boron co-doping. Ceram. Int. 2021, 47, 3070–3078. [Google Scholar] [CrossRef]
- Wang, J.; Nie, Y.; Miao, C.; Tan, Y.; Wen, M.; Xiao, W. Enhanced Electrochemical Properties of Ni-Rich Layered Cathode Materials via Mg2+ and Ti4+ Co-Doping for Lithium-Ion Batteries. J. Colloid Interface Sci. 2021, 601, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hai, C.; Shen, Y.; Zeng, J.; Zhang, L.; Li, X.; Ren, X.; Dong, S.; Zhang, G.; Sun, C.; et al. Improved Lithium Ion Diffusion and Stability of a LiNi0.8Co0.1Mn0.1O2 Cathode via the Synergistic Effect of Na and Mg Dual-Metal Cations for Lithium Ion Battery. J. Electrochem. Soc. 2020, 167, 020522. [Google Scholar]
- Jamil, S.; Yousaf, A.B.; Yoon, S.H.; Han, D.S.; Yang, L.; Kasak, P.; Wang, X. Dual Cationic Modified High Ni-Low Co Layered Oxide Cathode with a Heteroepitaxial Interface for High Energy-Density Lithium-Ion Batteries. Chem. Eng. J. 2021, 416, 129118. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, G.; Wang, W.; Peng, Z.; Fang, Z.; Cao, Y.; Huang, J.; Du, K. Enhancing Structural Stability and Electrochemical Properties of Co-Less Ni-Rich Layer Cathode Materials by Fluorine and Niobium Co-Doping. ACS Appl. Energy Mater. 2022, 5, 10927–10939. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, X.; Zhu, H.; Cao, K.; Liu, Q.; Liu, J.; Li, L.; Duan, J. High performance and structural stability of K and Cl co-doped LiNi0.5Co0.2Mn0.3O2 cathode materials in 4.6 voltage. Front. Chem. 2019, 6, 643. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, H.; Tong, J.; Li, W.; Liu, X.; Zhang, H.; Huang, H.; Zhou, W. Recent Progress on the Modification of High Nickel Content NCM: Coating, Doping, and Single Crystallization. Interdiscip. Mater. 2022, 1, 330–353. [Google Scholar] [CrossRef]
- Cao, Z.; Li, Y.; Shi, M.; Zhu, G.; Zhang, R.; Li, X.; Yue, H.; Yang, S. Improvement of the Cycling Performance and Thermal Stability of Lithium-Ion Batteries by Coating Cathode Materials with Al2O3 Nano Layer. J. Electrochem. Soc. 2017, 164, A475. [Google Scholar] [CrossRef]
- Lee, Y.S.; Shin, W.K.; Kannan, A.G.; Koo, S.M.; Kim, D.W. Improvement of the Cycling Performance and Thermal Stability of Lithium-Ion Cells by Double-Layer Coating of Cathode Materials with Al2O3 Nanoparticles and Conductive Polymer. ACS Appl. Mater. Interfaces 2015, 7, 13944–13951. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, M.; Qian, D.; Meng, Y.S. Ultrathin Al2O3 Coatings for Improved Cycling Performance and Thermal Stability of LiNi0.5Co0.2Mn0.3O2 Cathode Material. Electrochim. Acta 2016, 203, 154–161. [Google Scholar] [CrossRef]
- Wang, L.; Su, Q.; Shi, W.; Wang, C.; Li, H.; Wang, Y.; Du, G.; Zhang, M.; Zhao, W.; Ding, S.; et al. Optimized Structure Stability and Cycling Performance of LiNi0.8Co0.1Mn0.1O2 through homogeneous nano-thickness Al2O3 coating. Electrochim. Acta 2022, 435, 141411. [Google Scholar] [CrossRef]
- Fan, Q.; Lin, K.; Yang, S.; Guan, S.; Chen, J.; Feng, S.; Liu, J.; Liu, L.; Li, J.; Shi, Z. Constructing Effective TiO2 Nano-Coating for High-Voltage Ni-Rich Cathode Materials for Lithium Ion Batteries by Precise Kinetic Control. J. Power Sources 2020, 477, 228745. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Ren, D.; Hsu, H.; Xu, G.L.; Hou, J.; Wang, L.; Feng, X.; Lu, L.; Xu, W.; et al. Toward a High-Voltage Fast-Charging Pouch Cell with TiO2 Cathode Coating and Enhanced Battery Safety. Nano Energy 2020, 71, 104643. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Li, Z.; Huang, K.; Jiang, J.; Chen, Z.; Qi, X.; Dou, H.; Zhang, X. In Situ Tuning Residual Lithium Compounds and Constructing TiO2 Coating for Surface Modification of a Nickel-Rich Cathode toward High-Energy Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 12423–12432. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Sim, S.J.; Jin, B.S.; Kim, H.S.; Lee, S.H. Suppressed microcracking and F penetration of ni-rich layered cathode via the combined effects of titanium dioxide doping and coating. ACS Appl. Energy Mater. 2021, 4, 1743–1751. [Google Scholar] [CrossRef]
- Khalili Azar, M.; Razmjoo Khollari, M.A.; Esmaeili, M.; Heidari, E.; Hosseini-Hosseinabad, S.M.; Siavash Moakhar, R.; Dolati, A.; Ramakrishna, S. Enhanced Electrochemical Performance and Thermal Stability of ZrO2-and rGO–ZrO2-Coated Li[Ni0.8Co0.1Mn0.1]O2 Cathode Material for Li-Ion Batteries. ACS Appl. Energy Mater. 2020, 4, 934–945. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Y.; Wei, G.; Qiu, S.; Qu, J. Enhanced Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Cathode via Wet-Chemical Coating of MgO. J. Solid State Electrochem. 2019, 23, 2213–2224. [Google Scholar] [CrossRef]
- Sharifi-Asl, S.; Lu, J.; Amine, K.; Shahbazian-Yassar, R. Oxygen Release Degradation in Li-Ion Battery Cathode Materials: Mechanisms and Mitigating Approaches. Adv. Energy Mater. 2019, 9, 1900551. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Xue, B.; Wu, X.; Li, L.; Guo, Y.; Zhang, L. Synergistic Modification of Ni-Rich Full Concentration Gradient Materials with Enhanced Thermal Stability. Chem. Eng. J. 2023, 451, 138518. [Google Scholar] [CrossRef]
- Li, W.; Yang, L.; Li, Y.; Chen, Y.; Guo, J.; Zhu, J.; Pan, H.; Xi, X. Ultra-Thin AlPO4 Layer Coated LiNi0.7Co0.15Mn0.15O2 Cathodes with Enhanced High-Voltage and High-Temperature Performance for Lithium-Ion Half/Full Batteries. Front. Chem. 2020, 8, 597. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Wang, L.; Zhang, D.; Bao, D.; Yin, D.; Wang, L.; Cheng, Y.; Huang, G. A Universal Multifunctional Rare Earth Oxide Coating to Stabilize High-Voltage Lithium Layered Oxide Cathodes. Energy Storage Mater. 2023, 56, 155–164. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, G.T.; Guang, Y.; Bresser, D.; Diemant, T.; Huang, Y.; Copley, M.; Behm, R.J.; Passerini, S.; Shen, Z. Manganese Phosphate Coated Li[Ni0.6Co0.2Mn0.2]O2 Cathode Material: Towards Superior Cycling Stability at Elevated Temperature and High Voltage. J. Power Sources 2018, 402, 263–271. [Google Scholar] [CrossRef]
- He, X.; Xu, X.; Wang, L.; Du, C.; Cheng, X.; Zuo, P.; Ma, Y.; Yin, G. Enhanced Electrochemical Performance of LiNi0.8Co0.15Al0.05O2 Cathode Material via Li2TiO3 Nanoparticles Coating. J. Electrochem. Soc. 2019, 166, A143. [Google Scholar] [CrossRef]
- Yang, G.; Pan, K.; Lai, F.; Wang, Z.; Chu, Y.; Yang, S.; Han, J.; Wang, H.; Zhang, X.; Li, Q. Integrated Co-Modification of PO43− Polyanion Doping and Li2TiO3 Coating for Ni-Rich Layered LiNi0.6Co0.2Mn0.2O2 Cathode Material of Lithium-Ion Batteries. Chem. Eng. J. 2021, 421, 129964. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Chen, X.; Yang, X.; Xiang, F.; Lu, W. Enhancing the Stabilities and Electrochemical Performances of LiNi0.5Co0.2Mn0.3O2 Cathode Material by Simultaneous LiAlO2 Coating and Al Doping. Electrochim. Acta 2021, 376, 138038. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Gao, R.; Hu, Z.; Liu, X. High Rate Capability and Excellent Thermal Stability of Li+-Conductive Li2ZrO3-Coated LiNi1/3Co1/3Mn1/3O2 via a Synchronous Lithiation Strategy. J. Phys. Chem. C 2015, 119, 20350–20356. [Google Scholar] [CrossRef]
- Gan, Q.; Qin, N.; Wang, Z.; Li, Z.; Zhu, Y.; Li, Y.; Gu, S.; Yuan, H.; Luo, W.; Lu, L.; et al. Revealing Mechanism of Li3PO4 Coating Suppressed Surface Oxygen Release for Commercial Ni-Rich Layered Cathodes. ACS Appl. Energy Mater. 2020, 3, 7445–7455. [Google Scholar] [CrossRef]
- Tang, Z.F.; Wu, R.; Huang, P.F.; Wang, Q.S.; Chen, C.H. Improving the Electrochemical Performance of Ni-Rich Cathode Material LiNi0.815Co0.15Al0.035O2 by Removing the Lithium Residues and Forming Li3PO4 Coating Layer. J. Alloys Compd. 2017, 693, 1157–1163. [Google Scholar]
- Lee, S.W.; Kim, M.S.; Jeong, J.H.; Kim, D.H.; Chung, K.Y.; Roh, K.C.; Kim, K.B. Li3PO4 Surface Coating on Ni-Rich LiNi0.6Co0.2Mn0.2O2 by a Citric Acid Assisted Sol-Gel Method: Improved Thermal Stability and High-Voltage Performance. J. Power Sources 2017, 360, 206–214. [Google Scholar] [CrossRef]
- Kim, M.Y.; Song, Y.W.; Lim, J.; Park, S.J.; Kang, B.S.; Hong, Y.; Kim, H.S.; Han, J.H. LATP-Coated NCM-811 for High-Temperature Operation of All-Solid Lithium Battery. Mater. Chem. Phys. 2022, 290, 126644. [Google Scholar] [CrossRef]
- Qian, D.; Xu, B.; Cho, H.M.; Hatsukade, T.; Carroll, K.J.; Meng, Y.S. Lithium Lanthanum Titanium Oxides: A Fast Ionic Conductive Coating for Lithium-Ion Battery Cathodes. Chem. Mater. 2012, 24, 2744–2751. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, L.; Zhu, C.; Ren, W.; Kong, L.; Wan, Y. Nano LiFePO4 Coated Ni Rich Composite as Cathode for Lithium Ion Batteries with High Thermal Ability and Excellent Cycling Performance. J. Power Sources 2020, 464, 228235. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, T.F.; Jia, D.; Wang, Y.; Wu, Q.; Gu, H.T.; Wu, Y.M.; Tang, W.P. LiFePO4-Coated LiNi0.5Co0.2Mn0.3O2 Cathode Materials with Improved High Voltage Electrochemical Performance and Enhanced Safety for Lithium Ion Pouch Cells. J. Electrochem. Soc. 2019, 166, A5437. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, L.; Jia, D.; Jiang, X.; Wu, Y.; Hao, Q.; Xia, X.; Ouyang, Y.; Peng, L.; Tang, W.; et al. LiNi0.8Co0.15Al0.05O2 Cathodes Exhibiting Improved Capacity Retention and Thermal Stability due to a Lithium Iron Phosphate Coating. Electrochim. Acta 2019, 312, 179–187. [Google Scholar] [CrossRef]
- Duan, J.; Wu, C.; Cao, Y.; Du, K.; Peng, Z.; Hu, G. Enhanced Electrochemical Performance and Thermal Stability of LiNi0.80Co0.15Al0.05O2 via Nano-Sized LiMnPO4 Coating. Electrochim. Acta 2016, 221, 14–22. [Google Scholar] [CrossRef]
- Sun, Y.K.; Myung, S.T.; Park, B.C.; Prakash, J.; Belharouak, I.; Amine, K. High-Energy Cathode Material for Long-Life and Safe Lithium Batteries. Nat. Mater. 2009, 8, 320–324. [Google Scholar] [CrossRef]
- Sun, Y.K.; Chen, Z.; Noh, H.J.; Lee, D.J.; Jung, H.G.; Ren, Y.; Wang, S.; Yoon, C.S.; Myung, S.T.; Amine, K. Nanostructured High-Energy Cathode Materials for Advanced Lithium Batteries. Nat. Mater. 2012, 11, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.H.; Ryu, H.H.; Kim, J.H.; Mücke, R.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.K. Microstructure-Controlled Ni-Rich Cathode Material by Microscale Compositional Partition for Next-Generation Electric Vehicles. Adv. Energy Mater. 2019, 9, 1803902. [Google Scholar] [CrossRef]
- Xu, X.; Xiang, L.; Wang, L.; Jian, J.; Du, C.; He, X.; Huo, H.; Cheng, X.; Yin, G. Progressive Concentration Gradient Nickel-Rich Oxide Cathode Material for High-Energy and Long-Life Lithium-Ion Batteries. J. Mater. Chem. A 2019, 7, 7728–7735. [Google Scholar] [CrossRef]
- Shi, J.L.; Qi, R.; Zhang, X.D.; Wang, P.F.; Wang, P.F.; Fu, W.G.; Yin, Y.X.; Xu, J.; Wan, L.J.; Guo, Y.G. High-Thermal-and Air-Stability Cathode Material with Concentration-Gradient Buffer for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 42829–42835. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Guo, L.; Jin, H.; Du, B.; Cao, B.; Chen, Y.; Chen, Y. Building Nickel-Rich Cathodes with Large Concentration Gradient for High Performance Lithium-Ion Batteries. J. Power Sources 2020, 468, 228405. [Google Scholar] [CrossRef]
- Park, G.T.; Ryu, H.H.; Noh, T.C.; Kang, G.C.; Sun, Y.K. Microstructure-Optimized Concentration-Gradient NCM Cathode for Long-Life Li-Ion Batteries. Mater. Today 2022, 52, 9–18. [Google Scholar] [CrossRef]
- Zeng, X.; Jian, T.; Lu, Y.; Yang, L.; Ma, W.; Yang, Y.; Zhu, J.; Huang, C.; Dai, S.; Xi, X. Enhancing High-Temperature and High-Voltage Performances of Single-Crystal LiNi0.5Co0.2Mn0.3O2 Cathodes Through a LiBO2/LiAlO2 Dual-Modification Strategy. ACS Sustain. Chem. Eng. 2020, 8, 6293–6304. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, M.; Zhang, J.; Liu, R.; Wang, Y.; Xiao, H.; Huang, Y.; Yuan, G. Improving Electrochemical Performance of Ni-Rich LiNi0.8Co0.1Mn0.1O2 Cathode for Li-Ion Batteries by Dual-Conductive Coating Layer of PPy and LiAlO2. J. Alloys Compd. 2020, 848, 156387. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, M.; Zhang, L.; Wang, Y.; Yan, Y.; Li, Z.; Sun, X.; Zhang, D. Excellent High-Rate Cyclic Performance of LiNi0.8Co0.1Mn0.1O2 Cathodes via Dual Li2SiO3/PPy Coating. J. Alloys Compd. 2022, 938, 168575. [Google Scholar] [CrossRef]
- Wei, Z.; Liang, C.; Jiang, L.; Wang, L.; Cheng, S.; Peng, Q.; Feng, L.; Zhang, W.; Sun, J.; Wang, Q. In-Depth Study on Diffusion of Oxygen Vacancies in Li(NixCoyMnz)O2 Cathode Materials Under Thermal Induction. Energy Storage Mater. 2022, 47, 51–60. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, N.Y.; Seo, J.H.; Yu, Y.S.; Sharma, M.; Mücke, R.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.K. A Highly Stabilized Ni-Rich NCA Cathode for High-Energy Lithium-Ion Batteries. Mater. Today 2020, 36, 73–82. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.H.; Sun, Y.K.; Yoon, C.S. Evolution of a Radially Aligned Microstructure in Boron-Doped Li[Ni0.95Co0.04Al0.01]O2 Cathode Particles. ACS Appl. Mater. Interfaces 2022, 14, 17500–17508. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.H.; Park, G.T.; Son, B.K.; Nam, G.W.; Liu, J.; Kuo, L.Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.K. Heuristic Solution for Achieving Long-Term Cycle Stability for Ni-Rich Layered Cathodes at Full Depth of Discharge. Nat. Energy 2020, 5, 860–869. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kim, U.H.; Yoon, C.S.; Sun, Y.K. Enhanced Cycling Stability of Sn-Doped Li[Ni0.90Co0.05Mn0.05]O2 via Optimization of Particle Shape and Orientation. Chem. Eng. J. 2021, 405, 126887. [Google Scholar] [CrossRef]
- Du, B.; Mo, Y.; Jin, H.; Li, X.; Qu, Y.; Li, D.; Cao, B.; Jia, X.; Lu, Y.; Chen, Y. Radially Microstructural Design of LiNi0.8Co0.1Mn0.1O2 Cathode Material toward Long-Term Cyclability and High Rate Capability at High Voltage. ACS Appl. Energy Mater. 2020, 3, 6657–6669. [Google Scholar] [CrossRef]
- Harlow, J.E.; Ma, X.; Li, J.; Logan, E.; Liu, Y.; Zhang, N.; Ma, L.; Glazier, S.L.; Cormier, M.M.E.; Genovese, M.; et al. A Wide Range of Testing Results on an Excellent Lithium-Ion Cell Chemistry to be Used as Benchmarks for New Battery Technologies. J. Electrochem. Soc. 2019, 166, A3031–A3044. [Google Scholar] [CrossRef]
- Li, J.; Cameron, A.R.; Li, H.; Glazier, S.; Xiong, D.; Chatzidakis, M.; Allen, J.; Botton, G.A.; Dahn, J.R. Comparison of Single Crystal and Polycrystalline LiNi0.5Mn0.3Co0.2O2 Positive Electrode Materials for High Voltage Li-Ion Cells. J. Electrochem. Soc. 2017, 164, A1534. [Google Scholar] [CrossRef]
- Weber, R.; Fell, C.R.; Dahn, J.R.; Hy, S. Operando X-ray Diffraction Study of Polycrystalline and Single-Crystal LixNi0.5Mn0.3Co0.2O2. J. Electrochem. Soc. 2017, 164, A2992–A2999. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, N.; Stark, J.E.; Arab, P.; Li, H.; Dahn, J.R. Synthesis of Co-free Ni-rich Single Crystal Positive Electrode Materials for Lithium Ion Batteries: Part i. Two-Step Lithiation Method for Al-or Mg-doped LiNiO2. J. Electrochem. Soc. 2021, 168, 040531. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Stone, W.; Glazier, S.; Dahn, J.R. Development of Electrolytes for Single Crystal NMC532/Artificial Graphite Cells with Long Lifetime. J. Electrochem. Soc. 2018, 165, A626–A635. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Li, J.; Yang, H.; Dai, P.; Zeng, J.; Zhao, J. Single-Crystal Structure Helps Enhance the Thermal Performance of Ni-Rich Layered Cathode Materials for Lithium-Ion Batteries. Chem. Eng. J. 2022, 434, 134638. [Google Scholar] [CrossRef]
- Huang, B.; Wang, M.; Zhang, X.; Zhao, Z.; Chen, L.; Gu, Y. Synergistic coupling effect of single crystal morphology and precursor treatment of Ni-Rich cathode materials. J. Alloys Compd. 2020, 830, 154619. [Google Scholar] [CrossRef]
- Pang, P.; Tan, X.; Wang, Z.; Cai, Z.; Nan, J.; Xing, Z.; Li, H. Crack-Free Single-Crystal LiNi0.83Co0.10Mn0.07O2 as Cycling/Thermal Stable Cathode Materials for High-Voltage Lithium-Ion Batteries. Electrochim. Acta 2021, 365, 137380. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, L.; Huang, S.; Shang, W.; Kong, L.; Sun, M.; Chen, L.; Ren, W. Single-Crystal LiNi0.5Co0.2Mn0.3O2: A High Thermal and Cycling Stable Cathodes for Lithium-Ion Batteries. J. Mater. Sci. 2020, 55, 2913–2922. [Google Scholar] [CrossRef]
- Liu, G.; Li, M.; Wu, N.; Cui, L.; Huang, X.; Liu, X.; Zhao, Y.; Chen, H.; Yuan, W.; Bai, Y. Single-Crystalline Particles: An Effective Way to Ameliorate the Intragranular Cracking, Thermal Stability, and Capacity Fading of the LiNi0.6Co0.2Mn0.2O2 Electrodes. J. Electrochem. Soc. 2018, 165, A3040. [Google Scholar] [CrossRef]
- Ge, M.; Wi, S.; Liu, X.; Bai, J.; Ehrlich, S.; Lu, D.; Lee, W.K.; Chen, Z.H.; Wang, F. Kinetic Limitations in Single-Crystal High-Nickel Cathodes. Angew. Chem. Int. Ed. 2021, 60, 17350–17355. [Google Scholar] [CrossRef]
- Bi, Y.; Tao, J.; Wu, Y.; Li, L.; Xu, Y.; Hu, E.; Wu, B.; Hu, J.; Wang, C.; Zhang, J.; et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 2020, 370, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.H.; Lee, E.J.; Yoon, C.S.; Myung, S.T.; Sun, Y.K. Compositionally Graded Cathode Material with Long-Term Cycling Stability for Electric Vehicles Application. Adv. Energy Mater. 2016, 6, 1601417. [Google Scholar] [CrossRef]
- Feng, Z.; Rajagopalan, R.; Zhang, S.; Sun, D.; Tang, Y.; Ren, Y.; Wang, H. A Three in One Strategy to Achieve Zirconium Doping, Boron Doping, and Interfacial Coating for Stable LiNi0.8Co0.1Mn0.1O2 Cathode. Adv. Sci. 2021, 8, 2001809. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.; Wang, Q.; Hu, C.; Luo, B.; Liu, Y.; Xiao, Z.; Ou, X. Boosting High-Voltage and Ultralong-Cycling Performance of Single-Crystal LiNi0.5Co0.2Mn0.3O2 Cathode Materials via Three-in-One Modification. Energy Environ. Mater. 2023, 6, e12270. [Google Scholar] [CrossRef]
- Bao, W.; Qian, G.; Zhao, L.; Yu, Y.; Su, L.; Cai, X.; Zhao, H.; Zuo, Y.; Zhang, Y.; Li, H.; et al. Simultaneous Enhancement of Interfacial Stability and Kinetics of Single-Crystal LiNi0.6Mn0.2Co0.2O2 Through Optimized Surface Coating and Doping. Nano Lett. 2020, 20, 8832–8840. [Google Scholar] [CrossRef]
- Zhou, G.; Wei, Y.; Li, H.; Wang, C.; Huang, X.; Yang, D. Al2O3-Coated, Single Crystal Zr/Y co-Doped High-Ni NCM Cathode Materials for High-Performance Lithium-Ion Batteries. Part. Part. Syst. Charact. 2022, 39, 2200061. [Google Scholar] [CrossRef]
- Shen, J.; Deng, D.; Li, X.; Zhang, B.; Xiao, Z.; Hu, C.; Yan, X.; Ou, X. Realizing Ultrahigh-Voltage Performance of Single-Crystalline LiNi0.55Co0.15Mn0.3O2 Cathode Materials by Simultaneous Zr-Doping and B2O3-Coating. J. Alloys Compd. 2022, 903, 163999. [Google Scholar] [CrossRef]
- Fan, X.M.; Huang, Y.D.; Wei, H.X.; Tang, L.B.; He, Z.J.; Yan, C.; Mao, J.; Dai, K.H.; Zheng, J.C. Surface Modification Engineering Enabling 4.6 V Single-Crystalline Ni-Rich Cathode with Superior Long-Term Cyclability. Adv. Funct. Mater. 2022, 32, 2109421. [Google Scholar] [CrossRef]
- Han, Y.; Xu, J.; Wang, W.; Long, F.; Qu, Q.; Wang, Y.; Zheng, H. Implanting an Electrolyte Additive on a Single Crystal Ni-Rich Cathode Surface for Improved Cycleability and Safety. J. Mater. Chem. A 2020, 8, 24579–24589. [Google Scholar] [CrossRef]
- Lim, D.A.; Shin, Y.K.; Seok, J.H.; Hong, D.; Ahn, K.H.; Lee, C.H.; Kim, D.W. Cathode Electrolyte Interphase-Forming Additive for Improving Cycling Performance and Thermal Stability of Ni-Rich LiNixCoyMn1–x–yO2 Cathode Materials. ACS Appl. Mater. Interfaces 2022, 14, 54688–54697. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Q.; Sun, J. Electrochemical Performance and Thermal Stability Analysis of LiNixCoyMnzO2 Cathode Based on a Composite Safety Electrolyte. J. Hazard. Mater. 2018, 351, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, P.; Jiang, S. Experimental Study on the Effect of Electrolyte Flame Retardant Additives on the Nail Penetration Safety of Lithium-Ion Battery. Fire Sci. Technol. 2022, 41, 1209. [Google Scholar]
- Li, Y.; An, Y.; Tian, Y.; Fei, H.; Xiong, S.; Qian, Y.; Feng, J. Stable and Safe Lithium Metal Batteries with Ni-Rich Cathodes Enabled by a High Efficiency Flame Retardant Additive. J. Electrochem. Soc. 2019, 166, A2736. [Google Scholar] [CrossRef]
- An, K.; Tran, Y.H.T.; Kwak, S.; Han, J.; Song, S.W. Design of Fire-Resistant Liquid Electrolyte Formulation for Safe and Long-Cycled Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2106102. [Google Scholar] [CrossRef]
- Kim, A.Y.; Strauss, F.; Bartsch, T.; Teo, J.H.; Hatsukade, T.; Mazilkin, A.; Janek, J.; Hartmann, P.; Brezesinski, T. Stabilizing Effect of a Hybrid Surface Coating on a Ni-Rich NCM Cathode Material in All-Solid-State Batteries. Chem. Mater. 2019, 31, 9664–9672. [Google Scholar] [CrossRef]
- Wang, S.; Fang, R.; Li, Y.; Liu, Y.; Xin, C.; Richter, F.H.; Nan, C.W. Interfacial Challenges for All-Solid-State Batteries Based on Sulfide Solid Electrolytes. J. Mater. 2021, 7, 209–218. [Google Scholar] [CrossRef]
- Vadhva, P.; Hu, J.; Johnson, M.J.; Stocker, R.; Braglia, M.; Brett, D.J.; Rettie, A.J. Electrochemical Impedance Spectroscopy for All-Solid-State Batteries: Theory, Methods and Future Outlook. ChemElectroChem 2021, 8, 1930–1947. [Google Scholar] [CrossRef]
- Meng, F.; Gao, J.; Zhang, M.; Li, D.; Liu, X. Enhanced Safety Performance of Automotive Lithium-Ion Batteries with Al2O3-Coated Non-Woven Separator. Batter. Supercaps 2021, 4, 146–151. [Google Scholar] [CrossRef]
- Kim, P.S.; Le Mong, A.; Kim, D. Thermal, Mechanical, and Electrochemical Stability Enhancement of Al2O3 Coated Polypropylene/polyethylene/polypropylene Separator via Poly (vinylidene fluoride)-Poly (ethoxylated pentaerythritol tetraacrylate) Semi-Interpenetrating Network Binder. J. Membr. Sci. 2020, 612, 118481. [Google Scholar] [CrossRef]
- Pham, H.Q.; Lee, H.Y.; Hwang, E.H.; Kwon, Y.G.; Song, S.W. Non-Flammable Organic Liquid Electrolyte for High-Safety and High-Energy Density Li-Ion Batteries. J. Power Sources 2018, 404, 13–19. [Google Scholar] [CrossRef]
- Pham, H.Q.; Kim, G.; Jung, H.M.; Song, S.W. Fluorinated Polyimide as a Novel High-Voltage Binder for High-Capacity Cathode of Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1704690. [Google Scholar] [CrossRef]
- McKerracher, R.D.; Guzman-Guemez, J.; Wills, R.G.; Sharkh, S.M.; Kramer, D. Advances in Prevention of Thermal Runaway in Lithium-Ion Batteries. Adv. Energy Sustain. Res. 2021, 2, 2000059. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Li, H.; Cao, Y.; Yang, H.; Ai, X. Reversible Temperature-Responsive Cathode for Thermal Protection of Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 5236–5244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).