Preparation and Characterization of a LiFePO4- Lithium Salt Composite Cathode for All-Solid-State Li-Metal Batteries
Abstract
1. Introduction
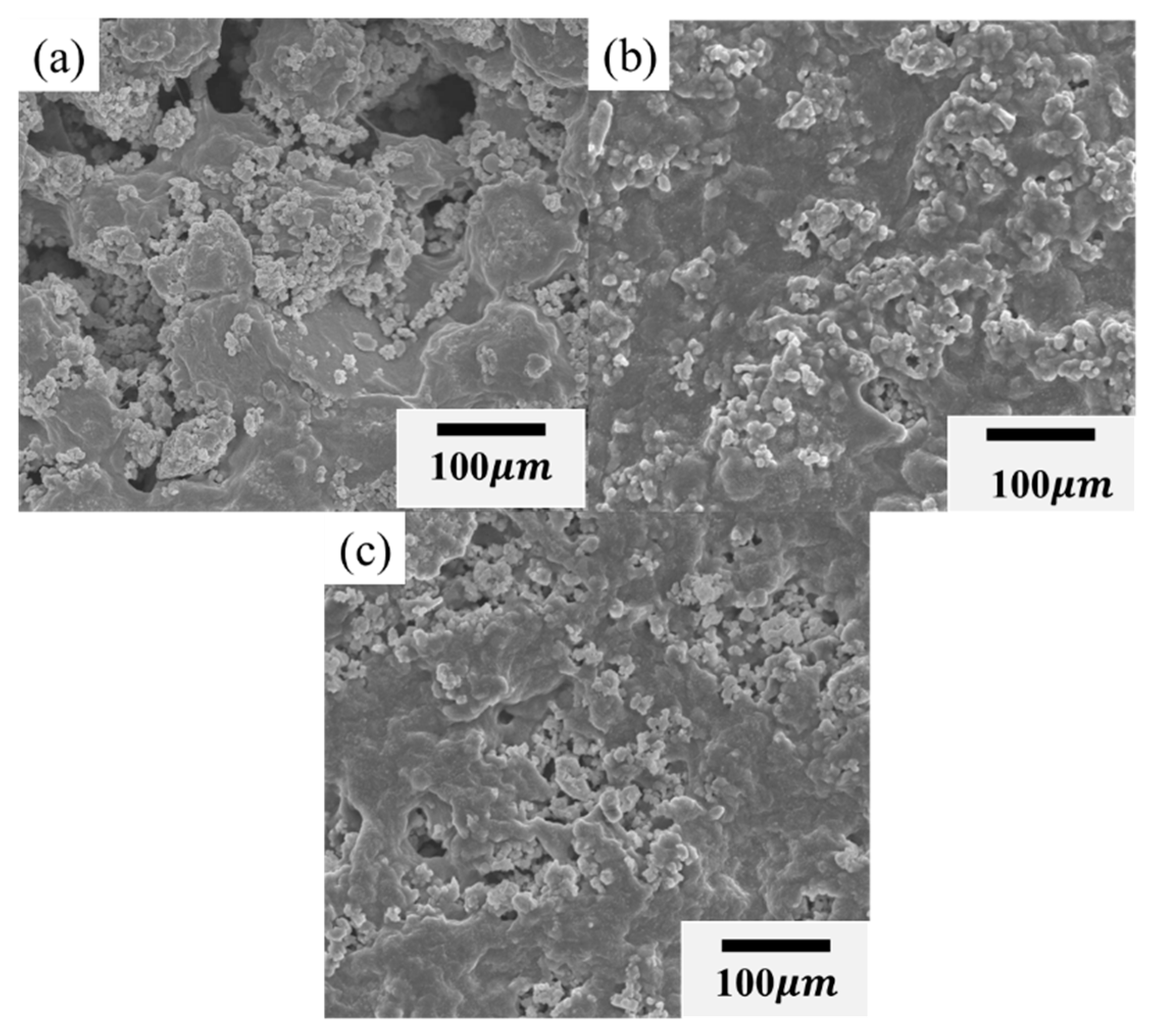
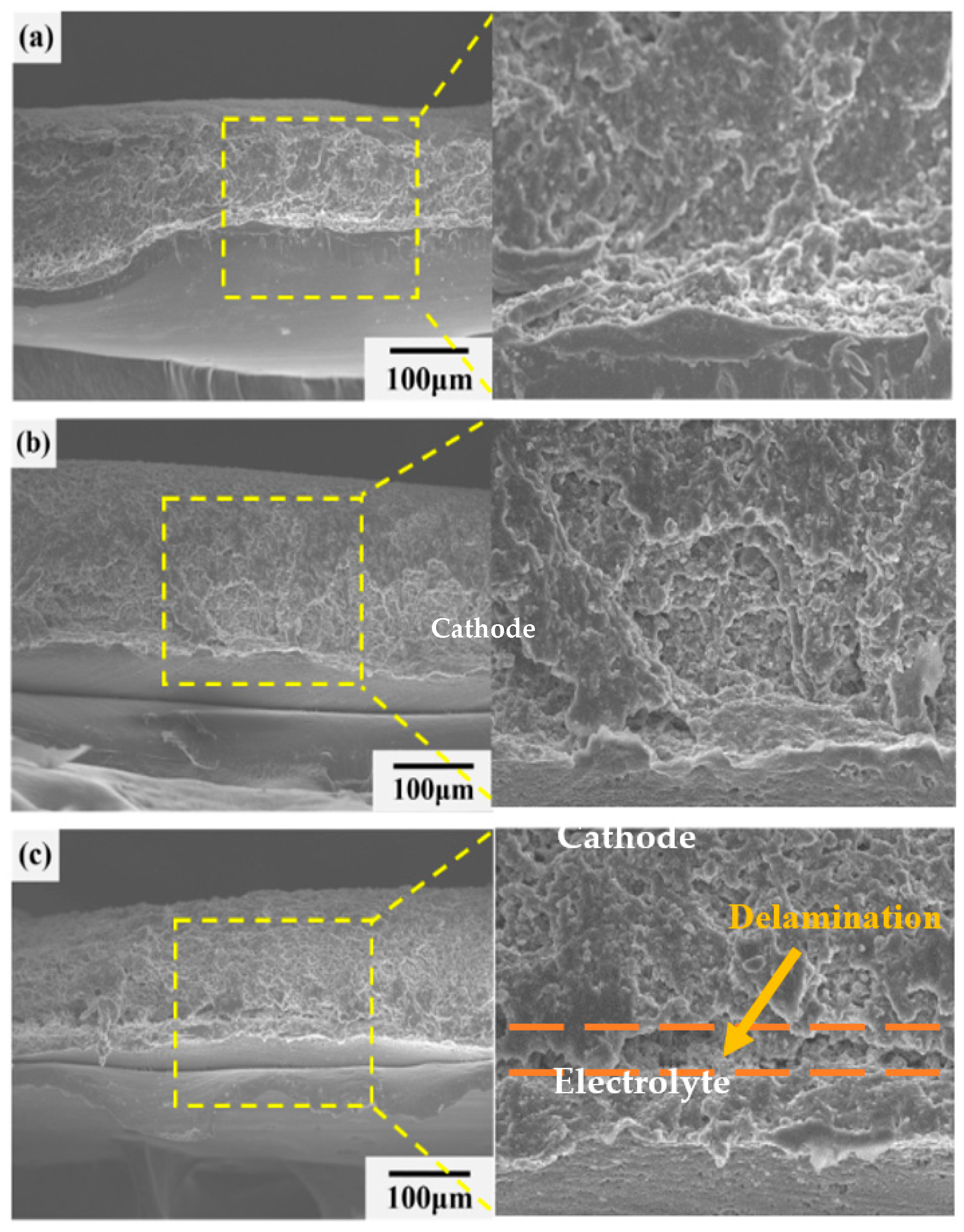
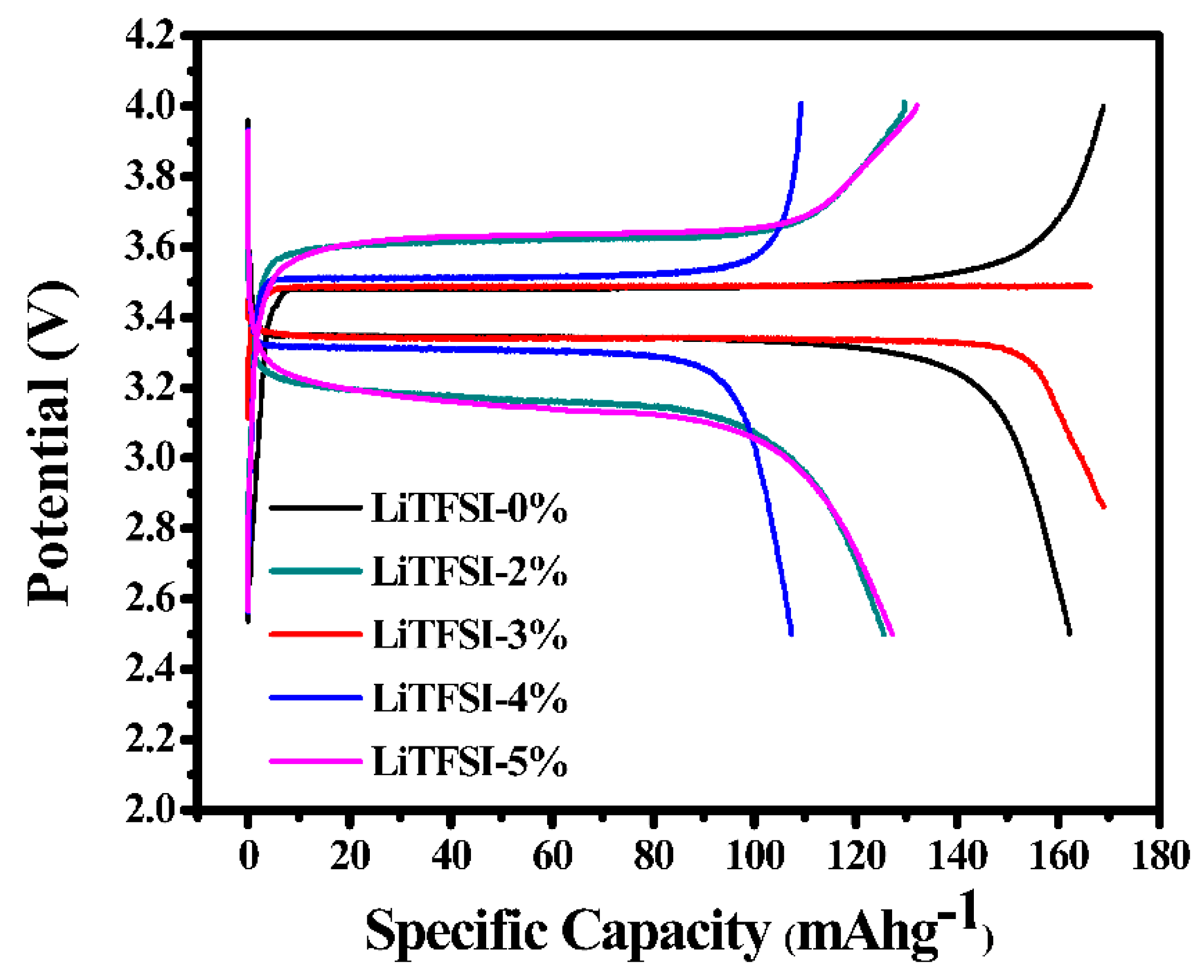
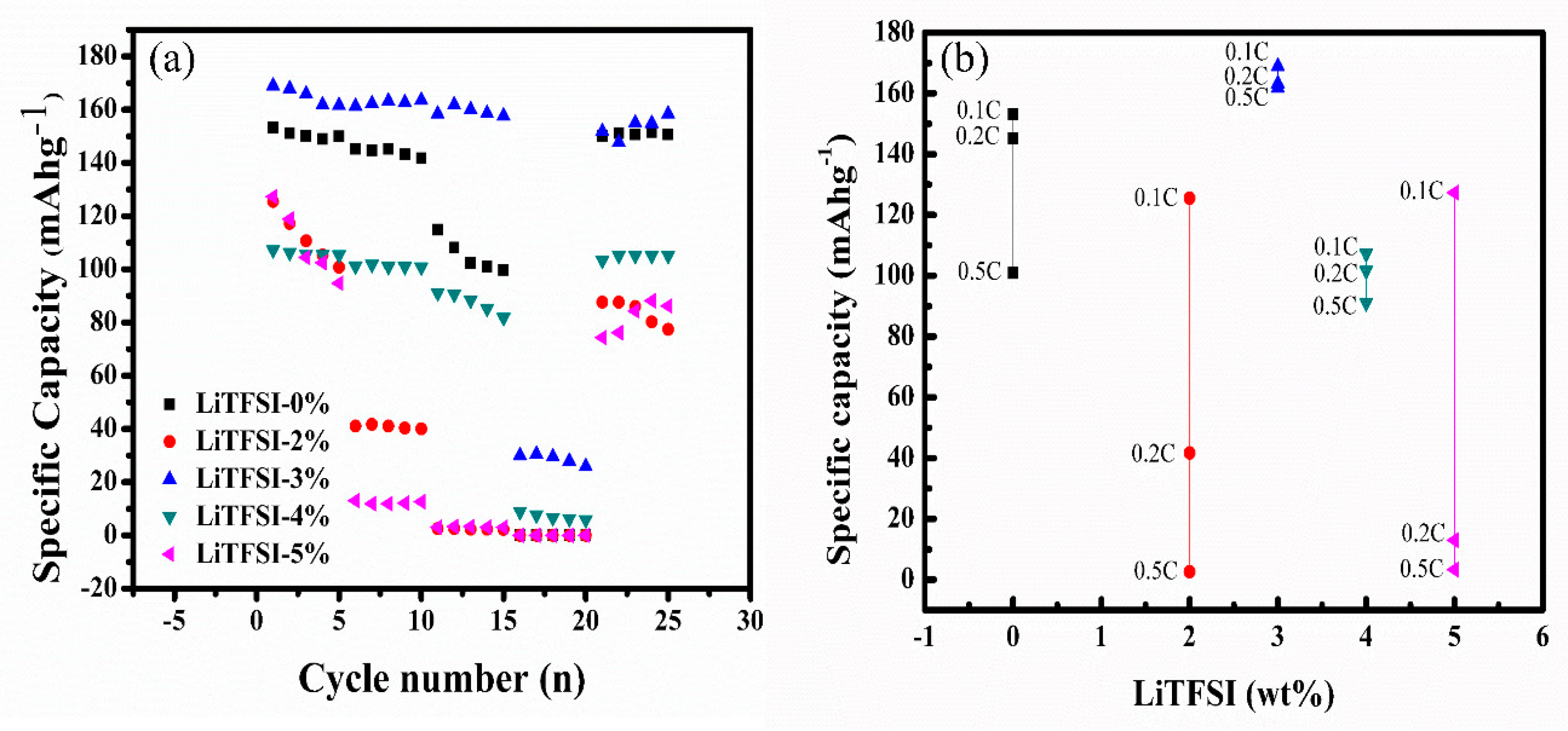
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of Lithium-Ion Batteries in Grid-Scale Energy Storage Systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef]
- Santamaria, C.; Morales, E.; Rio, C.D.; Herradon, B.; Amarilla, J.M. Studies on sodium-ion batteries: Searching for the proper combination of the cathode material, the electrolyte and the working voltage. The role of magnesium substitution in layered manganese-rich oxides, and pyrrolidinium ionic liquid. Electrochim. Acta 2023, 439, 141654–141663. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Li, Y.; Liu, Y.; Jing, Q.; Liu, X.; Liu, Z.; Zhang, X. Organosilicon-Based Functional Electrolytes for High-Performance Lithium Batteries. Adv. Energy Mater. 2021, 11, 2101057–2101082. [Google Scholar] [CrossRef]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332–102348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Kim, M.S.; Tu, Z.; Choudhury, S.; Tang, T.; Archer, L.A. Regulating electrodeposition morphology of lithium: Towards commercially relevant secondary Li metal batteries. Chem. Soc. Rev. 2020, 49, 2701–2750. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, K.; Liang, F.; Yao, Y.; Kong, L. High performance of LiFePO4 with nitrogen and phosphorus dual-doped carbon layer for lithium-ion batteries. J. Alloys Compd. 2022, 890, 161617–161624. [Google Scholar] [CrossRef]
- Nirmale, T.C.; Karbhal, I.; Kalubarme, R.S.; Shelke, M.V.; Varma, A.J.; Kale, B.B. Facile Synthesis of Unique Cellulose Triacetate Based Flexible and High Performance Gel Polymer Electrolyte for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 34773–34782. [Google Scholar] [CrossRef]
- He, P.; Yu, H.; Li, D.; Zhou, H. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J. Mater. Chem. 2012, 22, 3680–3695. [Google Scholar] [CrossRef]
- Vicente, N.; Haro, M.; Cintora-Juarez, D.; Perez-Vicente, C.; Tirado, J.L.; Ahmad, S.; Garcia-Belmonte, G. LiFePO4 particle conductive composite strategies for improving cathode rate capability. Electrochim. Acta 2015, 163, 323–329. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent Development in Carbon-LiFePO4 Cathodes for Lithium-Ion Batteries: A Mini Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Lei, W.; Li, H.; Tang, Y.; Shao, H. Progress and perspectives on electrospinning techniques for solid-state lithium batteries. Carbon Energy 2022, 4, 539–575. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Y.; Zhang, L.; Zhang, Q.; Cheng, Z.; Su, Y.; Shen, F.; Han, X. One step hot-pressing method for hybrid Li metal anode of solid-state lithium metal batteries. J. Mater. Sci. Technol. 2023, 153, 32–40. [Google Scholar] [CrossRef]
- Heng, S.; Shi, Q.; Zheng, X.; Wang, Y.; Qu, Q.; Liu, G.; Battaglia, V.S.; Zheng, H. An organic-skinned secondary coating for carbon-coated LiFePO4 cathode of high electrochemical performances. Electrochim. Acta 2017, 258, 1244–1253. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J. Synthesis of LiFePO4 Nanoparticles in Polyol Medium and Their Electrochemical Properties. Electrochem. Solid-State Lett. 2006, 9, A439–A442. [Google Scholar] [CrossRef]
- Moreno, M.G.; Fernandez, G.M.; Mysyk, R.; Carriazo, D. A high-energy hybrid lithium-ion capacitor enabled by a mixed capacitive-battery storage LiFePO4—AC cathode and a SnP2O7—rGO anode. Sustain. Energy Fuels 2023, 7, 965–976. [Google Scholar] [CrossRef]
- Rosaiah, P.; Kumar, P.J.; Babu, K.J.; Hussain, O.M. Electrical and electrochemical properties of nanocrystalline LiFePO4 cathode. Appl. Phys. A 2013, 113, 603–611. [Google Scholar] [CrossRef]
- Liu, X.; Sun, L.; Vu, N.H.; Linh, D.T.H.; Dien, P.T.; Hoa, L.T.; Lien, D.T.; Nang, H.X.; Dao, V.D. Synthesis of LiFePO4/carbon/graphene for high-performance Li-ion battery. J. Electroanal. Chem. 2023, 932, 117205–117210. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Guo, S. Composites of Graphene and LiFePO4 as Cathode Materials for Lithium-Ion Battery: A Mini-review. Nano-Micro Lett. 2014, 6, 316–326. [Google Scholar] [CrossRef]
- Balo, L.; Gupta, H.; Singh, S.K.; Singh, V.K.; Tripathi, A.K.; Srivastava, N.; Tiwari, R.K.; Mishra, R.; Meghnani, D.; Singh, R.K. Development of gel polymer electrolyte based on LiTFSI and EMIMFSI for application in rechargeable lithium metal battery with GO-LFP and NCA cathodes. J. Solid State Electrochem. 2019, 23, 2507–2518. [Google Scholar] [CrossRef]
- Wei, X.; Guan, Y.; Zheng, X.; Zhu, Q.; Shen, J.; Qiao, N.; Zhou, S.; Xu, B. Improvement on high rate performance of LiFePO4 cathodes using graphene as a conductive agent. Appl. Surf. Sci. 2018, 440, 748–754. [Google Scholar] [CrossRef]
- Falco, M.; Simari, C.; Ferrara, C.; Nair, J.R.; Meligrana, G.; Bella, F.; Nicotera, I.; Mustarelli, P.; Winter, M.; Gerbaldi, C. Understanding the Effect of UV-Induced Cross-Linking on the Physicochemical Properties of Highly Performing PEO/LiTFSI-Based Polymer Electrolytes. Langmuir 2019, 35, 8210–8219. [Google Scholar] [CrossRef] [PubMed]
- Karuppasamy, K.; Antony, R.; Alwin, S.; Balakumar, S.; Shajan, X.S. A Review on PEO Based Solid Polymer Electrolytes (SPEs) Complexed with LiX (X=Tf, BOB) for Rechargeable Lithium Ion Batteries. Mater. Sci. Forum 2015, 807, 41–63. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, X.; Bai, Z.; Fan, L.Z. Enhancing interfacial stability in solid-state lithium batteries with polymer/garnet solid electrolyte and composite cathode framework. J. Energy Chem. 2021, 52, 210–217. [Google Scholar] [CrossRef]
- Chen, H.; Adekoya, D.; Hencz, L.; Ma, J.; Chen, S.; Yan, C.; Zhao, H.; Cui, G.; Zhang, S. Stable Seamless Interfaces and Rapid Ionic Conductivity of Ca–CeO2/LiTFSI/PEO Composite Electrolyte for High-Rate and High-Voltage All-Solid-State Battery. Adv. Energy Mater. 2020, 10, 2000049–2000061. [Google Scholar] [CrossRef]
- Chen, X.; He, W.; Ding, L.X.; Wang, S.; Wang, h. Enhancing interfacial contact in all solid state batteries with a cathode-supported solid electrolyte membrane framework. Energy Environ. Sci. 2019, 12, 938–944. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Liang, X.; Yu, M.; Liu, B.; Sun, Z.; Hu, W.; Zhu, G. A single-ion gel polymer electrolyte based on polyimide grafted with lithium 3-chloropropanesulfonyl (trifluoromethanesulfonyl) imide for high performance lithium ion batteries. J. Mater. Chem. A 2023, 11, 1766–1773. [Google Scholar] [CrossRef]
- Stenina, I.; Pyrkova, A.; Yaroslavtsev, A. NASICON-Type Li1+xAlxZryTi2-x-y(PO4)3 Solid Electrolytes: Effect of Al, Zr Co-Doping and Synthesis Method. Batteries 2023, 9, 59. [Google Scholar] [CrossRef]
- Zhang, Q.K.; Zhang, X.Q.; Yuan, H.; Huang, J.Q. Thermally Stable and Nonflammable Electrolytes for Lithium Metal Batteries: Progress and Perspectives. Small Sci. 2021, 1, 2100058–2100071. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Sun, Y.; Wang, C.; Wang, Y.; Xia, Y. All-solid-state secondary lithium battery using solid polymer electrolyte and anthraquinone cathode. Solid State Ion. 2017, 300, 114–119. [Google Scholar] [CrossRef]
- Cheng, D.; Sun, C.; Lang, Z.; Zhang, J.; Hu, A.; Duan, J.; Chen, X.; Zang, H.Y.; Chen, J.; Zheng, M.; et al. Hybrid covalent organic-framework-based electrolytes for optimizing interface resistance in solid-state lithium-ion batteries. Cell Rep. Phys. Sci. 2022, 3, 100731–100743. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.Y.; He, Z.J.; Li, Y.J.; Mao, J.; Dai, K.H.; Yan, C.; Zheng, J.C. An advance review of solid-state battery: Challenges, progress and prospects. Sustain. Mater. Technol. 2021, 29, e00297–e00310. [Google Scholar] [CrossRef]
- Shen, S.P.; Tang, G.; Li, H.J.; Zhang, L.; Zheng, J.C.; Luo, Y.; Yue, J.P.; Shi, Y.; Chen, Z. Low-temperature fabrication of NASICON-type LATP with superior ionic conductivity. Ceram. Int. 2022, 48, 36961–36967. [Google Scholar] [CrossRef]
- Pogosova, M.A.; Krasnikova, I.V.; Sanin, A.O.; Lipovskikh, S.A.; Eliseev, A.A.; Sergeev, A.V.; Stevenson, K.J. Complex Investigation of Water Impact on Li-Ion Conductivity of Li1.3Al0.3Ti1.7(PO4)3—Electrochemical, Chemical, Structural, and Morphological Aspects. Chem. Mater. 2020, 32, 3723–3732. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, X.; Liu, Y.; Chen, S.; Chen, Y.; Lin, J.; Zhang, Y. Boosting the Performance of Solid-State Lithium Battery Based on Hybridizing Micron-Sized LATP in a PEO/PVDF-HFP Heterogeneous Polymer Matrix. Energy Technol. 2020, 8, 2000444–2000468. [Google Scholar] [CrossRef]
- Yao, Z.; Zhu, K.; Li, X.; Zhang, J.; Li, J.; Wang, J.; Yan, K.; Liu, J. Double-Layered Multifunctional Composite Electrolytes for High-Voltage Solid-State Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 11958–11967. [Google Scholar] [CrossRef] [PubMed]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141–16144. [Google Scholar] [CrossRef]
- Shi, X.; Ma, N.; Wu, Y.; Lu, Y.; Xiao, Q.; Li, Z.; Lei, G. Fabrication and electrochemical properties of LATP/PVDF composite electrolytes for rechargeable lithium-ion battery. Solid State Ion. 2018, 325, 112–119. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Huang, S.; Jiang, Y.; Chen, Z. Preparation and electrochemical study of PVDF-HFP/LATP/g-C3N4 composite polymer electrolyte membrane. Inorg. Chem. Commun. 2021, 131, 108793–108799. [Google Scholar] [CrossRef]
- Mohanty, D.; Lu, Z.L.; Hung, I.M. Effect of carbon coating on electrochemical properties of Li3V2(PO4)3 cathode synthesized by citric-acid gel method for lithium-ion batteries. J. Appl. Electrochem. 2022, 52, 1003–1013. [Google Scholar] [CrossRef]
- Mohanty, D.; Chen, S.Y.; Hung, I.M. Effect of Lithium Salt Concentration on Materials Characteristics and Electrochemical Performance of Hybrid Inorganic/Polymer Solid Electrolyte for Solid-State Lithium-Ion Batteries. Batteries 2022, 8, 173. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Pai, C.T.; Chen, Y.F.; Chen, I.L.; Chen, W.Y. Preparation of lithium iron phosphate cathode materials with different carbon contents using glucose additive for Li-ion batteries. J. Taiwan Inst. Chem. Eng. 2014, 45, 1501–1508. [Google Scholar] [CrossRef]
- Nien, Y.H.; Carey, J.R.; Chen, J.S. Physical and electrochemical properties of LiFePO4/C composite cathode prepared from various polymer-containing precursors. J. Power Source 2009, 193, 822–827. [Google Scholar] [CrossRef]
- Wu, H.C.; Wu, H.C.; Lee, E.; Wu, N.L. High-temperature carbon-coated aluminum current collector for enhanced power performance of LiFePO4 electrode of Li-ion batteries. Electrochem. Commun. 2010, 12, 488–491. [Google Scholar] [CrossRef]
- Jaumaux, P.; Liu, Q.; Zhou, D.; Xu, X.; Wang, Y.; Kang, F.; Li, B.; Wang, G. Deep-Eutectic-Solvent-Based Self-Healing Polymer Electrolyte for Safe and Long-Life Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 9134–9142. [Google Scholar] [CrossRef]
- Nilsson, V.; Bernin, D.; Brandell, D.; Edstrom, K.; Johansson, P. Interactions and Transport in Highly Concentrated LiTFSI-based Electrolytes. ChemphysChem 2020, 21, 1166–1176. [Google Scholar] [CrossRef]
- Atik, J.; Diddens, D.; Thienenkamp, J.H.; Brunklaus, G.; Winter, M.; Pillard, E. Cation-Assisted Lithium-Ion Transport for High-Performance PEO-based Ternary Solid Polymer Electrolytes. Angew. Chem. Int. Ed. 2021, 60, 11919–11927. [Google Scholar] [CrossRef]
- Chen, S.Y.; Hsieh, C.T.; Zhang, R.S.; Mohanty, D.; Gandomi, Y.A.; Hung, I.M. Hybrid solid state electrolytes blending NASICON-type Li1+xAlxTi2–x(PO4)3 with poly(vinylidene fluoride-co-hexafluoropropene) for lithium metal batteries. Electrochim. Acta 2022, 427, 140903. [Google Scholar] [CrossRef]

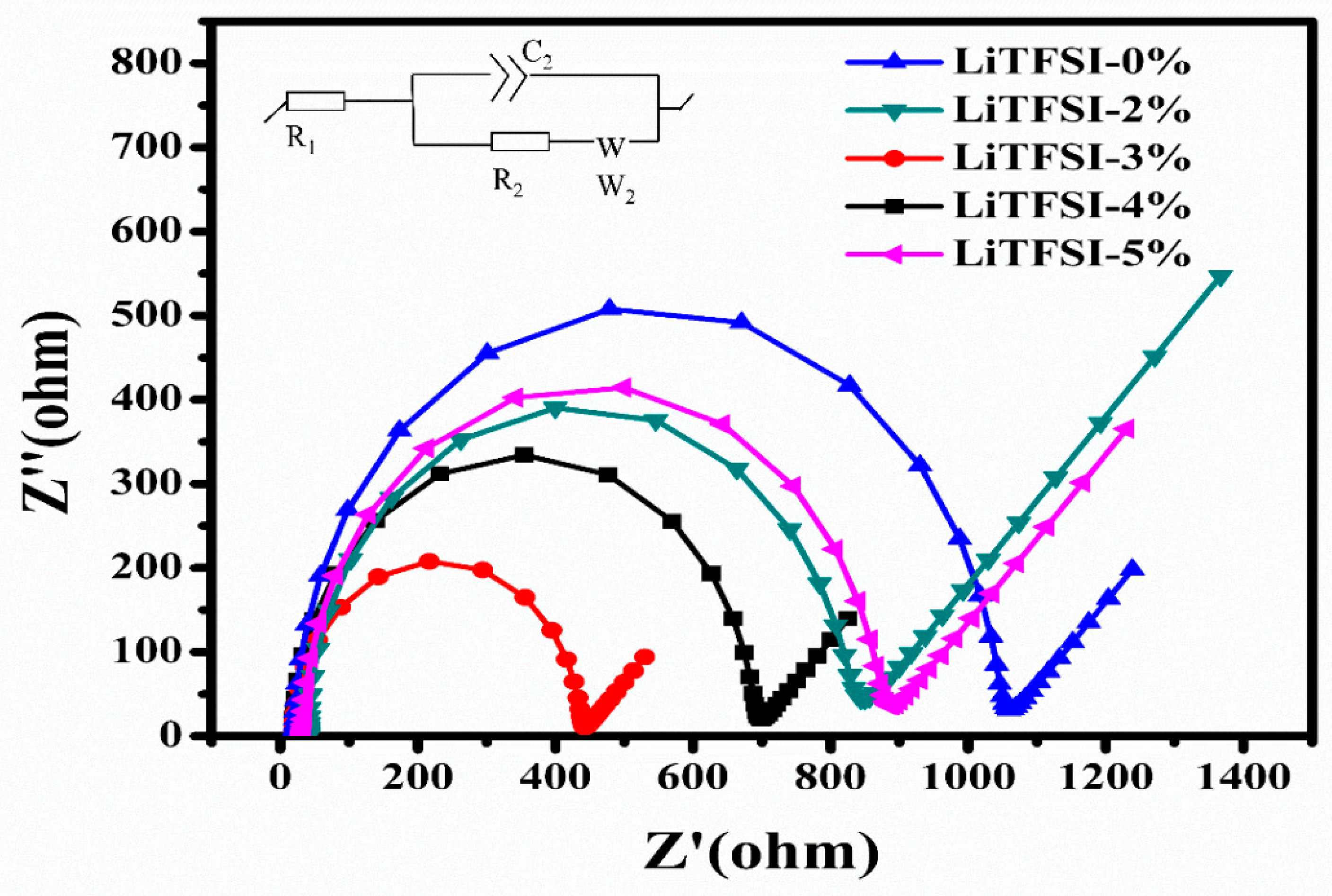
| Sample | R1 (Ω) | R2 (Ω) | Rtotal (Ω) |
|---|---|---|---|
| LiTFSI-0% | 21.4 | 1019.0 | 1040.4 |
| LiTFSI-2% | 40.8 | 819.3 | 860.1 |
| LiTFSI-3% | 20.2 | 415.7 | 435.9 |
| LiTFSI-4% | 18.7 | 666.2 | 685.9 |
| LiTFSI-5% | 33.9 | 831.6 | 865.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, D.; Huang, P.-H.; Hung, I.-M. Preparation and Characterization of a LiFePO4- Lithium Salt Composite Cathode for All-Solid-State Li-Metal Batteries. Batteries 2023, 9, 236. https://doi.org/10.3390/batteries9040236
Mohanty D, Huang P-H, Hung I-M. Preparation and Characterization of a LiFePO4- Lithium Salt Composite Cathode for All-Solid-State Li-Metal Batteries. Batteries. 2023; 9(4):236. https://doi.org/10.3390/batteries9040236
Chicago/Turabian StyleMohanty, Debabrata, Pin-Hsuan Huang, and I-Ming Hung. 2023. "Preparation and Characterization of a LiFePO4- Lithium Salt Composite Cathode for All-Solid-State Li-Metal Batteries" Batteries 9, no. 4: 236. https://doi.org/10.3390/batteries9040236
APA StyleMohanty, D., Huang, P.-H., & Hung, I.-M. (2023). Preparation and Characterization of a LiFePO4- Lithium Salt Composite Cathode for All-Solid-State Li-Metal Batteries. Batteries, 9(4), 236. https://doi.org/10.3390/batteries9040236







