1. Introduction
Sophisticated research on the safety of lithium-ion batteries (LIB) is an important challenge as these batteries are both currently in further development and already in wide-range use for customers in a variety of sectors and applications in everyday life. As well as the use in devices that improve day-to-day life, innovation in LIB to advance electric vehicles and grid storage systems is crucial to fight climate change and to contribute significantly to the energy transition. Thus, immediate progress is needed, as climate change is progressing towards a climate crisis worldwide.
Accelerating rate calorimetry (ARC) has been used to study thermal runaway and thermal abuse behavior [
1,
2]. Other laboratories are specialized for gaining similar understanding from thermal abuse experiments conducted in ovens or autoclaves instead of using calorimeters [
3]. It must be pointed out that both oven and ARC tests have benefits and disadvantages and each method enables different types of learning. Based on the specific scientific question, the appropriate technique should be chosen [
4].
The comparison of thermal behavior of different battery chemistries, such as LCO, LMO, NMC, NCA and LFP is widely covered in the literature e.g., [
5]. Nowadays, the most prominent LIB chemistry is NMC, together with NCA, whereas LFP cells are less common but have become more relevant recently due to their superior safety [
6].
Different LIB cell formats, such as cylindrical, prismatic and pouch, have been compared in terms of safety in [
7]. While pouch cells are mostly used in consumer electronics, they can also be found in electric vehicles [
8]. Prismatic cells are mostly used for electrical propulsion and for grid and home storage systems [
9]. Cylindrical cells have amongst the largest range of applications, ranging from consumer electronics and power tools to electric cars and energy storage systems [
10].
Considering cylindrical cells, the most prominent cell size is 18650. The safety behavior of this cell has, for example, been studied in [
10]. However, the cell size 21700 is becoming more and more prominent and is either replacing or complementing 18650 cells, both for electric vehicles and consumer goods. The safety of type 21700 cells was investigated in [
11]. The production of larger cylindrical cells, such as 26650 and 46800, is increasing and it has been announced they are to be used in several products, which shows that bigger cylindrical cells will play a relevant role in the near future [
12].
The events leading to thermal runaway and details of the cascade of reactions involved is described for NMC/NCA cells in [
13] and for LFP cells in [
4]. The thermal behavior of separate components of LIB, such as the electrolyte and separator, were studied by use of differential scanning calorimetry (DSC) in [
14]. The fire behavior of batteries in an open system and additionally for battery packs, full battery systems and electric vehicles was studied in [
7].
Thermal runaway behavior is impacted by cell chemistry and size as well as the SOC of cells, as evaluated in [
15], and by cell aging, as reported in [
16].
We have identified the following areas where there is a lack of knowledge and where there has been limited experimental work, which will be the focus of this study:
Data for cylindrical cells larger than 18650, especially type 21700 cells;
Results of mechanical abuse testing of cells, such as nail testing;
Comparison of safety for several states of charge, including lower and medium states of charge for both thermal and mechanical abuse scenarios;
Direct comparison between high power and high energy cells, considering the impact of the different construction of these cells on abuse behavior;
Safety and abuse study, coupled with profound material science investigation, into the components and structure of the cells using advanced analytical techniques.
2. Materials and Methods
For this investigation, commercial type 21700 lithium-ion cells with different cell chemistries and for high-energy (HE) and high-power (HP) cell designs were acquired from five different manufacturers. The cylindrical cells each had a diameter of 21 mm and a height of 70 mm and were intended for industrial applications, being sold in bulk packs after formation ready to use. The main differences in the cells were the cathode material and the cell design for high-energy or high-power applications. In this article, batteries with an NMC cathode are referred to as NMC cells or with NMC chemistry for all described effects and phenomena, both for anode and cathode processes, to facilitate identification and reading.
Characterizations of the full cells were performed by capacity measurement and by computed tomography scanning. After disassembly, the electrodes were analyzed by means of inductively coupled plasma optical emission spectroscopy (ICP-OES), carrier gas hot extraction (CGHE), carbon sulfur analysis (CS), digital and scanning electron microscopy (SEM), as well as gas chromatography coupled with mass spectroscopy (GC-MS). Each technique and the parameters are described in the following sections.
The main experiment consisted of thermal and mechanical abuse experiments at different states of charge of the cells in order to compare the performance of different chemistries and cell designs, as well as the impact of the state of charge.
2.1. Electrical Characterization
To determine the capacity a Biologic BCS 815-128 system was used. All cells were tested using a standardized test program at an isothermal temperature of 25 °C in an incubator. Therefore, the cells with condition at delivery after formation and a state of charge (SOC) of about 30, were discharged to SOC 0 and subsequently fully charged with constant current (CC) and constant voltage (CV), followed by a full discharge with constant current (CC) and another CC-CV charge and CC discharge. All charging heretofore was performed with a C rate of C/3. With the second full discharge, the capacity value was determined. All values were compared between at least 160 cells that were acquired per type, and capacity was averaged over all acquired cells per type. The cells were stored at SOC 30. Shortly before each abuse experiment, cells were discharged to SOC 0 and then charged to the scheduled SOC for SOC 100 with CC-CV and for all other SOC levels with a time-limited CC charging protocol.
2.2. Elemental Composition Analysis
For the different analytical investigations, all cells were discharged to SOC 0 and subsequently opened in a glovebox under argon atmosphere. The cell casing was removed without damaging the active material; this was followed by unrolling of the electrodes and division of each electrode into pieces. The pieces were cleaned of electrolyte and LiPF6 remains by three washings with dimethyl carbonate (DMC) on each side of the active material, inside the glovebox. The process was followed by an evaporation phase of the DMC under vacuum for 12 h. The samples were stored and transported under argon atmosphere in sealed films.
Elemental composition analysis was carried out by ICP-OES with an iCAP 7600DUO from Thermo Fisher Scientific. Oxygen content was obtained by CGHE on a Bruker AXS G8 Galileo instrument and carbon content with a CS analyzer LECO CS600, all three methods were validated with internal standards. For the analysis, three samples were used to obtain the metal, oxygen and carbon content of the anode and cathode, respectively. Consequently, per cell, nine sections of each electrode were analyzed.
2.3. Microscopy Characterization
A Keyence VHX 7000 digital light microscope, placed inside a glovebox with argon atmosphere, was used to analyze the anodes and cathodes for two cells of each type. The cells were disassembled, and the electrodes were unwound and cut to provide 7 cm by 7 cm samples for analysis; magnifications of up to 5000× were used.
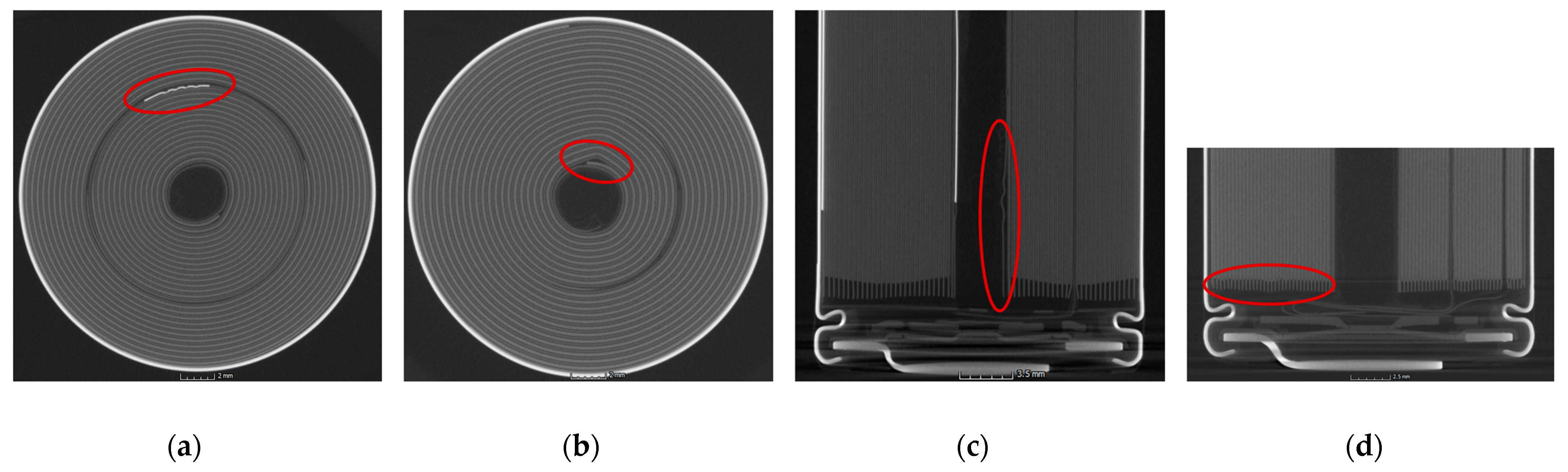
2.4. CT Analysis
The CT system used was a Phoenix v|tome|x s 240, with voltage set to 180 kV, a current of 170 µA and 1600 single images per scan, and a voxel size for x and y of 0.031 mm. CT scans were obtained for two samples of each cell type. All cells were charged to SOC 30 and with an SEI layer already formed at the time of scanning. myVGL software was used to overlay the cross-sectional images from the scans at varying depths of the material so that the resulting three-dimensional representation of the cell could be analyzed. It was possible to distinguish between features in the cells due to variations in the atomic numbers of the different materials and corresponding brightness in the scan. For each scan, the anode, cathode, positive tab, negative tab, and casing thicknesses were measured, along with the size of the cavity at the jelly roll center. Additionally, any irregularities, manufacturing defects, and variations in safety devices were captured and recorded.
2.5. Further Components Analysis
To determine the electrolyte composition, cells were opened under argon atmosphere in a glovebox and placed within a sealed plastic tube in a centrifuge to extract electrolyte from the cell. The recovered liquid was then analyzed by means of gas chromatography coupled with mass spectroscopy (GC-MS) using an internal standard for validation with a Clarus 690 Arnel 4019 gas chromatography system by Perkin-Elmer; a similar approach is described in [
17].
The separator was identified to be made of polyethylene (PE) by FTIR spectroscopy, performed on a Nicolet™ iS™ 5 FT-IR-spectrometer from Thermo Fisher Scientific. The coating of each separator was found to be made of Al2O3, as determined by energy dispersive X-ray spectroscopy (EDX), using a scanning electron microscope of type Phenom XL by Thermo Fisher Scientific.
From the material safety data sheet (MSDS) of each cell, the conducting salt was identified to be LiPF6 for all cells. The binder used was polyvinylidene fluoride (PVDF) for the cathode and styrene butadiene rubber (SBR) for the anode.
2.6. Accelerating Rate Calorimetry
2.6.1. Calorimeters for Abuse Testing
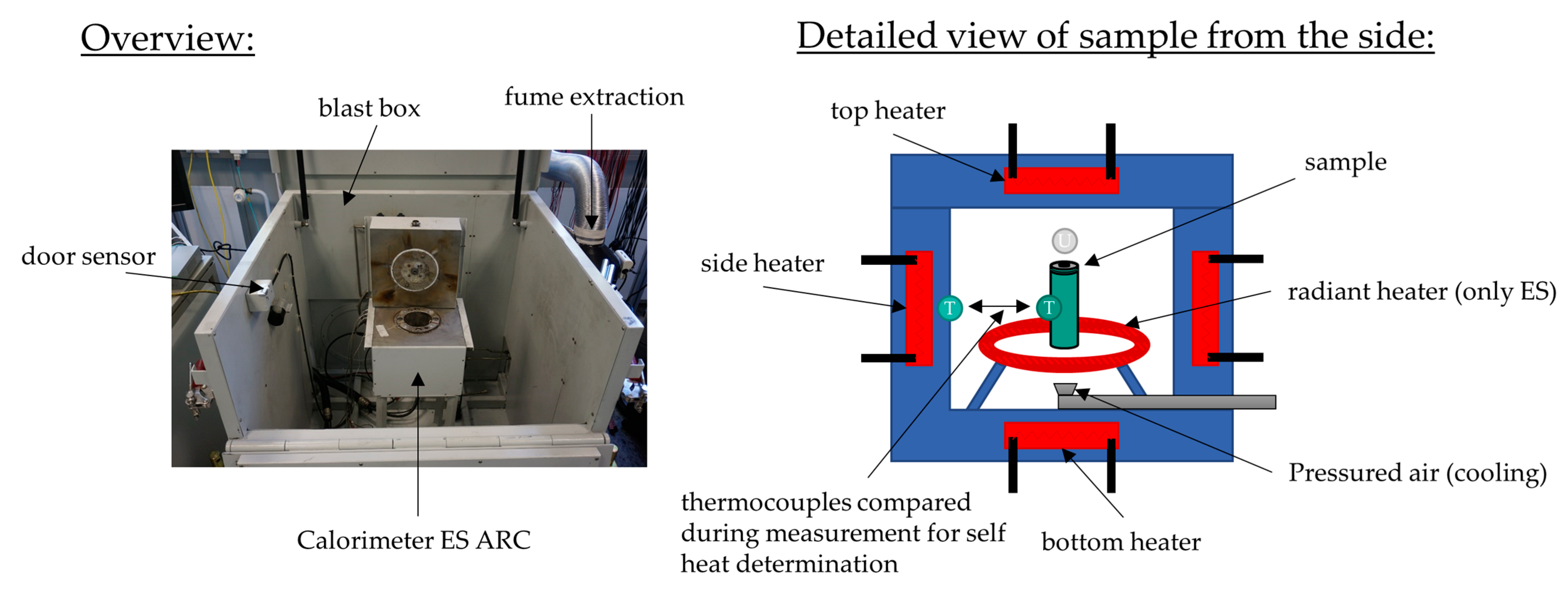
Accelerating rate calorimeters (ARCs) were originally built for the testing of explosives and have been adapted for battery abuse tests. They are equipped with safety systems and are built to withstand battery fire and explosions. For the experiments in this study, we used calorimeters manufactured by Thermal Hazard Technology (THT). Depending on the cell size and the experiment, different calorimeters were appropriate: for the thermal abuse test on type 21700 cylindrical cells, a small calorimeter with low thermal inertia and a small chamber to capture all thermal effects and temperature rates precisely was ideal; therefore, the type ES ARC was used (shown in
Figure 1), which was able to track the experiment with a sensitivity of up to 0.005 °C/min. For the mechanical abuse test, a larger calorimeter was required to accommodate the set-up. Therefore, the EV+ ARC was utilized (shown in Figure 3b). It had a larger experimental chamber and all controls for the nail penetration device. For the EV+ ARC, the sensitivity was 0.02 °C/min.
Both ARC models allow tracking of the temperature and temperature rates precisely and can operate in (quasi-)adiabatic mode. This means that the cell ideally cannot transfer heat to the walls of the calorimeter chamber, providing a test atmosphere for batteries that represents the worst case and is consequently ideal for safety assessment.
The ARC was calibrated following the recommendations of the manufacturer and using the calibration mode. As recommended by the manufacturer, a dummy made of aluminum with the same thermocouple set-up was used for the experiments and the heating parameters were calibrated up to 350 °C. The heat capacity of this dummy was comparable to that of the battery. For aluminum, it was given at 298 K with 0.89 J/g·K in [
18]; for the batteries, it was given at between 0.83 and 1.26 J/g·K as described in chapter 3.5.1. During calibration, the power settings for the heaters were optimized for uniform heating, and the self-heating or self-cooling from temperature gradients or thermal stabilizing of the system was recorded. This assured a correct determination of the self-heating rate during the actual test. Subsequently, using a so-called drift check, the calibration was validated. This test consisted of a heat-wait-seek test until 350 °C and was passed when the self-heating and self-cooling of the dummy was below 0.02 °C/min. When the thermocouple had to be replaced, a new calibration and drift check was performed.
2.6.2. Thermocouples and Uncertainty Estimation
All abuse tests were performed with two types of thermocouples by Omega with the following specifications. The so-called bomb thermocouple, which controlled the experiment and was placed on the center of the side of the battery, was always a type N thermocouple. The auxiliary thermocouples to obtain temperature information at other cell areas were of type K.
The following measurement-induced uncertainties were identified: A temperature measurement uncertainty could be induced depending on the thermocouples and their accuracy, which is given in
Table 1. In addition, as the heating step size was 5 K, the onset temperature could be overshot by heating by a maximum of 4.9 K in the worst case. The lowest data recording rate was 0.05 min; therefore, effects faster than this could not be captured. Delayed exothermal behavior, after the waiting time of 15–20 min, was also only found during the next seek phase in the worst case of 4.9 K higher. An uncertainty introduced by the cables for electrical measurement and the thermocouples could be neglected, as the set-up was calibrated with thermocouples and the cables attached and their diameters were small, allowing for no significant heat transfer. The activation energy accuracy depends on the quality of the Arrhenius fit. For enthalpy calculation, uncertainty is introduced by averaging the c
p value, by the temperature inaccuracy, and by the uncertainty in mass determination. The cells were weighed before the experiment. Weight changes during the running experiment due to decomposition of the active material and venting could not be measured as the ARC was not a closed system.
2.6.3. Thermal Abuse Test
For the thermal abuse experiments, the so-called heat-wait-seek (HWS) test was used. This test consisted of a heating interval, followed by a waiting and a seeking interval. The first heating interval started after direct heating from the laboratory temperature to 50 °C. The waiting phase enabled thermal equilibrium to be established after each heating step. The seek phase involved comparison of the temperature of two thermocouples, one on the side of the heater and one on the side of the sample, which are shown in
Figure 1. When these two thermocouples showed a difference during the seek phase, the heating rate was determined. If it exceeded the threshold set to 0.02 °C/min, self-heating was detected and the system switched to a (quasi-)adiabatic mode, which is called the exotherm mode, preventing heat transfer to the chamber, allowing exothermal reaction of the cell. Thus, the cell was increasingly heating up until thermal runaway occurred or the chemicals for the exothermal reaction were completely consumed. Some events, such as venting or endothermal reactions, might stop the self-heating of the cell, which would then result in another heating phase, until the cell was exothermal again or went to thermal runaway. A more detailed description of this experimental procedure can be found in [
19].
The test was stopped at 350 °C, if no thermal runaway was attained before, to prevent damage to the calorimeter. If the cell did not go into runaway by this temperature, it was not expected to do so afterwards. The parameters of each phase of investigation are presented in
Table 2.
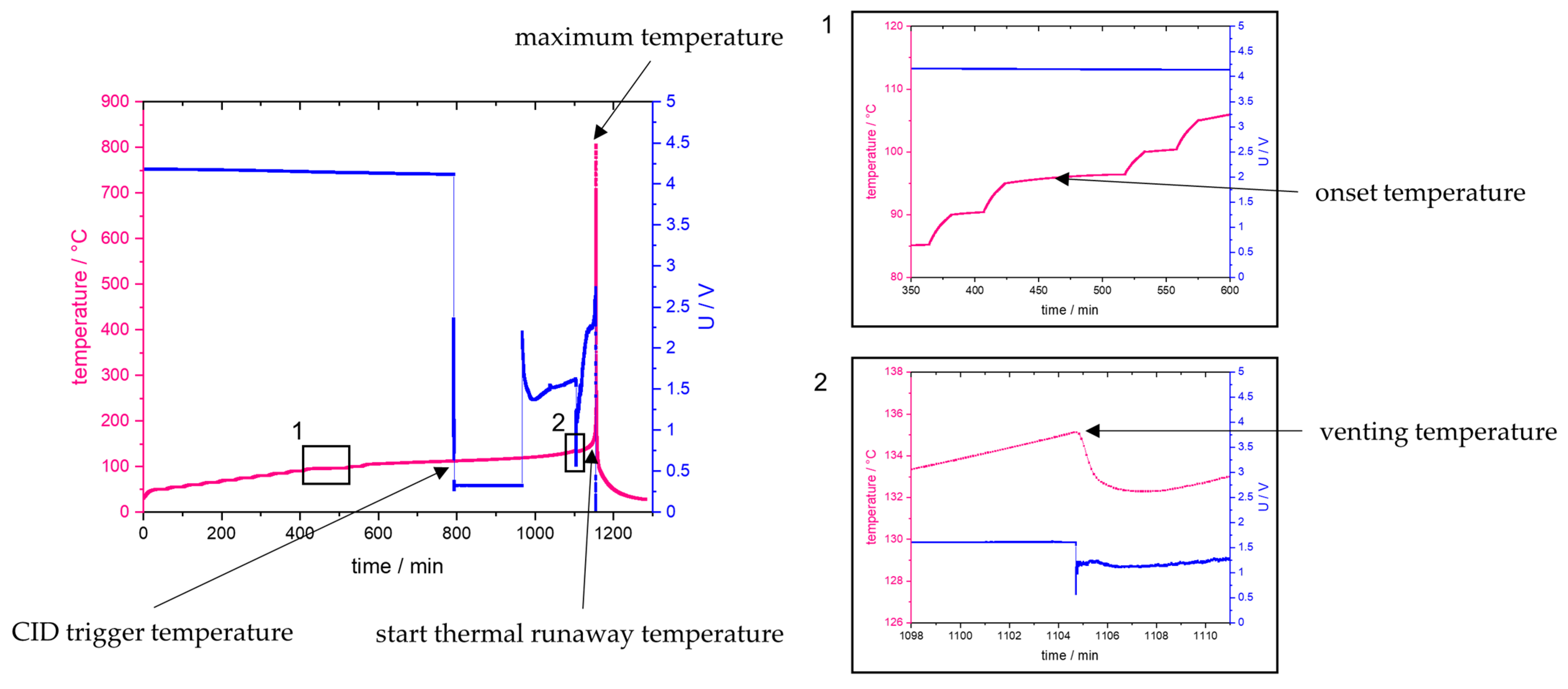
For identification of cell venting, a short decrease in the temperature was looked for in the data files. This occurs due to the Joule–Thomson effect caused during outgassing of the electrolyte, leading to immediate endothermal evaporation.
In the literature, different definitions for thermal runaway are used: some definitions use an exothermal temperature rate of 1, 5 or 10 °C/min as an indication of thermal runaway. The problem here is that, at low SOC and for LFP cells in some experiments, these rates are not reached at all, but the cells nonetheless experience thermal runaway from thermal abuse testing. Additionally, the rate where cells go into thermal runaway shows a lot of variation from experiment to experiment and can be hard to track because of the immediate change in the reaction velocity at the start of the runaway. Therefore, a different definition is used here. In order to analytically determine the moment just before the sharp temperature rise, the following definition is used: The cell must be in an exothermal state and the exothermal data log file is checked. This file logs one data point at every 1 K or every 0.05 min. When there is more than 1 K between two logged steps, this indicates the beginning of thermal runaway, as the cell is heating faster than our measurement device can log, and, thus, it is a runaway situation in this experimental set-up.
The definition of all critical temperatures is given in
Table 3.
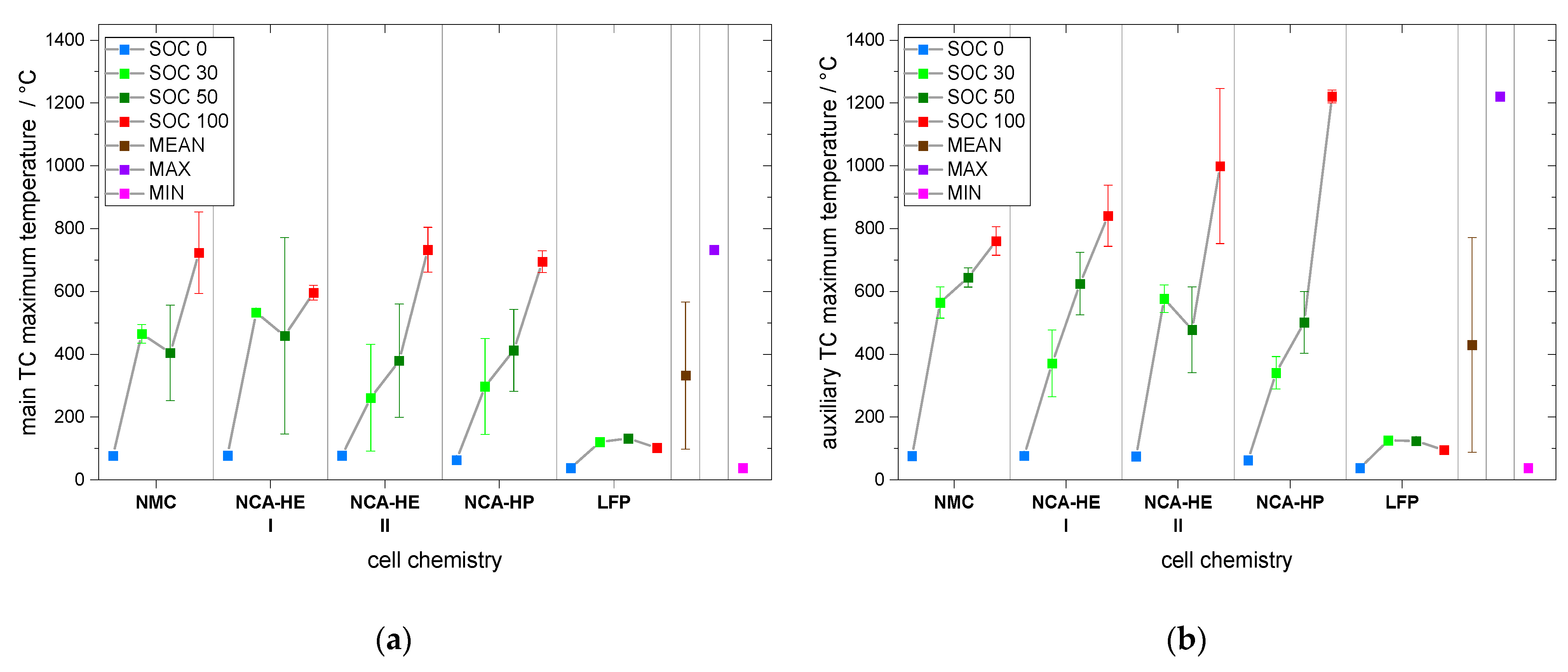
All critical temperatures presented in this paper are mean values of at least two experiments and are plotted together with their standard deviation (SD) as an uncertainty bar.
An overview of an experiment, showing the temperature and voltage over time for an NCA SOC 100 cell with all critical temperatures indicated is given in
Figure 2.
From
Figure 2, the location in time and the identification of the critical temperatures during the experiment can be seen, for which the definitions are given in
Table 3.
2.6.4. Mechanical Abuse Test
The so-called nail penetration test was used for mechanical abuse testing. This experiment consists of a steel nail puncturing the cell inducing internal short circuits as well as compression and a degree of internal crushing of the cell.
In this investigation, the ARC-EV+ was utilized, as it accommodates a so-called nail penetration and crushing option unit (NPCO) specifically built for this test. Once the experiment was started, after 30 s, a steel nail was forced into the cell in a transversal direction in the middle of the cell. The temperature and a video of the reaction was recorded.
Table 4 shows the parameters of the experiment that were applied.
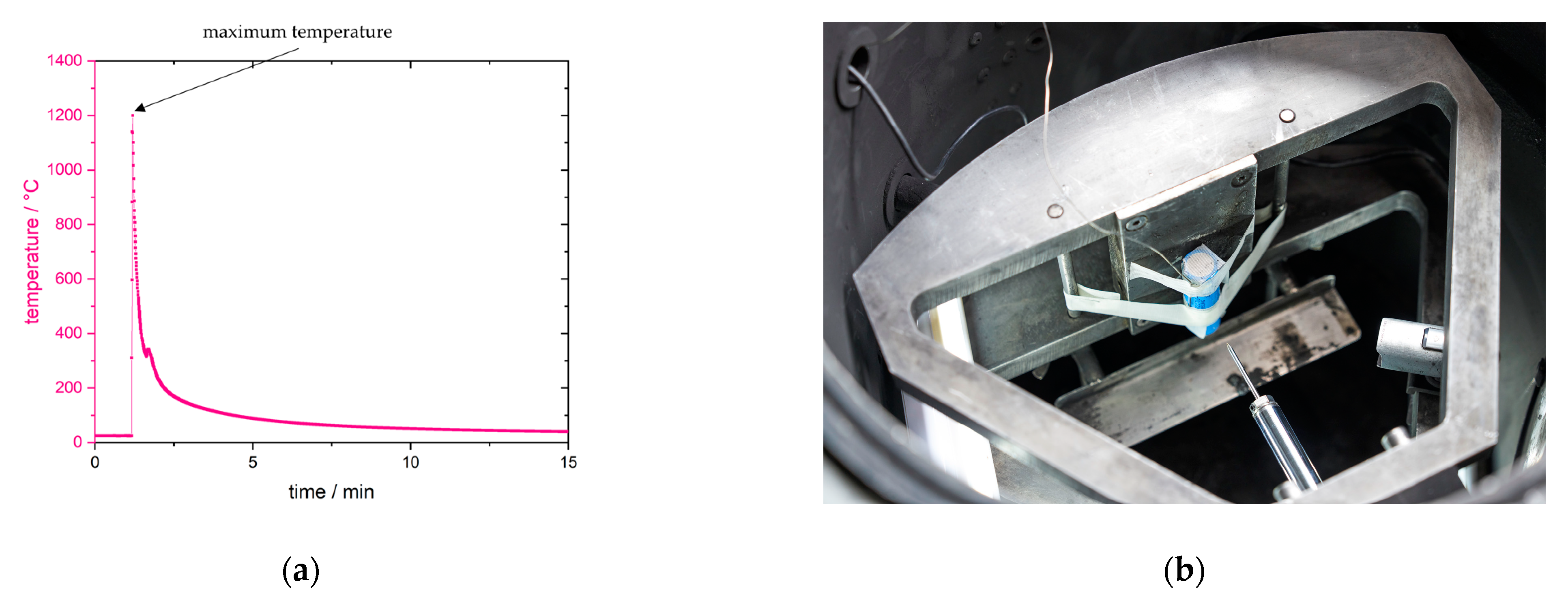
Only one critical temperature can be obtained from mechanical abuse, which is the maximum temperature obtained shortly after penetration during thermal runaway or exothermal reaction because of the short circuit through and crushing by the nail. An overview of the experiment is given in
Figure 3a using a temperature over time representation.
The nail penetration system and location of the cell inside the calorimeter, as well as a simplified set-up with one thermocouple at the center of the lateral side of the cell, is shown in
Figure 3b. The maximum temperature should be compared at different locations of the cell; therefore, a more complex set-up was used with several auxiliary thermocouples. Thermocouples were attached in the center (main TC, type N), on the top and bottom of the cell, on the side, and on the cathode and anode (auxiliary TC type K). Their accuracy is given in
Section 2.6.2.
4. Discussion
4.1. Influence of Cell Chemistry
When comparing the different cell chemistries, one has to take into account that the cells have different capacities. In particular, the LFP cells had a lower capacity than the other chemistries, as the highest available capacity for type 21700 cells was 3 Ah. Therefore, the exothermal data is always presented in a capacity standardized way. For NCA cells, we compared between HE and HP and can attribute the difference between these two cells, respectively, on one side to the capacity difference and, on the other, to the construction difference between the cells. As the impact on the temperature was found to be low between these two different cells, the difference between LFP and the other cells should also mainly have come from the different cathode chemistry and not from capacity difference.
Additionally, the electrolyte composition also showed little difference; moreover, the difference was mainly in LFP, for NCA-HE, both cells had very similar electrolyte composition, and between NMC and NCA-HP, there was little disparity, whether EMC, FEC or EC were used. It is assumed, as commercial cells were investigated, that the manufacturers had always chosen the best electrolyte composition for the performance of each cathode material and that the electrolyte was chosen in accordance with this, and that we can, thus, make our deductions based on the cathode material.
Consequently, based on these experimental results, the safety behavior of the different cell chemistries, mainly influenced by the cathode material for type 21700 LIB, can be compared.
The cells studied were high nickel cells, for NMC with a chemistry close to 811, and, for NCA, close to 90 mass % nickel in the cathode, 10 mass % cobalt and about 0.15 mass % aluminum, for all three NCA cells studied. Furthermore, the LFP cells shared a common LiFePO4 composition. The separator was made of PE with an Al2O3 coating, which was the same for all cells. Moreover, the anode was built of carbon and, with the exception of LFP, also contained between 1 to 1.6 mass % silicon. Subsequently, the main difference and focus of comparison was the cathode active material; therefore, the cells were defined by cathode chemistry.
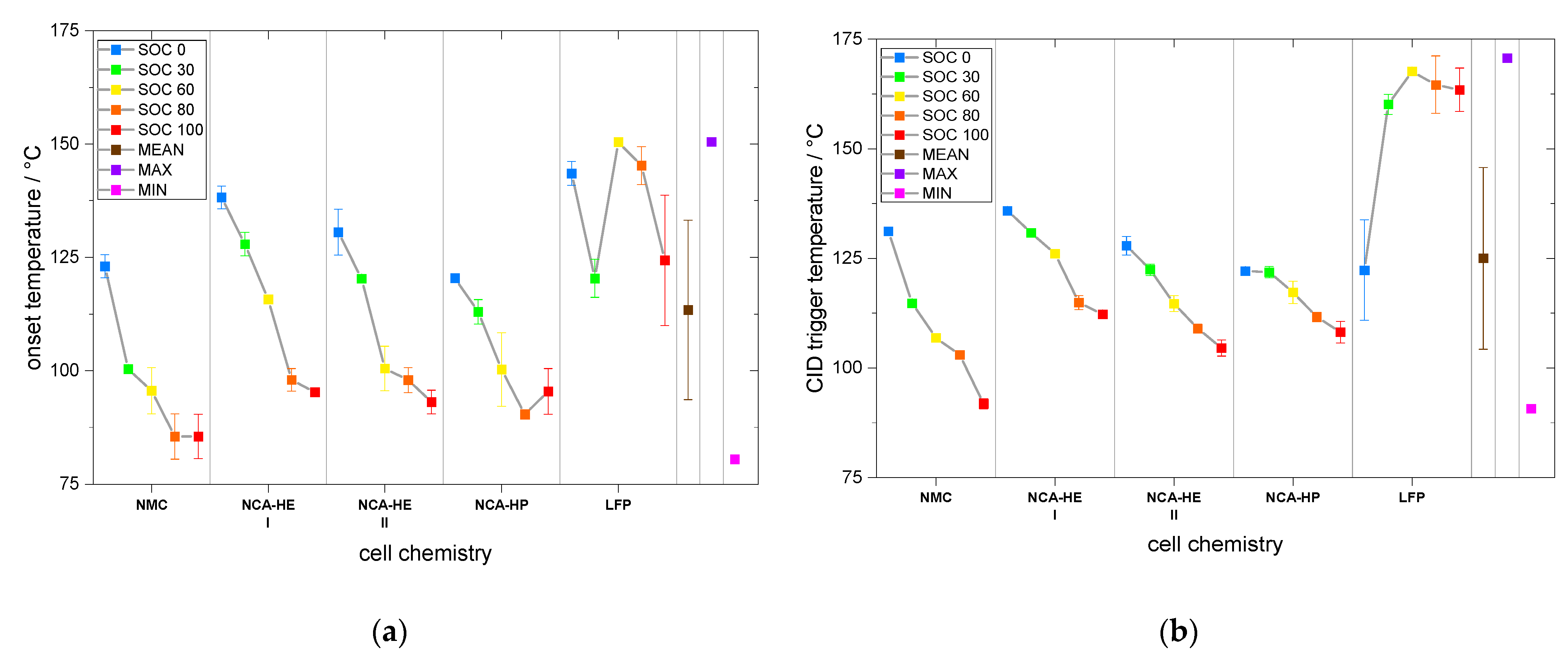
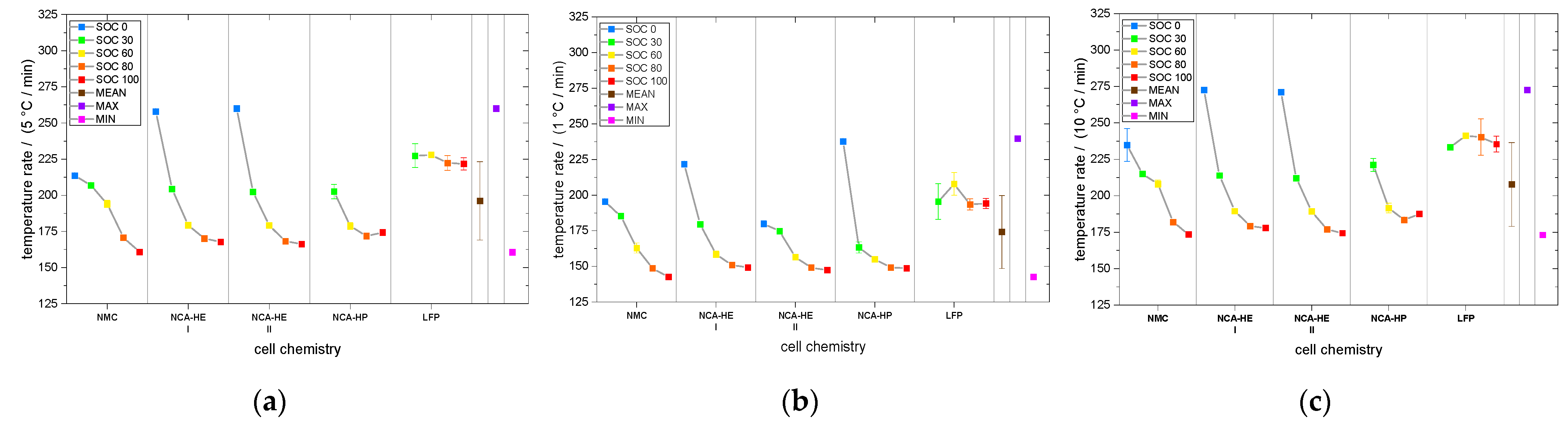
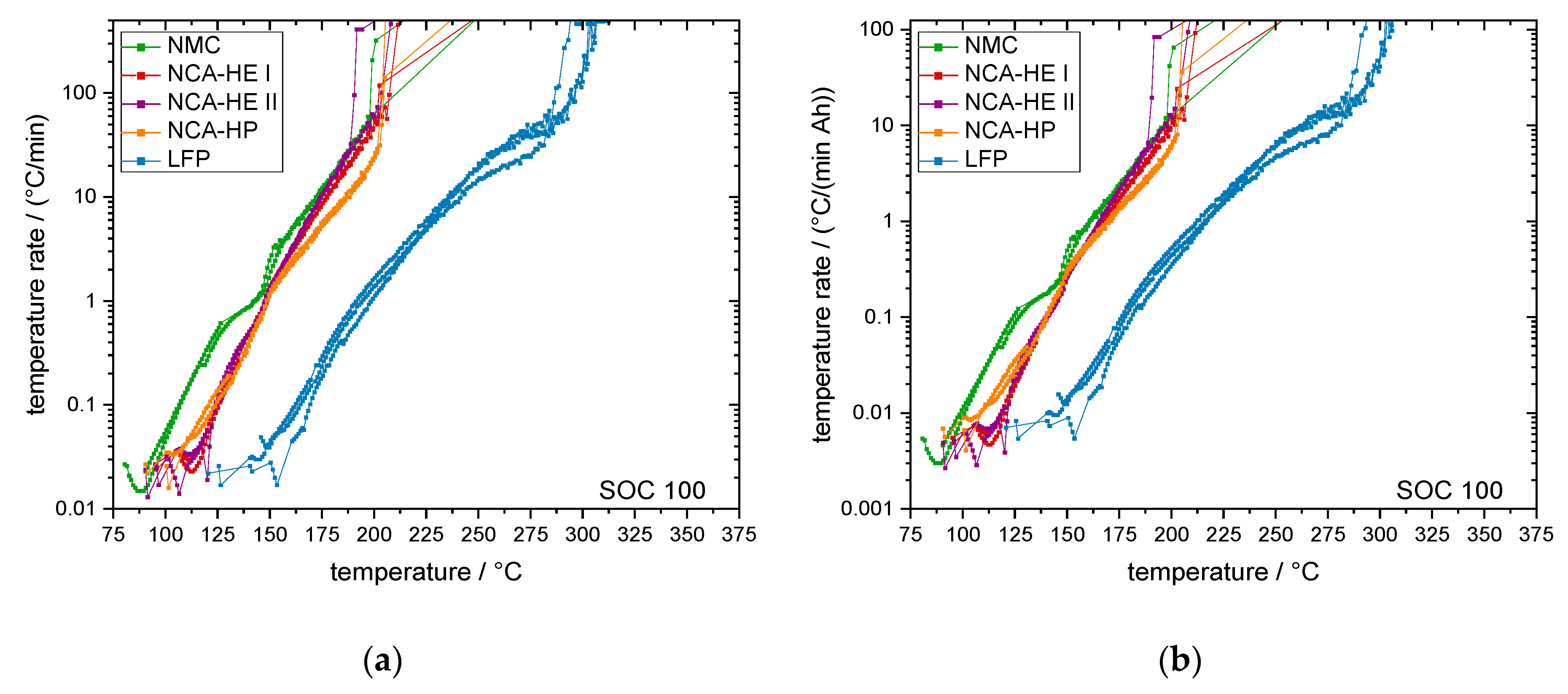
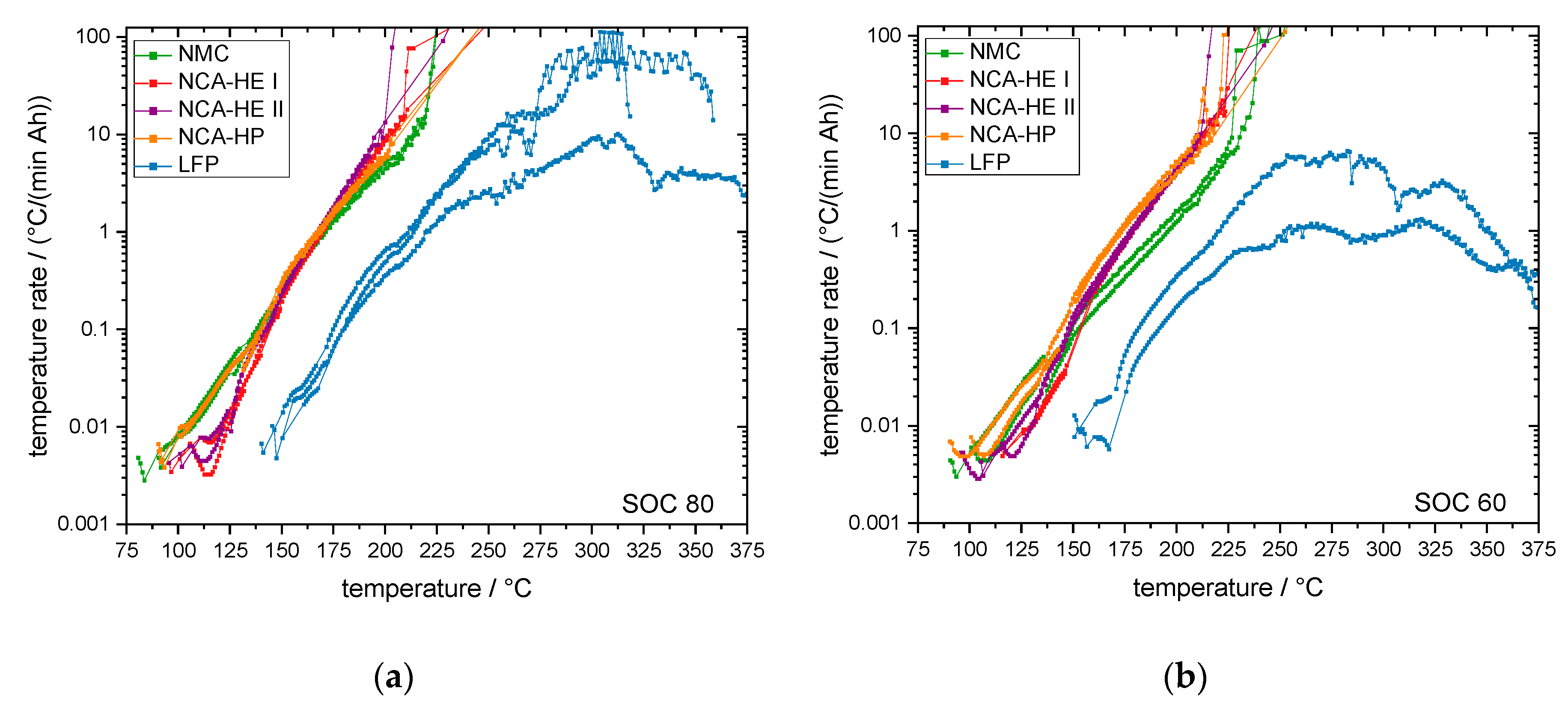
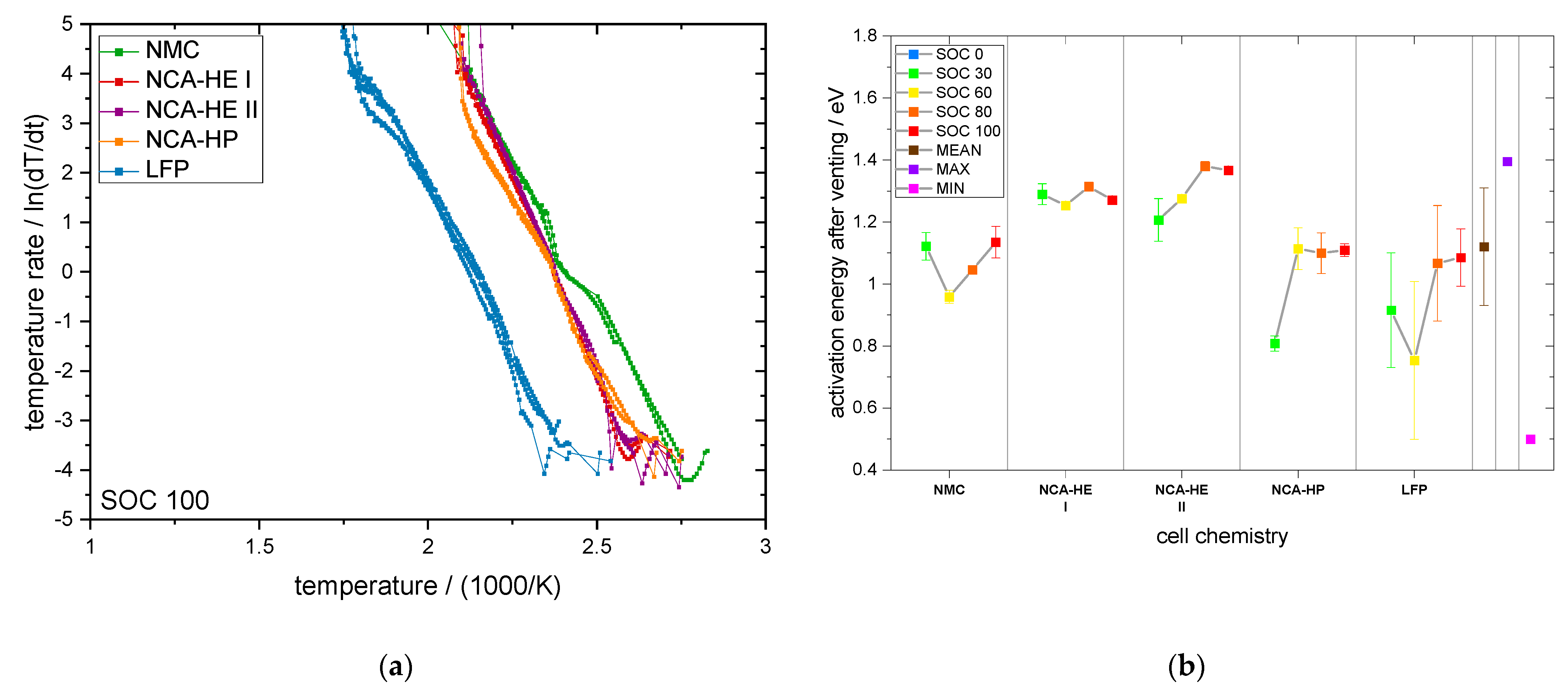
For most SOCs, and in the predominant regions of reaction, NMC cells showed the highest reactivity and reached critical temperatures the earliest. They had an earlier onset, CID trigger and venting temperature than NCA and LFP. The NCA cells were, depending on the temperature rate of the reaction, less reactive than the NMC cells below rates of 1 °C/min.
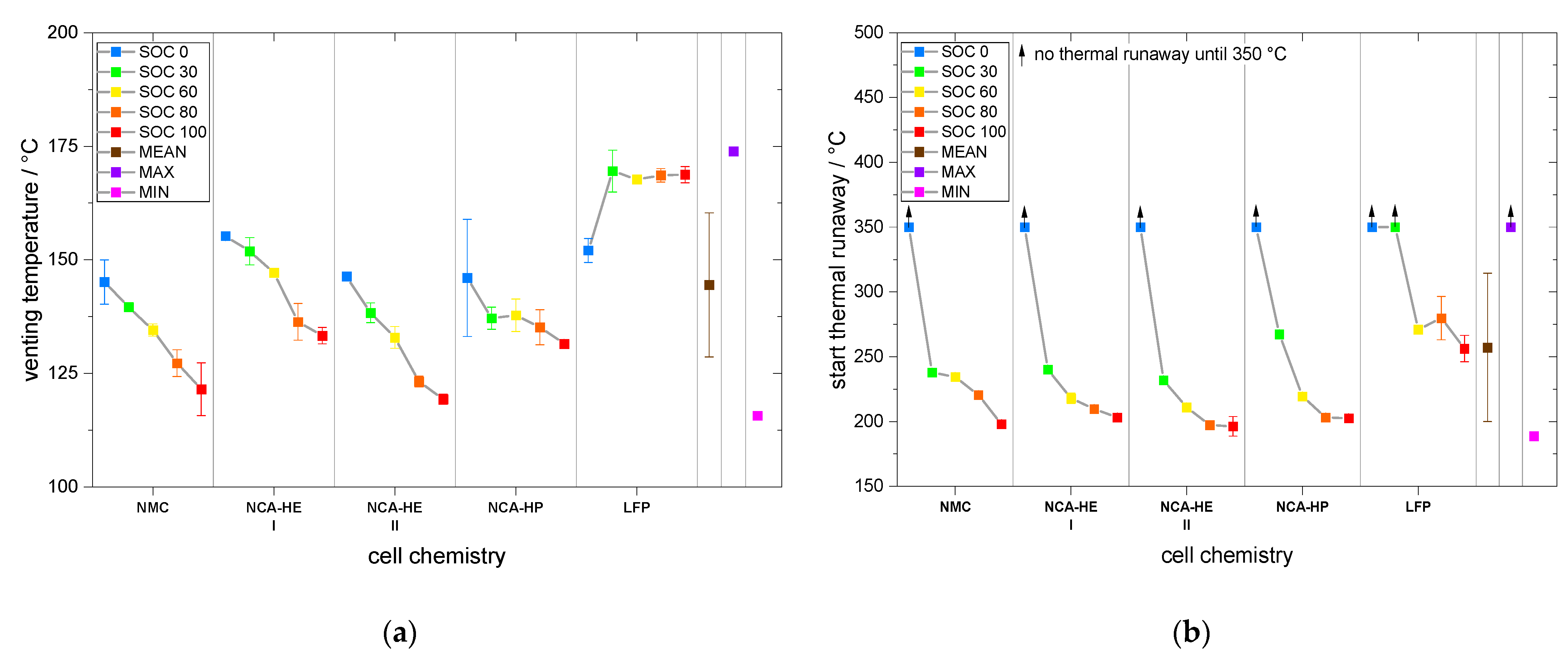
The start of thermal runaway occurred first for NCA, followed closely by NMC cells. The LFP cells always reacted at higher temperatures and at lower rates, and all critical points were undoubtedly later in time and at higher temperature. Thus, no thermal runaway occurred for SOC 0 under thermal abuse and for all tested SOCs under mechanical abuse. The latter is an outstanding behavior of LFP, making it significantly safer than the other chemistries.
Furthermore, we found through CT analysis important construction differences between NCA-HE and NCA-HP cells, the latter having thicker current collectors and more tabs. Additionally, the maximum capacity difference between the cells was 1 Ah, with about 20 % more capacity in NCA-HE cells, but comparing those cells under thermal abuse showed only small differences.
In addition, through CT analysis, we also found differences in the construction of the safety devices located near the positive terminal, such as the safety vent, CID, and PTC device. This raises the question of the influence of the construction on the cell behavior. All the cells were from different commercial suppliers, and, for NCA-HE, two cells with the same chemistry and capacity, but from two different manufacturers, were directly compared. Both cells reacted very similarly in their exothermal behavior and at the start of thermal runaway. The differences found mostly lay inside the variation range from cell to cell, indicated by the standard deviation in the provided graphs. However, the CID trigger temperature and the venting temperature over different SOCs were shifted between NCA-HE I and NCA-HE II, indicating that the different manufacturers have chosen different pressures to trigger these safety features, whereas NCA-HE II had lower critical temperatures. Interestingly, the different temperatures resulted in the same thermal runaway behavior seen in the exothermal data and a very close start of thermal runaway temperature. Further, for mechanical abuse, the cells behaved similarly, with only slightly higher maximum temperatures for NCA-HE II.
4.2. Influence of SOC
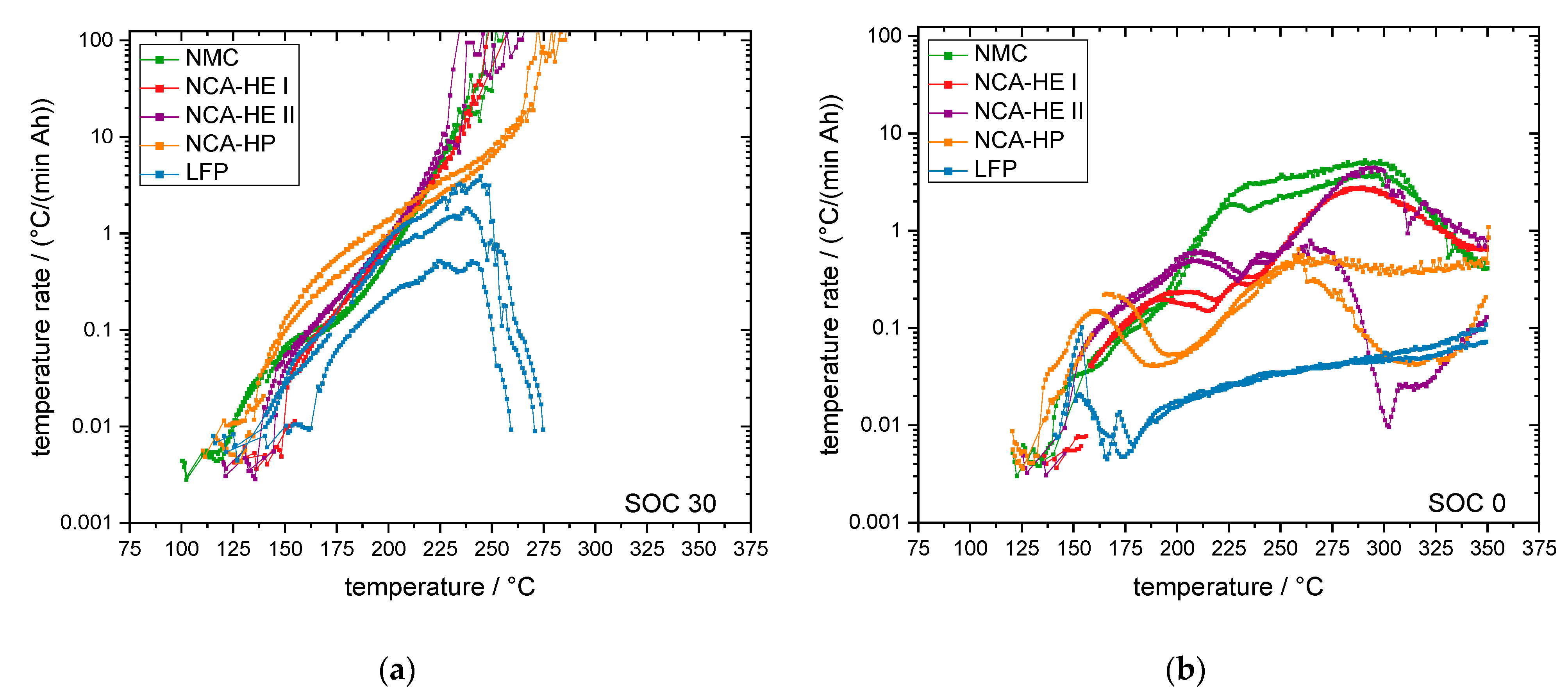
A cell at SOC 100 has a fully lithiated anode and a significant amount of electrical energy stored. However, a cell at SOC 0 has a fully delithiated anode and just a small amount of electrical energy stored, together with its chemical energy. For SOC 0, we found no thermal runaway for all chemistries with all abuse methods, and can, thus, conclude that thermal runaway requires a lithiated anode.
Additionally, analyzing NMC and NCA, for a higher SOC, most parameters were found to be at lower temperatures; however, for LFP the SOC influence was not always clear and sometimes a higher SOC resulted in a lower temperature, meaning higher reactivity. As the anode was the same for LFP cells, this indicates that here the lithiation state of the LFP cathode was relevant and influenced the behavior. A summary of the SOC influence on critical temperatures is provided in
Table 19.
With a higher lithiation level of the anode, more hydrocarbons can be formed during the decomposition reactions. This results in a more severe thermal runaway reaction at high SOC and the earlier reaching of critical points, such as venting or the start of thermal runaway. While most parameters had a negative temperature coefficient with the SOC, meaning they occurred at lower temperatures for a higher SOC, there were some exceptions.
As mentioned, LFP stood out and had either no clear SOC influence or even a positive temperature coefficient, meaning low SOCs were more reactive, which was the case for the CID trigger temperature and the maximum temperature. For SOC 60, one of two LFP cells reached a rate of above 10 °C/min (not capacity standardized rate), and, additionally, met the criterion used to define thermal runaway given in
Table 3, whereas the other cell tested did not. Heat damage, discoloration and soot was found on these cells after the test. We conclude that, at SOC 60, LFP cells went into thermal runaway but with very low rates; however, with a different definition of thermal runaway, a different conclusion is possible. At SOC 30, one of three LFP cells exceeded 10 °C/min (not capacity standardized rate) shortly, but none of the LFP cells reached the criterion defined for thermal runaway in
Table 3. Reaction of all LFP cells stopped between 250 °C and 275 °C without further heating; therefore, we conclude that no thermal runaway occurred for SOC 30 for LFP cells. Post-mortem analysis showed blank aluminum casing but no internal cell components on the outside; thermal damage was expected to come from heating up to 350 °C during test and not from thermal runaway.
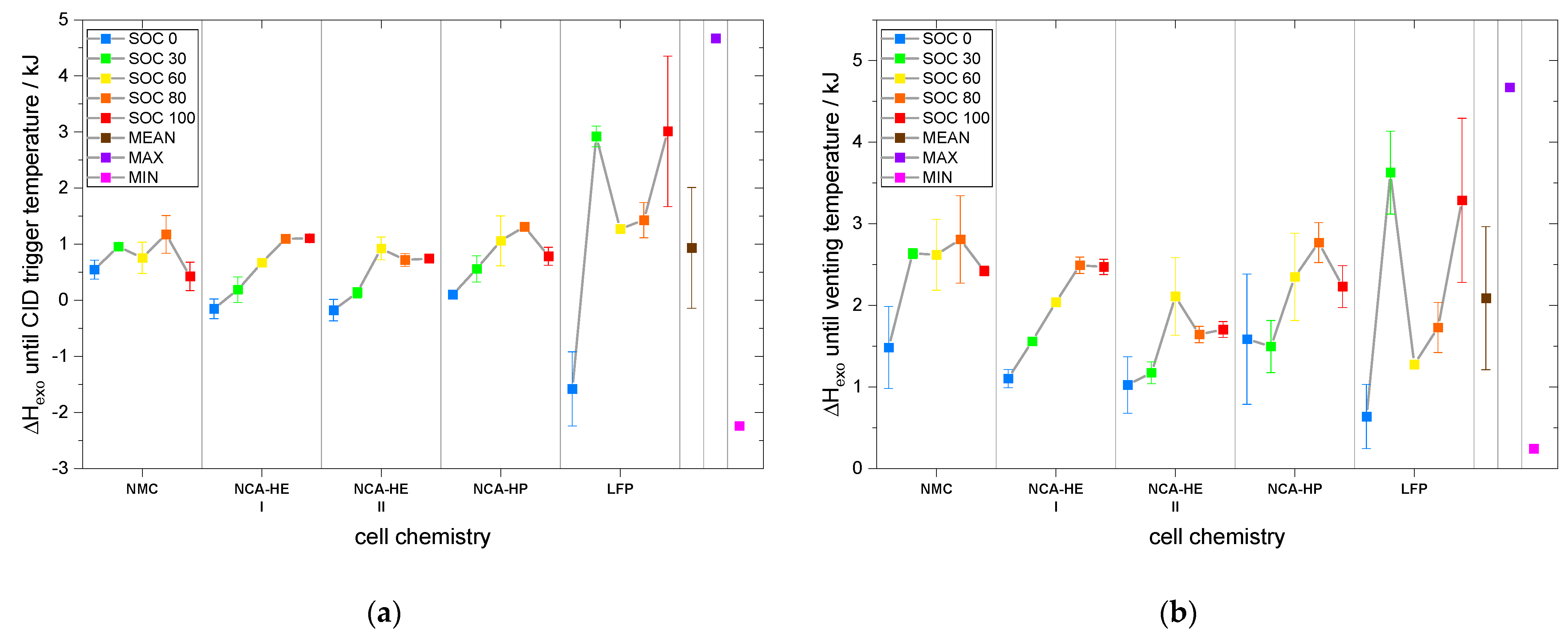
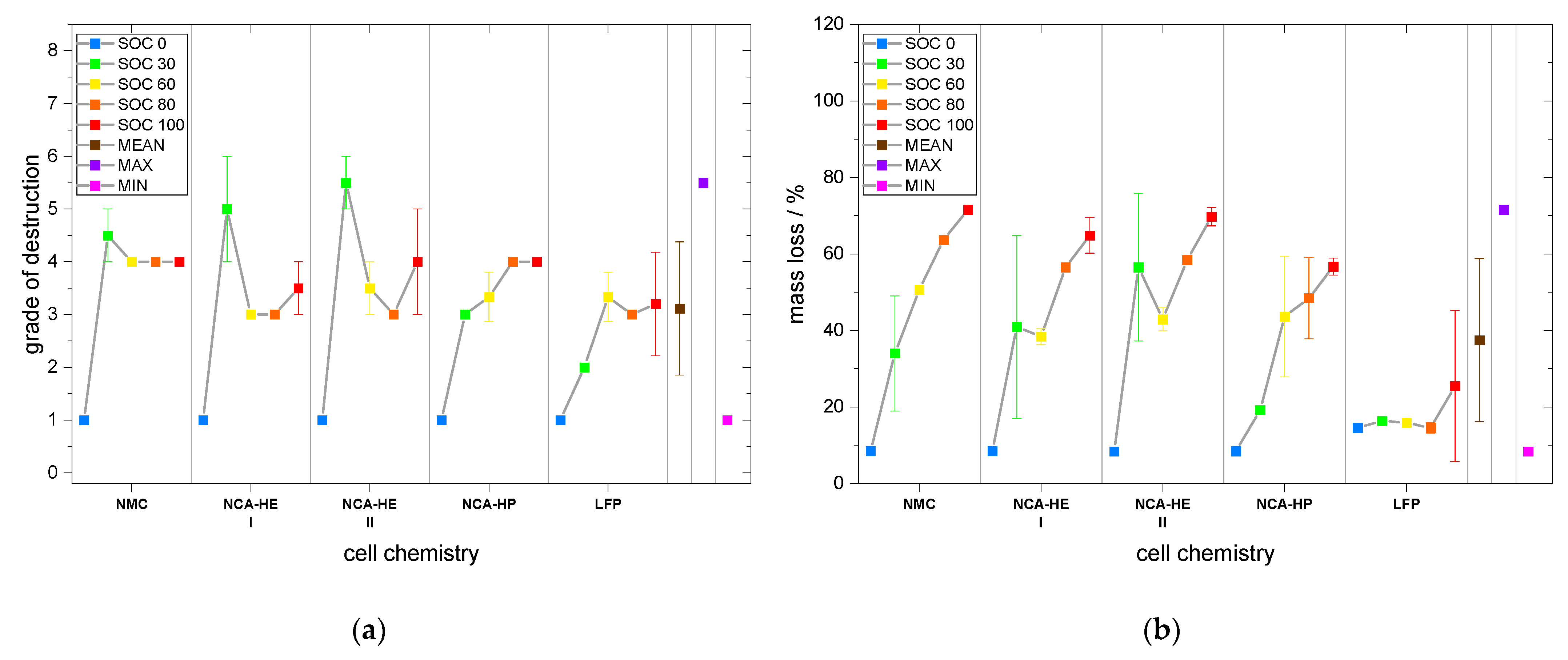
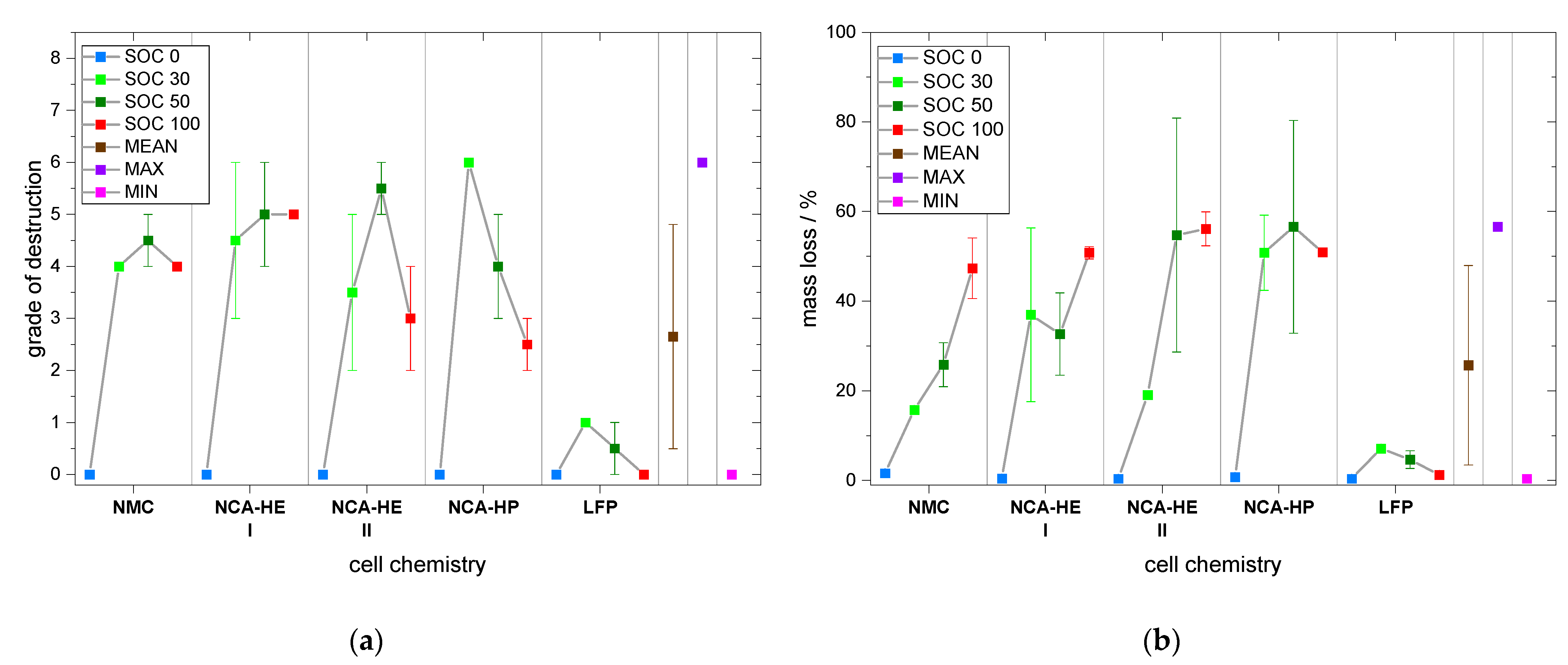
Moreover, for the grade of destruction for both abuse methods and mass loss for nail penetration, the highest was found for either SOC 30 or 50 for nearly all chemistries and tests. The grade of destruction was due to the explosion occurring during thermal runaway; thus, at lower SOC, we estimate a higher amount of hydrogen to be released or a different amount of oxygen, creating a more explosive gas mix than for SOC 100. It is also possible that at SOC 100 ignition occurred earlier with less hydrogen and was, thus, less violent than for SOC 30/50, where explosion could be triggered at a moment where more hydrogen was present. Thus, jelly roll ejection occurred more often at SOC 30 than at other SOC, which might indicate the formation of a more flammable vapor inside the cell. Moreover, the low reaction speeds at the beginning might have led to a different degradation process that resulted in a more powerful thermal runaway at the end for SOC 30. The mass loss indicates the combustion intensity as well as whether a lot of mass was ejected from the cell during runaway. It is partially coupled to the grade of destruction, e.g., with jelly roll ejection, a high grade of destruction goes hand-in-hand with extensive mass loss. We observed that mass loss was highest at SOC 100 for thermal abuse and conclude that combustion intensity and the quantity of components reacting during thermal runaway is higher for a more lithiated anode, resulting in higher mass loss during the experiment.
Furthermore, in the exothermal behavior of the cells with SOC 0, several decreases in exothermal behavior could be the consequence of endothermal reactions taking place. The influence of separator melting, and electrolyte evaporation was considered to be a possible factor; therefore, the melting point of PE at about 125–132 °C (which, however, was delayed by the Al
2O
3 coating), as well as the boiling point of all individual carbonates in the electrolytes, given in
Table 20, was compared with the points of diminishing rates found in the exothermal data of SOC 0. However, it was not possible to correlate the observed slowing of rates with any of the known boiling or melting points. Therefore, we conclude that the cascade of reactions and the decomposition of the active material at SOC 0 itself consisted of several phases with diminishing rates and would stop by itself if no more heat was added.
The fact that at SOC 0 no thermal runaway occurred for all chemistries and abuse methods is useful for recycling processes and analysis that need cells to be disassembled. Additionally, the storage of a system that will not be put into service again should be undertaken at SOC 0 to prevent thermal runaway.
However, as prolonged SOC 0 storage can damage systems because of a further self-discharge of the cells over time, this state is not an option for the transport or shipping of new batteries and systems. Therefore, SOC 30 is more favorable, as, at this SOC, reactivity is slower and critical temperatures occur at higher points compared to a higher SOC. Nevertheless, at this SOC, a fire risk is present for mechanical and thermal abuse and preventive measures need to be considered to prevent and to detect a thermal runaway event.
4.3. Influence of Abuse Method
When comparing the results of thermal and mechanical abuse in this study, one must consider that not the exact same SOCs were tested. In consequence, the average SOC for thermal abuse was SOC 54 and the average SOC for mechanical abuse was SOC 45. Therefore, the average values cannot be perfectly compared for the impact of the abuse method on each chemistry. They can, however, be compared at specific SOC and for the overall behavior for all chemistries and SOC.
Furthermore, comparing the maximum temperatures, the exceptional maximum temperature value was found for mechanical abuse with 1220 °C, but also the lowest maximum temperature occurred for a mechanical abuse experiment at only 37 °C. While the average for mechanical abuse was 314 °C, over all tests at the center, and 439 °C at the auxiliary thermocouple location, for thermal abuse, it was found to be 492 °C, in a range of 359 °C to 837 °C. Thus, the average was close and a battery fire burned at similar temperatures for both abuse methods, but the extreme temperature above 1000 °C was only found for mechanical abuse.
Moreover, the grade of destruction, on average, was of grade 2 for mechanical abuse; hence, it was lower than for thermal abuse, with an average grade of 3. This makes sense, because of the longer time the thermal abuse takes (several days compared to a few minutes) and since grade 3 represents a loss of components, such as molten aluminum on the top.
Subsequently, mass loss by nail penetration was on average 26%, also smaller than the 37 % we found for thermal abuse.
4.4. System Safety Design Based on the Critical Temperatures
It can be inferred from this paper, that SOC 100 is not always the worst case, and that the SOC impact is different from chemistry to chemistry. In addition, in an emergency, the exact SOC of each cell and the chemistry of the system might not be known. Thus, for all measured and calculated values, the average, minimum and maximum values are given in this paper to enable them to be used for system design or safety analysis, which needs to be adapted to all SOC. Likewise, the critical temperatures are relevant and have an impact on the integrity of a system where the battery is included. Indeed, when reaching the CID trigger temperature of several cells, the high volt system of an electric car can shut down and significantly reduce the relevant functions of the car. Thereafter, at the venting temperature, toxic gases are emitted from the battery and there is an explosion hazard. The given critical temperatures for reaching temperature rates of 1, 5 and 10 °C/min can be used to trigger an emergency cooling system. Finally, at the start of the thermal runaway temperature, where a battery fire is inevitable, systems to stop thermal propagation need to be triggered.
4.5. Consequence for the Extinguishing of Battery Fires
To consider fire extinguishing based on this data, the maximum temperature is of most relevance, but also the start of the thermal runaway temperature.
The latter indicates when a battery fire can be anticipated and the former gives indications about the suitable extinguishing agents; both are presented in
Section 3.3.
Hence, for LFP cells, the thermal runaway start occurred between 250 °C and 301 °C, and for the other chemistries between 189 °C and 270 °C; the average for all SOC and chemistry was 257 °C. Thus, a system heated to this temperature containing batteries can be expected to have a battery fire through thermal abuse.
Subsequently, for thermal abuse, an average maximum temperature for all SOC and chemistries was found to be 492 °C, with a minimum of 359 °C and a maximum of 837 °C. Furthermore, for mechanical abuse, the overall maximum temperature ranged from 38 °C to 1220 °C, averaging at 439 °C. It is remarkable that LFP cells did not go into thermal runaway from mechanical abuse.
Moreover, the recorded 1220 °C from mechanical abuse may have been due to the fire of metal components and this should be considered for battery fire extinguishing.
5. Conclusions
We can summarize that accelerating rate calorimetry is a proficient way to quantitatively determine the critical temperatures of thermal runaway and other safety relevant events during cell degradation through thermal and mechanical abuse. We can, therefore, assess cell safety and compare the different thermal runaway behavior observed for different active materials, SOC, cell construction and abuse method.
The severity and occurrence of thermal runaway depends on the active material and the state of charge. With the same active material, the SOC governs the thermal runaway reactions—the overall energy of cells, such as their capacity, is less relevant. The difference between NCA-HE and NCA-HP cells in terms of construction and capacity has only a little impact on thermal runaway behavior.
Moreover, comparing the chemistry, NMC cells were the most reactive and started exothermal reactions the earliest, experiencing most critical temperatures at lower values than for the other chemistries. Interestingly, this led to thermal runaway a little later than NCA because of a lower slope of the reaction or change in slope, indicating the reaction slowing down.
NCA cells reacted faster than NMC cells above 1 °C/min and, thus, reached thermal runaway a little earlier, but reacted slower than NMC cells below 1 °C/min. Thus, most critical temperatures were reached later by NCA. For mechanical abuse, NMC and NCA cells behaved comparably, except for a very high temperature, reached only by NCA-HP cells.
Further, the thermal runaway behavior of LFP cells was different; they only reacted at higher temperatures and much more slowly. The SOC corresponds to the lithiation state of the anode and cathode of the cell and, therefore, the ability to go into thermal runaway, which did not occur for thermal abuse and mechanical abuse at SOC 0 for all cells tested. For the LFP cathode material, no thermal runaway was observed for mechanical abuse and at SOC 0 and SOC 30 for thermal abuse. However, the higher temperatures and lower temperature rates for LFP cell reaction can make the detectability of runaway events harder and battery management systems need to be adapted.
Therefore, it can be concluded that LFP cells are inherently safer than NCA and NMC cells. This comes at the price of a lower capacity; the cells of same size 21700 of LFP cathode are only commercially available at a capacity of 3 Ah, while NMC and NCA are available with 5 Ah. This represents 2 Ah less and even 8.1 Wh less as the nominal voltage is lower as well:
Hence, redesign of the energy storage is needed when LFP cells are used. However, considering that during mechanical abuse for the NCA-HP cell a temperature of 1220 °C was measured for high SOC, and LFP cells did not reach temperatures above 136 °C, this creates room for a different, lightweight overall system design, with inclusion of more cells to obtain comparable capacity.
To conclude, whilst the critical temperatures were lower and the temperature rates highest at SOC 100, because of an overall higher reactivity at this SOC, the grade of destruction was observed to be higher at SOC 30 or 50, respectively. These parameters are relevant to assess the thermal propagation risk in a battery pack. Additionally, the maximum temperature of thermal runaway, which is higher for mechanical abuse than for thermal abuse, is relevant for full system design and safety evaluation.





.png)