1. Introduction
The use of electric vehicles (EVs) is becoming increasingly relevant to reduce oil consumption and avoid environmental pollution. Nevertheless, the primary challenge for clean energy vehicles is to improve the performance of their energy storage system, typically comprised of lithium-ion (Li-ion) batteries. A weak point of Li-ion batteries (LIBs) is that their behavior is strongly dependent on temperature, which advisable range of operation is between 15
and 35
[
1]. Exploring the performance of the models for LIBs has potential utilization in several fields such as: condition monitoring, health assessment, remaining useful life (RUL) prediction and battery thermal management systems (BTMS).
In order to estimate the temperature of a LIB, there exist some models such as: thermal, electrochemical, equivalent circuit model (ECM) or a combination among them. A thermal model can be a lumped, one-dimensional (1D), two-dimensional (2D), or three-dimensional (3D) system, where the main input requirements for modeling are thermal properties and the heat generation rate of the battery.
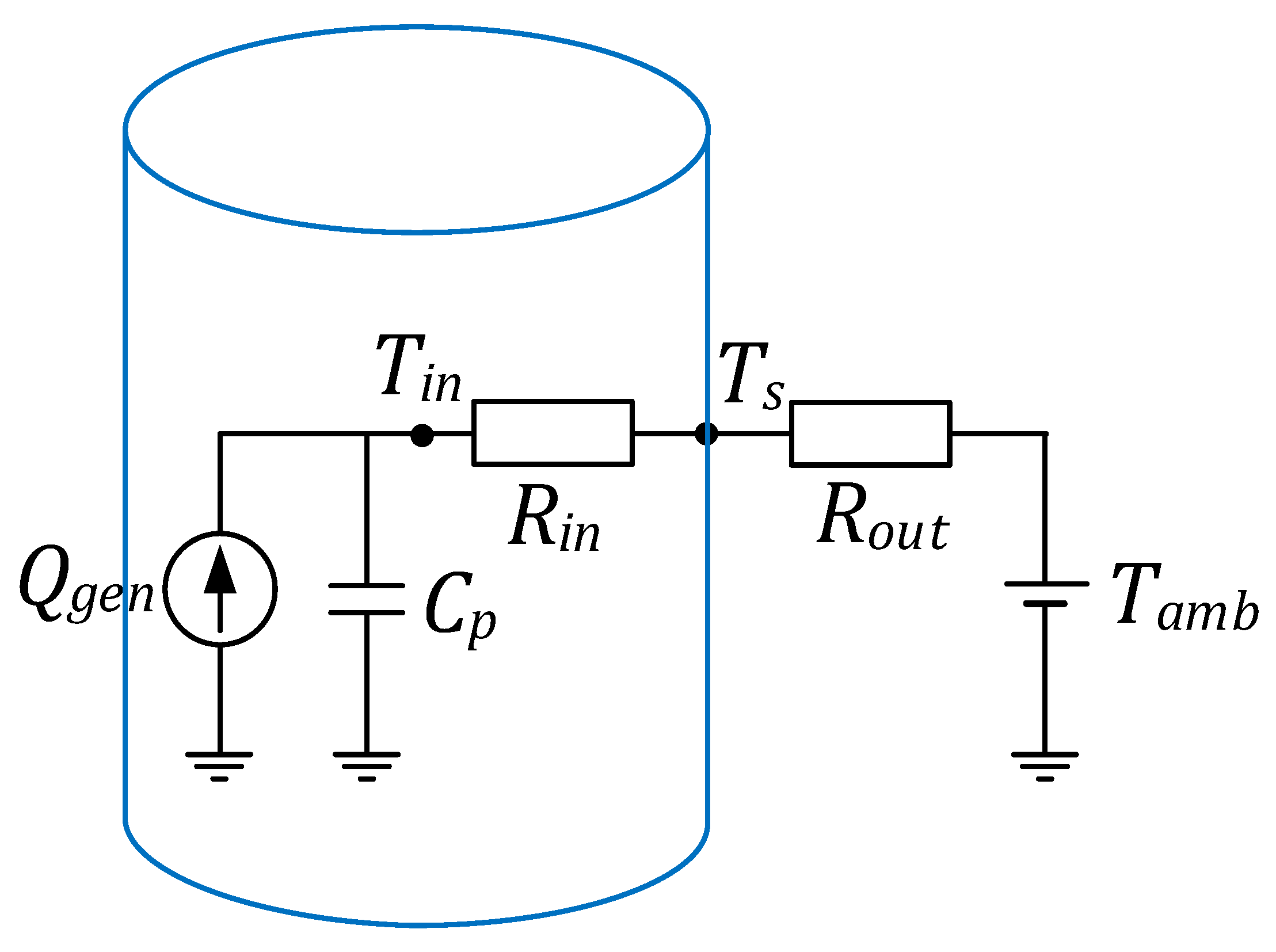
Regarding the thermal lumped model, it can simulate the whole cell at a uniform temperature taking into consideration that the gradient temperature within the battery is negligible [
2], and is also capable to estimate the center and surface temperature of the cell considering an internal thermal resistance [
3] and the degradation of a battery pack [
4]. Moreover, there exists many other thermal studies based on a lumped system. For instance, Bryden et al. [
1] determined the heat capacity of many cells, Coman et al. [
5] studied thermal abuse conditions, Lin et al. [
6] formulated an electro-thermal model for a battery, and Gao et al. [
7] proposed a reduced order model to control a battery pack. Most of the aforementioned studies on lumped models were developed employing small size batteries such as 18650 (18 mm diameter and 65 mm height) and 26650 (26 mm diameter and 65 mm height). For large format batteries, it is appropriate to study the cooling parameters to maintain a maximum temperature and uniformity of the cell [
8] since the temperature gradient is considerable. For instance, in Reference [
9], the authors reported the non-uniform temperature distribution for a 20 Ah LIB at discharging rates of 2C, 3C, and 4C. C-rate is the measurement of the charging or discharging current with respect to the rated capacity, for example, for a 1 Ah battery, 1C rate represents the current that completely discharges the battery in one hour.
Samba et al. [
10] studied the thermal distribution for a pouch battery using a 2D computational fluid dynamics (CFD) model. Besides, some studies have employed analytical techniques such as polynomial approximation in order to estimate the heat conduction inside the battery for 1D and 2D models [
11,
12].
Three-dimensional thermal model is generally based on CFD solvers, and has been widely utilized by researchers to study the thermal performance of a single cell [
13] and battery packs such as the work developed in References [
14,
15,
16].
On the other hand, electrochemical models can be solved using multi-scale multi-domain (MSMD) approach by using electrochemical equations according to the physics of Li-ion transport in the battery at different domains and different scales [
17]. This approach is useful to determine not only the temperature profile, but also relevant characteristics such as: heat generation, voltage, current flow field, and so forth. MSMD approach is implemented into Commercial software ANSYS Fluent through three sub-models: an equivalent circuit model (ECM) [
18], a full electrochemical model based on Newman Pseudo-2D equations [
19], and a Newman, Tiedemann, Gu and Kim (NTGK) model, which is a semi-empirical electrochemical model that requires discharging tests as input data [
20]. When using NTGK model, the differences between simulated and measured cell temperatures could be less than 1
[
21]. Furthermore, MSMD model is capable to simulate a battery pack with the cells connected electrically [
22,
23] and thermal runaway [
24,
25]. Panchal et al. [
19] applied MSMD framework by solving a Newman model to determine the temperature and voltage of a 26650 LFP lithium-ion battery discharged at constant discharge rates, whereas G. Li and S. Li [
17] simulated a prismatic battery under a driving cycle by the same approach.
The aforementioned models have been studied independently. For instance, Xu et al. [
26] presented a comparative study oriented to thermal control for cylindrical LIBs without considering electrochemical and CDF models. A suitable thermal management system is a priority for optimal performance and safety of the batteries [
27]. In particular, comparisons between an electrochemical model and the other approaches are very scarce. The main contributions of this paper are:
To provide a methodology to characterize the electrothermal behavior of LIBs studying the heat transfer coefficients under free convection including thermal radiation effects, heat generation, and the estimation of thermal parameters.
To compare the main approaches used for thermal modeling of a cylindrical LIB with experimental validation for a same case study.
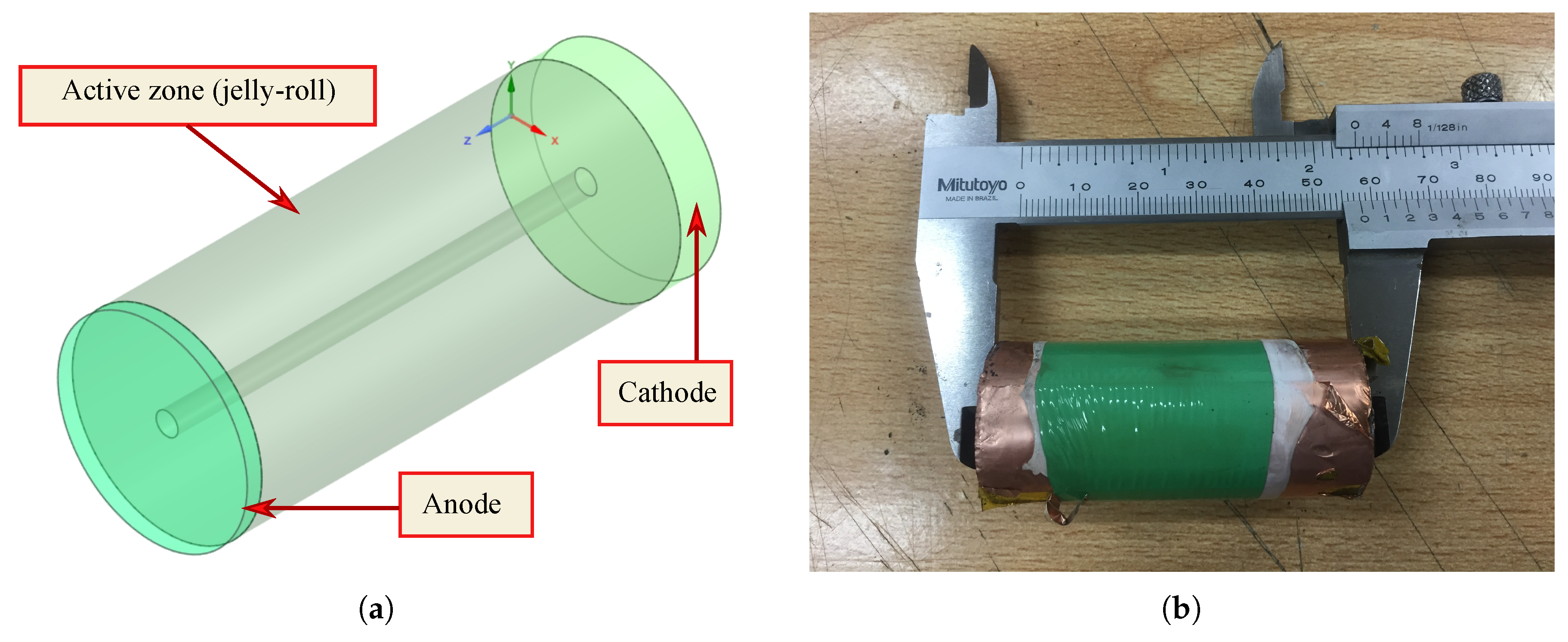
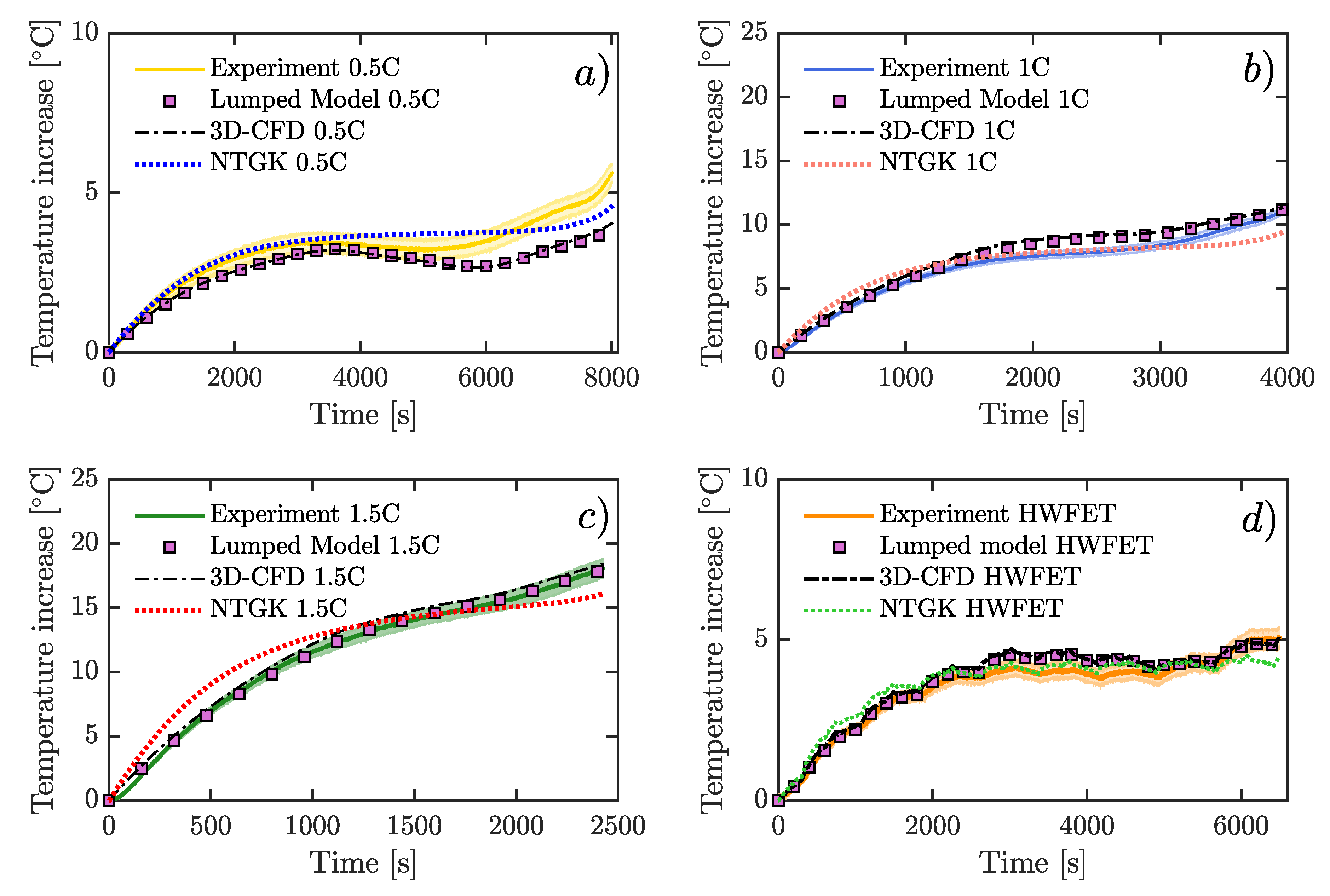
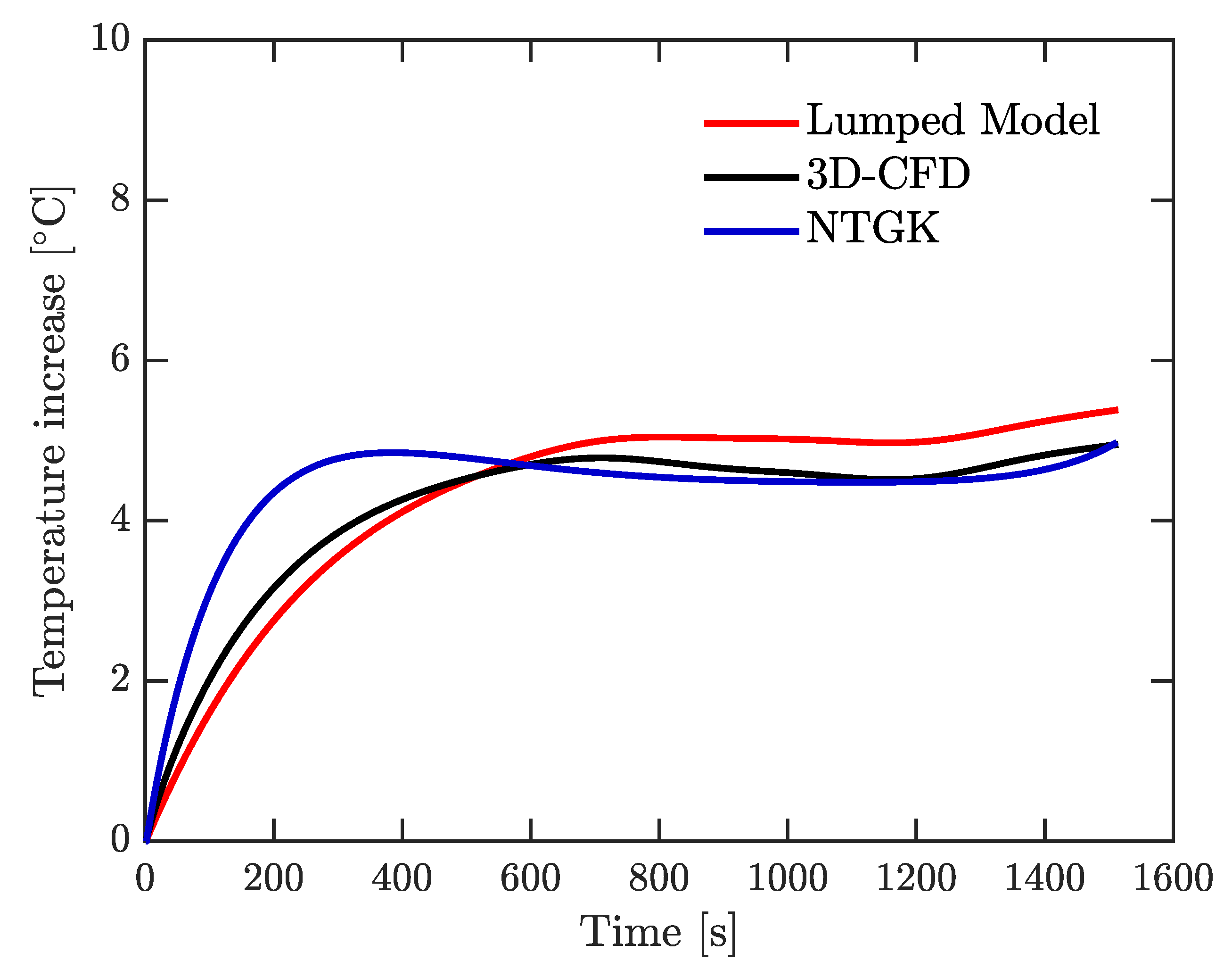
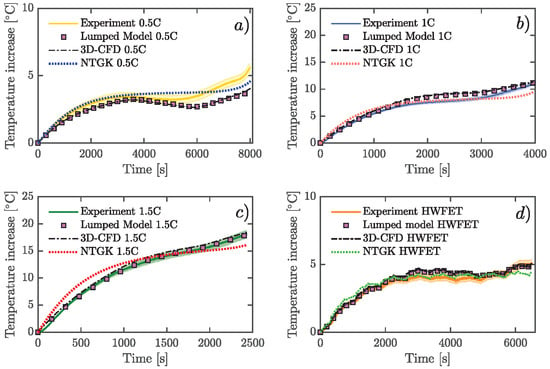
A 26650 lithium cobalt oxide (LCO) battery was used. Three of the most employed models to study the thermal behavior of LIBs were solved numerically: a lumped model, a 3D-CFD model, and an electrochemical approach using the NTGK model. Furthermore, voltage was simulated using NTGK formulation. All of these simulations were done at the same physical conditions of the experimental tests, at constant current discharge rate, and under the highway fuel economy test (HWFET) driving cycle. Moreover, a real driving-cycle of a Nissan Leaf was studied using the lumped model.
This article is organized as follows:
Section 2 presents a theoretical framework on the methods for battery modeling utilized in this work.
Section 3 describes the procedure to estimate thermal parameters and heat generation rate. Comparison among the models is provided in
Section 4. Conclusions are given in
Section 5.
3. Materials and Methods
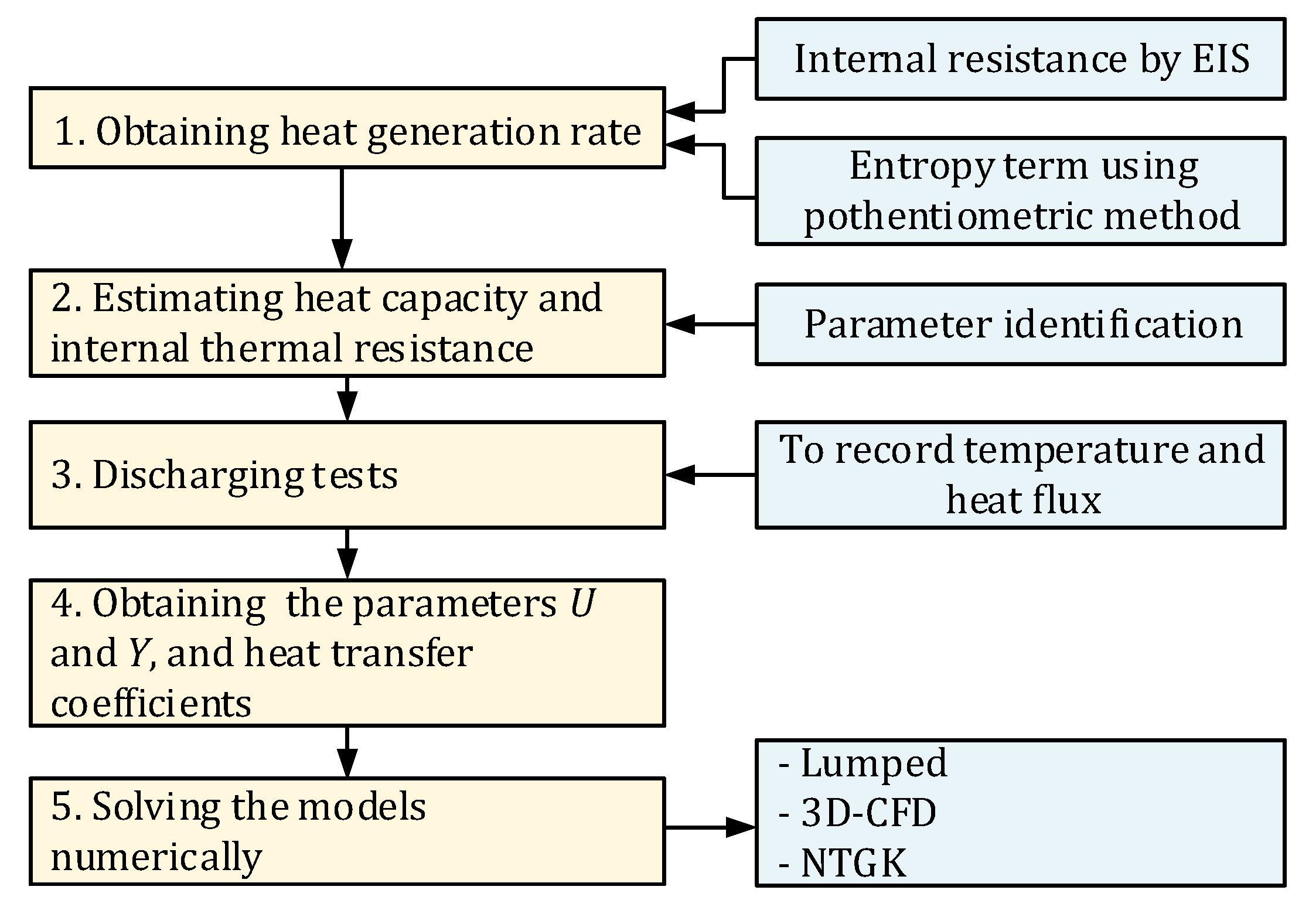
The contributions in this paper are as follows—firstly, to characterize the heat transfer coefficients of a battery under free convection including thermal radiation effects; secondly, to estimate the parameters; and thirdly, to compare the main approaches used for thermal modeling of a LIB with experimental validation at constant and variable current rates. Besides, heat generation and voltage are solved.
Figure 4 depicts the general procedure to develop the present study. The first step is to obtain the heat generation rate of the battery by estimating the internal resistance
using EIS, and the entropic coefficient applying the potentiometric method. Next stage is to estimate heat capacity and internal thermal resistance of the cell
via parameter identification. Next step is to perform battery discharging tests while ambient temperature, cell surface temperature, and heat flux from the LIB are recorded. Discharging tests allow to obtain
U and
Y functions using parameter estimation tool in Fluent. Finally, the models are numerically solved using MATLAB for lumped model, and ANSYS Fluent 19.2 based on finite volume method, for both 3D-CFD and NTGK models.
3.1. Experimental Set-Up
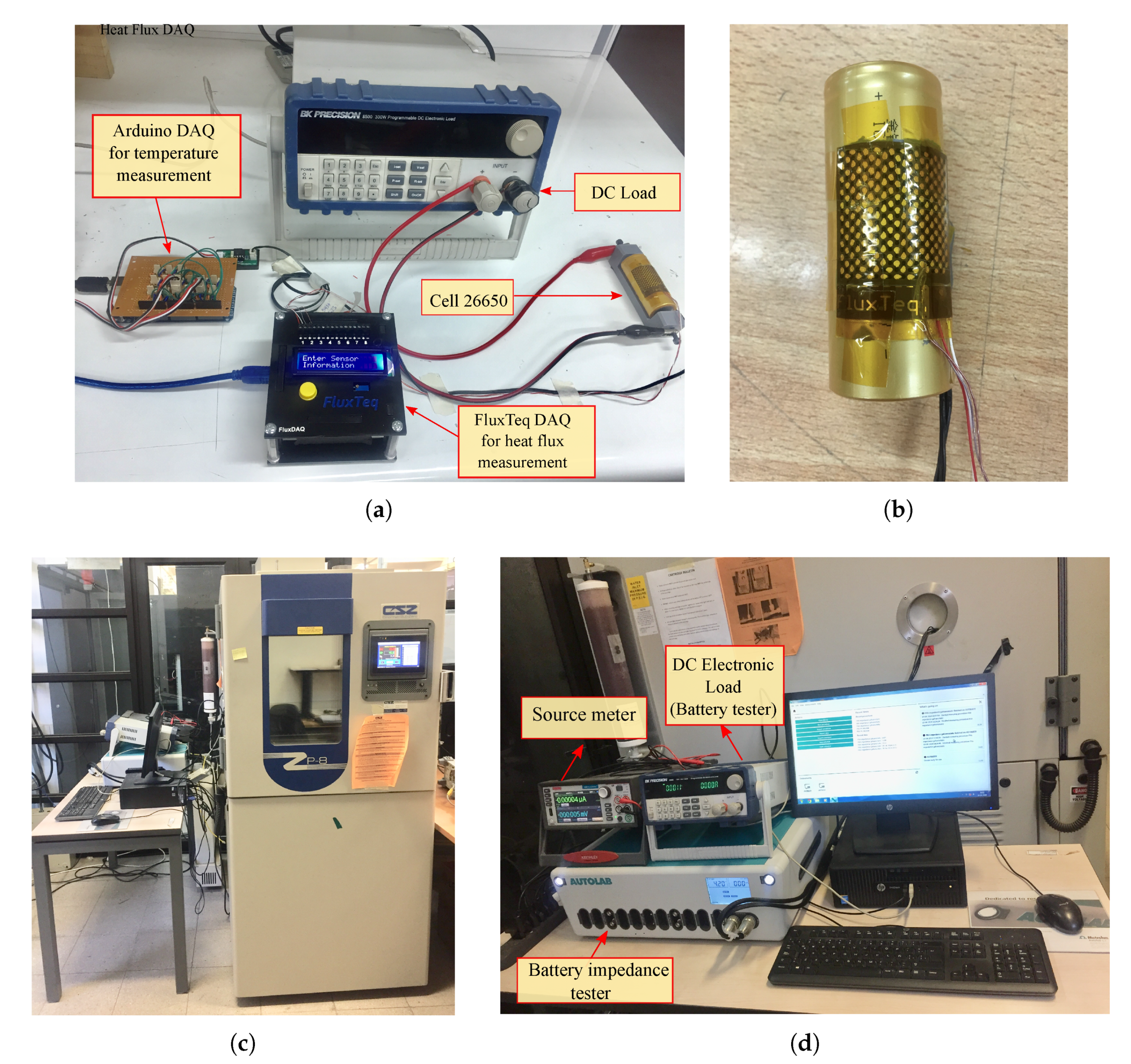
An ICR 26650 battery (see specifications in
Table 5) was fully charged following the constant-current constant-voltage (CC-CV) protocol using a battery charger (iCharger 208B). The first stage (constant-current) was at 2 A, then the charger turns to constant voltage. Subsequently, the battery was discharged using a BK Precision 8500 programmable DC load (
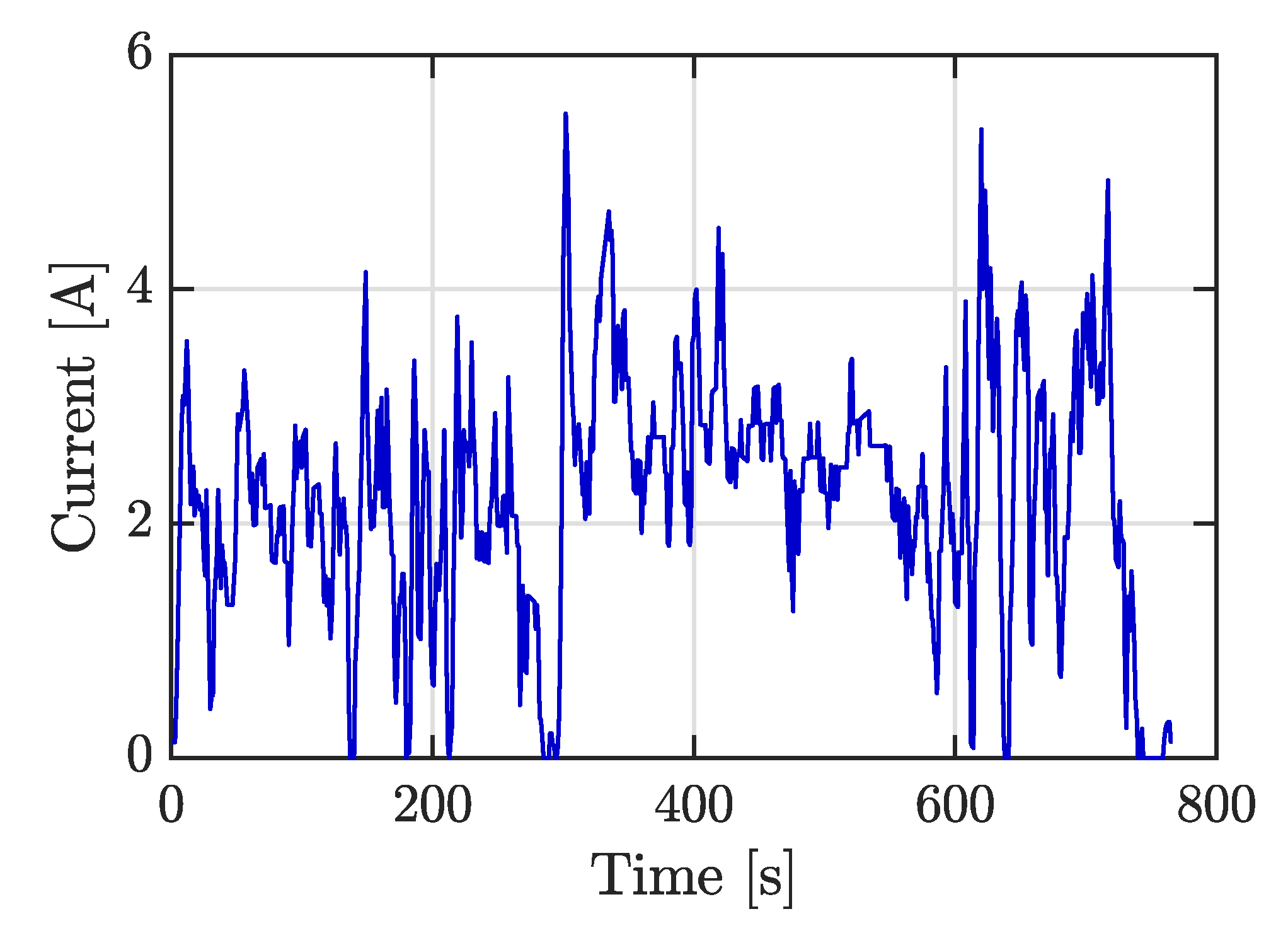
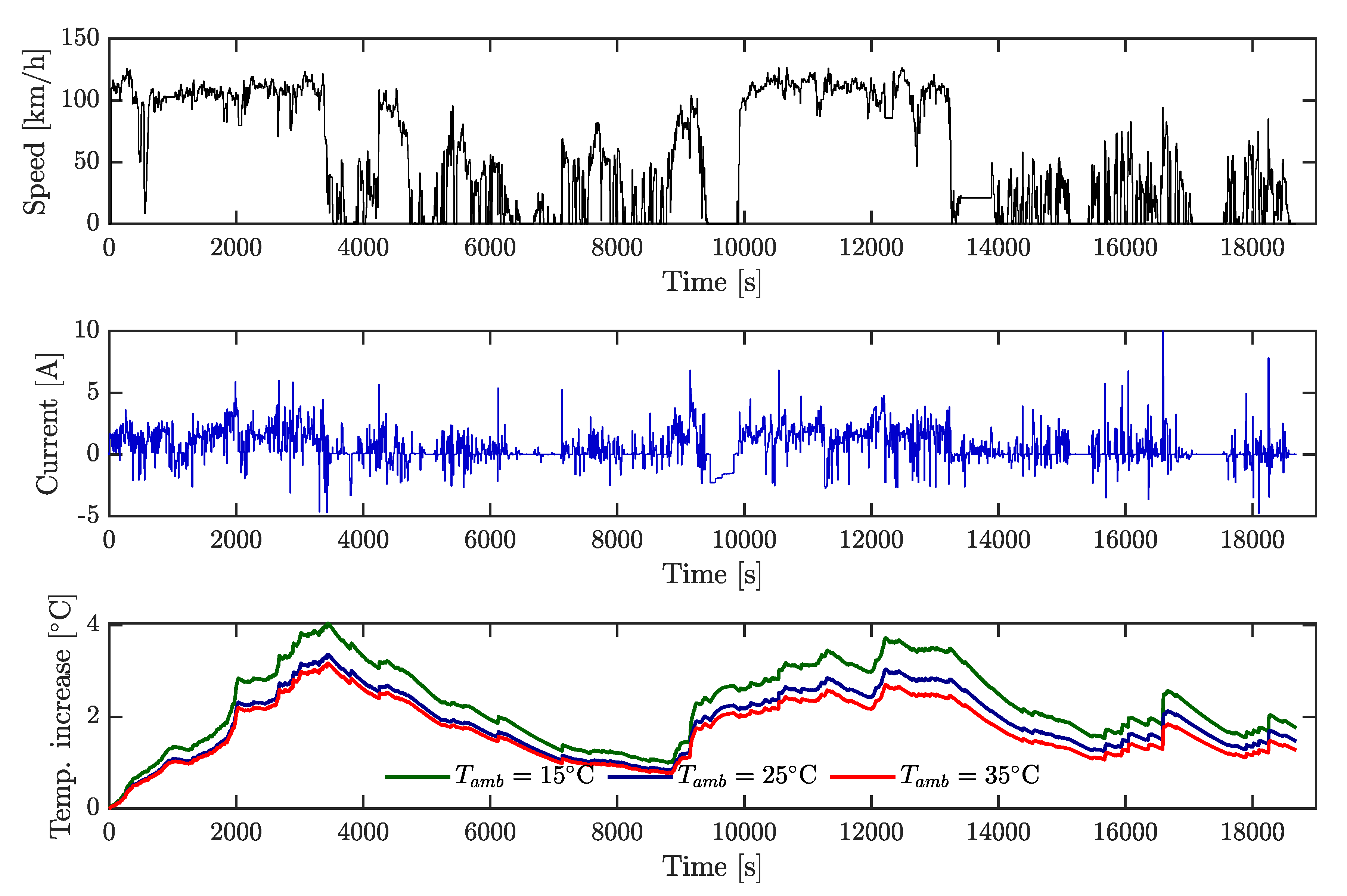
Figure 5a). Current rates applied were 2 A (0.5C), 4 A (1C), 6 A (1.5C), and also a variable current profile. This last profile was obtained by ADVISOR software selecting a highway HWFET-driving cycle as velocity input for an electric vehicle. The simulated current was scaled at a maximum of 5.5 A, as shown in
Figure 6, and this cycle was repeatedly applied to the cell until the energy extracted was 4 Ah. Regeneration current was not considered.
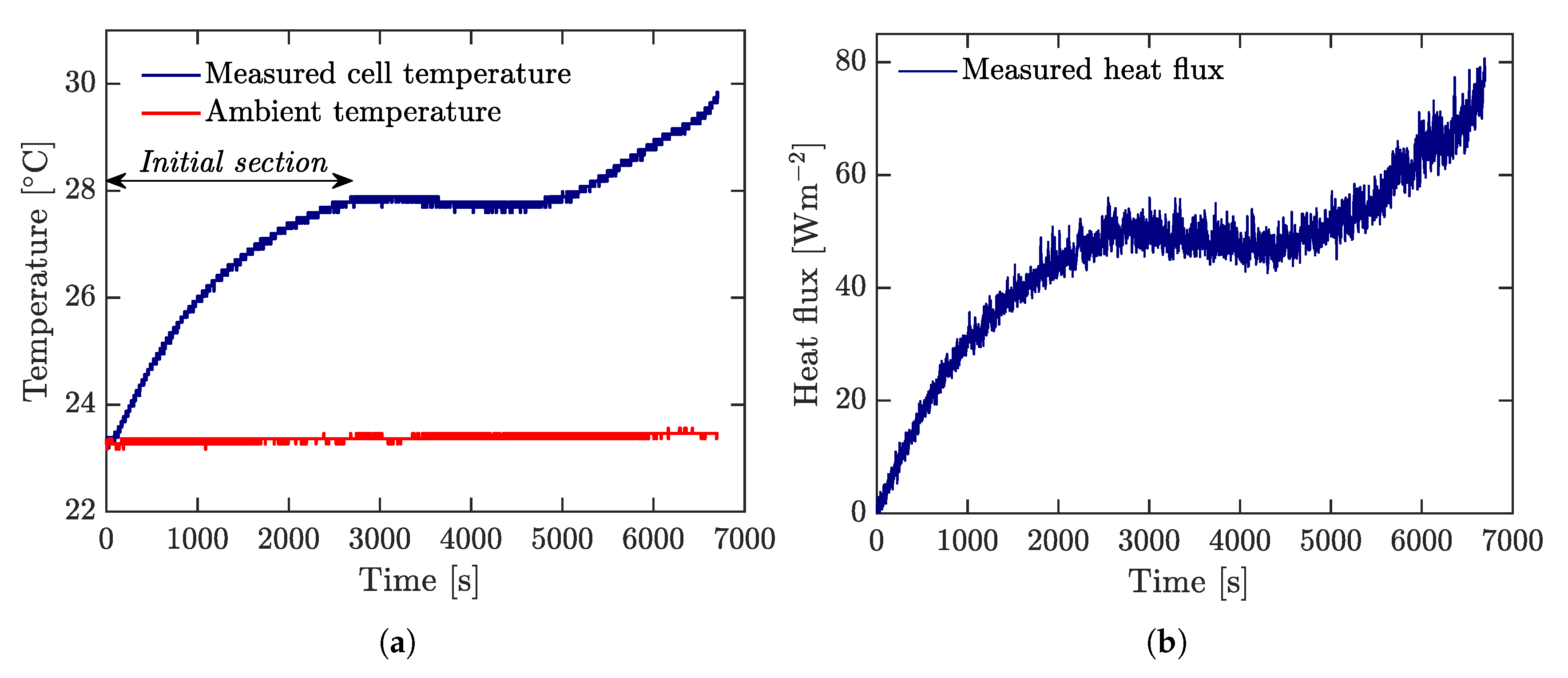
All discharging tests were repeated five times to ensure repeatability measurements. Ambient temperature and cell temperature were recorded through a negative temperature coefficient (NTC) thermistor (ZX-Thermometer) connected to an Arduino processor board. This sensor operates between
and 85
with an uncertainty of ±
. It requires a direct-current supply voltage between +1.8 V and +5.0 V. A code was developed using MATLAB software to convert voltage input (from Arduino board) into temperature. Since ambient temperature fluctuations were less than 1
during all tests, this value was assumed to be constant. Heat flux from the cell was measured by a PHFS-01 FluxTeq heat flux sensor (
Figure 5b) connected to a FluxTeq DAQ (see
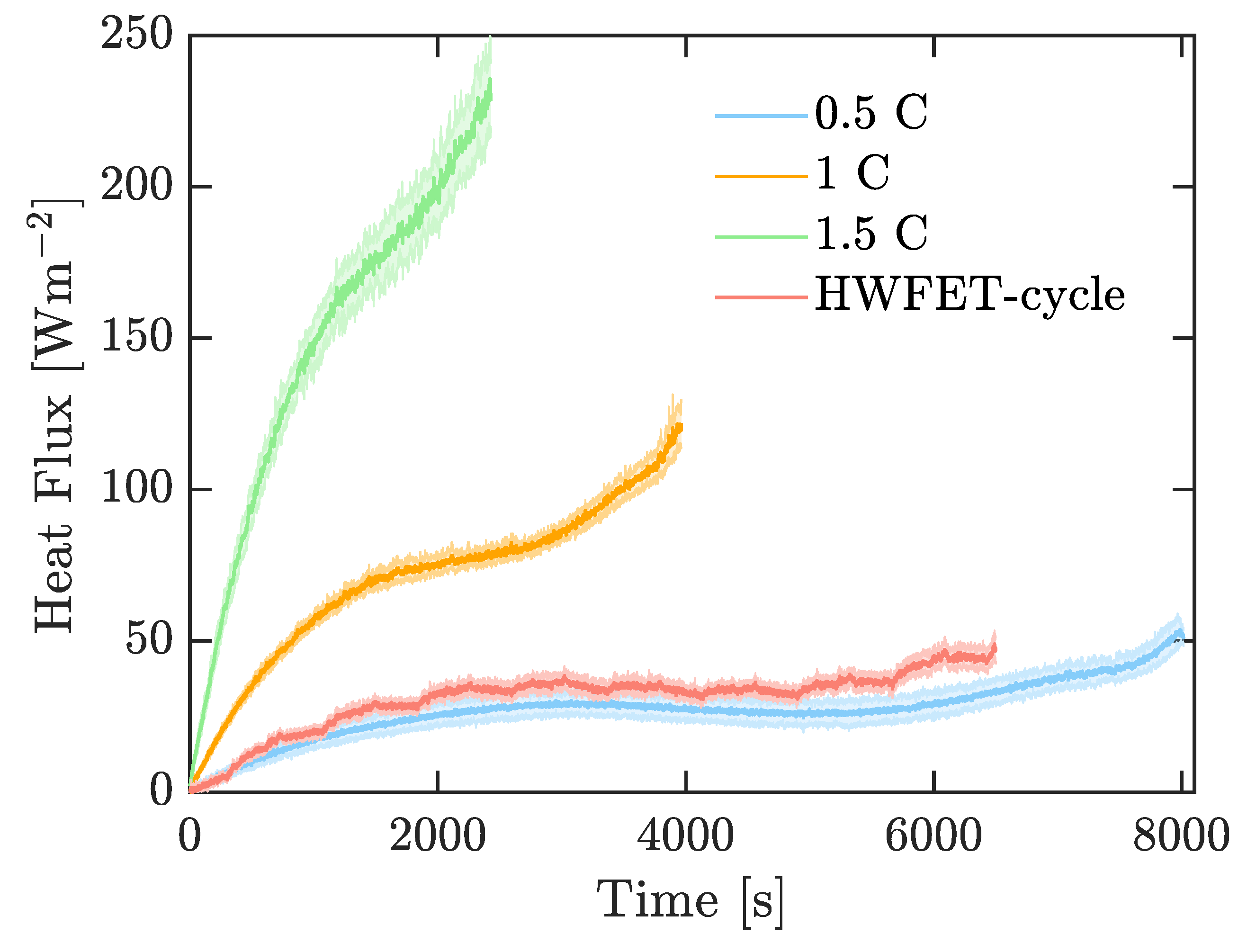
Figure 5a). Measurements of heat flux are plotted in
Figure 7.
3.2. Heat Generation Estimation
Heat generation in the cell was computed from Equation (
12) that includes internal resistance and entropy change.
3.2.1. Experiment for Internal Resistance
Electrochemical impedance spectroscopy (EIS) was carried out to estimate the internal resistance of the battery. First, the fully charged battery was placed in a thermal chamber EZT-570i (see
Figure 5c) at 15
. After 2 h of rest, galvanostatic EIS was conducted by using an impedance tester equipment PGSTAT302N (see
Figure 5d). Details of the setup are listed in
Table 6. This process was repeated at 25
, 35
, and 45
. After performing the experiment at 45
, the battery was discharged at 90% SOC and overnighted. The whole process was repeated until the cell was completely discharged (0% SOC), decreasing the battery SOC in intervals of 10% between each of the EIS tests.
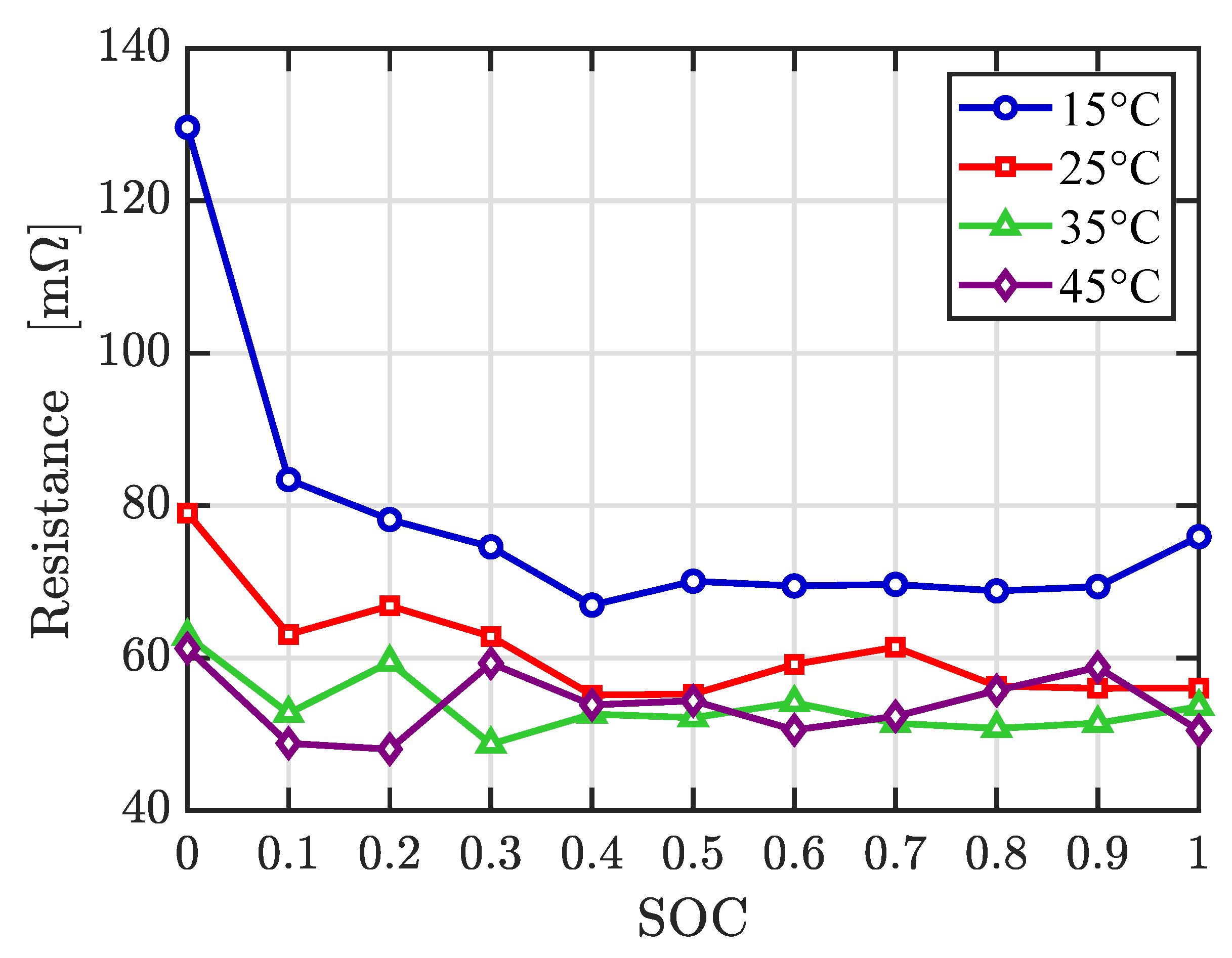
The experimental characterization of the battery internal resistance at different temperatures, obtained from EIS tests, is depicted in
Figure 8. It reveals that at lower temperature, and near to 0% of SOC, the cell resistance increases. At 35
and 45
, the resistance has practically the same value.
Average battery resistance for each discharging rate test is detailed in
Table 7. It is evident that at lower discharge rates, the resistance increases. Each value represents
in Equation (
12), that is, it allows to compute the irreversible heating.
3.2.2. Entropy Coefficient Experiment
Entropy change term is expressed as a relationship between open circuit voltage and cell temperature, and was obtained by applying the potentiometric method: the battery 100% charged was put into a thermal chamber at 15
. After it rested for two hours to reach thermal equilibrium [
15], and the voltage was measured by using a KEITHLEY 2460 sourcemeter. In order to obtain the relationship
, the same process was performed progressively at 25, 35, and 45
, respectively. Next, the cell was discharged at 90% SOC and overnighted. The whole test described before was repeated with intervals of 10% of SOC until the charge of the battery drops to 0% of SOC [
39].
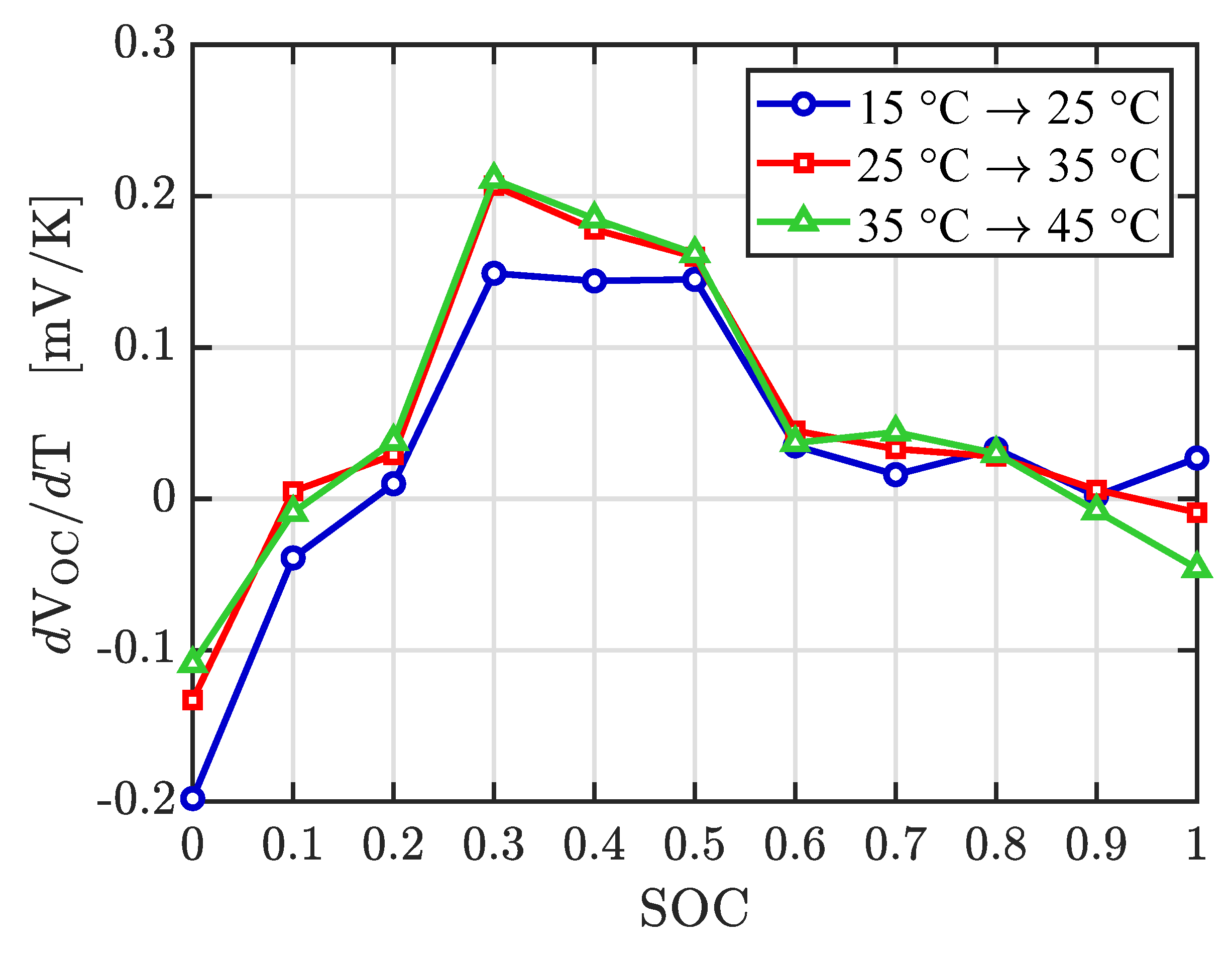
Experimental values for the entropy term are presented in
Figure 9. There does not exist a significant variation of entropy change at the different temperatures. In the interval of 20% to 80%, the entropy change is positive with a maximum value near to 0.2 mV/K. A similar entropy profile was reported by Zhang et al. [
40].
3.3. Heat Capacity and Internal Thermal Resistance
To estimate heat capacity and internal thermal resistance, a consecutive current pulse of 0.4 A, 2 A, 4 A, and 3 A, each one of them with a duration of 10 s, was continuously applied until the battery was discharged. Ambient and battery temperatures, and heat flux were recorded as depicted in
Figure 10a, and
Figure 10b, respectively. These measurements allow to compute
by least squares regression from Equation (
6), and then,
from Equation (
9). After obtaining
, it is possible to figure out an experimental
from Equation (
7).
Following a similar procedure proposed by Bryden et al. [
1], the next stage was to estimate
and
from Equation (
3) by fitting the
initial section of the measured cell temperature (see
Figure 10a), where the temperature gradient is greater. This region was taken for the first 2700 s. Parameters obtained from this section are given in
Table 8. Then, specific heat is obtained by
. The aforementioned authors reported values of
and
for a 26650 LFP battery.
3.4. Physical Properties and Solving
Properties detailed in
Table 9 were used to solve the lumped and 3D-CFD models. For NTGK approach, parameters and properties are given in
Table 3 and
Table 4, respectively. Besides, for all three proposed models, there were utilized experimental heat transfer coefficients based on heat flux and temperature measurements of the cell surface. These coefficients are discussed in the
Section 4.2, and were obtained following a similar procedure described in the
Section 3.3. Furthermore, it was assumed that the external radiation temperature is the same as the ambient temperature.. The nominal capacity of the cell is 4 Ah; however, the average tested capacity was 4.3 Ah, and this last value was employed.
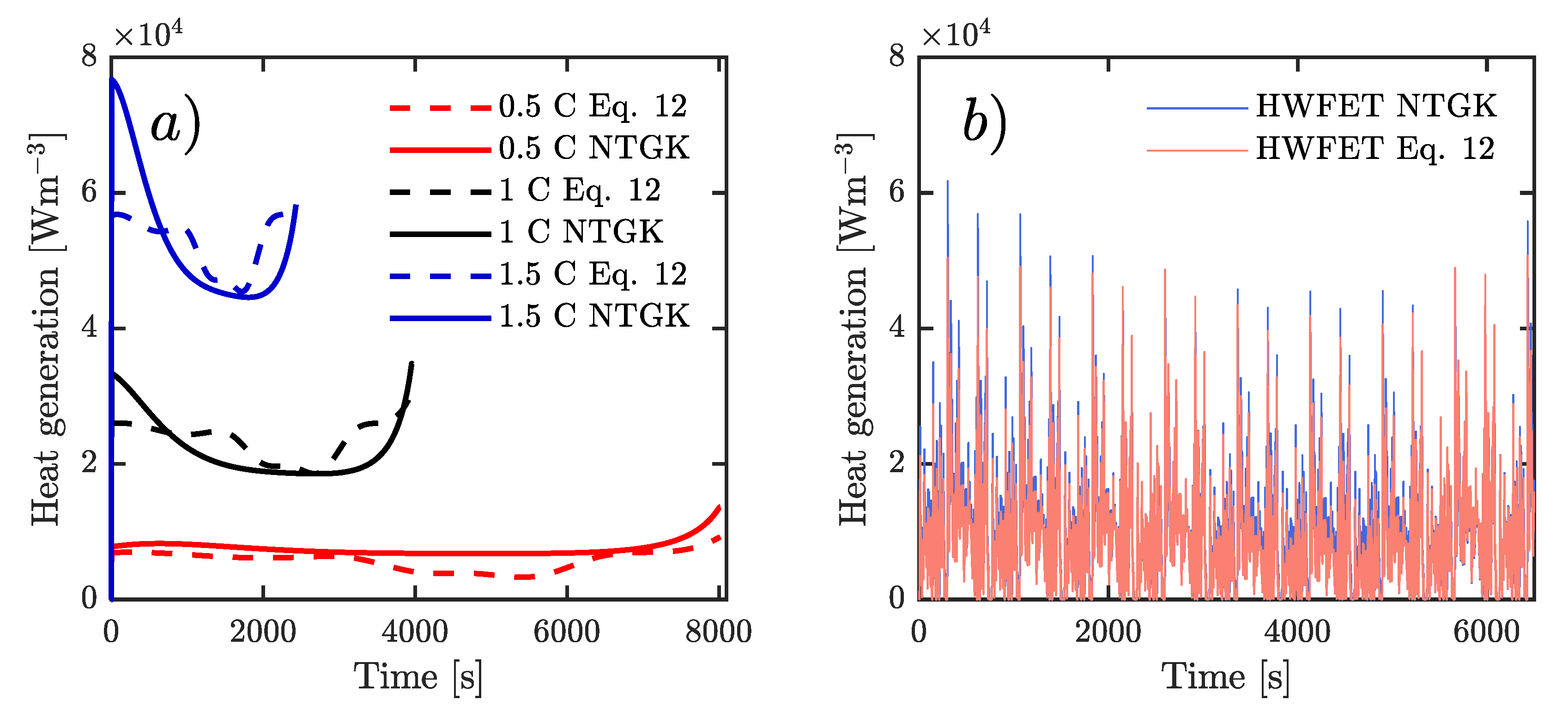
According to the current profile applied to simulate the 3D-CFD approach, there are two considerations to couple the heat generation per unit volume to the energy equation. Firstly, for constant current rates, a User Define Function (UDF) was utilized. Secondly, for variable currents (HWFET-cycle), a transient table was employed. In contrast, the NTGK model automatically computes the heat generation rate.
Numerically, the lumped model was solved by Runge Kutta Fourth order method, and the other two models were computed through a Semi-Implicit Method for Pressure Linked Equations (SIMPLE) scheme. For current rates of 2 A, 4 A, 6 A, and HWFET-cycle, the simulation time was 8015 s, 3964 s, 2430 s, and 6500 s, respectively. Furthermore, 1 s time step was utilized in all cases.