Comparison of Single-Ion Conducting Polymer Gel Electrolytes for Sodium, Potassium, and Calcium Batteries: Influence of Polymer Chemistry, Cation Identity, Charge Density, and Solvent on Conductivity
Abstract
1. Introduction
2. Results and Discussion
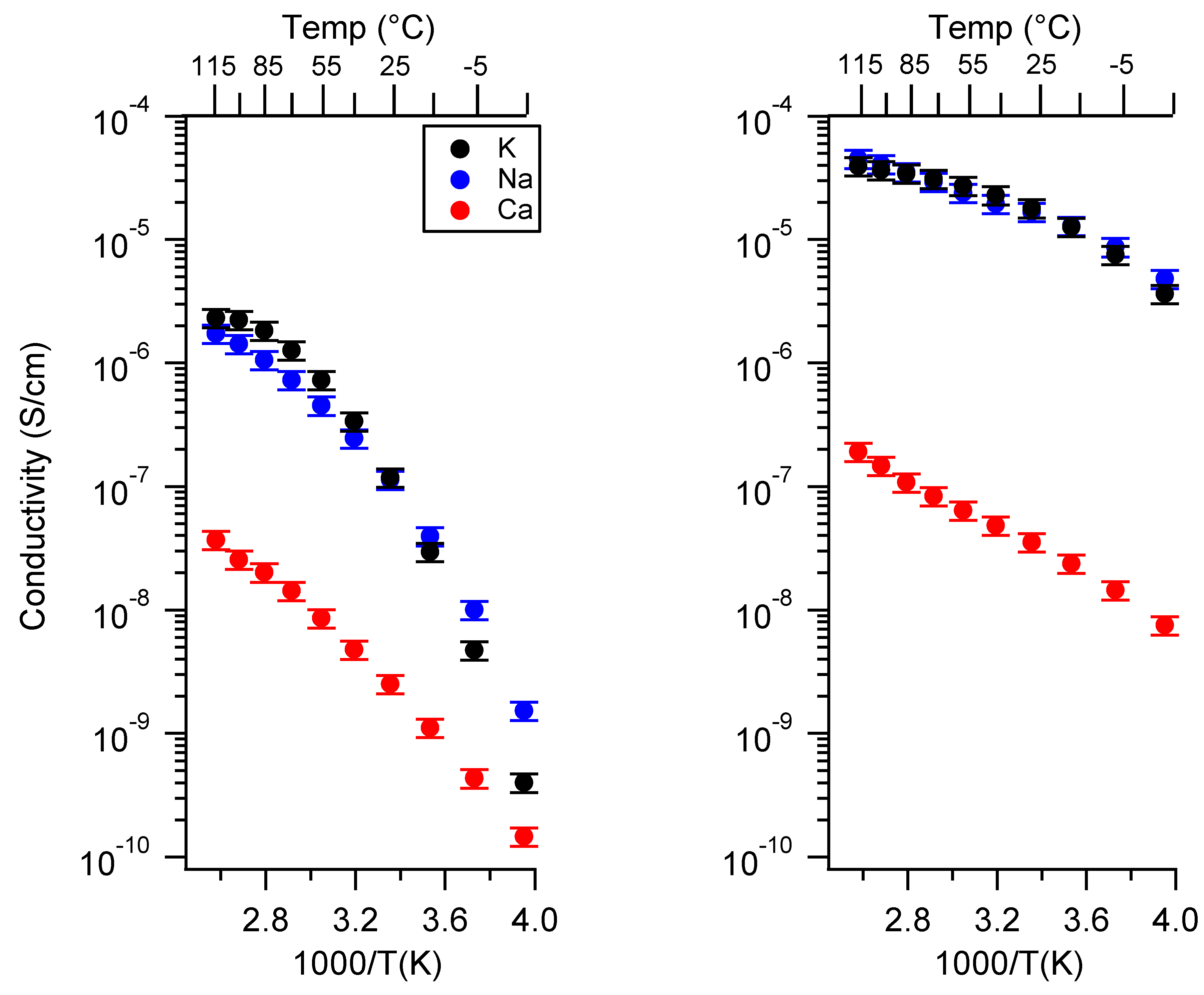
2.1. Effect of the Cation
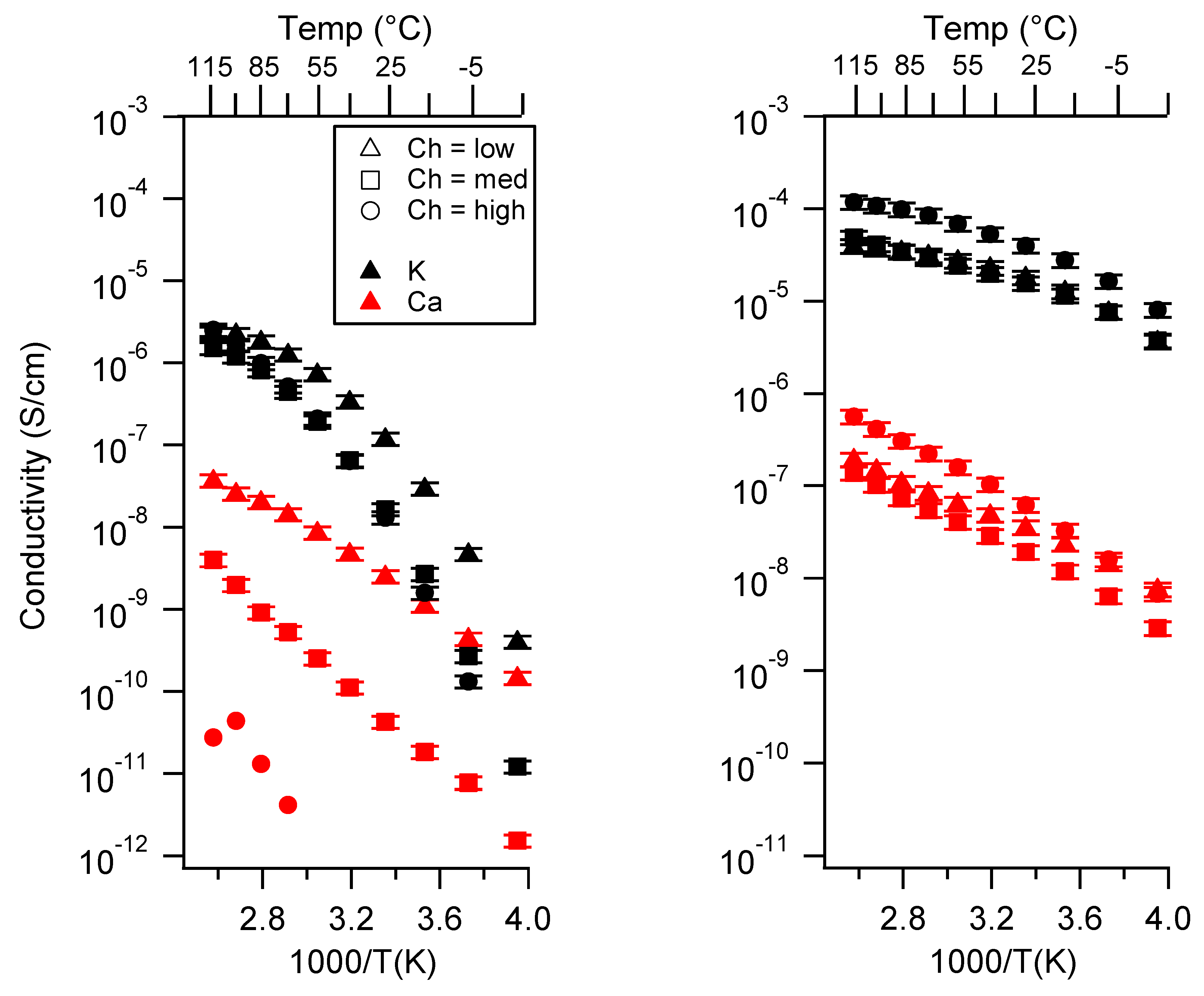
2.2. Effect of Charge Density
2.3. Effect of Swelling Condition
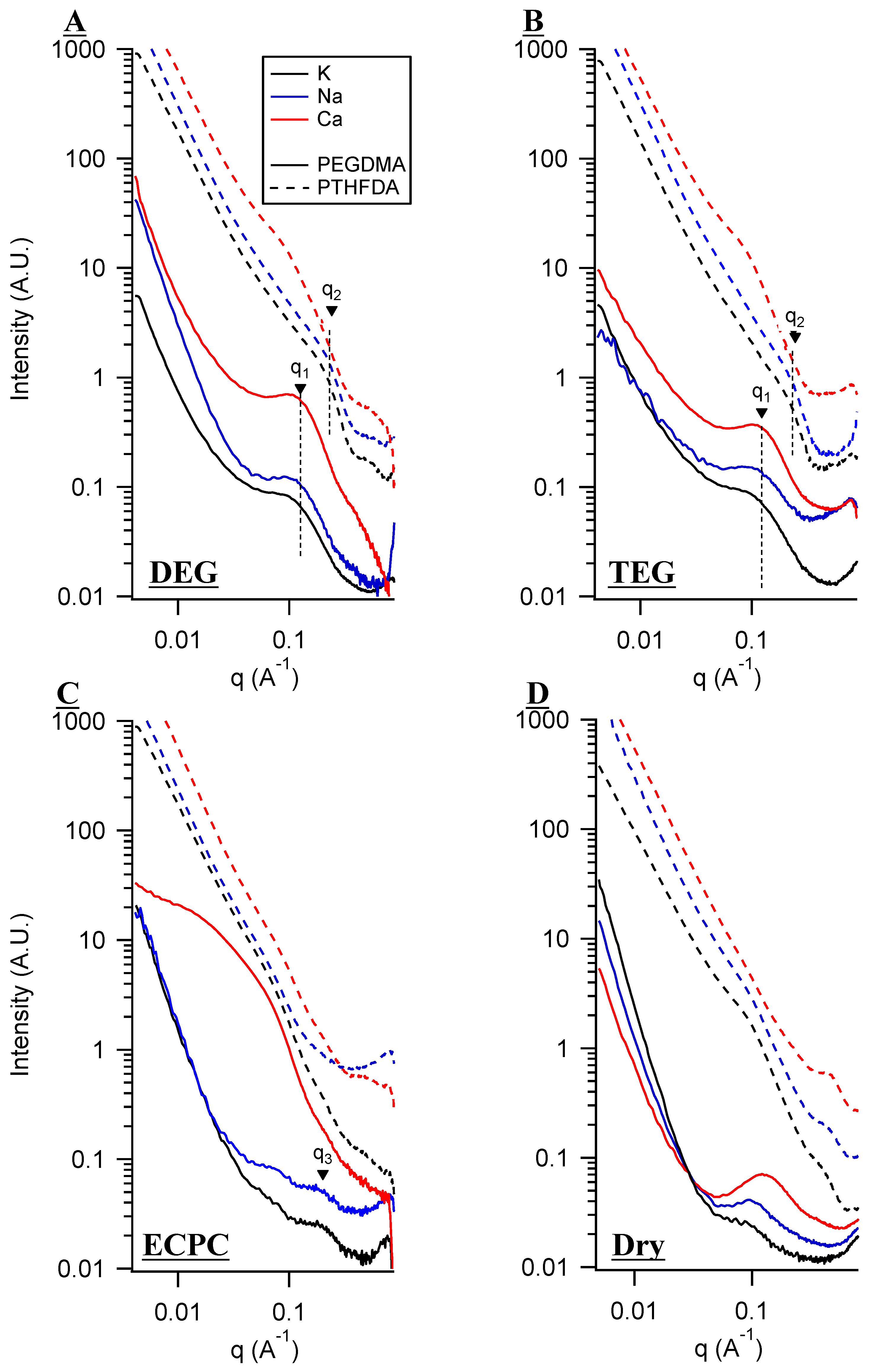
3. Small Angle X-Ray Scattering (SAXS) and Solvent Uptake
4. Conclusions
5. Experimental
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
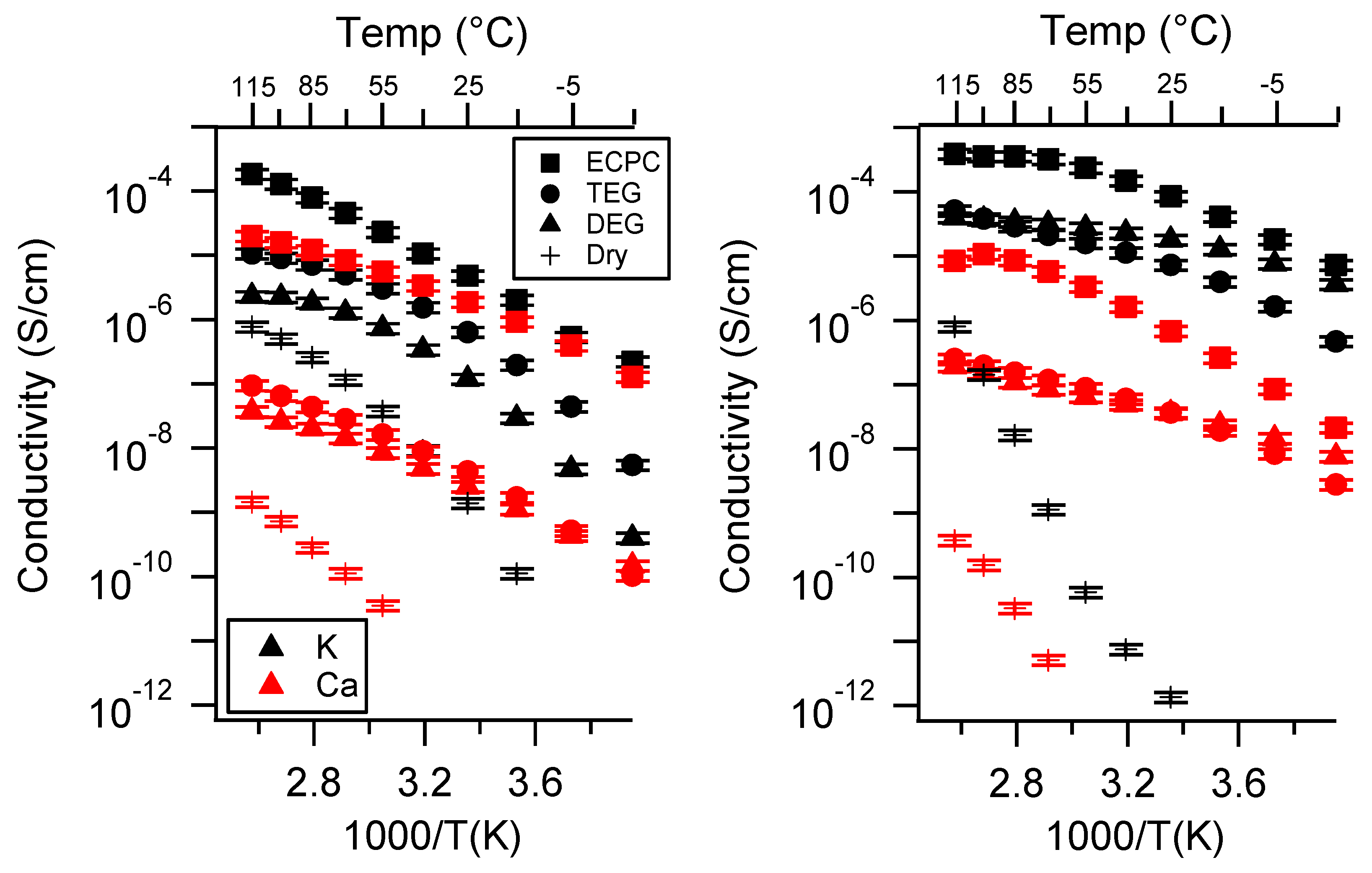
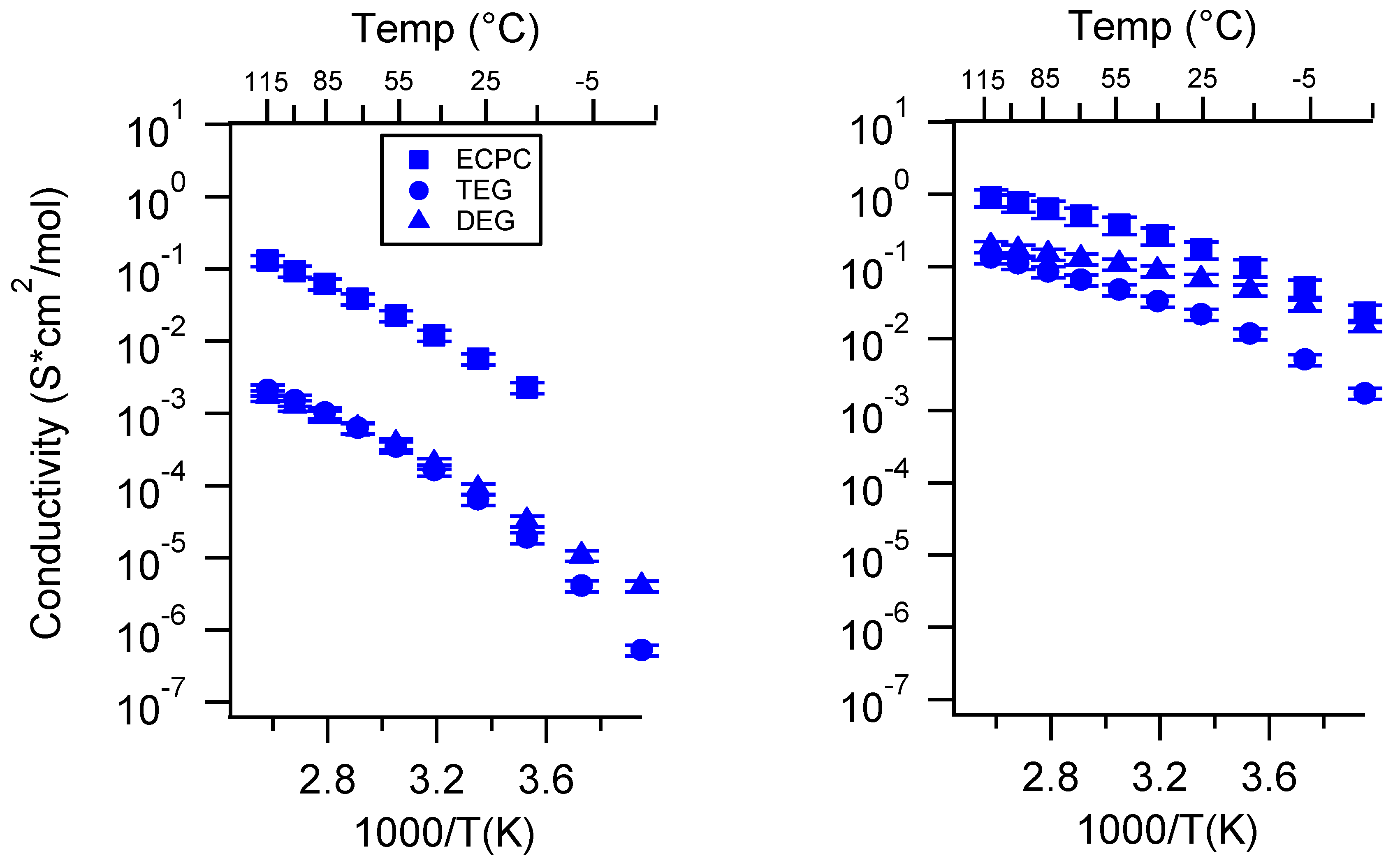
Appendix A. All SIPE Conductivity as a Function of Cation
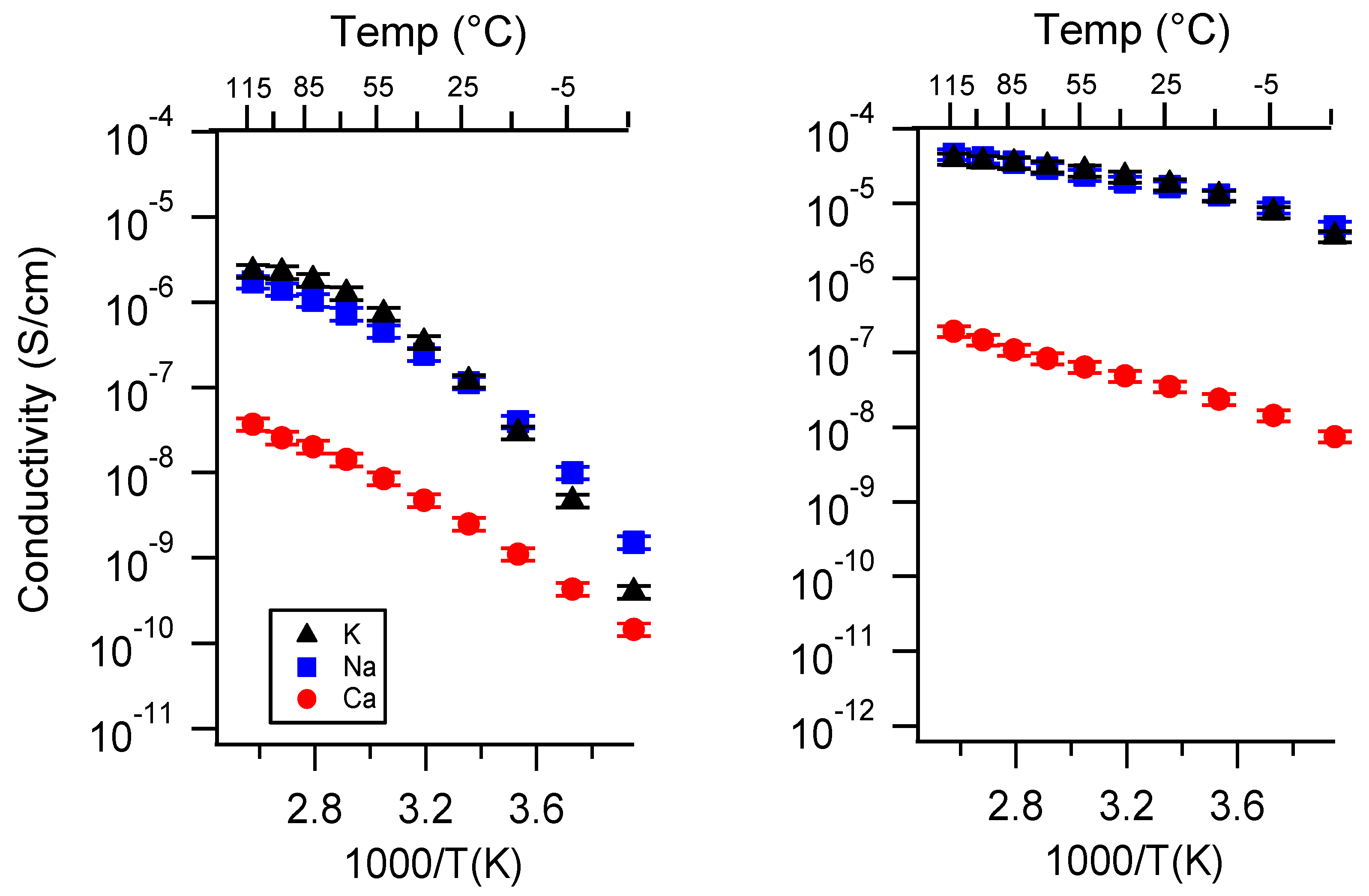
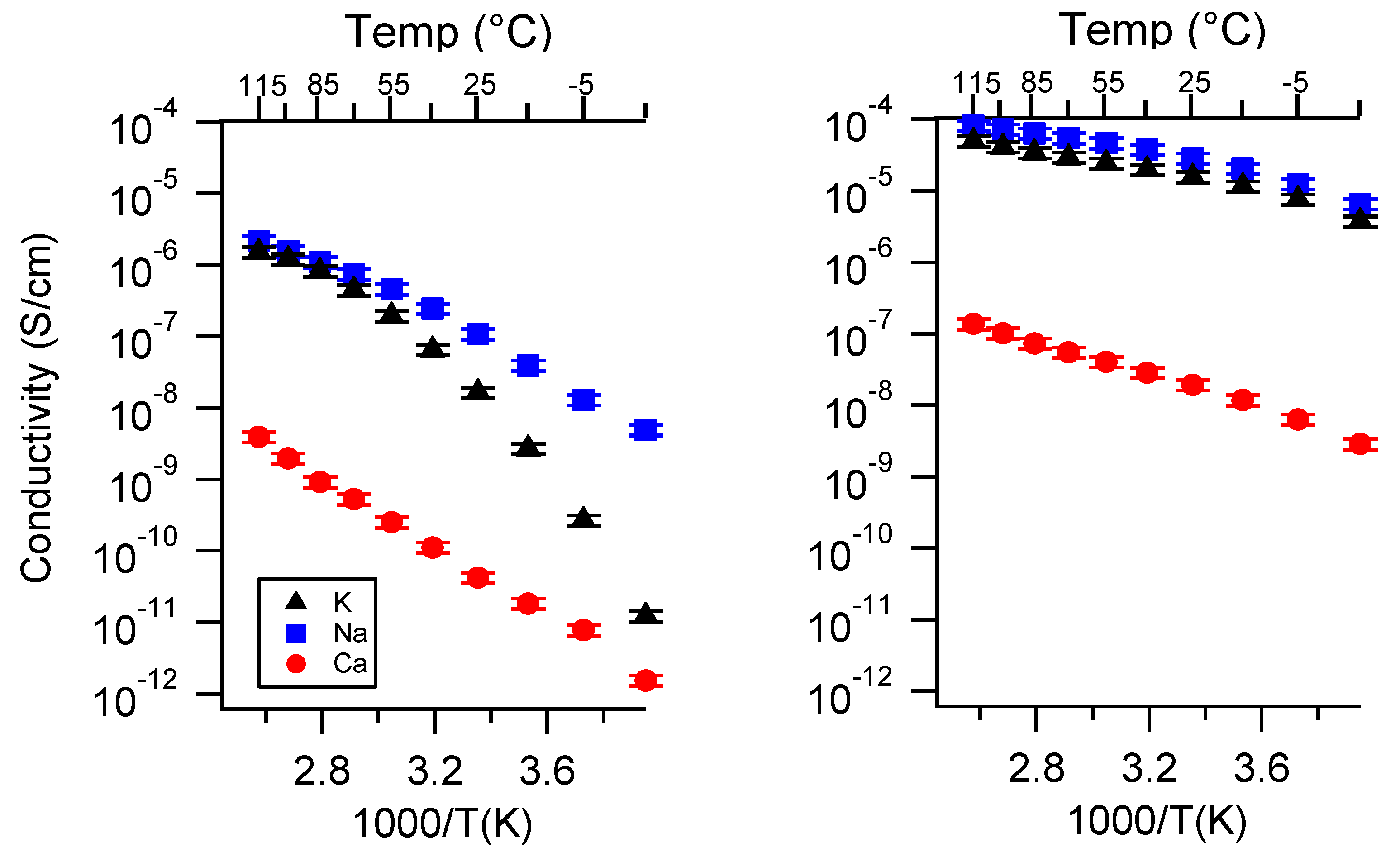
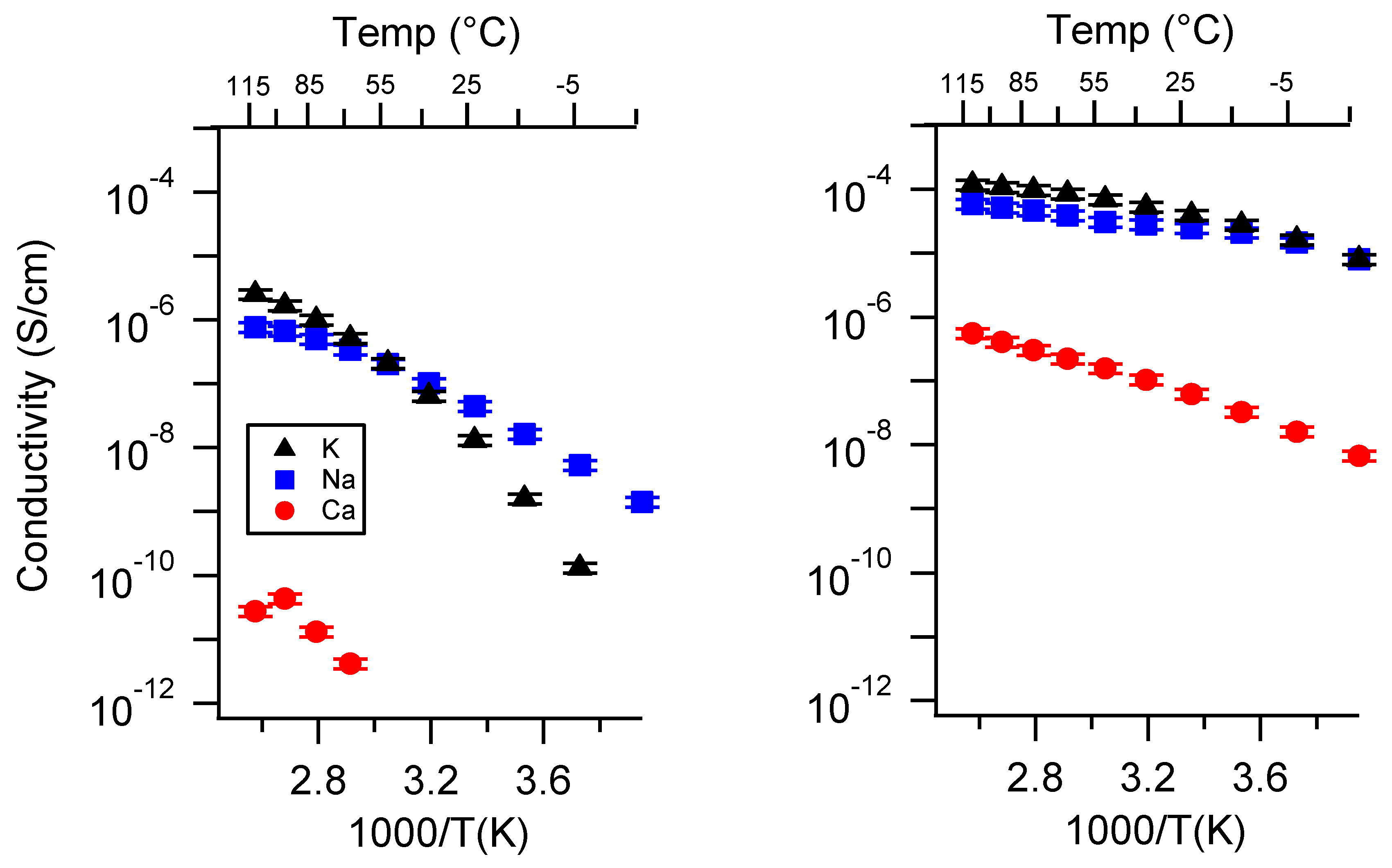
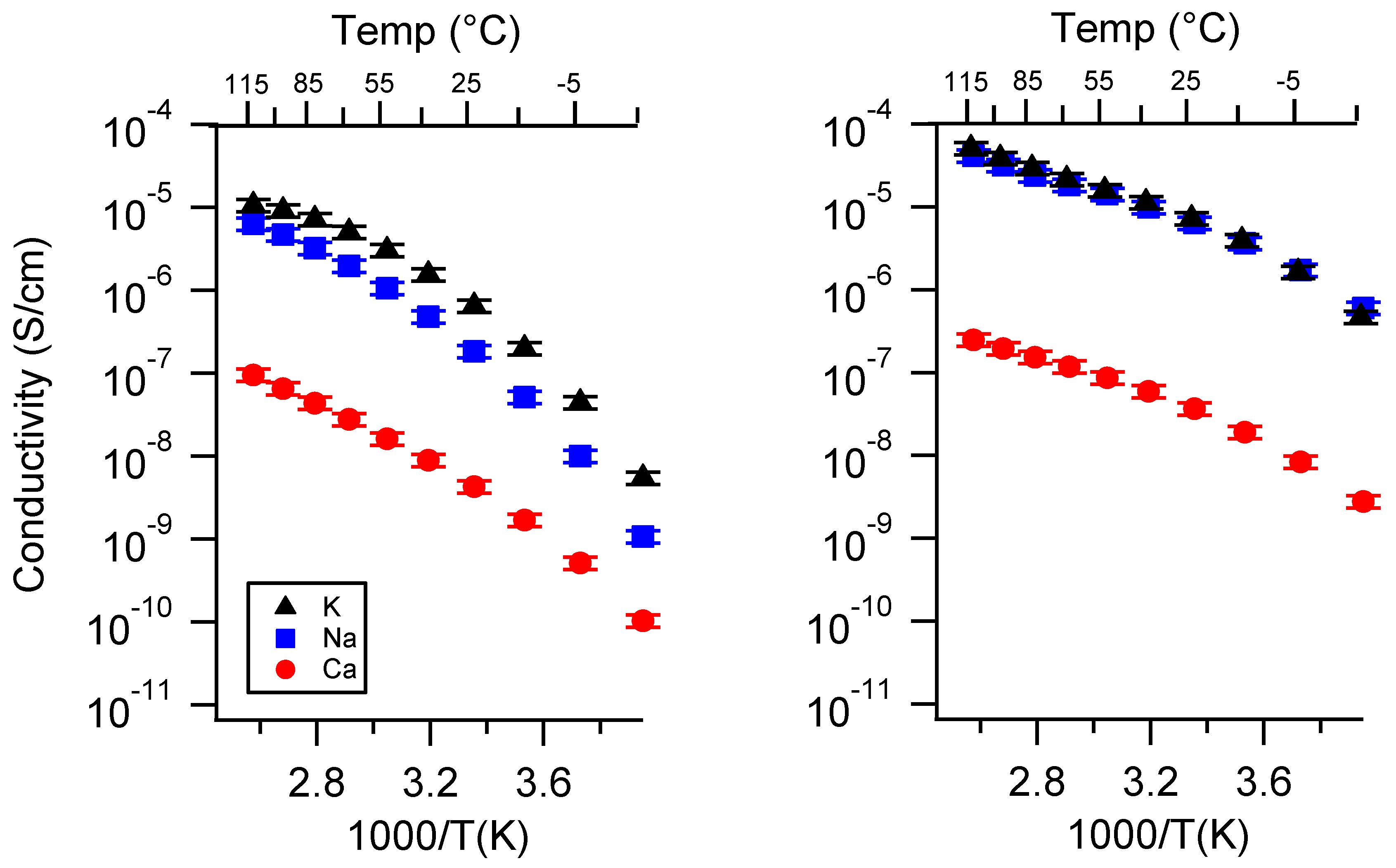
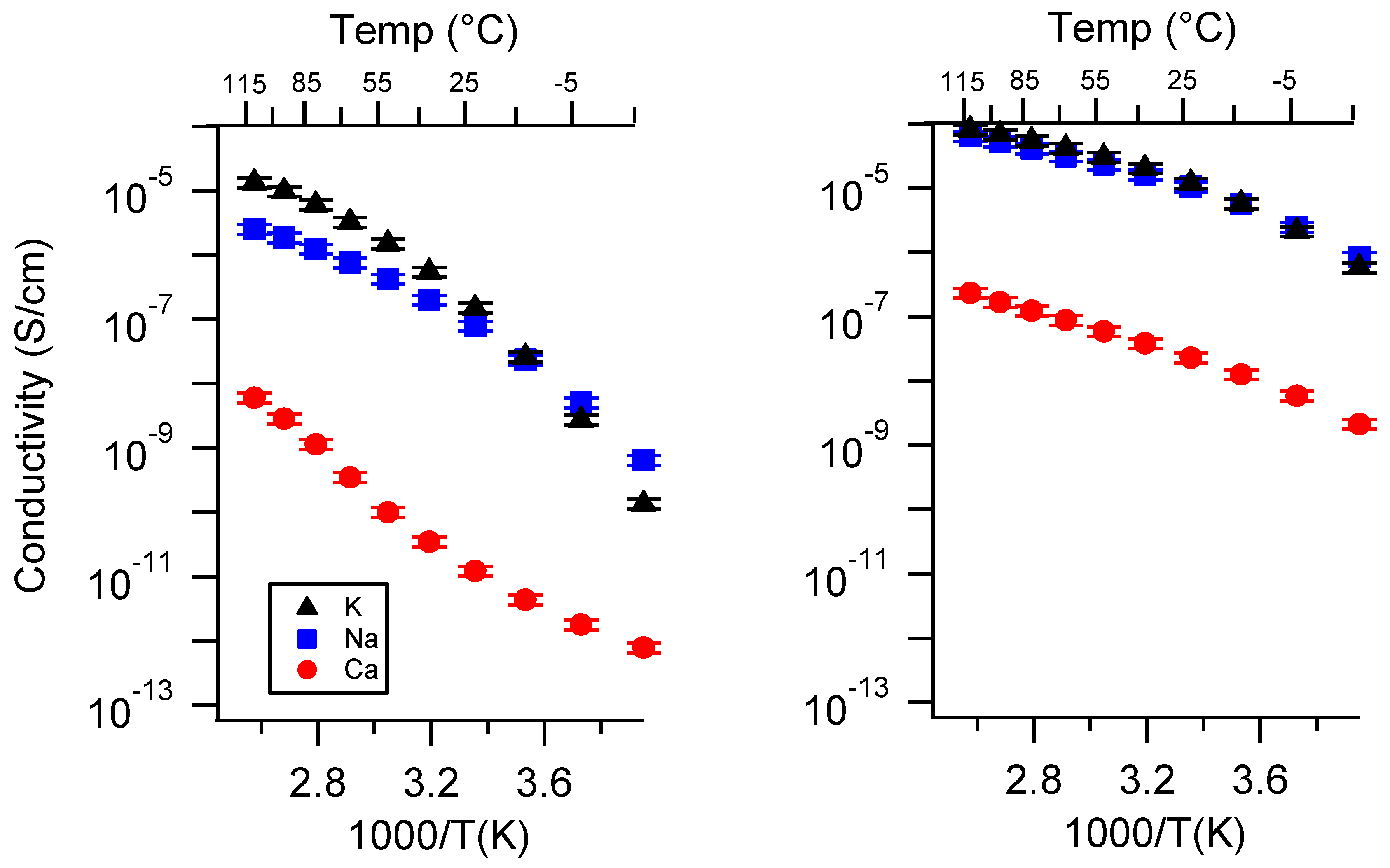
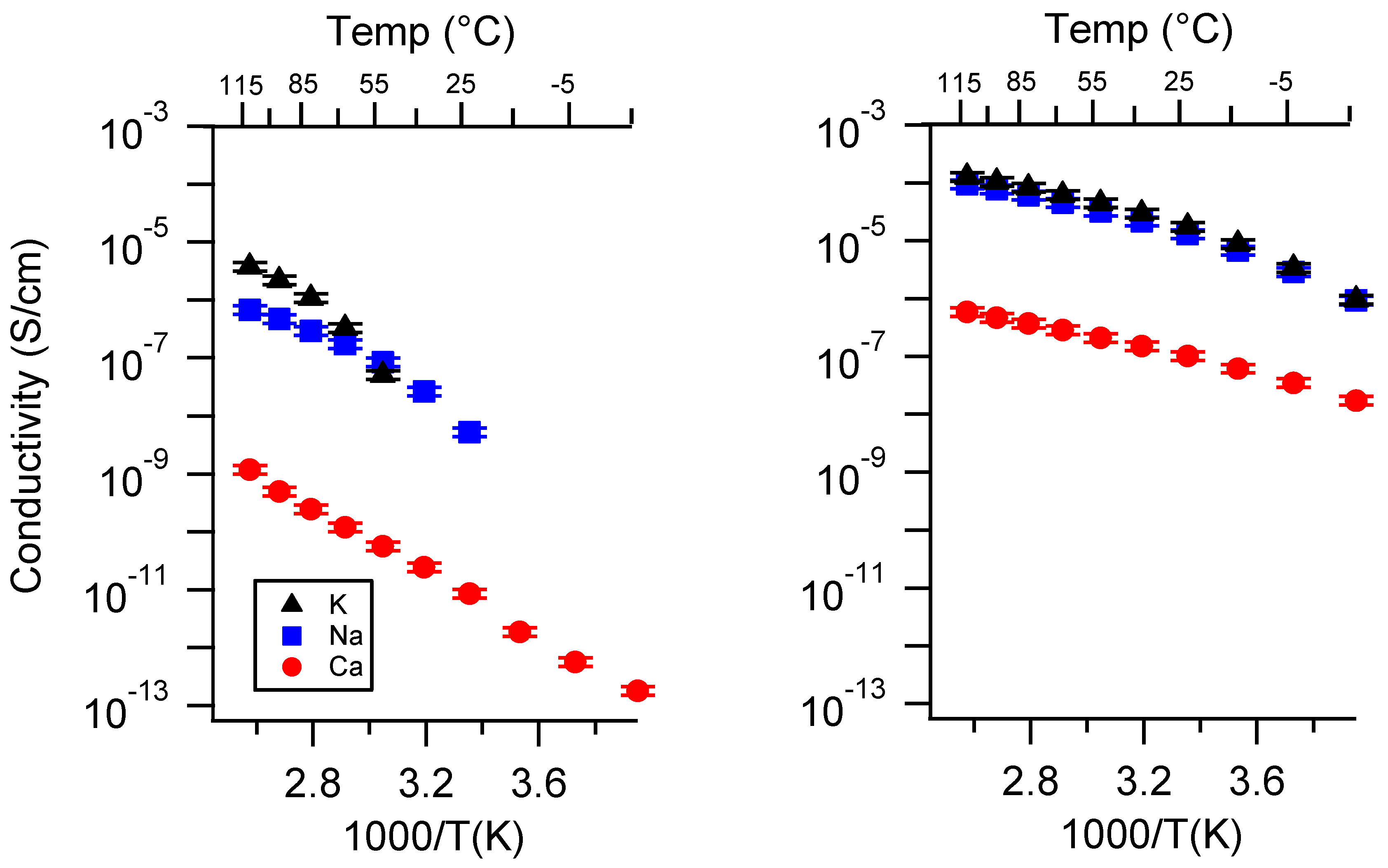
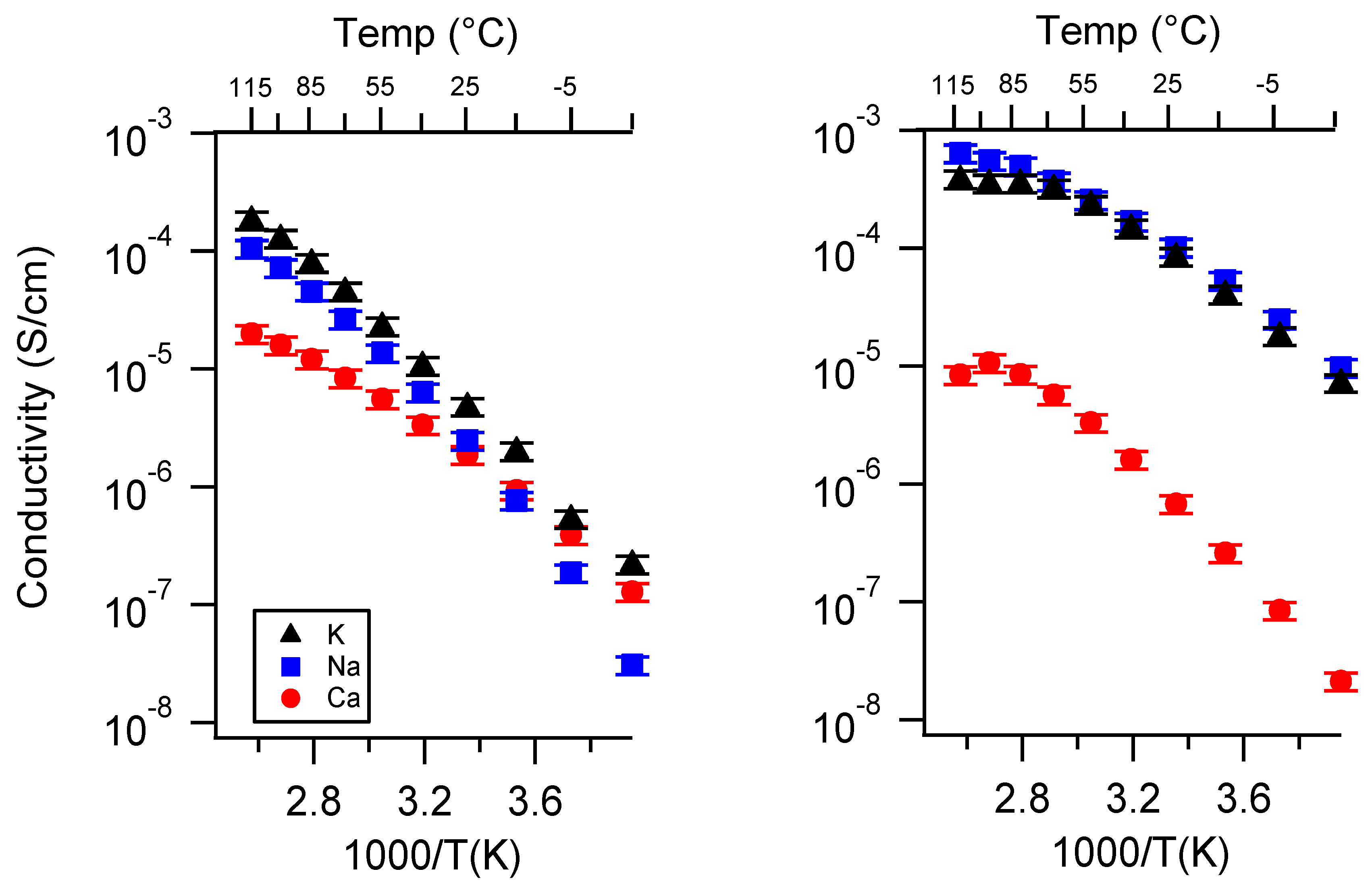
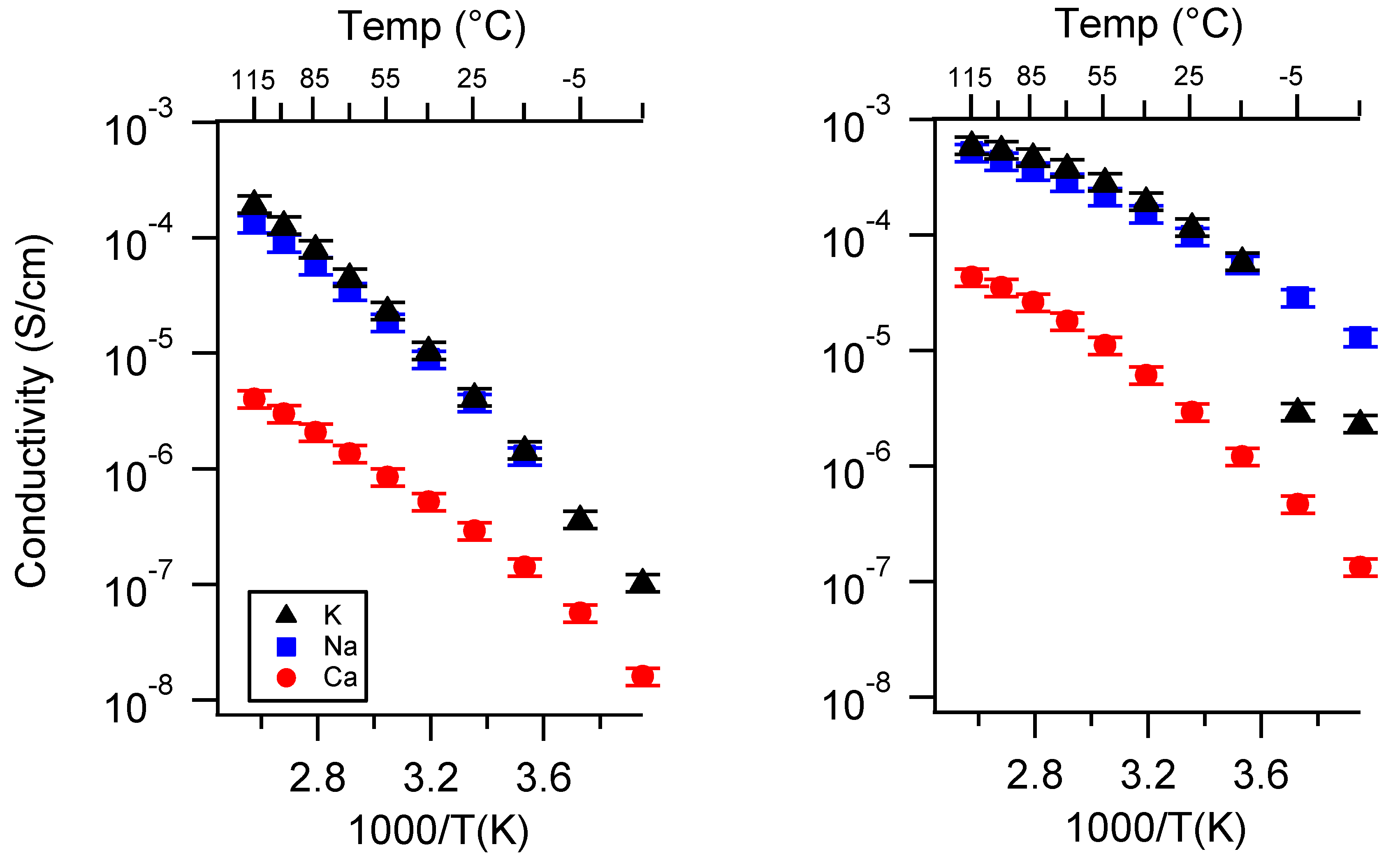
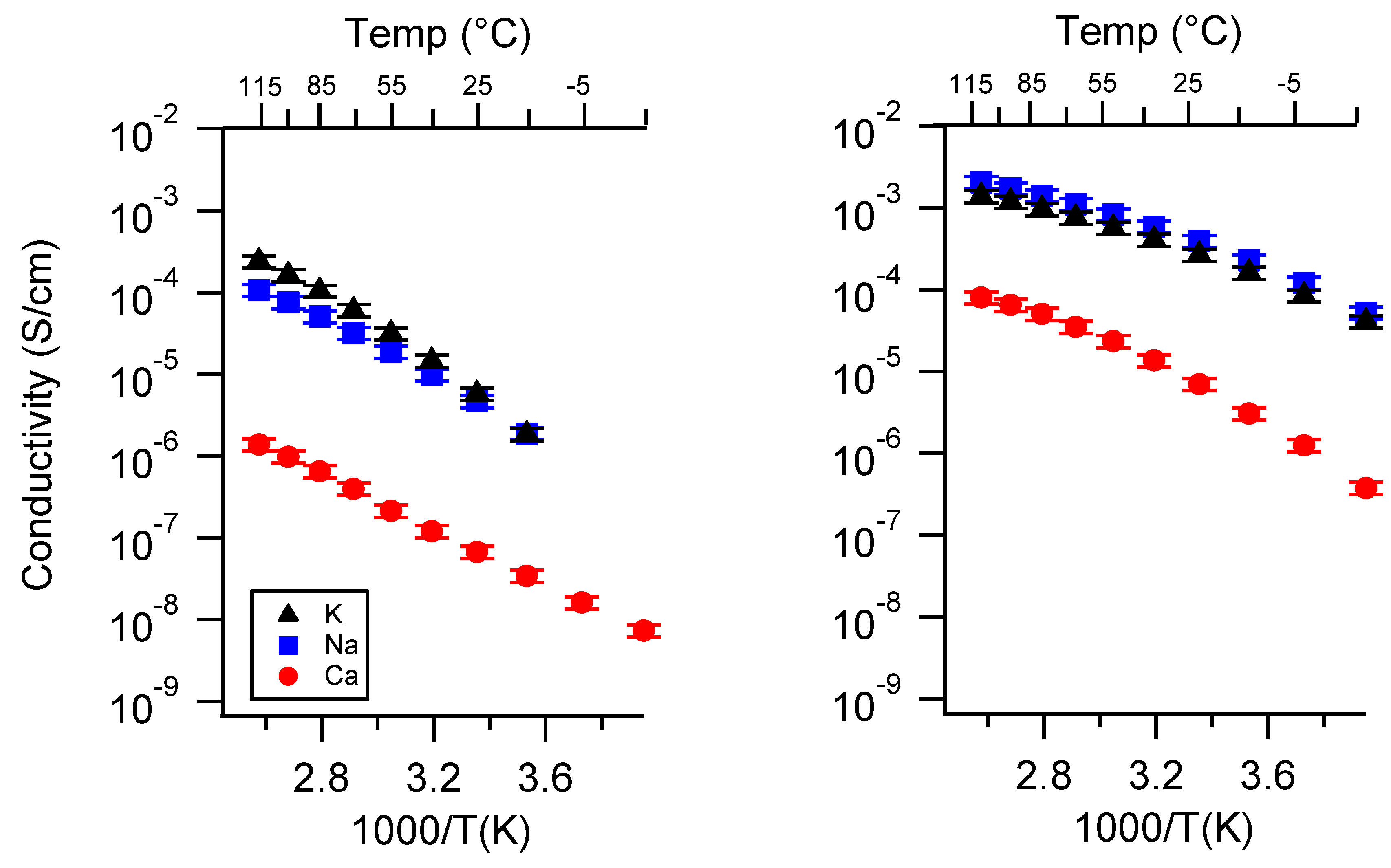
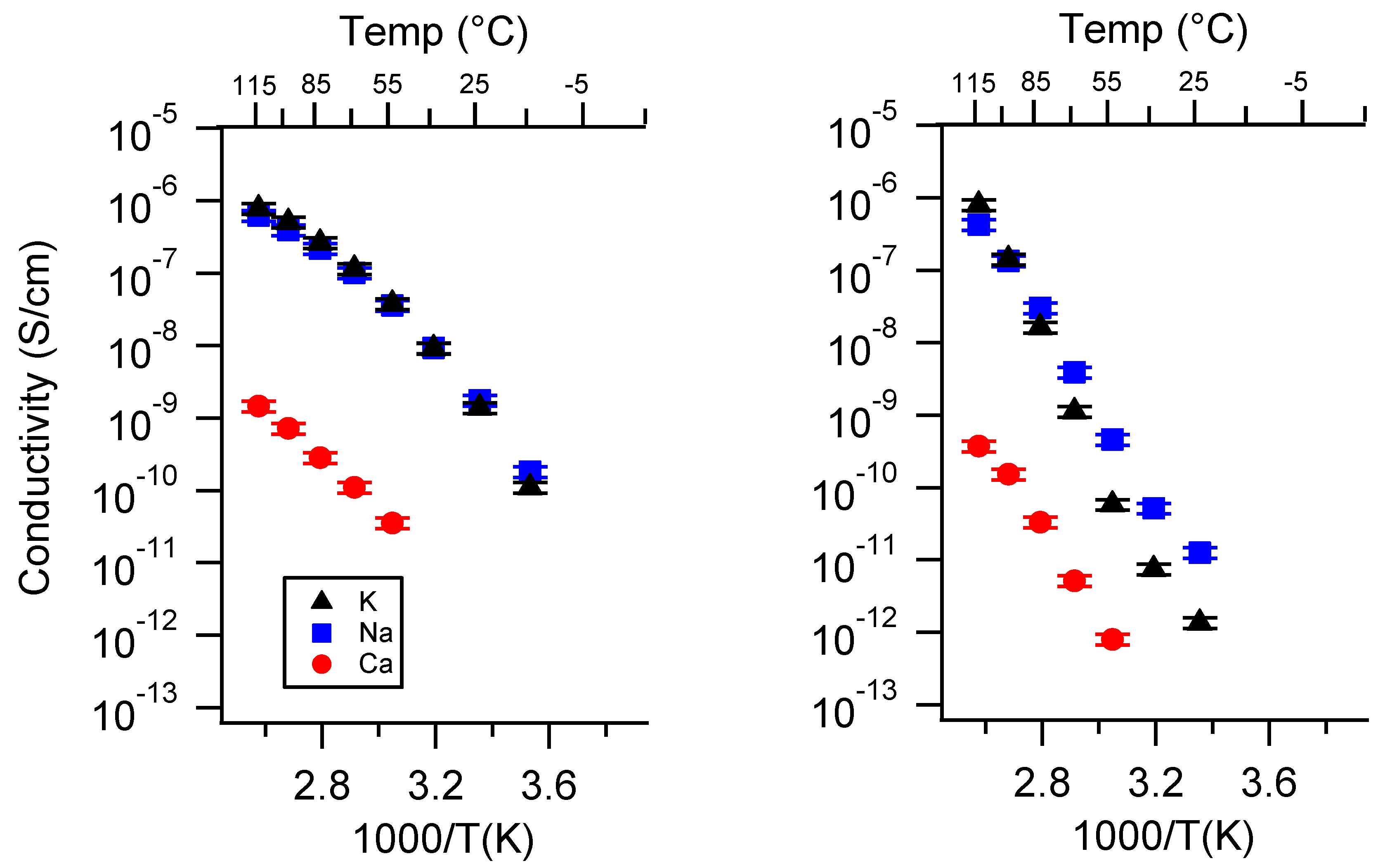
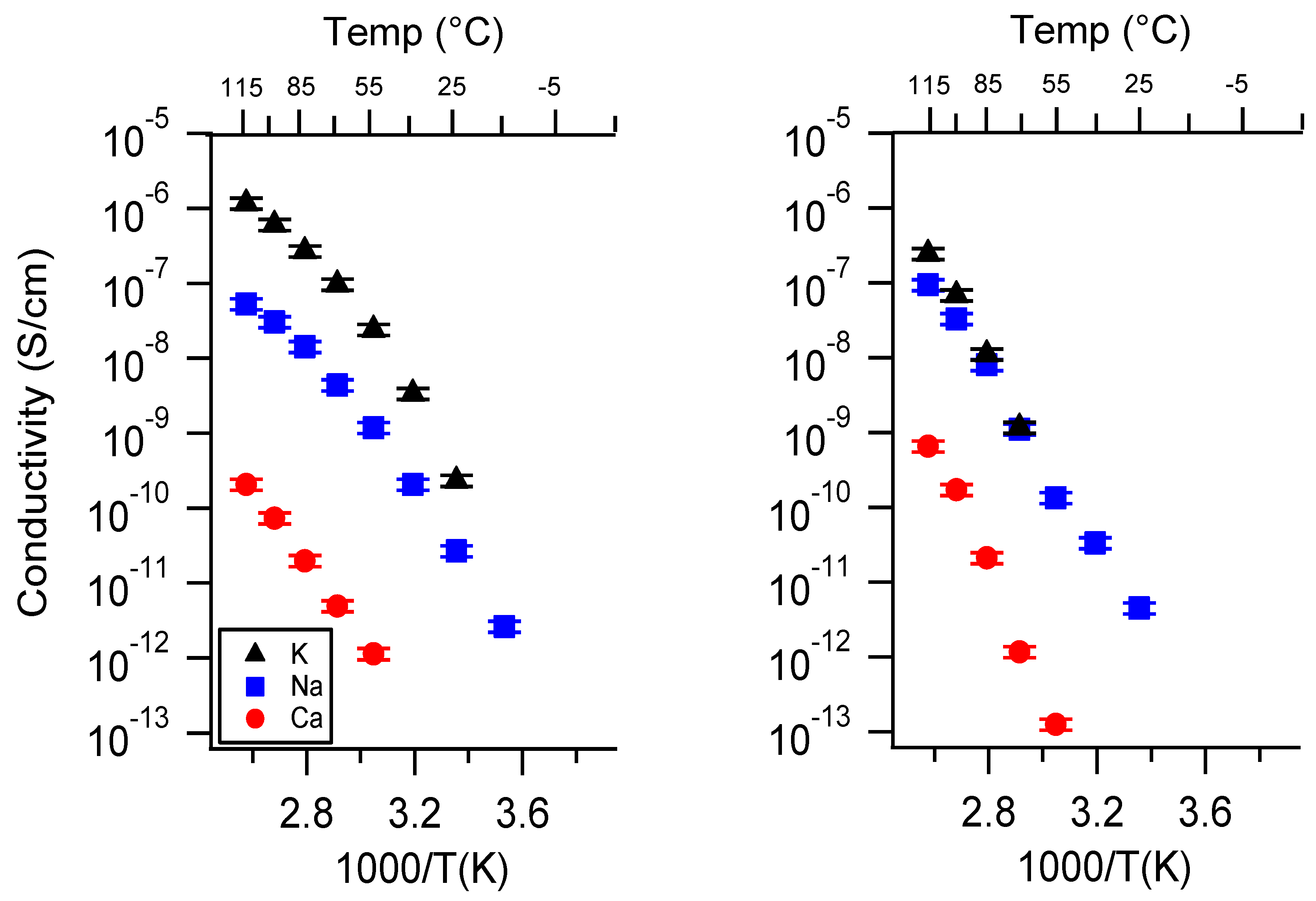
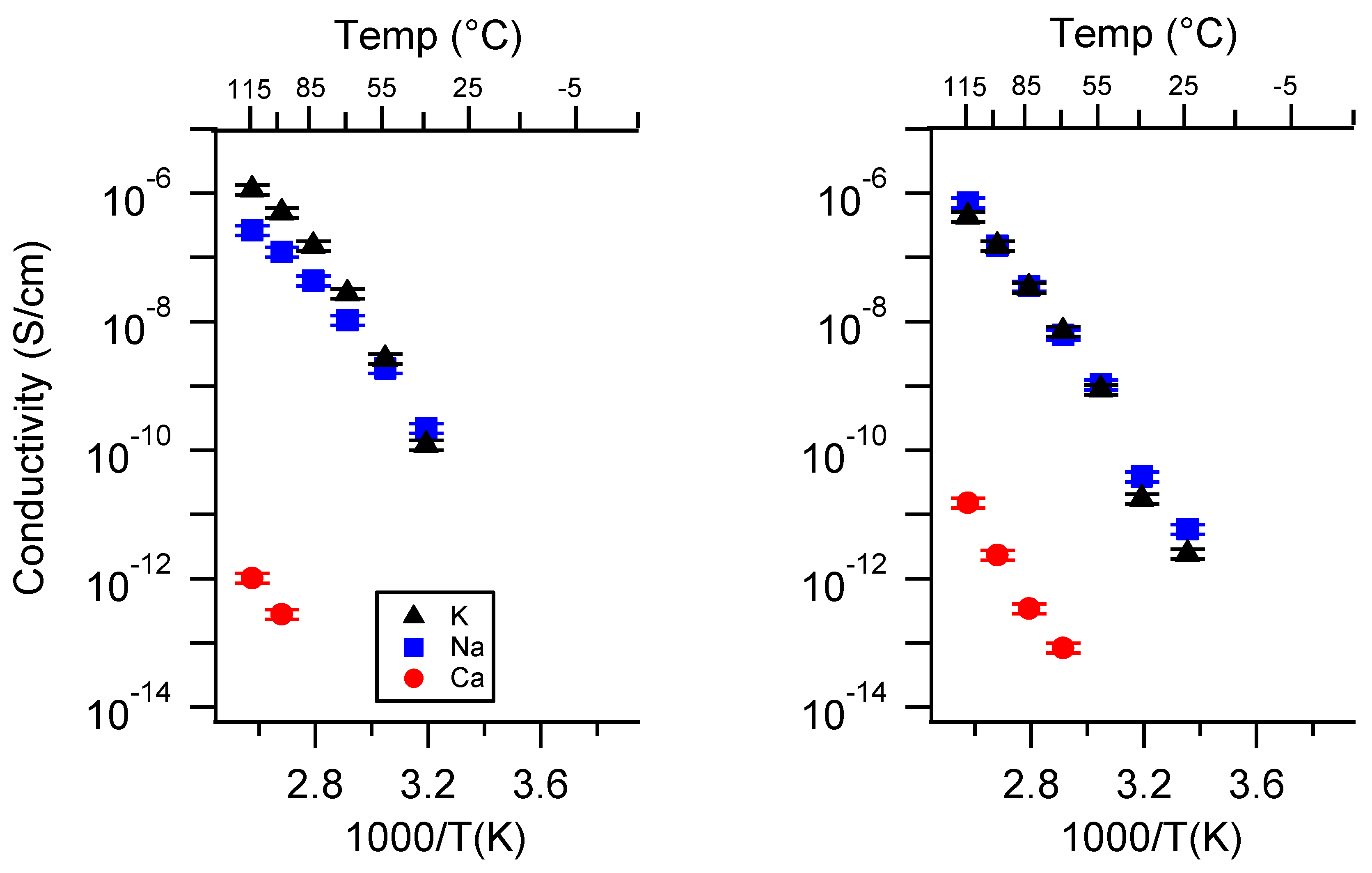
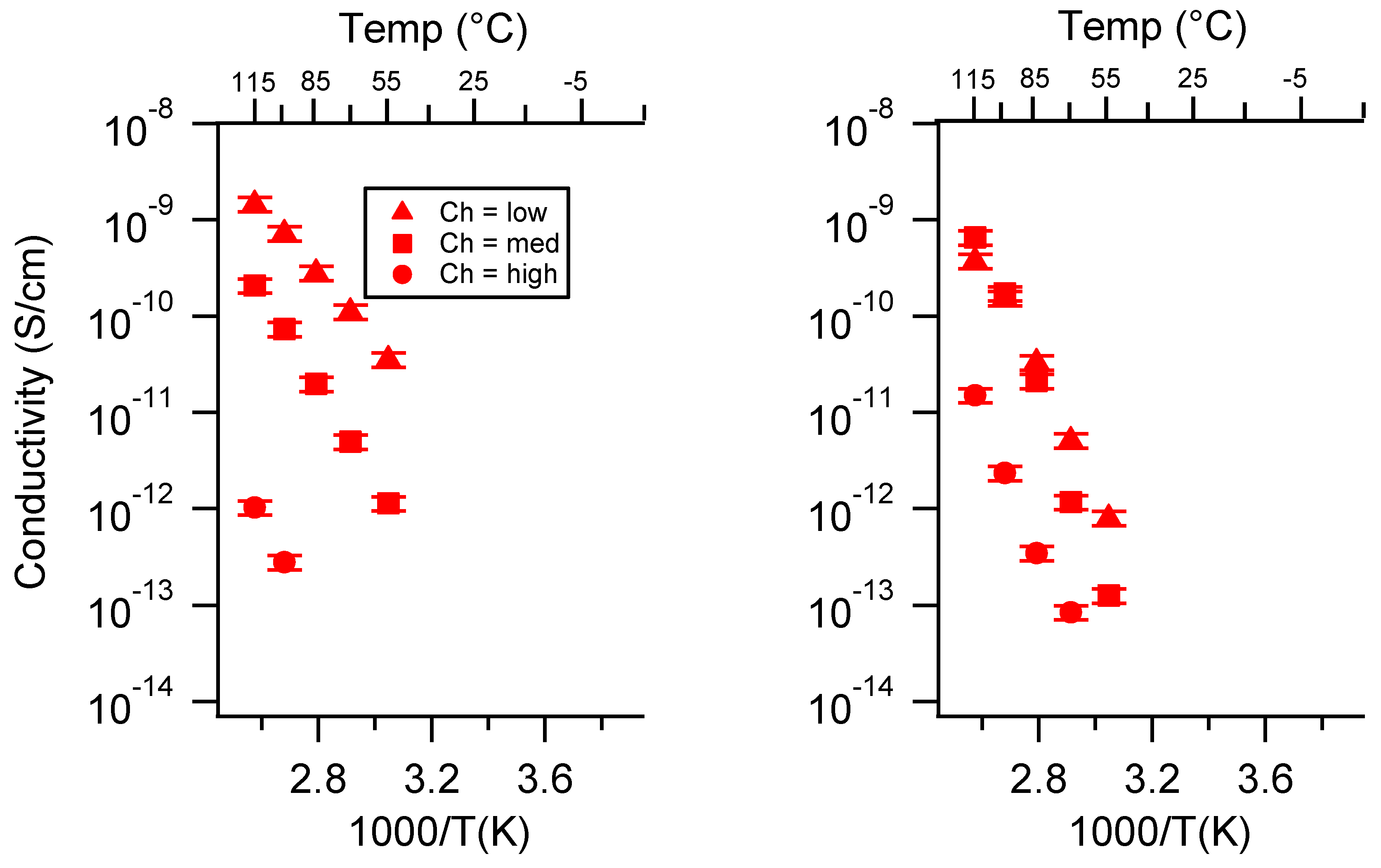
Appendix B. All SIPE Conductivity as a Function of Charge Density
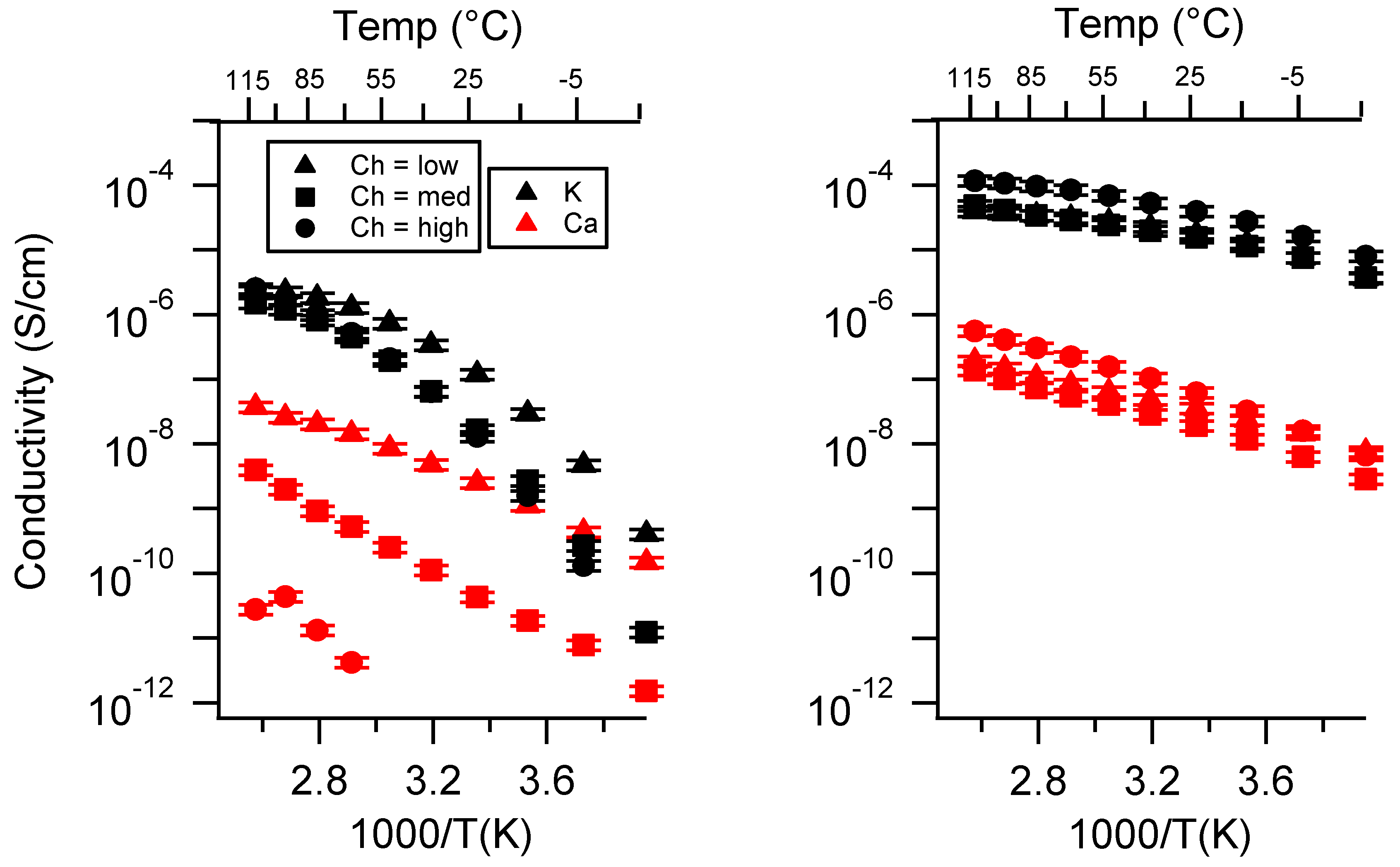
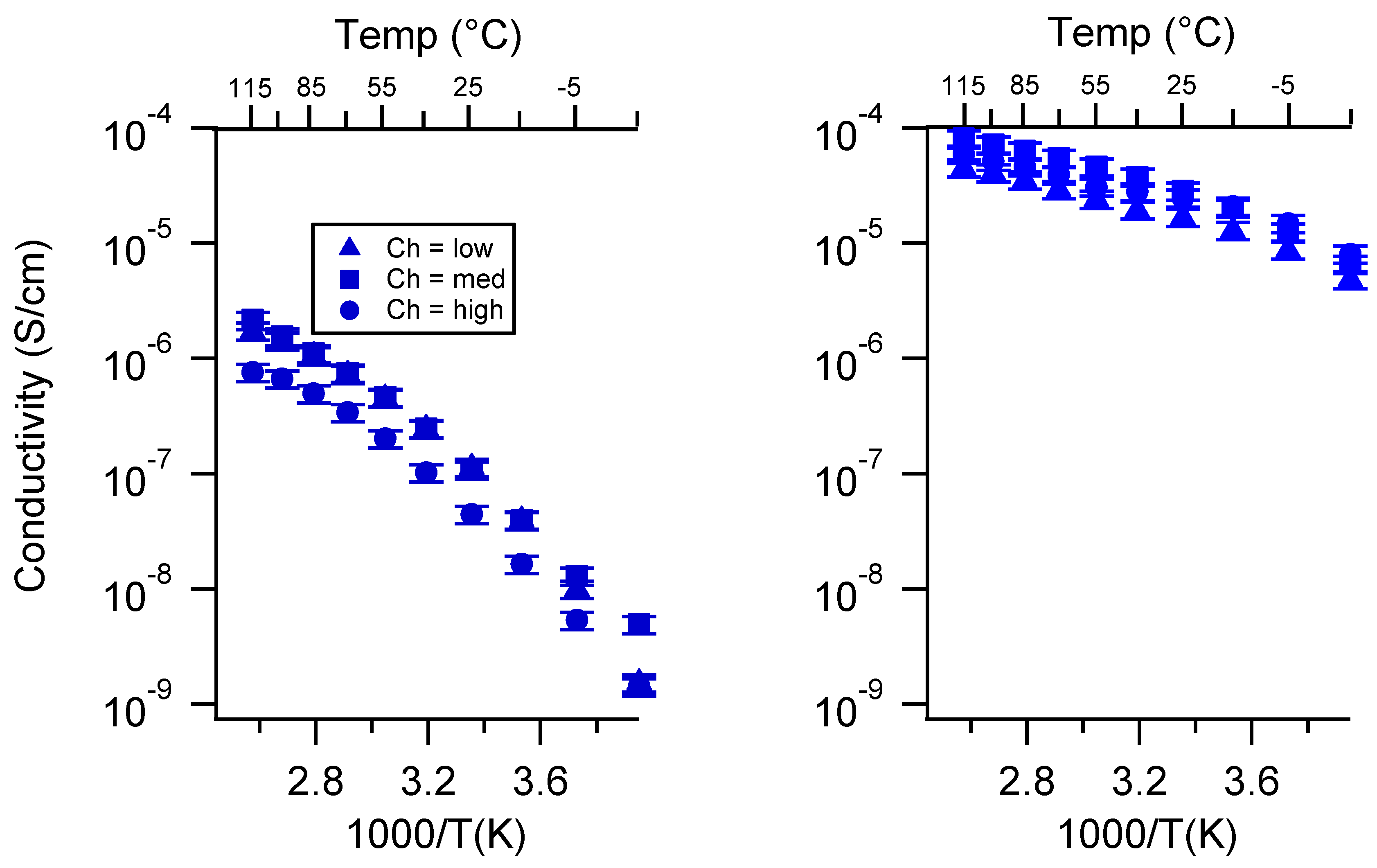

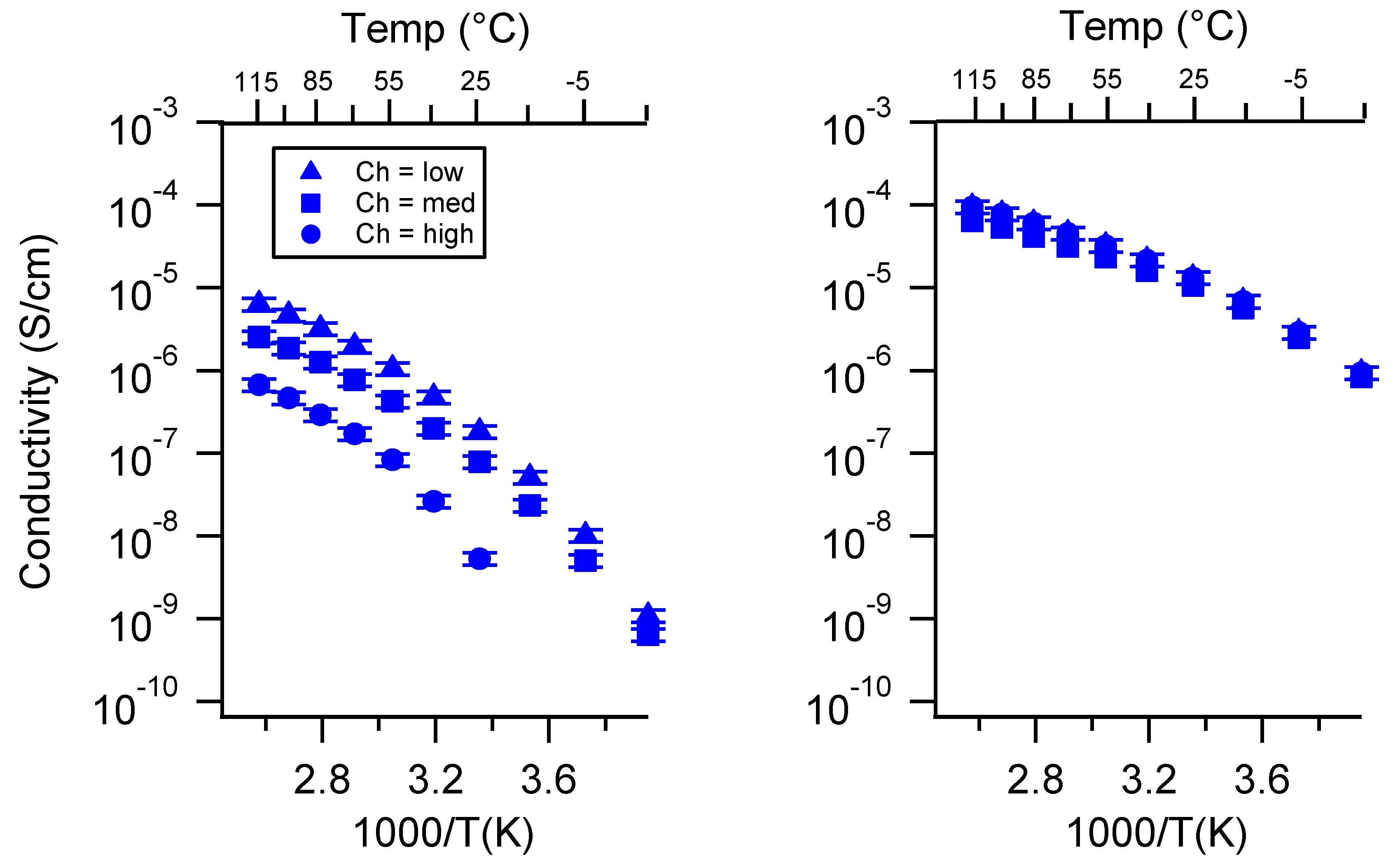

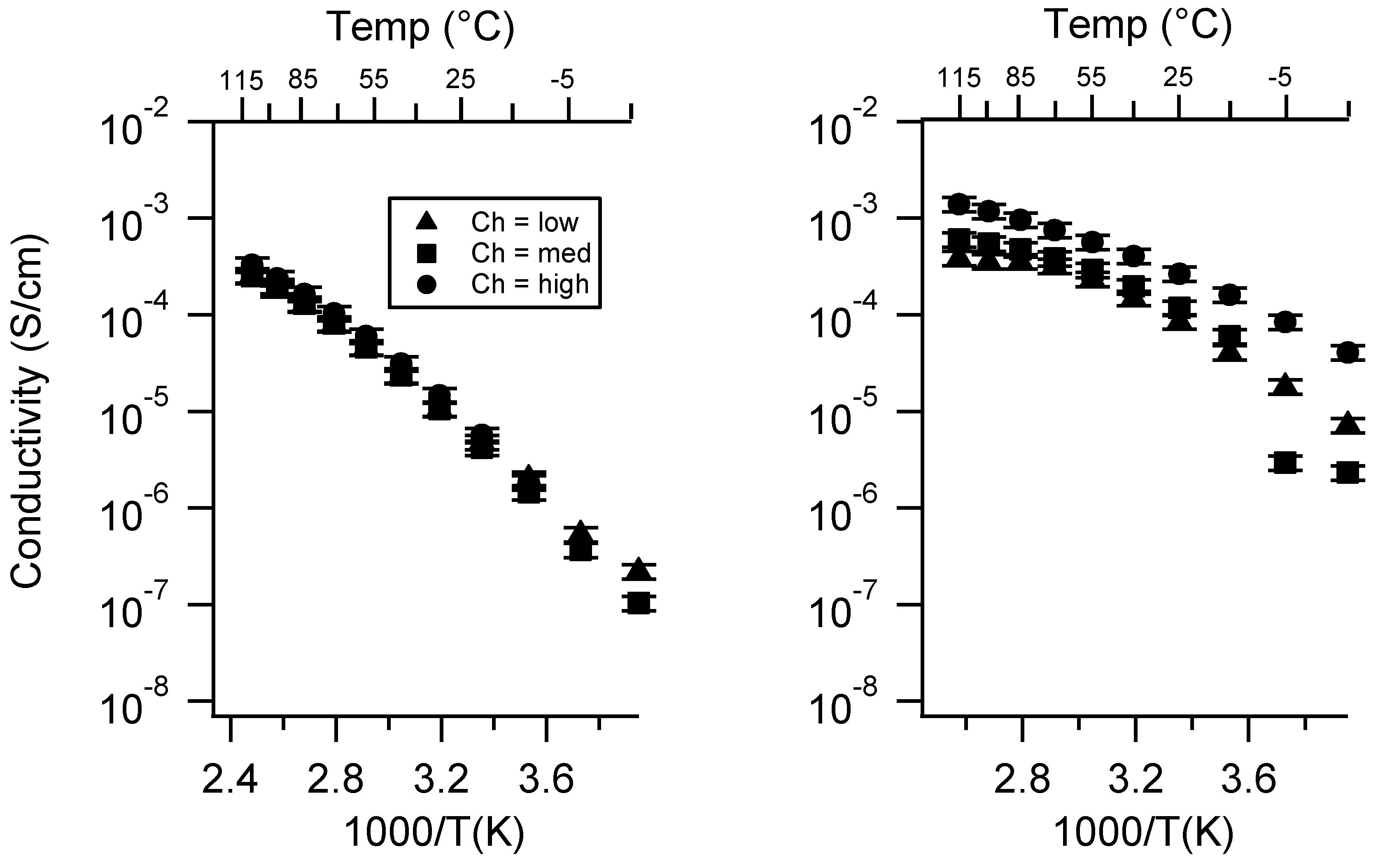
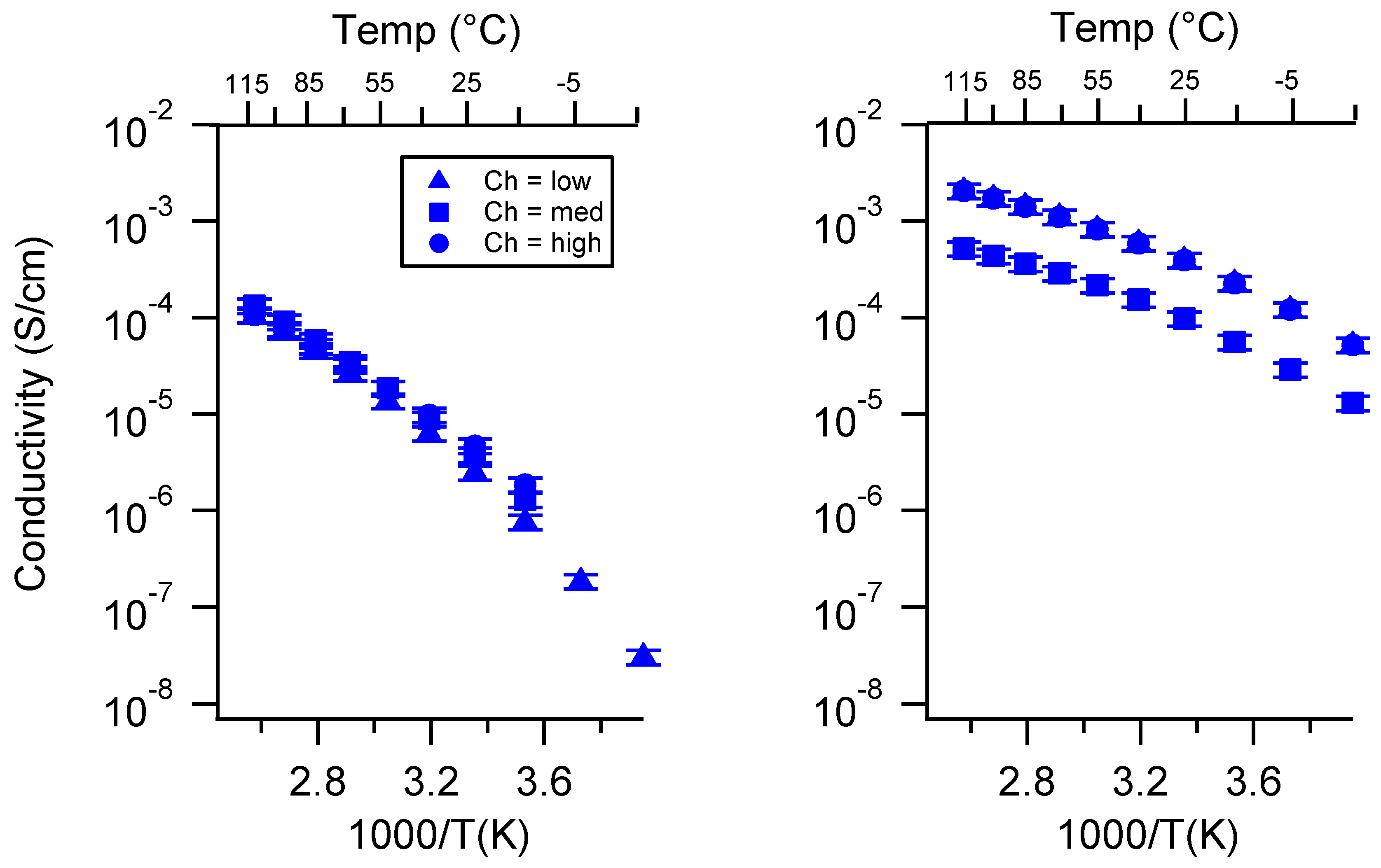
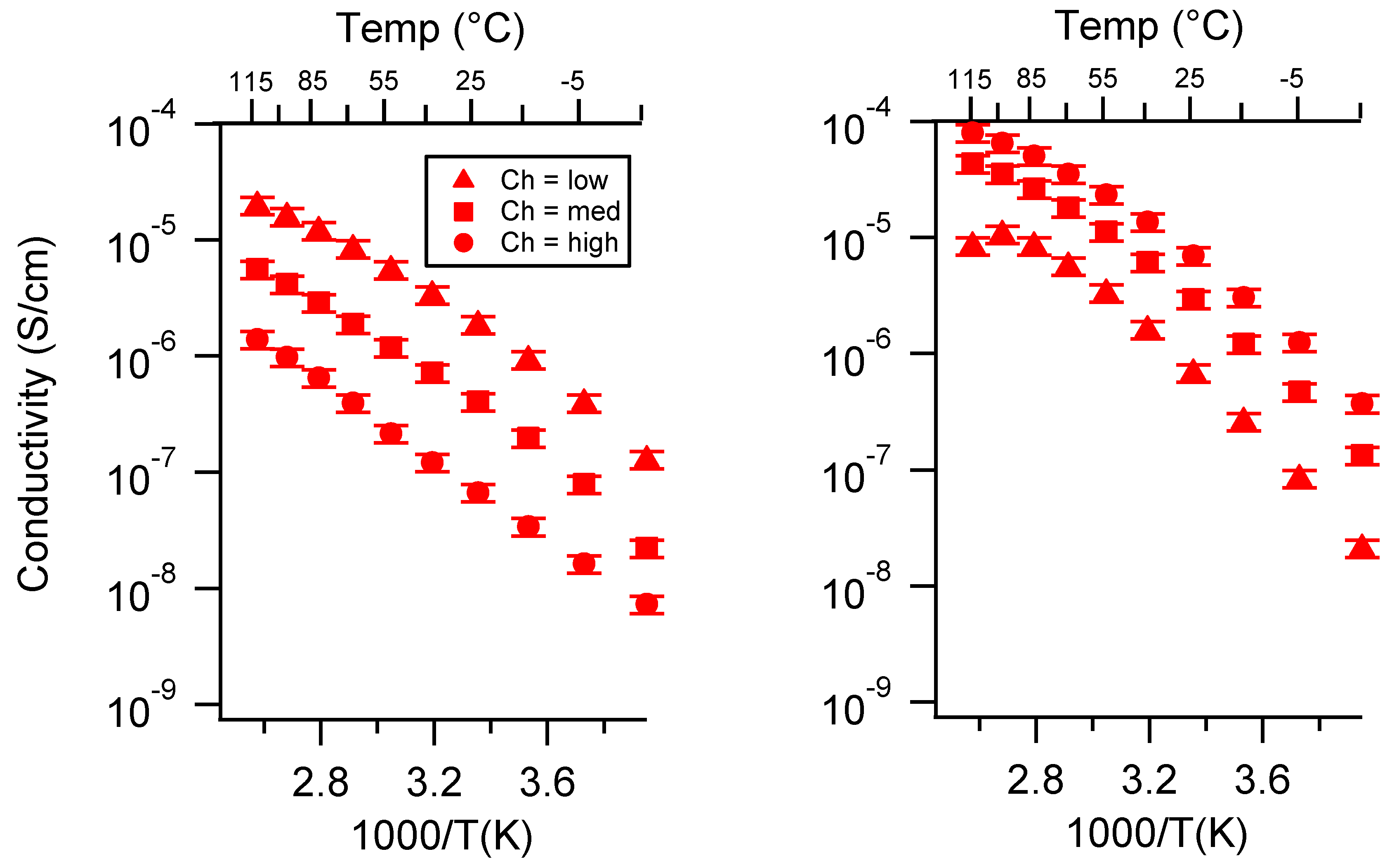
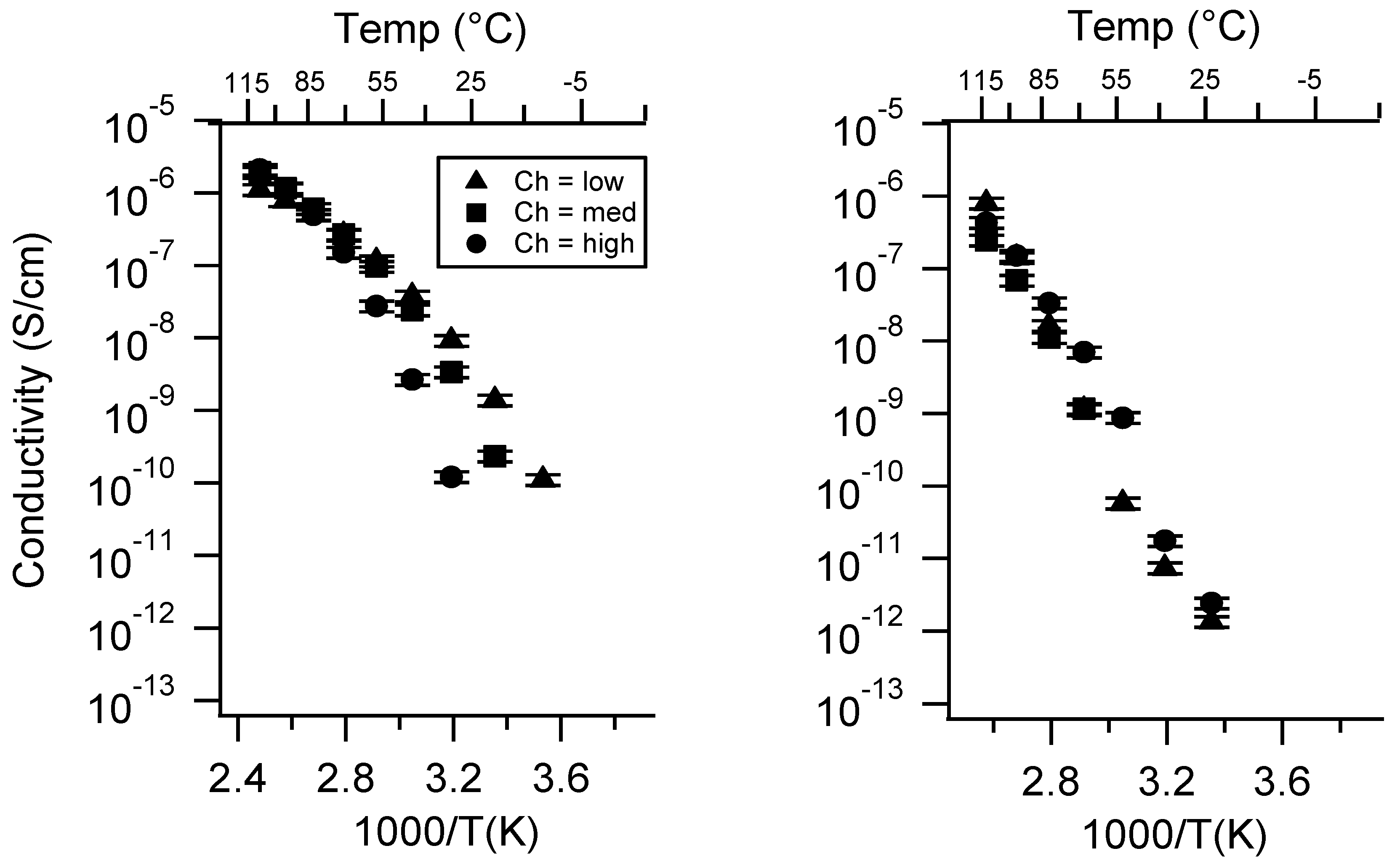
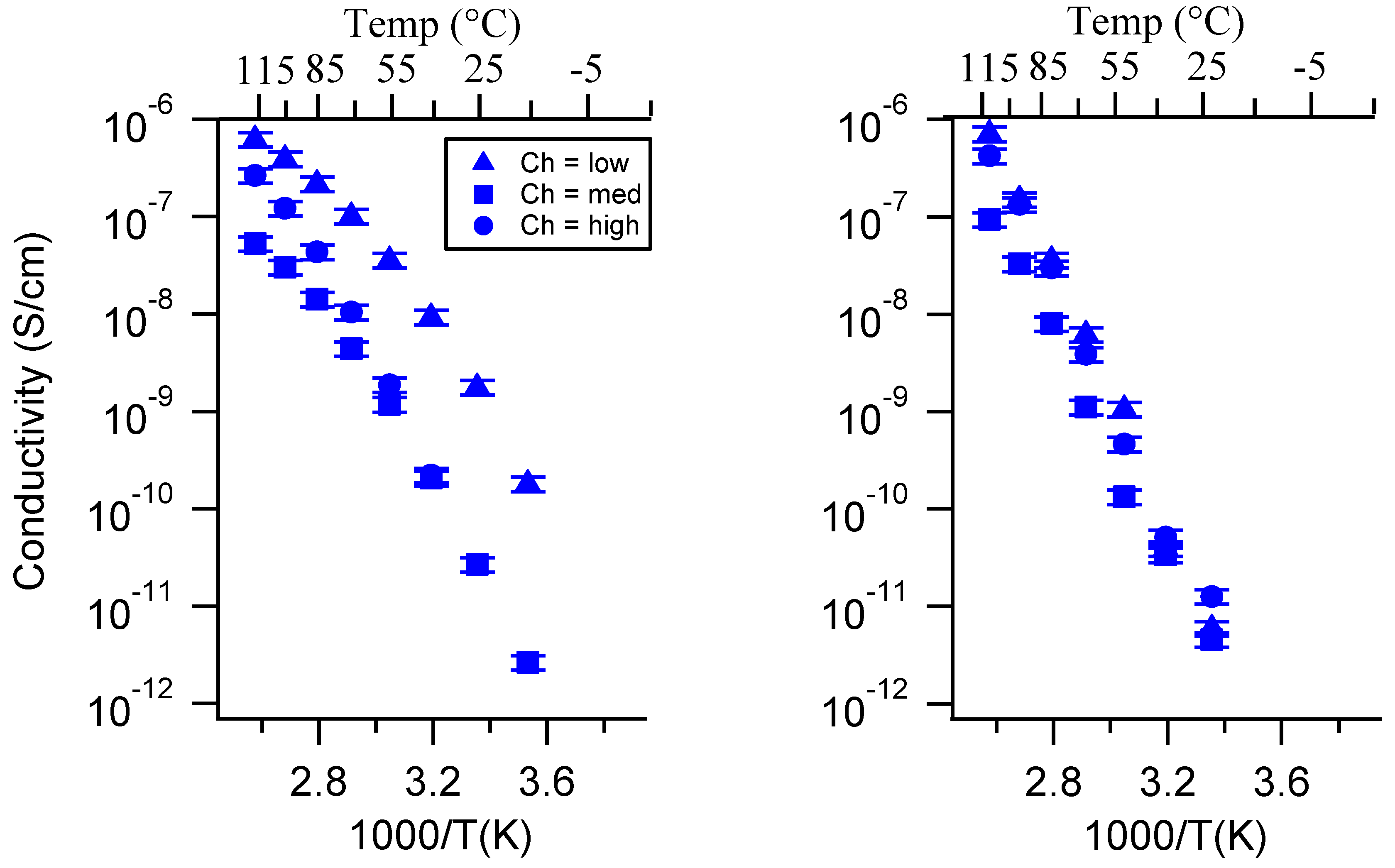
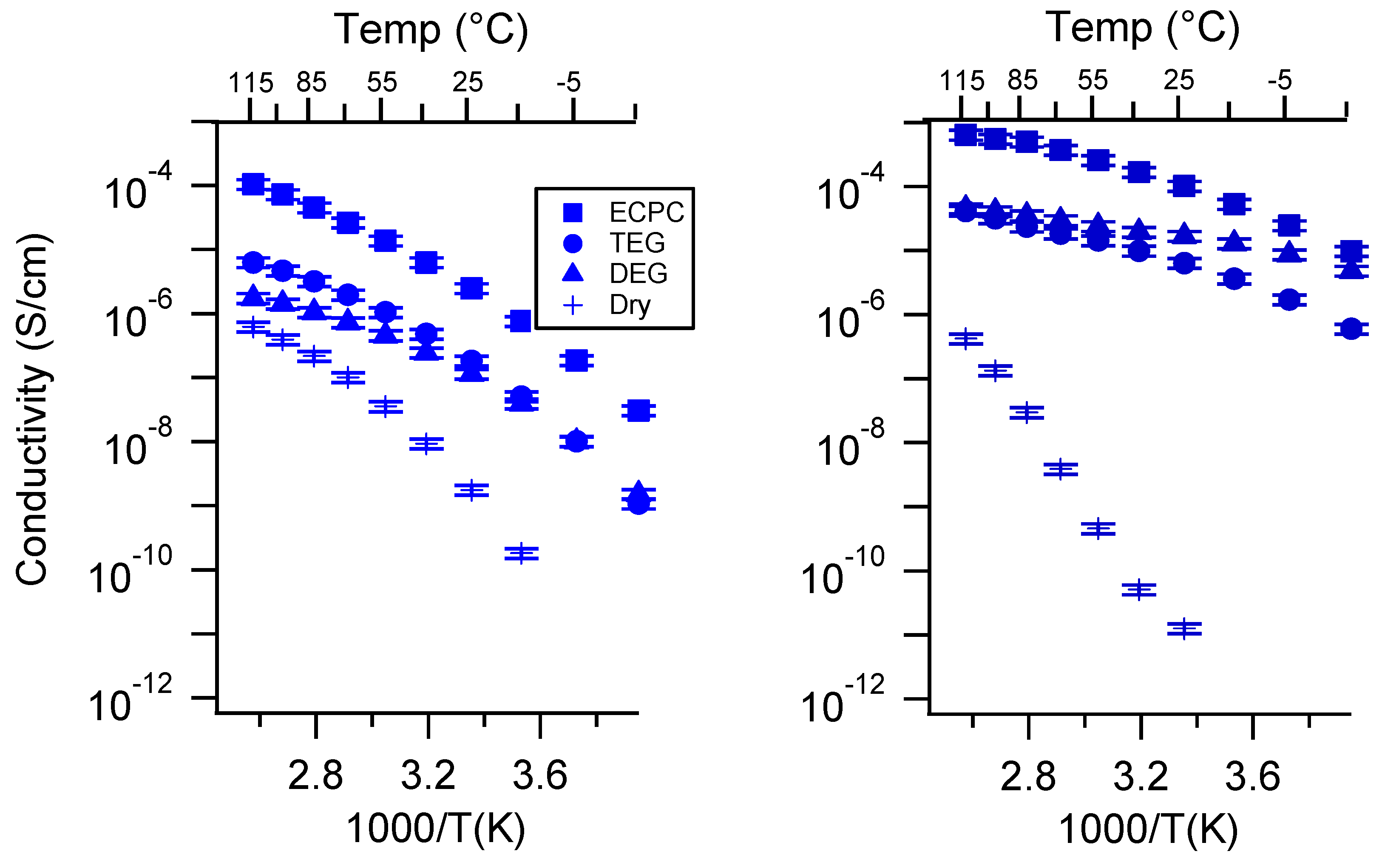
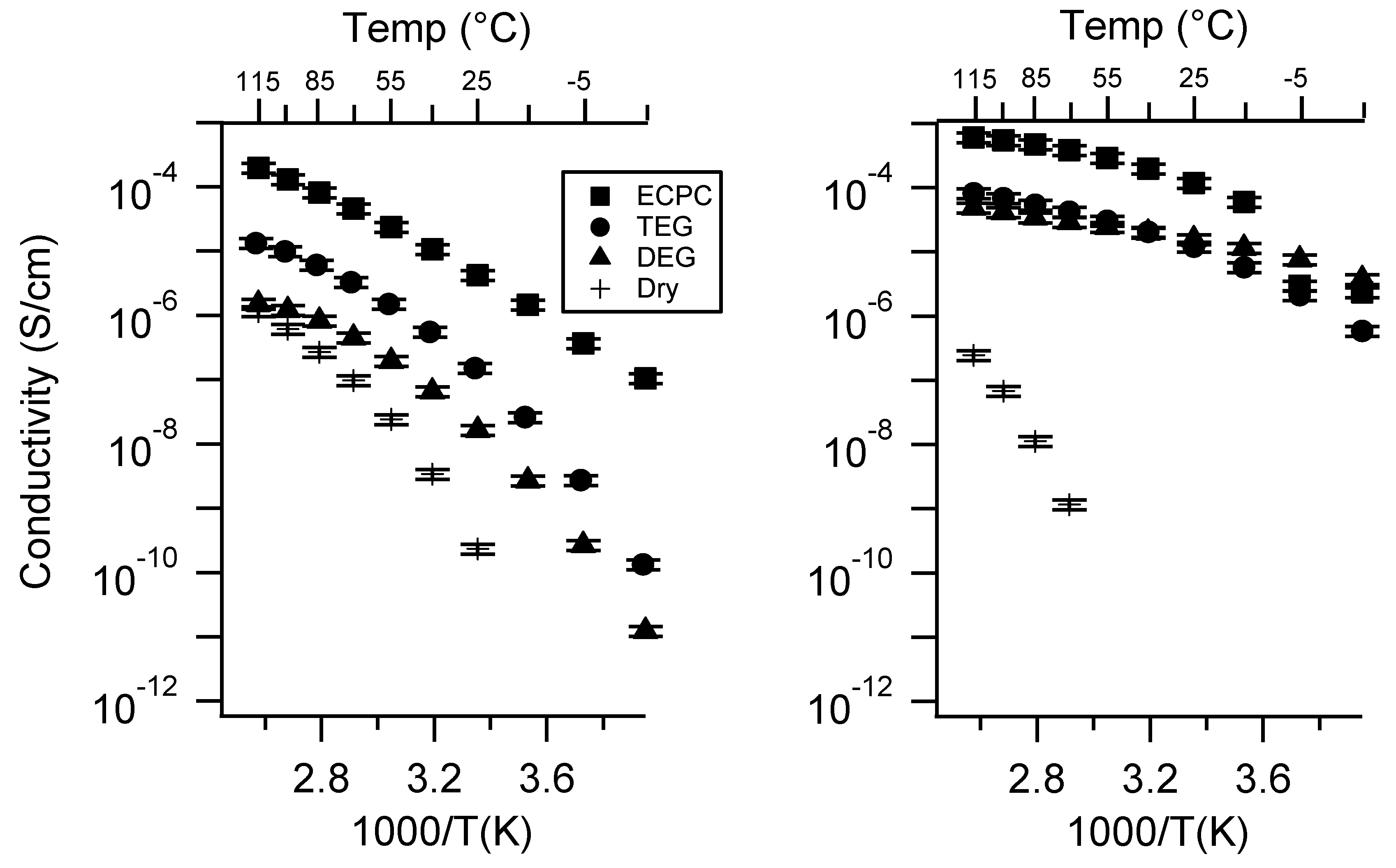
Appendix C. All SIPE Conductivity as a Function of Swelling State
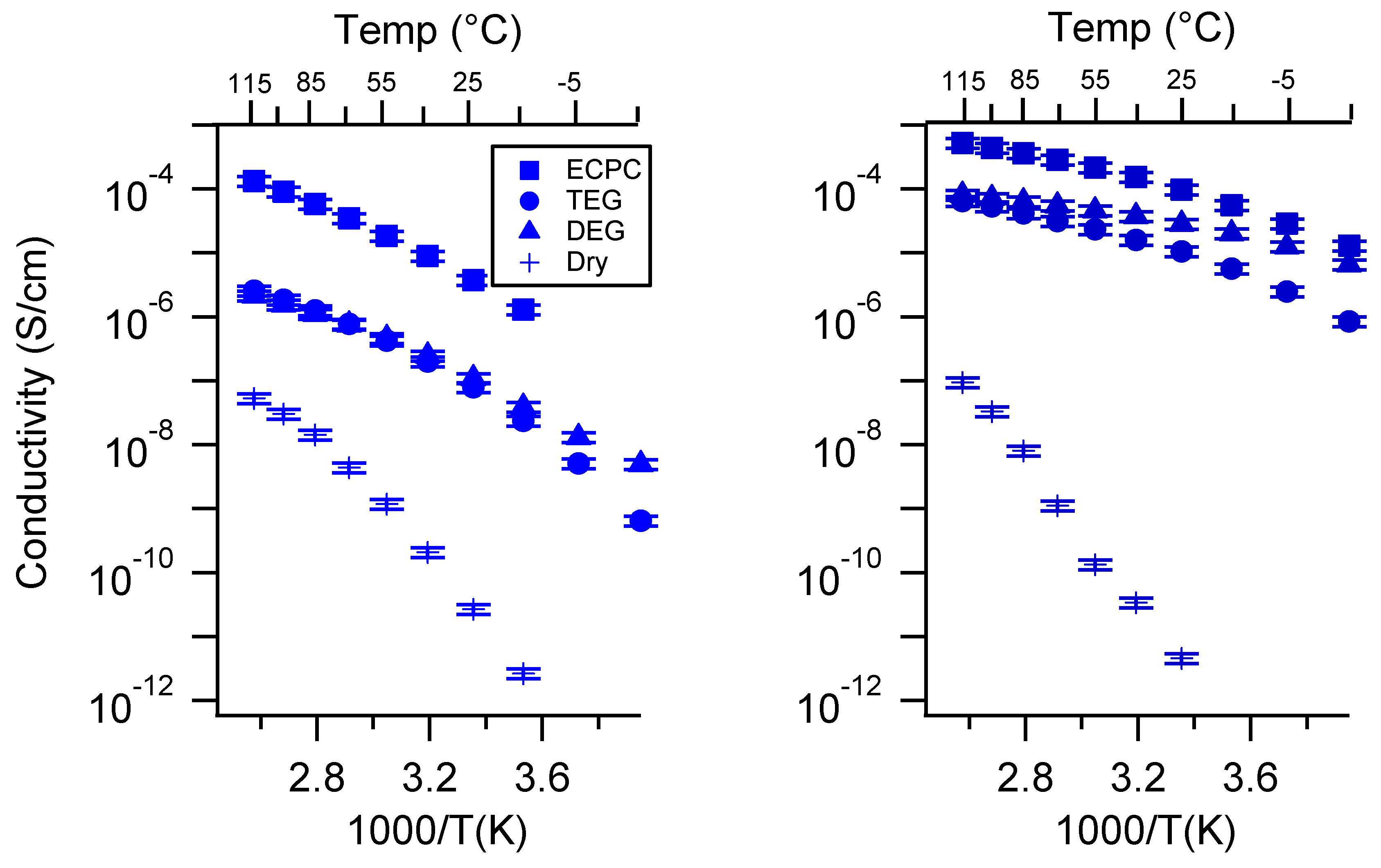
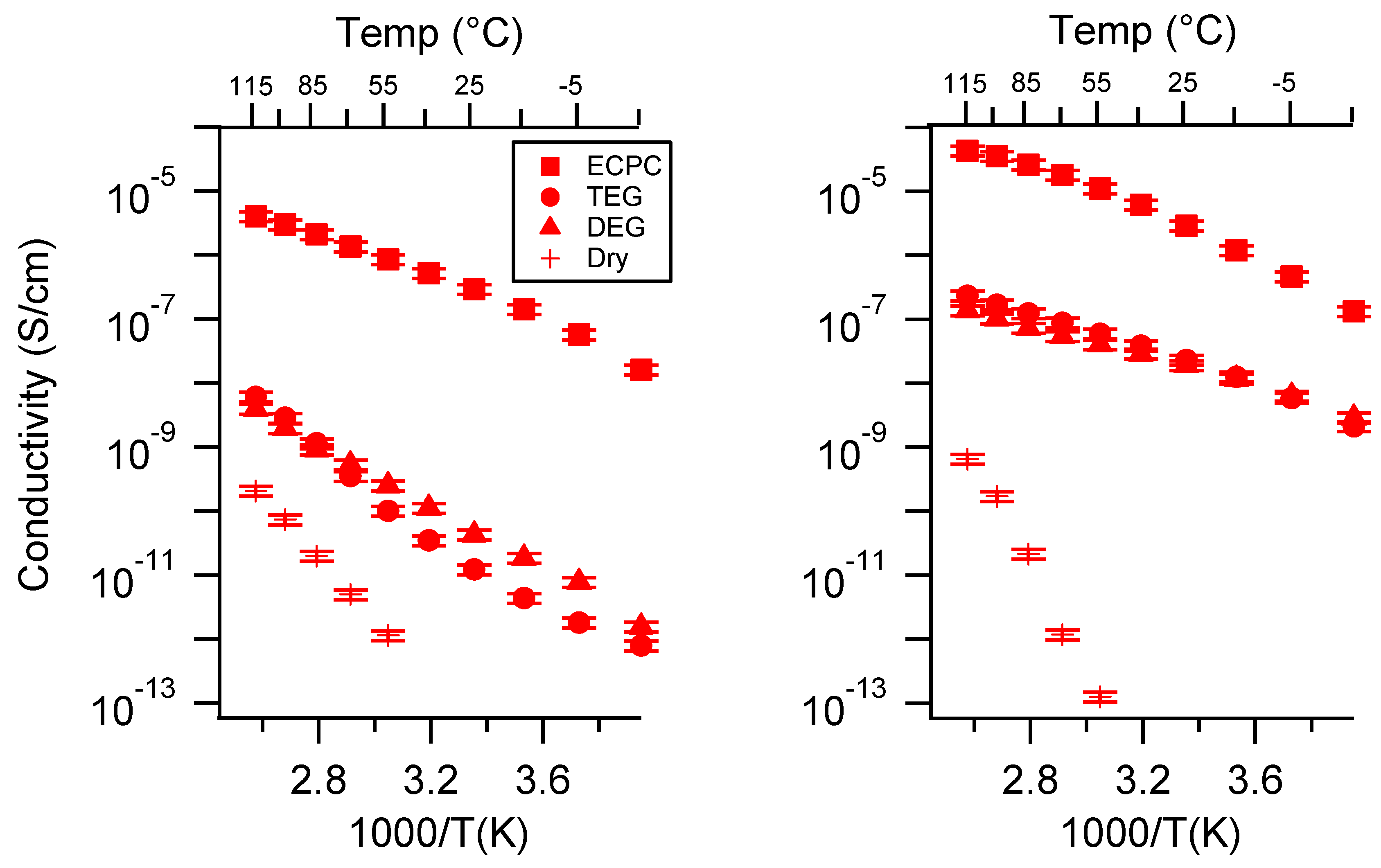
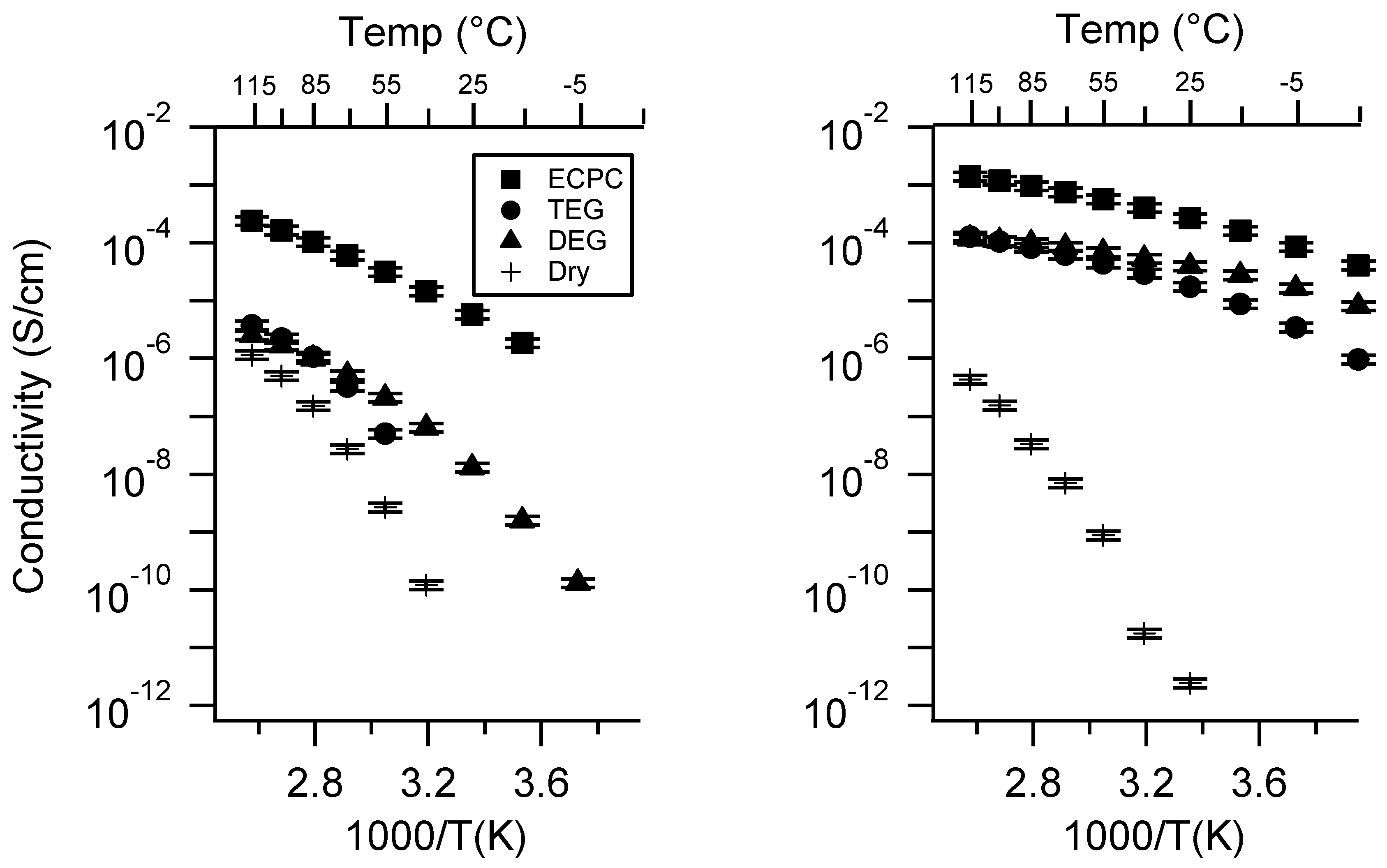
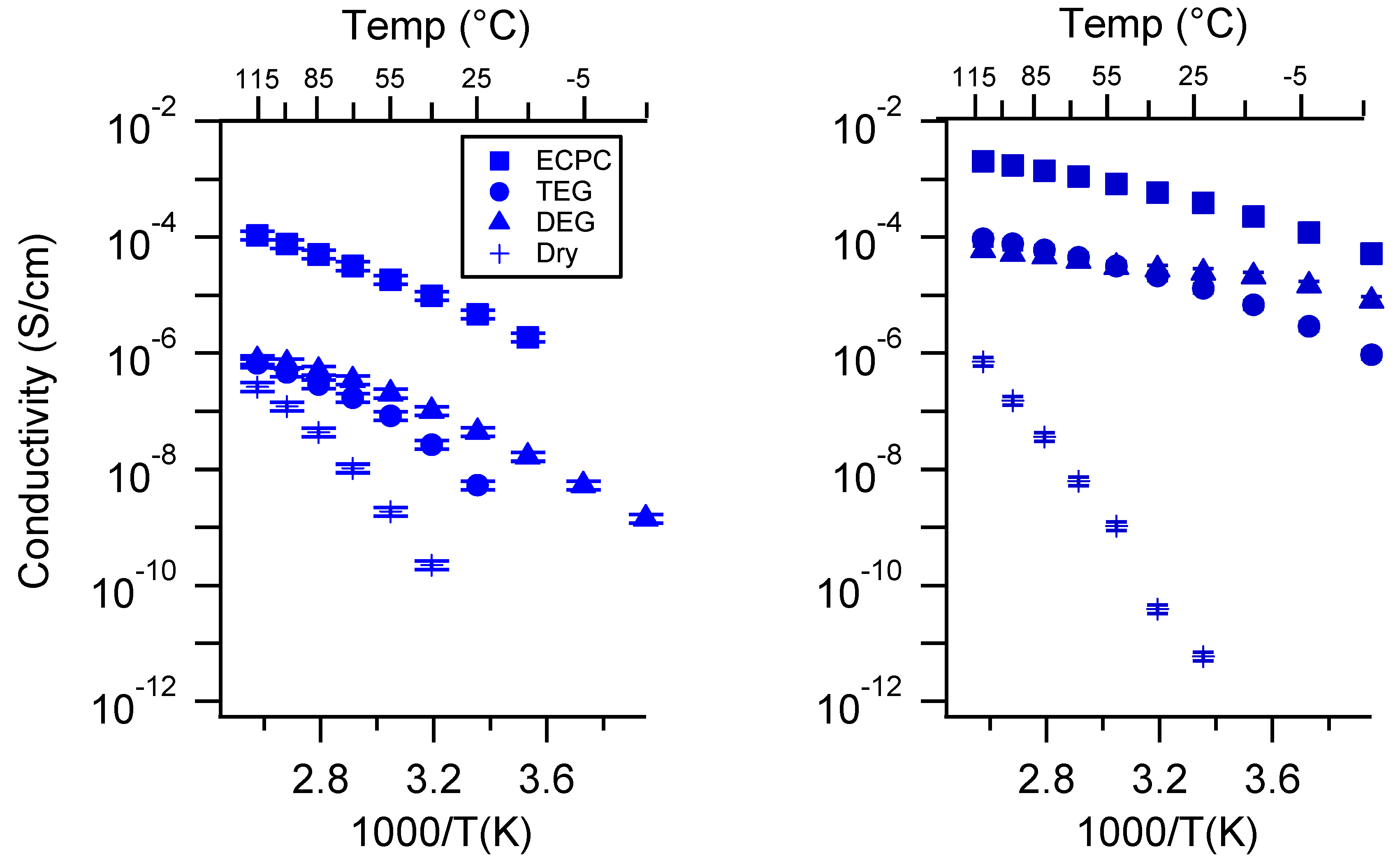

Appendix D. Volume Change with Swelling for all Other SIPEs and Select Molar Conductivity
| Sample | Solvent | Volume Change % | Estimated Cation Concentration (mol Charge/cm3 Polymer) |
|---|---|---|---|
| PEGDMA1000_low_SSK | DEG | 5.8 ± 0.3 | 0.00093 |
| TEG | 34.9 ± 2.0 | 0.00073 | |
| EC:PC | 90.7 ± 5.2 | 0.00052 | |
| PEGDMA1000_med_SSK | DEG | 15.4 ± 0.9 | 0.00127 |
| TEG | 28.1 ± 1.6 | 0.00114 | |
| EC:PC | 92.0 ± 5.2 | 0.00076 | |
| PEGDMA1000_high_SSK | DEG | 12.2 ± 0.7 | 0.00173 |
| TEG | 20.5 ± 1.2 | 0.00162 | |
| EC:PC | 93.4 ± 5.3 | 0.00101 | |
| PEGDMA1000_low_SSNa | DEG | 37.9 ± 2.2 | 0.00071 |
| TEG | 8.3 ± 0.5 | 0.00091 | |
| EC:PC | 109.7 ± 6.3 | 0.00047 | |
| PEGDMA1000_high_SSNa | DEG | 4.6 ± 0.2 | 0.00186 |
| TEG | 6.4 ± 0.4 | 0.00183 | |
| EC:PC | 99.6 ± 5.7 | 0.00098 | |
| PEGDMA1000_low_SSCa | DEG | 0.0 | 0.00049 |
| TEG | 0.0 | 0.00049 | |
| EC:PC | 16.1 ± 0.9 | 0.00042 | |
| PEGDMA1000_med_SSCa | DEG | 0.0 | 0.00073 |
| TEG | 0.0 | 0.00073 | |
| EC:PC | 61.0 ± 3.5 | 0.00045 | |
| PEGDMA1000_high_SSCa | DEG | 0.0 | 0.00097 |
| TEG | 8.3 ± 0.5 | 0.00090 | |
| EC:PC | 20.1 ± 1.1 | 0.00081 | |
| PTHFDA1000_low_STFSIK | DEG | 99.1 ± 5.6 | 0.00042 |
| TEG | 61.8 ± 3.5 | 0.00052 | |
| EC:PC | 38.2 ± 2.2 | 0.00061 | |
| PTHFDA1000_med_STFSIK | DEG | 79.4 ± 4.5 | 0.00069 |
| TEG | 50.5 ± 2.9 | 0.00082 | |
| EC:PC | 27.0 ± 1.5 | 0.00097 | |
| PTHFDA1000_high_STFSIK | DEG | 86.5 ± 4.9 | 0.00084 |
| TEG | 86.5 ± 4.9 | 0.00084 | |
| EC:PC | 111.7 ± 6.4 | 0.00074 | |
| PTHFDA1000_low_STFSINa | DEG | 133.1 ± 7.6 | 0.00036 |
| TEG | 99.1 ± 5.6 | 0.00042 | |
| EC:PC | 65.1 ± 3.7 | 0.00051 | |
| PTHFDA1000_high_STFSINa | DEG | 109.7 ± 6.3 | 0.00075 |
| TEG | 49.7 ± 2.8 | 0.00105 | |
| EC:PC | 165.1 ± 9.4 | 0.00059 | |
| PTHFDA1000_low_STFSICa | DEG | 145.5 ± 8.3 | 0.00017 |
| TEG | 141.1 ± 8.0 | 0.00017 | |
| EC:PC | 30.6 ± 1.7 | 0.00032 | |
| PTHFDA1000_med_STFSICa | DEG | 107.8 ± 6.1 | 0.00030 |
| TEG | 107.8 ± 6.1 | 0.00030 | |
| EC:PC | 48.8 ± 2.8 | 0.00042 | |
| PTHFDA1000_high_STFSICa | DEG | 164.5 ± 9.4 | 0.00030 |
| TEG | 117.7 ± 6.7 | 0.00036 | |
| EC:PC | 108.7 ± 6.2 | 0.00038 |
Appendix E. Electrochemical Stability Window of SIPEs

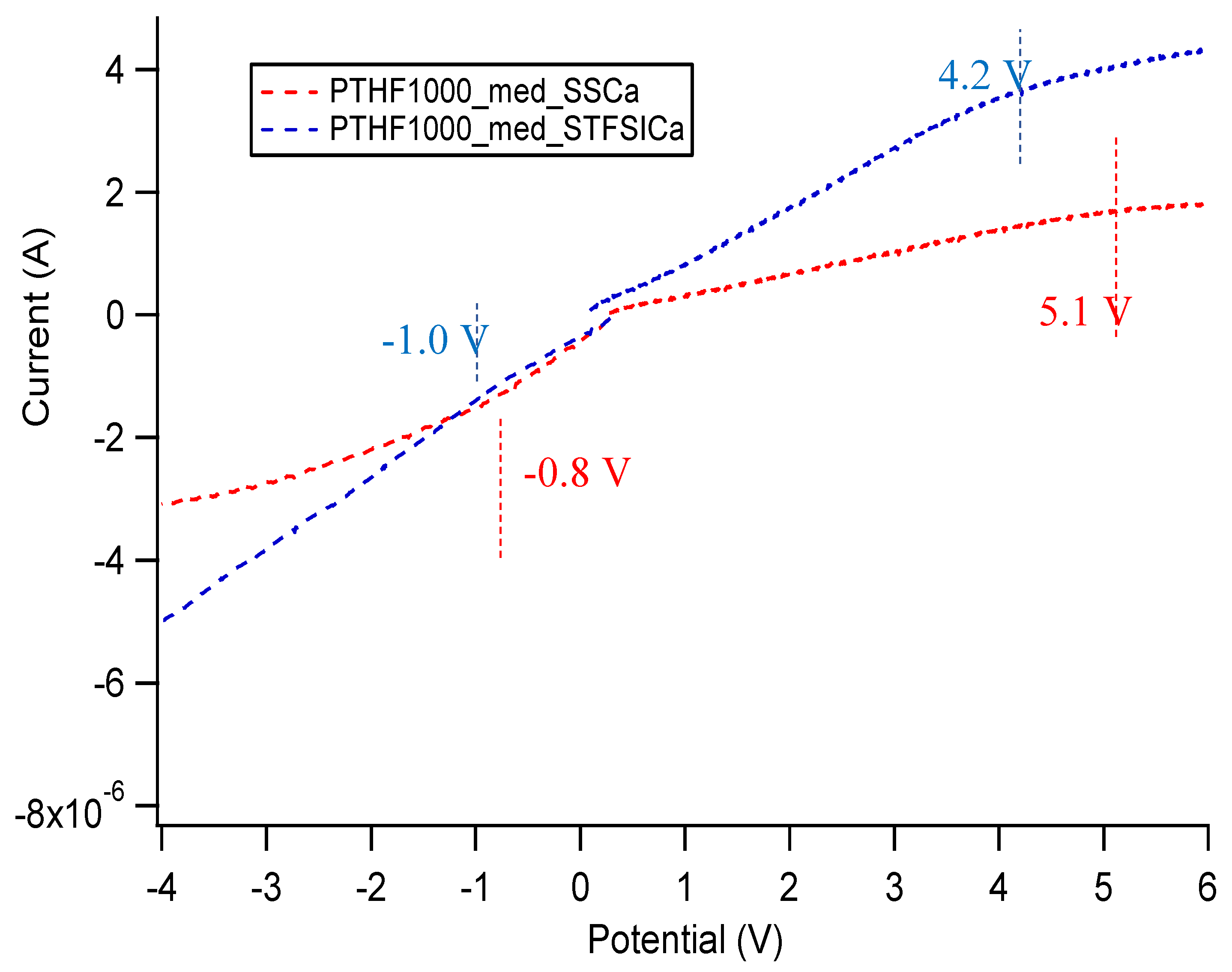

| Sample | Approximate Stability Window (V) |
|---|---|
| PTHF1000_med_SSCa DEG | 5.2 |
| PTHF1000_med_STFSICa DEG | 5.9 |
| PTHF1000_med_SSNa DEG | 5.5 |
| PTHF1000_med_STFSINa DEG | 3.6 |
| PTHF1000_med_SSNa EC/PC | 4.6 |
| PTHF1000_med_STFSINa EC/PC | 4.6 |
References
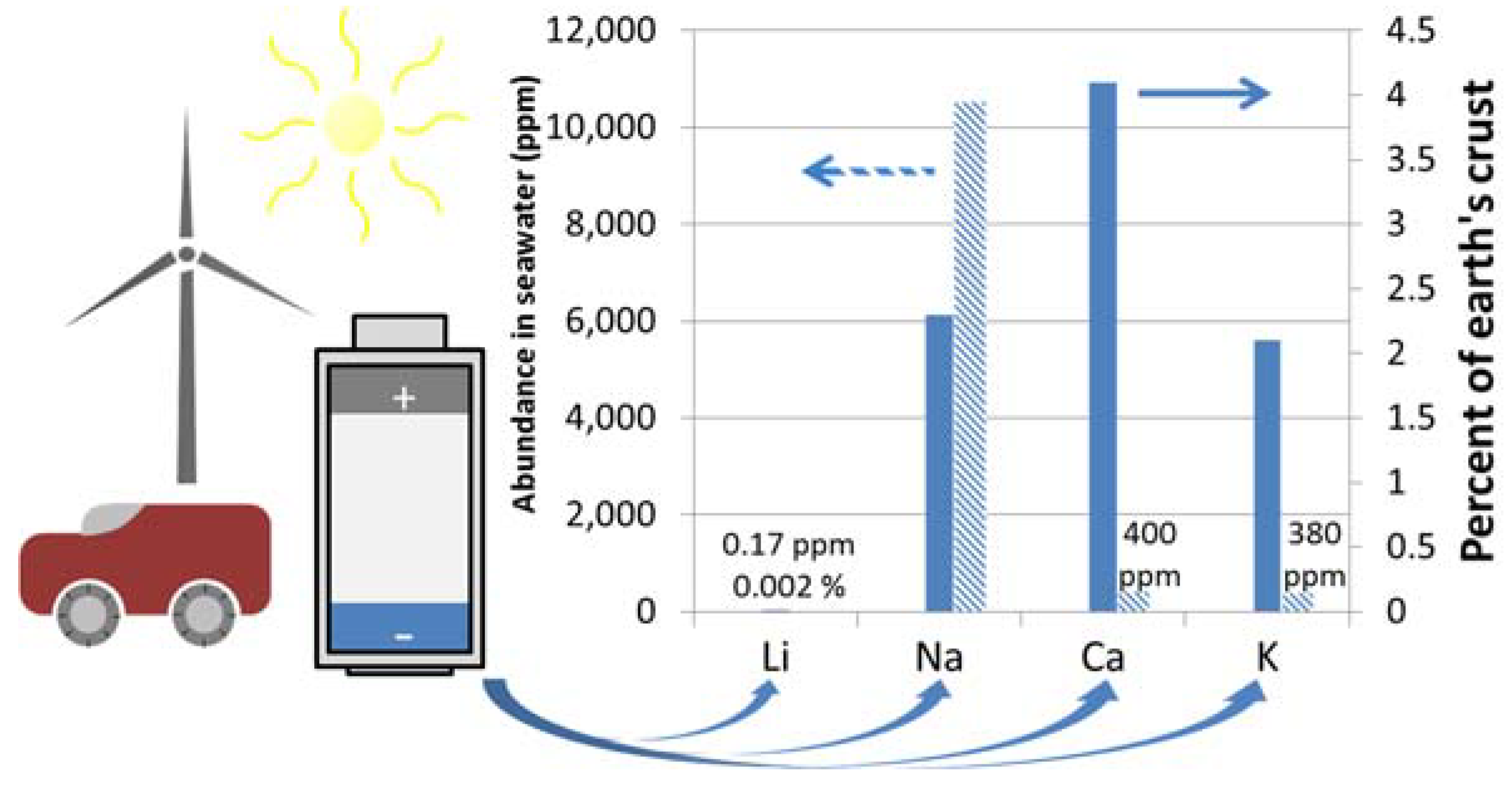
- Haynes, W.M. Abundance Of Elements In The Earth’s Crust And In The Sea. In CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 14–17. [Google Scholar]
- Duxbury, A.; Mackenzie, F.; Byrne, R. Seawater; Encyclopædia Britannica, Inc.: Chicago, IL, USA, 2017. [Google Scholar]
- Diederichsen, K.M.; McShane, E.J.; McCloskey, B.D. Promising Routes to a High Li+ Transference Number Electrolyte for Lithium Ion Batteries. ACS Energy Lett. 2017, 2, 2563–2575. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. The Importance of the Lithium Ion Transference Number in Lithium/Polymer Cells. Electrochim. Acta 1994, 39, 2073–2081. [Google Scholar] [CrossRef]
- Every, H.; Forsyth, M.; MacFarlane, D.R. Plasticized Single Conductung Polyelectrolytes Based on Poly(AMPS). Ionics 1996, 2, 53–62. [Google Scholar] [CrossRef]
- Doyle, M.; Lewittes, M.E.; Roelofs, M.G.; Perusich, S.A.; Lowrey, R.E. Relationship between Conductivity of Perfluorinated Ionomeric Membranes and Nonaqueous Solvent Properties. J. Membr. Sci. 2001, 184, 257–273. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Wohlfarth, A.; De Araujo, C.C.; Fuchs, A.; Maier, J. Single Alkaline-Ion (Li+, Na+) Conductors by Ion Exchange of Proton-Conducting Ionomers and Polyelectrolytes. Chem. Phys. Chem. 2011, 12, 2558–2560. [Google Scholar] [CrossRef]
- Pourcelly, G.; Oikonomou, A.; Gavach, C.; Hurwitz, H.D. Influence of the Water Content on the Kinetics of Counter-Ion Transport in Perfluorosulphonic Membranes. J. Electroanal. Chem. 1990, 287, 43–59. [Google Scholar] [CrossRef]
- Cao, C.; Wang, H.; Liu, W.; Liao, X.; Li, L. Nafion Membranes as Electrolyte and Separator for Sodium-Ion Battery. Int. J. Hydrog. Energy 2014, 39, 16110–16115. [Google Scholar] [CrossRef]
- Sanginov, E.A.; Kayumov, R.R.; Shmygleva, L.V.; Lesnichaya, V.A.; Karelin, A.I.; Dobrovolsky, Y.A. Study of the Transport of Alkali Metal Ions in a Nonaqueous Polymer Electrolyte Based on Nafion. Solid State Ion. 2017, 300, 26–31. [Google Scholar] [CrossRef]
- Cao, C.; Liu, W.; Tan, L.; Liao, X.; Li, L. Sodium-Ion Batteries Using Ion Exchange Membranes as Electrolytes and Separators. Chem. Commun. 2013, 49, 11740–11742. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Chai, J.; Liu, T.; Hu, R.; Zhang, Z.; Li, G.; Cui, G. A Novel Single-Ion Conducting Gel Polymer Electrolyte Based on Polymeric Sodium Tartaric Acid Borate for Elevated-Temperature Sodium Metal Batteries. Solid State Ion. 2019, 337, 140–146. [Google Scholar] [CrossRef]
- Pan, Q.; Li, Z.; Zhang, W.; Zeng, D.; Sun, Y.; Cheng, H. Single Ion Conducting Sodium Ion Batteries Enabled by a Sodium Ion Exchanged Poly(Bis(4-Carbonyl Benzene Sulfonyl)Imide-Co-2,5-Diamino Benzesulfonic Acid) Polymer Electrolyte. Solid State Ion. 2017, 300, 60–66. [Google Scholar] [CrossRef]
- Villaluenga, I.; Bogle, X.; Greenbaum, S.; Gil De Muro, I.; Rojo, T.; Armand, M. Cation Only Conduction in New Polymer-SiO2 Nanohybrids: Na + Electrolytes. J. Mater. Chem. A 2013, 1, 8348–8352. [Google Scholar] [CrossRef]
- Dou, S.; Zhang, S.; Klein, R.J.; Runt, J.; Colby, R.H. Synthesis and Characterization of Poly(Ethylene Glycol)-Based Single-Ion Conductors. Chem. Mater. 2006, 18, 4288–4295. [Google Scholar] [CrossRef]
- Benrabah, D.; Sylla, S.; Alloin, F.; Sanchez, J.Y.; Armand, M. Perfluorosulfonate-Polyether Based Single Ion Conductors. Electrochim. Acta 1995, 40, 2259–2264. [Google Scholar] [CrossRef]
- Yamazaki, K.; Tomoo, S. Single-Ion Conduction in UV-Crosslinked Films of Poly(Urethane-Co- (Alkali-Metal Methacrylates)). Int. J. Syst. Bacteriol. 1995, 40, 565–571. [Google Scholar]
- Elmore, C.T.; Seidler, M.E.; Ford, H.O.; Merrill, L.C.; Upadhyay, S.P.; Schneider, W.F.; Schaefer, J.L. Ion Transport in Solvent-Free, Crosslinked, Single-Ion Conducting Polymer Electrolytes for Post-Lithium Ion Batteries. Batteries 2018, 4, 28. [Google Scholar] [CrossRef]
- Wang, S.W.; Colby, R.H. Linear Viscoelasticity and Cation Conduction in Polyurethane Sulfonate Ionomers with Ions in the Soft Segment-Single Phase Systems. Macromolecules 2018, 51, 2757–2766. [Google Scholar] [CrossRef]
- Sinha, K.; Maranas, J. Does Ion Aggregation Impact Polymer Dynamics and Conductivity in PEO-Based Single Ion Conductors? Macromolecules 2014, 47, 2718–2726. [Google Scholar] [CrossRef]
- Doan, K.E.; Ratner, M.A.; Shriver, D.F. Synthesis and Electrical Response of Single-Ion Conducting Network Polymers Based on Sodium Poly(Tetraalkoxyaluminates). Chem. Mater. 1991, 3, 418–423. [Google Scholar] [CrossRef]
- Tsuchida, E.; Kobayashi, N.; Ohno, H. Single-Ion Conduction in Poly[(Oligo(Oxyethylene) Methacrylate)-Co-(Alkali-Metal Methacrylates)]. Macromolecules 1988, 21, 96–100. [Google Scholar] [CrossRef]
- Kim, H.T.; Park, J.K. Effects of Cations on Ionic States of Poly(Oligo-Oxyethylene Methacrylate-Co-Alkali Metal Acrylamidocaproate) Single-Ion Conductor. Solid State Ion. 1997, 98, 237–244. [Google Scholar] [CrossRef]
- Fumkawa, T.; Yoneya, K.; Takahashi, Y.; Ito, K.; Ohno, H. Correlation between Ionic and Dipolar Motions in a Single-Ion Conducting Polymer P[MEO9-MAM]. Electrochim. Acta 2000, 45, 1443–1448. [Google Scholar]
- Wang, J.H.H.; Yang, C.H.C.; Masser, H.; Shiau, H.S.; O’Reilly, M.V.; Winey, K.I.; Runt, J.; Painter, P.C.; Colby, R.H. Ion States and Transport in Styrenesulfonate Methacrylic PEO9 Random Copolymer Ionomers. Macromolecules 2015, 48, 7273–7285. [Google Scholar] [CrossRef]
- Rank, C.; Yan, L.; Mecking, S.; Winey, K.I. Periodic Polyethylene Sulfonates from Polyesterification: Bulk and Nanoparticle Morphologies and Ionic Conductivities. Macromolecules 2019, 52, 8466–8475. [Google Scholar] [CrossRef]
- Yan, L.; Rank, C.; Mecking, S.; Winey, K.I. Gyroid and Other Ordered Morphologies in Single-Ion Conducting Polymers and Their Impact on Ion Conductivity. J. Am. Chem. Soc. 2019. [Google Scholar] [CrossRef]
- Li, J.; Zhu, H.; Wang, X.; Armand, M.; Macfarlane, D.R.; Forsyth, M. Synthesis of Sodium Poly[4-Styrenesulfonyl(Trifluoromethylsulfonyl)Imide]-Co-Ethylacrylate] Solid Polymer Electrolytes. Electrochim. Acta 2015, 175, 232–239. [Google Scholar] [CrossRef]
- Noor, S.A.M.; Sun, J.; Macfarlane, D.R.; Armand, M.; Gunzelmann, D.; Forsyth, M. Decoupled Ion Conduction in Poly(2-Acrylamido-2-Methyl-1-Propane-Sulfonic Acid) Homopolymers. J. Mater. Chem. A 2014, 2, 17934–17943. [Google Scholar] [CrossRef]
- Yue, X.; He, Q.; Lim, H.D.; Liu, P. Hierarchical Structural Designs of Ion Exchange Membranes for Flow Batteries. J. Mater. Chem. A 2019, 7, 5794–5802. [Google Scholar] [CrossRef]
- Guo, D.; Wang, J.; Lei, J. Synthesis and Characterization of an Acrylate-Copolymer-Based Antistatic Agent Composed of a Single-Ion Conductive Polymer Electrolyte. J. Appl. Polym. Sci. 2011, 119, 2674–2682. [Google Scholar] [CrossRef]
- Wang, Y.P.; Feng, H.Y.; Fu, Z.S.; Tsuchida, E.; Takeoka, S.; Ohta, T. Ion Dissociation and Conduction of Nafion/Modified Oligo(Oxyethylene) Composite Films. Polym. Adv. Technol. 1991, 2, 295–299. [Google Scholar] [CrossRef]
- Ren, X.; Lau, K.C.; Yu, M.; Bi, X.; Kreidler, E.; Curtiss, L.A.; Wu, Y. Understanding Side Reactions in K-O2 Batteries for Improved Cycle Life. ACS Appl. Mater. Interfaces 2014, 6, 19299–19307. [Google Scholar] [CrossRef] [PubMed]
- Genier, F.S.; Burdin, C.V.; Biria, S.; Hosein, I.D. A Novel Calcium-Ion Solid Polymer Electrolyte Based on Crosslinked Poly(Ethylene Glycol) Diacrylate. J. Power Sources 2019, 414, 302–307. [Google Scholar] [CrossRef]
- Wang, J.; Genier, F.S.; Li, H.; Biria, S.; Hosein, I.D. A Solid Polymer Electrolyte from Cross-Linked Polytetrahydrofuran for Calcium Ion Conduction. ACS Appl. Polym. Mater. 2019, 1, 1837–1844. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-Ion BAB Triblock Copolymers as Highly Efficient Electrolytes for Lithium-Metal Batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Ramanujapuram, A.; Gordon, D.; Magasinski, A.; Ward, B.; Nitta, N.; Huang, C.; Yushin, G. Degradation and Stabilization of Lithium Cobalt Oxide in Aqueous Electrolytes. Energy Environ. Sci. 2016, 9, 1841–1848. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Passerini, S. Non-Aqueous Potassium-Ion Batteries: A Review. Curr. Opin. Electrochem. 2018, 9, 41–48. [Google Scholar] [CrossRef]
- Arroyo-De Dompablo, M.E.; Ponrouch, A.; Johansson, P.; Palacín, M.R. Achievements, Challenges, and Prospects of Calcium Batteries. Chem. Rev. 2019. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Recent Progress in Rechargeable Potassium Batteries. Adv. Funct. Mater. 2018, 28, 1802938. [Google Scholar] [CrossRef]
- Che, H.; Chen, S.; Xie, Y.; Wang, H.; Amine, K.; Liao, X.Z.; Ma, Z.F. Electrolyte Design Strategies and Research Progress for Room-Temperature Sodium-Ion Batteries. Energy Environ. Sci. 2017, 10, 1075–1101. [Google Scholar] [CrossRef]
- Bommier, C.; Ji, X. Electrolytes, SEI Formation, and Binders: A Review of Nonelectrode Factors for Sodium-Ion Battery Anodes. Small 2018, 14, 1703576. [Google Scholar] [CrossRef]
- Chen, L.; Fiore, M.; Wang, J.E.; Ruffo, R.; Kim, D.-K.; Longoni, G. Readiness Level of Sodium-Ion Battery Technology: A Materials Review. Adv. Sustain. Syst. 2018, 2, 1700153. [Google Scholar] [CrossRef]
- Merrill, L.C.; Ford, H.O.; Schaefer, J.L. Application of Single-Ion Conducting Gel Polymer Electrolytes in Magnesium Batteries. ACS Appl. Energy Mater. 2019, 2, 6355–6363. [Google Scholar] [CrossRef]
- Ford, H.O.; Merrill, L.C.; He, P.; Upadhyay, S.P.; Schaefer, J.L. Cross-Linked Ionomer Gel Separators for Polysulfide Shuttle Mitigation in Magnesium-Sulfur Batteries: Elucidation of Structure-Property Relationships. Macromolecules 2018, 51, 8629–8636. [Google Scholar] [CrossRef]
- Ford, H.O.; Park, B.; Jiang, J.; Seidler, M.E.; Schaefer, J.L. Enhanced Li + Conduction within Single-Ion Conducting Polymer Gel Electrolytes via Reduced Cation- Polymer Interaction. ACS Materials Lett. 2020. [Google Scholar] [CrossRef]
- Klein, R.J.; Zhang, S.; Dou, S.; Jones, B.H.; Colby, R.H.; Runt, J. Modeling Electrode Polarization in Dielectric Spectroscopy: Ion Mobility and Mobile Ion Concentration of Single-Ion Polymer Electrolytes. J. Chem. Phys. 2006, 124, 144903. [Google Scholar] [CrossRef]
- Seki, S.; Susan, M.A.B.H.; Kaneko, T.; Tokuda, H.; Noda, A.; Watanabe, M. Distinct Difference in Ionic Transport Behavior in Polymer Electrolytes Depending on the Matrix Polymers and Incorporated Salts. J. Phys. Chem. B 2005, 109, 3886–3892. [Google Scholar] [CrossRef]
- Petrowsky, M.; Frech, R. Temperature Dependence of Ion Transport: The Compensated Arrhenius Equation. J. Phys. Chem. B 2009, 113, 5996–6000. [Google Scholar] [CrossRef]
- Marcus, Y. Ionic Radii in Aqueous Solutions. Chem. Rev. 1988, 88, 1475–1498. [Google Scholar] [CrossRef]
- Mähler, J.; Persson, I. A Study of the Hydration of the Alkali Metal Ions in Aqueous Solution. Inorg. Chem. 2012, 51, 425–438. [Google Scholar] [CrossRef]
- Hall, L.M.; Seitz, M.E.; Winey, K.I.; Opper, K.L.; Wagener, K.B.; Stevens, M.J.; Frischknecht, A.L. Ionic Aggregate Structure in Ionomer Melts: Effect of Molecular Architecture on Aggregates and the Ionomer Peak. J. Am. Chem. Soc. 2012, 134, 574–587. [Google Scholar] [CrossRef]
- Zheng, Q.; Pesko, D.M.; Savoie, B.M.; Timachova, K.; Hasan, A.L.; Smith, M.C.; Miller, T.F.; Coates, G.W.; Balsara, N.P. Optimizing Ion Transport in Polyether-Based Electrolytes for Lithium Batteries. Macromolecules 2018, 51, 2847–2858. [Google Scholar] [CrossRef]
- Pesko, D.M.; Webb, M.A.; Jung, Y.; Zheng, Q.; Miller, T.F.; Coates, G.W.; Balsara, N.P. Universal Relationship between Conductivity and Solvation-Site Connectivity in Ether-Based Polymer Electrolytes. Macromolecules 2016, 49, 5244–5255. [Google Scholar] [CrossRef]
- Webb, M.A.; Jung, Y.; Pesko, D.M.; Savoie, B.M.; Yamamoto, U.; Coates, G.W.; Balsara, N.P.; Wang, Z.G.; Miller, T.F. Systematic Computational and Experimental Investigation of Lithium-Ion Transport Mechanisms in Polyester-Based Polymer Electrolytes. ACS Cent. Sci. 2015, 1, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Yarusso, D.J.; Cooper, S.L. Microstructure of Ionomers: Interpretation of Small-Angle X-Ray Scattering Data. Macromolecules 1983, 16, 1871–1880. [Google Scholar] [CrossRef]
- Li, Y.; Peiffer, D.G.; Chu, B. Long-Range Inhomogeneities in Sulfonated Polystyrene Ionomers. Macromolecules 1993, 26, 4006–4012. [Google Scholar] [CrossRef]
- Elliott, J.A.; Hanna, S.; Elliott, A.M.S.; Cooley, G.E. Interpretation of the Small-Angle X-Ray Scattering from Swollen and Oriented Perfluorinated Ionomer Membranes. Macromolecules 2000, 33, 4161–4171. [Google Scholar] [CrossRef]
- Gebel, G.; Diat, O. Neutron and X-Ray Scattering: Suitable Tools for Studying Lonomer Membranes. Fuel Cells 2005, 5, 261–276. [Google Scholar] [CrossRef]
- Hamley, I.W.; Castelletto, V. Small-Angle Scattering of Block Copolymers in the Melt, Solution and Crystal States. Prog. Polym. Sci. 2004, 29, 909–948. [Google Scholar]
- Meziane, R.; Bonnet, J.P.; Courty, M.; Djellab, K.; Armand, M. Single-Ion Polymer Electrolytes Based on a Delocalized Polyanion for Lithium Batteries. Electrochim. Acta 2011, 57, 14–19. [Google Scholar] [CrossRef]

| Chemistry | Conductivity (S/cm) | Solvent | Ref |
|---|---|---|---|
| Poly(tetrahydrofuran) diacrylate-x-4-styrenesulfonyl (trifluoromethylsulfonyl) imide (PTHFDA-STFSI) sodium exchanged | 2.4 × 10−5 (25 °C) | DEG | This work |
| 1.3 × 10−5 (25 °C) | TEG | ||
| 3.9 × 10−4 (25 °C) | EC:PC | ||
| 5.9 × 10−12 (25 °C) | Dry | ||
| Poly(2-acrylamido-2-methyl-propane- 1-sulphonic acid) (PAMPS) | 10−2 (20 °C) * | 50H2O/SO3 | [5] |
| 10−5 (20 °C) * | Dimethyl Sulfoxide (DMSO) | ||
| PFSA (Nafion 117) sodium exchanged | 1.8 × 10−2 (23 °C) | Water | [6] |
| 3.2 × 10−4 (23 °C) | Triethyl phosphate | ||
| 3.8 × 10−3 (23 °C) | N-methyl Formamide | ||
| 5.1 × 10−4 (23 °C) | g-butyrolactone | ||
| 5.0 ×10−4(23 °C) | DMSO | ||
| Fully sulfonated poly phenylene-sulfone | 1 × 10−3 (25 °C) * | DMSO | [7] |
| 2 × 10−3 (70 °C) * | |||
| PFSA (Nafion 117) sodium exchanged | 10−3 (RT) * | 14H2O/SO3 | [8] |
| 10−6 (RT) * | 6H2O/SO3 | ||
| PFSA (Nafion 115) sodium exchanged | 3.52 × 10−4 (RT) | EC/PC 1:1 | [9] |
| 1.52 × 10−3 (70 °C) | |||
| PFSA (Nafion 115) sodium exchanged | 3.5 × 10−4 (30 °C) | DMSO | [10] |
| Perfluorinated sulfonic sodium membrane (PFSA) Nafion-sodium exchanged | 2.5 × 10−4 (25 °C) | EC/PC 1:1 | [11] |
| 1.5 × 10−3 (70 °C) | |||
| 1.5 × 10−4 (25 °C) | |||
| 1.1 × 10−3 (70 °C) | |||
| Poly(sodium tartaric acid borate) blend poly(vinylene carbonate) | 1 × 10−4 (RT) * | EC/DEC 1:1 | [12] |
| poly(bis(4-carbonyl benzene sulfonyl)imide-co-2.5-diamino benzesulfonic acid) (NaPA) + PVDF/HFP | 0.91 × 10−4 (20 °C) | EC/DMC 1:1 | [13] |
| 4.1 × 10−4 (80 °C) | |||
| SiO2 particles grafted with Poly(ethylene glycol) (PEG) and NaSTFSI dispersed in PEO | 10−5 (RT) * | Poly(ethylene glycol) dimethyl ether (PEGDME) 250 g/mol | [14] |
| 10−4 (75 °C) * | |||
| PEG900 and dimethyl 5-sulfoisophthalate sodium salt | 1.07 × 10−6 (25 °C) | Dry | [15] |
| N,N-Diallyl-l-amido-tetrafluoroethanesulfonate (DaaRFSO3Na) | 4 × 10−7 (25 °C) * | Dry | [16] |
| Poly(ethylene glycol-co-2,4-tolyldiisocyanate-co-(alkali-metal methacrylates)) (PEG-PU-AMMA) | 3 × 10−7 (25 °C) * | Dry | [17] |
| 9 × 10−6 (80 °C) * | |||
| Poly(ethylene glycol) diacrylate (PEGDA, 1500 g/mol)-co-STFSI Na | 2.0 × 10−7 (25 °C) | Dry | [18] |
| 5.0 × 10−6 (85 °C) | |||
| Polyurethane sulfonate | 10−7 (25 °C) * | Dry | [19] |
| PEO-co-benzenesulfonate | 10−7 (25 °C) * | Dry | [20] |
| Network Polymers Based on Sodium Poly(tetraalkoxyaluminates) with Poly(ethylene oxide) (PEO) blend | 10−7 (50 °C) * | Dry | [21] |
| 10−6 (80 °C) * | |||
| Poly[ (oligo(oxyethylene) methacrylate)-co-(alkali-metal methacrylates)] | 8 × 10−8 (25 °C) * | Dry | [22] |
| Poly(oligo-oxyethylenemethacrylate-co-alkali metal acrylamidocaproate) | 6.9 × 10−8 (RT) | Dry | [23] |
| Nona-oxyethylene Methacrylate-co-alkali-metal methacrylate | 10−8 (25 °C) * | Dry | [24] |
| Methacrylic PEO-co-sodium stytene sulfonate (PEO9M-r-NaSS) | 10−8 (RT) * | Dry | [25] |
| 10−7 (80 °C)* | |||
| Polyethylene-like 48 carbon sodium dimethylsulfosuccinate (PES48Na) Precise ionomer | 10−10 (100 °C) * | Dry | [26] |
| 10−6 (160 °C) * | |||
| Polyethylene-like sulfonated precise ionomer–gyroid phase | 10−11 (80 °C) * | Dry | [27] |
| 10−7 (120 °C) * | |||
| Sodium Poly(4-styrenesulfonyl (trifluoromethylsulfonyl)imide) (NaPSTFSI) NaPSTFSI Poly ethyl acrylate blends | 10−14 to 10−11 (40 °C) * | Dry | [28] |
| 10−12 to 10−9 (40 °C) * | Dry | ||
| Poly(2-acrylamido-2-methyl-1-propane-sulfonic acid sodium salt) homopolymer | 10−11 (130 °C) * | Dry | [29] |
| Chemistry | Conductivity (S/cm) | Solvent | Ref |
|---|---|---|---|
| Poly(tetrahydrofuran) diacrylate-x-4-styrenesulfonyl (trifluoromethylsulfonyl) imide (PTHFDA-STFSI) potassium exchanged | 4.0 × 10−5 (25 °C) | DEG | This work |
| 1.8 × 10−5 (25 °C) | TEG | ||
| 2.7 × 10−4 (25 °C) | EC:PC | ||
| 2.4 × 10−12 (25 °C) | Dry | ||
| PFSA (Nafion 117) potassium exchanged | 1.4 × 10−2 (23 °C) | Water | [6] |
| 1.4 × 10−4 (23 °C) | Triethyl phosphate | ||
| 4.1 × 10−3 (23 °C) | N-methylFormamide | ||
| 2.3 × 10−5 (23 °C) | g-butyrolactone | ||
| 8.0 × 10−4 (23 °C) | DMSO | ||
| PFSA (Nafion 117) potassium exchanged | 8.0 × 10−3 (RT) | Water, fully hydrated | [30] |
| PFSA (Nafion 117) potassium exchanged | 10−3 (RT) * | 9H2O/SO3 | [8] |
| 10−6 (RT) * | 6H2O/SO3 | ||
| PFSA (Nafion 115) potassium exchanged | 3.3 × 10−4 (30 °C) | DMSO | [10] |
| Acrylic acid and methoxy poly(ethylene glycol)s coordinating K+ | 6 × 10−6 (30 °C) * | 10% RH | [31] |
| N,N-Diallyl-l-amido-tetrafluoroethanesulfonate (DaaRFSO3K) | 3 × 10−6 (25 °C) * | Dry | [16] |
| PFSA (Nafion) potassium exchanged | 10−6 (100 °C) * | Dry | [32] |
| Poly(ethylene glycol)-co-2,4-tolyl diisocyanate-co-(alkali-metal methacrylates))(PEG-PU-AMMA) | 6 × 10−7 (25 °C) * | Dry | [17] |
| 1 × 10−5 (80 °C) * | |||
| Poly(oligo-oxyethylenemethacrylate-co-alkali metal acrylamidocaproate) | 2 × 10−7 (RT) * | Dry | [23] |
| Poly(oligo(oxyethylene) methacrylate)-co-(alkali-metal methacrylates) | 2 × 10−7 (25 °C) * | Dry | [22] |
| Nona-oxyethylene methacrylate and alkali-metal methacrylate-K | 10−7 (25 °C) * | Dry | [24] |
| PEGDA(1500 g/mol)-co-STFSI K | 9 × 10−8 (25 °C) | Dry | [18] |
| 5 × 10−6 (85 °C) | |||
| PFSA (Nafion) 211 potassium exchanged | Unreported | Water | [33] |
| Chemistry | Conductivity (S/cm) | Solvent | Ref |
|---|---|---|---|
| Poly(tetrahydrofuran) diacrylate-x-4-styrenesulfonyl (trifluoromethylsulfonyl) imide (PTHFDA-STFSI) calcium exchanged | 6.2 × 10−7 (25 °C) | DEG | This work |
| 1.0 × 10−7 (25 °C) | TEG | ||
| 7.0 × 10−6 (25 °C) | EC:PC | ||
| 3.5 × 10−13 (85 °C) | Dry | ||
| PFSA (Nafion 117) calcium exchanged | 9.1 × 10−3 (23 °C) | Water | [6] |
| 1.0 × 10−6 (23 °C) | Triethyl phosphate | ||
| 2.7 × 10−3 (23 °C) | N-methyl Formamide | ||
| 1.1 × 10−5 (23 °C) | g-butyrolactone | ||
| 3.5 × 10−4(23 °C) | DMSO | ||
| PFSA (Nafion 117) calcium exchanged | 10−3 (RT) * | 14H2O/SO3 | [8] |
| 10−6 (RT) * | 8H2O/SO3 | ||
| PEGDA 1500 g/mol -co-STFSI Ca | 2.0 × 10−9 (25 °C) | Dry | [18] |
| 9.0 × 10−8 (85 °C) | |||
| PEGDA 575 g/mol + Ca(NO3)2 4H2O | 5 × 10−6 (25 °C) * | Dry | [34] ‡ |
| Poly(tetrahydrofuran) (PTHF)-Epoxy + Ca(NO3)2 | 2 × 10−4 (25 °C) * | Dry | [35] ‡ |
| Sample | Cation | Charge Density × 104 (mol ch/g Monomer) | Solvent | Conductivity (S/cm) 25 °C | Conductivity (S/cm) 85 °C |
|---|---|---|---|---|---|
| PEGDMA1000-SS | K | 8 (low) | DEG | 1.2 × 10‒7 | 1.8 × 10‒6 |
| TEG | 6.4 × 10‒7 | 7.1 × 10‒6 | |||
| EC:PC | 4.8 × 10‒6 | 8.0 × 10‒5 | |||
| Dry | 1.4 × 10‒9 | 2.6 × 10‒7 | |||
| 12 (med) | DEG | 1.7 × 10‒8 | 8.2 × 10‒7 | ||
| TEG | 1.5 × 10‒7 | 6.1 × 10‒6 | |||
| EC:PC | 4.2 × 10‒6 | 8.1 × 10‒5 | |||
| Dry | 2.4 × 10‒10 | 2.7 × 10‒7 | |||
| 16 (high) | DEG | 1.3 × 10‒8 | 9.9 × 10‒7 | ||
| TEG | 1.1 × 10‒6 | ||||
| EC:PC | 5.7 × 10‒6 | 1.0 × 10‒4 | |||
| Dry | 1.5 × 10‒7 | ||||
| PEGDMA1000-SS | Na | 8 (low) | DEG | 1.1 × 10‒7 | 1.1 × 10‒7 |
| TEG | 1.8 × 10‒7 | 3.2 × 10‒6 | |||
| EC:PC | 2.5 × 10‒6 | 4.6 × 10‒5 | |||
| Dry | 1.8 × 10‒9 | 2.2 × 10‒7 | |||
| 12 (med) | DEG | 1.1 × 10‒7 | 1.1 × 10‒6 | ||
| TEG | 7.9 × 10‒8 | 1.3 × 10‒6 | |||
| EC:PC | 3.8 × 10‒6 | 5.8 × 10‒5 | |||
| Dry | 2.7 × 10‒11 | 1.4 × 10‒8 | |||
| 16 (high) | DEG | 4.4 × 10‒8 | 5.0 × 10‒7 | ||
| TEG | 5.3 × 10‒9 | 2.9 × 10‒7 | |||
| EC:PC | 4.7 × 10‒6 | 5.1 × 10‒5 | |||
| Dry | 4.4 × 10‒8 | ||||
| PEGDMA1000-SS | Ca | 8 (low) | DEG | 2.5 × 10‒9 | 2.0 × 10‒8 |
| TEG | 4.3 × 10‒9 | 4.4 × 10‒8 | |||
| EC:PC | 1.9 × 10‒6 | 1.2 × 10−5 | |||
| Dry | 2.8 × 10−10 | ||||
| 12 (med) | DEG | 4.3 × 10‒11 | 9.2 × 10−10 | ||
| TEG | 1.2 × 10‒11 | 1.2 × 10−9 | |||
| EC:PC | 2.9 × 10‒7 | 2.1 × 10−6 | |||
| Dry | 2.0 × 10−11 | ||||
| 16 (high) | DEG | 1.3 × 10−11 | |||
| TEG | 8.5 × 10‒12 | 2.5 × 10−10 | |||
| EC:PC | 6.7 × 10−8 | 6.5 × 10−7 | |||
| Dry | 2.8 × 10−13 (100 °C) | ||||
| PTHFDA-STFSI | K | 7 (low) | DEG | 1.8 × 10−5 | 3.4 × 10−5 |
| TEG | 7.3 × 10−6 | 2.9 × 10−5 | |||
| EC:PC | 8.5 × 10−5 | 3.6 × 10−4 | |||
| Dry | 1.4 × 10−12 | 1.6 × 10−8 | |||
| 10 (med) | DEG | 1.6 × 10−5 | 3.4 × 10−5 | ||
| TEG | 1.2 × 10−5 | 5.4 × 10−5 | |||
| EC:PC | 1.2 × 10−4 | 4.7 × 10−4 | |||
| Dry | 1.1 × 10−8 | ||||
| 13 (high) | DEG | 4.0 × 10−5 | 9.8 × 10−5 | ||
| TEG | 1.8 × 10−5 | 8.3 × 10−5 | |||
| EC:PC | 2.7 × 10−4 | 9.7 × 10−4 | |||
| Dry | 2.4 × 10−12 | 3.4 × 10−8 | |||
| PTHFDA-STFSI | Na | 7 (low) | DEG | 1.7 × 10−5 | 3.5 × 10−5 |
| TEG | 6.4 × 10−6 | 2.4 × 10−6 | |||
| EC:PC | 1.0 × 10−4 | 5.0 × 10−4 | |||
| Dry | 1.3 × 10−11 | 3.0 × 10−8 | |||
| 10 (med) | DEG | 2.8 × 10−5 | 6.3 × 10−5 | ||
| TEG | 1.1 × 10−5 | 4.1 × 10−5 | |||
| EC:PC | 9.8 × 10−5 | 3.6 × 10−4 | |||
| Dry | 4.6 × 10−12 | 8.0 × 10−9 | |||
| 13 (high) | DEG | 2.5 × 10−5 | 4.6 × 10−5 | ||
| TEG | 1.3 × 10−5 | 6.0 × 10−5 | |||
| EC:PC | 3.9 × 10−4 | 1.4 × 10−3 | |||
| Dry | 5.9 × 10−12 | 3.6 × 10−8 | |||
| PTHFDA-STFSI | Ca | 7 (low) | DEG | 3.5 × 10−8 | 1.1 × 10−7 |
| TEG | 3.7 × 10−8 | 1.5 × 10−7 | |||
| EC:PC | 6.8 × 10−7 | 8.5 × 10−6 | |||
| Dry | 3.3 × 10−11 | ||||
| 10 (med) | DEG | 1.9 × 10−8 | 7.3 × 10−8 | ||
| TEG | 2.3 × 10−8 | 1.2 × 10−7 | |||
| EC:PC | 2.9 × 10−6 | 2.6 × 10−5 | |||
| Dry | 2.1 × 10−11 | ||||
| 13 (high) | DEG | 6.2 × 10−8 | 3.1 × 10−7 | ||
| TEG | 1.0 × 10−7 | 3.7 × 10−7 | |||
| EC:PC | 7.0 × 10−6 | 5.1 × 10−5 | |||
| Dry | 3.5 × 10−13 |
| Sample | Solvent | Mass Change % | Volume Change % | Cation Conc. (mol Charge/cm3 Polymer) |
|---|---|---|---|---|
| PEGDMA1000_med_SSNa | DEG | 24 ± 3.6 | 24 ± 2.6 | 0.00121 |
| PTHFDA1000_med_STFSINa | DEG | 164 ± 14 | 175 ± 2.3 | 0.00043 |
| PEGDMA1000_med_SSNa | TEG | 21 ± 5.7 | 23 ± 4.3 | 0.00122 |
| PTHFDA1000_med_STFSINa | TEG | 142 ± 7.8 | 157 ± 4.8 | 0.00048 |
| PEGDMA1000_med_SSNa | EC:PC | 97 ± 9.1 | 91 ± 4.9 | 0.00082 |
| PTHFDA1000_med_STFSINa | EC:PC | 169 ± 14.9 | 135 ± 19.8 | 0.00057 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ford, H.O.; Cui, C.; Schaefer, J.L. Comparison of Single-Ion Conducting Polymer Gel Electrolytes for Sodium, Potassium, and Calcium Batteries: Influence of Polymer Chemistry, Cation Identity, Charge Density, and Solvent on Conductivity. Batteries 2020, 6, 11. https://doi.org/10.3390/batteries6010011
Ford HO, Cui C, Schaefer JL. Comparison of Single-Ion Conducting Polymer Gel Electrolytes for Sodium, Potassium, and Calcium Batteries: Influence of Polymer Chemistry, Cation Identity, Charge Density, and Solvent on Conductivity. Batteries. 2020; 6(1):11. https://doi.org/10.3390/batteries6010011
Chicago/Turabian StyleFord, Hunter O., Chuanchuan Cui, and Jennifer L. Schaefer. 2020. "Comparison of Single-Ion Conducting Polymer Gel Electrolytes for Sodium, Potassium, and Calcium Batteries: Influence of Polymer Chemistry, Cation Identity, Charge Density, and Solvent on Conductivity" Batteries 6, no. 1: 11. https://doi.org/10.3390/batteries6010011
APA StyleFord, H. O., Cui, C., & Schaefer, J. L. (2020). Comparison of Single-Ion Conducting Polymer Gel Electrolytes for Sodium, Potassium, and Calcium Batteries: Influence of Polymer Chemistry, Cation Identity, Charge Density, and Solvent on Conductivity. Batteries, 6(1), 11. https://doi.org/10.3390/batteries6010011





